Administración de heparina subcutánea lenta versus rápida para la prevención de la equimosis y la intensidad del dolor en el sitio de inyección
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Design: randomised and self‐controlled trial | |
| Participants | Baseline: 150 participants ‐ 102 female and 48 male; mean age of participants was 74.8 (SD 12.37) years Setting: patients who were hospitalised in the 2 orthopaedic units of a teaching hospital in northern Italy Country: Italy Injection site: left or right side of lower abdomen Injection protocol: needle gauge 27.5, 5/8 inch, syringe volume 0.4 mL, enoxaparin 4000 IU Inclusion criteria: received SC injection of LMWH, were monitored for at least 3 days after first injection Exclusion criteria: already received SC heparin injection; had haematological, cardiologic, or liver disease; were pregnant; were taking oral anticoagulant or antiaggregate drugs; had altered integrity of abdominal skin | |
| Interventions | Slow injection (30 seconds) vs fast injection of heparin (10 seconds) Time between 2 injections was 24 hr. | |
| Outcomes | Extent of injection site bruising was evaluated at 48 hr after each injection with a plastic ruler to measure maximum horizontal diameter of bruise recorded as mm | |
| Notes | Funding sources: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was reported but how sequence was generated was not described. Quote: "the order of the treatment (A or B) was randomly selected." The CIS contacted trial authors to ask for information about randomisation method; trial authors stated that random sequence was generated by computer. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel blinding was impossible in this trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The decision (treatment A, 10 seconds left hypochondrium; treatment B, 30 seconds right hypochondrium) was written in a paper and placed in an envelope and kept in a locked safe." |
| Incomplete outcome data (attrition bias) | Low risk | Trial authors reported no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No potential other bias was identified. |
| Methods | Randomised parallel controlled trial | |
| Participants | Baseline: 60 participants were divided into 3 groups. We reported the results of 20 participants in the 10‐second heparin injection group and 20 participants in the 30‐second heparin injection group ‐ 13 male and 27 female; mean age in the intervention group was 62.7 (SD 8.83) years and in the control group 57.9 (SD 12.5) years. Setting: patients who were hospitalised in the orthopedic wards of a university hospital in Turkey Country: Turkey Injection site: LMWH was injected SC into the tissue of the lower abdominal wall (umbilical region) Injection protocol: insertion angle 90°, grasping of tissue at injection site, injection without drug aspiration Inclusion criteria: 18 years of age or older, received SC injections of LMWH once a day, had normal platelet values, were conscious, did not have any complications in the perioperative days, did not have acute painful disease Exclusion criteria: were pregnant, had abnormalities of coagulation or haematologic and allergic diseases, received any other injections at the abdominal site during the days of the research, had any incision or scar tissue at the abdominal site | |
| Interventions | Slow injection (30 seconds) vs fast injection of heparin (10 seconds) | |
| Outcomes | Site pain intensity was assessed by VAS (0 to 100 mm) immediately, and at 48, 60, and 72 hr after injection. Injection site bruising was evaluated at 48, 60, and 72 hr after each injection with a transparent mm ruler to measure the surface area of the bruise and record it as mm2. | |
| Notes | Study authors were contacted about any use of anticoagulant drugs; they reported that participants were excluded if participants took any anticoagulant drugs before the start of the study. Funding sources: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerized randomisation program was used to allocate the patients." |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "non‐blinded study design was used." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "non‐blinded study design was used." |
| Incomplete outcome data (attrition bias) | High risk | 6.25% dropout after randomisation in the intervention group, as 4 participants were hospitalised in intensive care unit after randomisation. Use of intention‐to‐treat analysis was not reported. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Other aspects of heparin injection (e.g. heparin temperature, syringe size, injection volume, air bubble in the syringe) were not clearly described. This may have affected study outcomes. |
| Methods | Design: randomised self‐controlled trial | |
| Participants | Baseline: 50 participants ‐ 25 male and 25 female; mean age of participants was 55.25 (SD 12.37) years Setting: patients who were hospitalised in the neurology, orthopaedics, and cardiology units of a university hospital in Izmir, Turkey Country: Turkey Injection site: right or left side of abdomen Injection protocol: insertion angle 90°, grasping the tissue of the injection site, injection without drug aspiration Inclusion criteria: received SC injection of LMWH, were conscious, platelet values were within normal limits before the trial started Exclusion criteria: were pregnant; had haematological disease, abnormal coagulation, or any allergic disease; received any other injections at the abdominal site during the trial, had any incision or scar tissue at the abdominal site | |
| Interventions | Slow injection (30 seconds) versus fast injection of heparin (10 seconds) Time between 2 injections was 12 hr. | |
| Outcomes | Site pain intensity was assessed by VAS (0 to 100 mm) immediately after injection. Injection site bruising was evaluated at 48 and 72 hr after each injection with millimetric measuring paper to measure area of the bruise recorded as mm2. | |
| Notes | Study authors were contacted, but no response was received. Funding sources: Research Foundation of Ege University, Izmir, Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was reported but how sequence was generated was not described. Quote: "Each of participant randomised into intervention or control group according to treatment order." |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel was impossible in this trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Another rater who was blind to the research operated the stop‐watch to determine the pain period." Assessor blinding for measurement of bruise was not reported. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts or losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Other aspects of heparin injection (e.g. heparin temperature, syringe size, needle gauge, injection volume, air bubble in the syringe) were not clearly described. This may have affected outcomes. |
| Methods | Design: RCT, factorial design | |
| Participants | Baseline: 60 participants were divided into 6 groups. We reported the results of 9 participants in the 10‐second heparin injection group and 10 participants in the 30‐second heparin injection group ‐ 13 male and 6 female; mean age in the intervention group was 61.5 (SD 11.5) years and in the control group 61.6 (SD 6.5) years. Setting: patients who were hospitalised in the cardiology unit of a university hospital in China Country: China Injection site: about 5 cm up and down the navel Injection protocol: injection without drug aspiration Inclusion criteria: received SC injection of enoxaparin sodium 4100 IU; were conscious; platelet values were within the limits of 100,000 to 300,000/dL; activated partial thromboplastin time (APTT) in the reference range 25 to 37.5 seconds; no large abdominal skin bruising, induration, or skin disease; not taking antiplatelet drugs such as aspirin or clopidogrel before the start of the study | |
| Interventions | Slow injection (30‐second injection) as intervention vs fast injection of heparin (10‐second injection) as control | |
| Outcomes | Site pain intensity was assessed by VAS (0 to 100 mm) immediately after each injection. Extent of injection site bruising was evaluated at 48 hr after injection with ruler to measure the maximum diameter of bruise recorded as mm. | |
| Notes | Funding sources: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel was impossible in this trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Researcher who evaluated outcomes was blinded to the group. |
| Incomplete outcome data (attrition bias) | High risk | One participant in the control group (10%) was lost to follow‐up. Study did not report intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Other aspects of heparin injection (e.g. heparin temperature, syringe size, needle gauge, injection volume, air bubble in the syringe) were not clearly described. This may have affected outcomes. |
APTT: activated partial thromboplastin time.
CIS: Cochrane Vascular Information Specialist.
cm: centimetres.
hr: hours.
IU: international units.
LMWH: low molecular weight heparin.
mm: millimetres.
RCT: randomised controlled trial.
SC: subcutaneous.
SD: standard deviation.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Intervention: comparing 4 different techniques of heparin injection | |
| Quasi‐experimental design | |
| Quasi‐experimental design | |
| Quasi‐experimental design | |
| Participants used anticoagulant drugs during the study | |
| Quasi‐randomised design | |
| Quasi‐randomised design | |
| Non‐random design | |
| Intervention: comparing 10‐second heparin injection vs 30‐second heparin injection plus air lock | |
| Quasi‐experimental design | |
| Intervention: comparing 4 administration techniques of heparin injection | |
| Intervention: comparing 2 administration techniques of heparin injection | |
| Non‐random design | |
| Intervention: comparing 4 administration techniques of heparin injection with speeds of less than 20 seconds | |
| Participants used anticoagulant drugs during the study | |
| Intervention: comparing 10‐second injection and waiting for 10 seconds before withdrawal of the needle vs 10‐second injection | |
| Quasi‐experimental design | |
| Quasi‐experimental design | |
| Non‐random design | |
| Intervention: comparing 3 different techniques of heparin injection | |
| Intervention: comparing 2 administration techniques of heparin injection |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intensity of injection pain Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 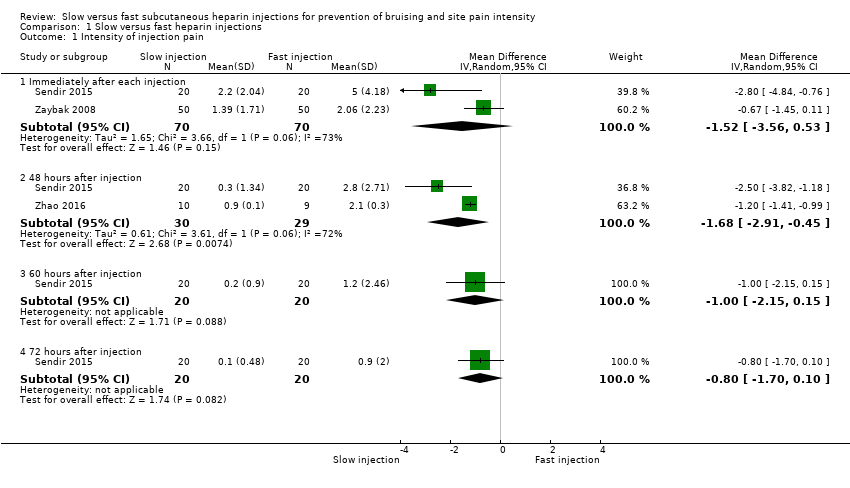 Comparison 1 Slow versus fast heparin injections, Outcome 1 Intensity of injection pain. | ||||
| 1.1 Immediately after each injection | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.56, 0.53] |
| 1.2 48 hours after injection | 2 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.91, ‐0.45] |
| 1.3 60 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.15, 0.15] |
| 1.4 72 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.8 [‐1.70, 0.10] |
| 2 Bruise size 48 hours after injection Show forest plot | 4 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.24, 0.04] |
| Analysis 1.2 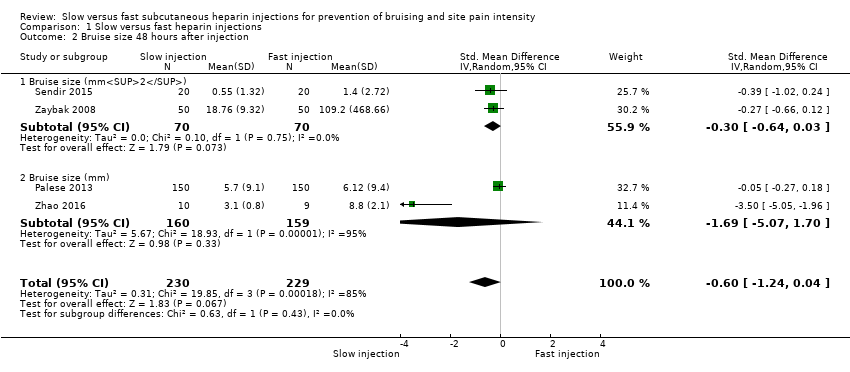 Comparison 1 Slow versus fast heparin injections, Outcome 2 Bruise size 48 hours after injection. | ||||
| 2.1 Bruise size (mm2) | 2 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.64, 0.03] |
| 2.2 Bruise size (mm) | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐5.07, 1.70] |
| 3 Bruise size Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Slow versus fast heparin injections, Outcome 3 Bruise size. | ||||
| 3.1 60 hours after injection (mm2) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐3.85 [‐8.99, 1.29] |
| 3.2 72 hours after injection (mm2) | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐6.57, 1.99] |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Slow versus fast heparin injections, Outcome 1 Intensity of injection pain.

Comparison 1 Slow versus fast heparin injections, Outcome 2 Bruise size 48 hours after injection.

Comparison 1 Slow versus fast heparin injections, Outcome 3 Bruise size.
| Slow vs fast subcutaneous heparin injection for prevention of bruising and site pain intensity | |||||
| Patient or population: patients treated with subcutaneous heparin injections Settings: hospital outpatient and inpatient units Intervention: slow injection (injection speed of 20 or more seconds) Comparison: fast injection (injection speed of less than 20 seconds) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk with fast injection | Corresponding risk with slow injection | ||||
| Intensity of injection pain immediately after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) | Mean pain intensity reported by the 2 studies ranged across fast injection groups from 2 to 5. | Mean pain intensity in the slow injection group was 1.52 points less than in the fast group (3.56 lower to 0.53 higher; P = 0.15). | 140 | ⊕⊕⊝⊝a | |
| Intensity of injection pain 48 hours after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) | Mean pain intensity ranged across fast injection groups from 2.1 to 2.8. | Mean pain intensity in the slow injection group was 1.68 points less than in the fast group (2.91 lower to 0.45 lower; P = 0.007). | 59 | ⊕⊕⊝⊝b | |
| Bruise size 48 hours after injection (mm/mm2) | See comment. | Mean bruising size in the slow injection group was 0.6 SD lower than in the fast injection group (1.24 lower to 0.04 higher; P = 0.07). | 459 | ⊕⊕⊝⊝c | Bruise size was measured on different scales; therefore we used the SMD to pool data. |
| Haematoma at injection site | See comment. | No studies measured this outcome. | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) for pain intensity was the range of mean pain score reported following fast injection by the 2 studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the mean difference of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aWe downgraded the quality of evidence by two steps owing to study limitations, as we identified few studies with small numbers of participants (imprecision) and high heterogeneity (I2 = 73%) (inconsistency). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intensity of injection pain Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediately after each injection | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.56, 0.53] |
| 1.2 48 hours after injection | 2 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.91, ‐0.45] |
| 1.3 60 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.15, 0.15] |
| 1.4 72 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.8 [‐1.70, 0.10] |
| 2 Bruise size 48 hours after injection Show forest plot | 4 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.24, 0.04] |
| 2.1 Bruise size (mm2) | 2 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.64, 0.03] |
| 2.2 Bruise size (mm) | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐5.07, 1.70] |
| 3 Bruise size Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 60 hours after injection (mm2) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐3.85 [‐8.99, 1.29] |
| 3.2 72 hours after injection (mm2) | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐6.57, 1.99] |


