Aspirina con o sin antiemético para la migraña aguda en adultos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, double‐dummy, three‐period, cross‐over. Single oral dose of each treatment for each of three migraine attacks Assessments at 0 and 2 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine without aura. At least 12‐month history of migraine, with age of onset before 50 years and two to six attacks per month. Prophylaxis permitted if stable for ≥ 2 months Excluded participants with other types of headache. Included participants with 'slight' migraine at baseline, but reported primary outcomes for those with ≥ moderate pain separately N = 247 (198 treated three attacks and analysed for efficacy) M = 57, F = 190 Mean age = 40 years 36.8% of randomised participants were taking prophylactic therapy | |
| Interventions | Aspirin 1000 mg, n = 198 Acetaminophen 400 mg plus codeine 25 mg, n = 198 Placebo, n = 198 | |
| Outcomes | Headache relief at 2 hours Pain‐free at 2 hours PI: 100 mm VAS Mean PID at 2 hours (from baseline) Relief of nausea and vomiting Use of rescue medication Patient preference for medication Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy design |
| Incomplete outcome data (attrition bias) | Unclear risk | Completer analysis (participants treating all three attacks), but adequate reasons for exclusion given for all others |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose per attack. Participants treated two migraine attacks Medication taken when migraine headache pain of moderate or severe intensity Assessments at 0 and 2 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine with or without aura. At least 12‐month history of migraine, with two to six attacks per month for three months prior to inclusion in study Prophylaxis permitted if stable for ≥ 3 months Excluded participants whose migraine headache was never accompanied by nausea or vomiting N = 266 (250 analysed for efficacy, 16 did not take medication) M = 46, F = 220 Mean age 37 years | |
| Interventions | Lysine acetylsalicylate 1650 mg (equivalent to 900 mg aspirin) plus metoclopramide 10 mg, n = 126 Placebo, n = 124 | |
| Outcomes | Headache relief at 2 hours Pain‐free at 2 hours PI: 4‐point scale Presence of nausea and vomiting Headache recurrence at 24 hours PGE: 4‐point scale Use of rescue medications | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, three‐arm, parallel‐group, double‐dummy. Single oral dose Medication taken when migraine headache pain of moderate or severe intensity Assessments at 0, 0.5, 1, 1.5, 2 and 24 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine with and without aura. At least 12‐month history of migraine, with one to six attacks per month N = 433 M = 66, F = 367 Mean age 42 years | |
| Interventions | Effervescent acetylsalicylic acid 1000 mg, n = 146 Sumatriptan 50 mg, n = 135 Placebo, n = 152 | |
| Outcomes | Headache relief at 1 and 2 hours Pain‐free at 2 hours 24‐hour sustained relief Adverse events Remission of associated symptoms: nausea, photophobia, phonophobia Overall impression of study medication Need for rescue medication | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "Matching effervescent or tablet placebo" |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs described |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, three‐fold cross‐over, double‐dummy. Single oral dose per attack. Each participant treated three migraine attacks with different treatments. Medication taken when migraine headache pain of moderate or severe intensity Assessments at 0, 0.5, 1, 1.5, 2 and 24 hours If pain not controlled, participants encouraged to wait 2 hours before taking rescue medication Participants instructed to leave a minimum of 48 hours between consecutive study treatments to ensure that new attack and not migraine recurrence was being treated | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine with and without aura. At least 12‐month history of migraine, with one to six attacks per month N = 312 M = 59, F = 253 Mean age 38 years | |
| Interventions | Effervescent acetylsalicylic acid 1000 mg, n = 222 Ibuprofen 400 mg, n = 212 Sumatriptan 50 mg, n = 226 Placebo, n = 222 | |
| Outcomes | Pain intensity at 0.5, 1, 1.5 and 2 hours Nausea, vomiting, photophobia and phonophobia at same time points Global assessment of medication on 4‐point categorical scale Use of 'escape medication' Time when headache disappeared Recurrence within 24 hours Adverse events | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment was assigned by a predetermined randomisation code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double dummy design |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Low risk | > 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, parallel‐group, double‐dummy. Single oral dose. Each participant treated three migraine attacks. Medication taken when migraine headache pain of moderate or severe intensity, provided it was within 6 hours of headache onset, and participants had been free from any previous migraine attack for at least 24 hours Assessments at 0 and 2 hours If pain not controlled, participants encouraged to wait 2 hours before taking rescue medication Patients returned to study centre after treating first attack, and were then given medication and diary cards for treating two further attacks | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine with and without aura. At least 12‐month history of migraine, with age of onset before 50 years and one to six attacks per month of moderate to severe intensity, for three months prior to inclusion in study Excluded participants with basilar, ophthalmoplegic or hemiplegic migraine, and those with non‐migraine headache on more than 10 days per month for preceding 6 months Approximately half of participants in each treatment arm had previously been treated with acetylsalicylic acid plus metoclopramide, with 'good' or 'fair' response reported in roughly 55% of participants in each group Approximately 80% of participants had previously received or were currently receiving acetylsalicylic acid or NSAIDs alone, with 'good' or 'fair' response reported in approximately 45% of participants in both groups N = 666 M = 100, F = 566 Mean age 41 years | |
| Interventions | Acetylsalicylic acid 900 mg plus metoclopramide 10 mg, n = 340 Zolmitriptan 2.5 mg, n = 326 | |
| Outcomes | Headache response at 2 hours in all three attacks Pain‐free at 2 hours after first dose in all three attacks Relief of migraine‐associated symptoms (nausea, vomiting, photophobia, phonophobia) PI: standard 4‐point scale Use of escape medication Headache recurrence Adverse events PGE: standard 4‐point scale Time to onset of meaningful migraine relief | |
| Notes | Oxford Quality Scale: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy design |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Low risk | > 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, parallel‐group. Single oral dose Medication taken when migraine headache pain was of moderate or severe intensity Assessments at 0, 2 and 24 hours | |
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine Excluded attacks of migraine with aura. Excluded participants with tension headache N = 303 M = 35, F = 268 Mean age = 40 years | |
| Interventions | Effervescent aspirin 900 mg plus metoclopramide 10 mg, n = 152 Placebo, n = 151 | |
| Outcomes | Headache relief at 2 hours Pain‐free at 2 hours 24‐hour sustained relief Use of rescue medication Relief of associated symptoms Functional disability PGE Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised after drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs described, but 16% (aspirin) and 13% (placebo) not included in 2 h efficacy analyses because they were asleep |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose Participants instructed to take medication only if attack of at least moderate intensity, and within 6 hours of onset of symptoms Assessments at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6 and 24 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine. At least 12‐month history of migraine, with one to six attacks per month Excluded participants usually so incapacitated as to require bed rest during attacks, and those who vomited more than 20% of time during attacks N = 374 (343 analysed for efficacy, 31 did not take medication) M = 62, F = 312 Mean age = 42 years | |
| Interventions | Effervescent acetylsalicylic acid 2 x 500 mg, n = 169 Placebo, n = 174 | |
| Outcomes | Headache relief at 2 hours Pain‐free after 2 hours Relief of migraine‐associated symptoms: nausea, vomiting, photophobia, phonophobia (4‐point scale) PI : 4‐point scale PGE: 4‐point scale Headache recurrence within 24 hours Use of rescue medication Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, double‐dummy, parallel‐group. Single oral dose Each participant to treat two attacks, as soon as intensity was moderate or severe Assessments at 0, 2 and 24 hours If pain not controlled, participants encouraged to wait 2 hours before taking rescue medication | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine with and without aura. At least 12‐month history of migraine, with one to six attacks moderate or severe attacks per month. Prophylaxis permitted if stable for ≥3 months N = 296 randomised (268 took study medication) M = 47, F = 249 Mean age 37 years | |
| Interventions | Calcium carbasalate 1145 mg (equivalent to 900 mg aspirin) plus metoclopramide 10 mg, n = 136 Ergotamine 1 mg plus metoclopramide 10 mg, n = 132 | |
| Outcomes | Headache relief at 2 hours Pain‐free after 2 hours Relief of nausea PGE: 4‐point scale Headache recurrence within 24 hours Use of rescue medication Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method, using tablet and sachet |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose Medication administered when migraine headache pain of moderate or severe intensity Assessments at 0, 0.5, 1, 2, 3, 4, 5, 6 and 24 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Aged 18‐50 years, meeting IHS criteria for migraine with and without aura. At least 12‐month history of migraine, with one to six attacks per month of at least moderate pain intensity. Prophylaxis permitted if stable for ≥ 3 months N = 409 (401 with confirmed migraine) M = 85, F = 316 Mean age 38 years | |
| Interventions | Aspirin 1000 mg, n = 205 Placebo, n = 204 | |
| Outcomes | Headache relief at 1 and 2 hours Pain‐free at 2 hours 24 hour sustained relief Adverse events Relief of associated symptoms: nausea, photophobia, phonophobia Need for rescue medication PI: standard 4‐point scale | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "Matched placebo" |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Low risk | > 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, two‐period cross‐over. Single oral dose of each medication for each of two attacks Medication administered when migraine headache pain of moderate or severe intensity Assessments at 0, 0.25, 0.5, 0.75, 1, 2, 3, 4, and 6 hours post‐treatment If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Aged > 18 years, meeting IHS criteria for migraine with and without aura. At least 12‐month history of migraine, with one to six attacks per month within previous three months Excluded participants who vomited during the majority of their migraine attacks Excluded participants who regularly used NSAIDs or other drugs that could interact with trial medications N = 101 (73 treated two attacks and analysed for efficacy) M = 11, F = 90 Mean age 44 years | |
| Interventions | Mouth‐dispersible aspirin 900 mg, n = 73 Placebo, n = 73 | |
| Outcomes | Headache relief at all assessment time points Pain‐free at all assessment time points PI: standard 4‐point scale Functional disability: standard 4‐point scale Presence/absence of associated symptoms Headache recurrence at 24 hours Use of rescue medication Participants' and investigators' global assessments Palatability and convenience of study medication | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "Placebo tablets formulated and manufactured to be indistinguishable from aspirin tablets, with respect to appearance, taste and dispersion in mouth" |
| Incomplete outcome data (attrition bias) | Unclear risk | Completer analysis (participants treating both attacks), but adequate reasons for exclusion given for others |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, three parallel groups. Single oral dose per attack. Two attacks treated Medication taken when migraine headache pain of moderate or severe intensity Assessments at 0 and 2 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine with or without aura. At least 12‐month history of migraine, with two to six attacks per month during three months prior to study N = 421 (385 experienced ≥ 1 attack and analysed for efficacy) M = 94, F = 327 Mean age = 39 years | |
| Interventions | Lysine acetylsalicylate 1620 mg (equivalent to 900 mg aspirin) plus metoclopramide 10 mg, n = 137 Sumatriptan 100 mg, n = 122 Placebo, n = 126 | |
| Outcomes | Headache relief at 2 hours PI: 4‐point scale Pain‐free at 2 hours 24‐hour sustained relief Headache recurrence at 24 hours Relief of nausea and vomiting Use of rescue medication PGE: 4‐point scale Adverse effects | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy design |
| Incomplete outcome data (attrition bias) | Low risk | Missing data < 3% |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, parallel‐group, double‐dummy. Single oral dose per attack Participants treated up to three migraine attacks Assessments at 0 and 2 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication Participants instructed to leave minimum interval of 48 hours between consecutive study treatments to ensure adequate washout | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine. At least 12‐month history of migraine, with one to six attacks per month of moderate or severe intensity Excluded participants with need for continuing migraine prophylaxis N = 358 (355 analysed for at least one attack) M = 72, F = 283 Mean age = 41 years | |
| Interventions | Aspirin 900 mg plus metoclopramide 10 mg, n = 183 Sumatriptan 100 mg, n = 172 | |
| Outcomes | Headache relief at 2 hours PI: 4‐point scale Presence of nausea, vomiting, photophobia, phonophobia Functional disability Headache recurrence at 48 hours Use of rescue medication PGE: 4‐point scale Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐dummy design |
| Incomplete outcome data (attrition bias) | Unclear risk | Discrepancy between numbers in text and in Table 2. Numbers in text used: up to 15% missing data |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, parallel‐group Medication taken up to three times at 2‐hour intervals, if adequate relief not obtained, to treat a single migraine attack Rescue medication taken if no relief of symptoms after three doses of trial medication. Usual medication allowed if attack persisted 2 hours after second dose of trial medication Assessments at 0 and 2 hours | |
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine Excluded participants with tension headache N = 227 M = 34, F = 193 Mean age 34 years | |
| Interventions | Lysine acetylsalicylate 1620 mg (equivalent to 900 mg aspirin) plus metoclopramide 10 mg, n = 112 Ergotamine 2 mg plus caffeine 200 mg, n = 115 | |
| Outcomes | No nausea or vomiting 2 hours after first intake Headache intensity (4‐point categorical scale) Headache relief Complete resolution of headache Use of rescue medication Patients' global evaluation of treatment | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central randomisation list generated with SAS (PC version) 6.08" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "Maintained by providing an equivalent number of placebo sachets or capsules" |
| Incomplete outcome data (attrition bias) | Low risk | No useful efficacy data. Safety population accounted for |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Abstract (full data in Chabriat 1994) | |
| Mixed migraine and tension‐type headaches | |
| Intravenous administration ‐ self‐administration not possible | |
| Mixed migraine and tension‐type headaches | |
| Not randomised or double‐blind | |
| Included patients with mild headaches, headaches not IHS classified |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.70, 2.55] |
| Analysis 1.1  Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 1 Pain free at 2 hours. | ||||
| 2 Headache relief at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.48, 1.83] |
| Analysis 1.2  Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 2 Headache relief at 2 hours. | ||||
| 3 Headache relief at 1 hour Show forest plot | 4 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.72, 2.63] |
| Analysis 1.3  Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 3 Headache relief at 1 hour. | ||||
| 4 24‐hour sustained headache relief Show forest plot | 3 | 1142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.37, 1.95] |
| Analysis 1.4  Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 4 24‐hour sustained headache relief. | ||||
| 5 Pain free at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 5 Pain free at 2 hours ‐ effect of formulation. | ||||
| 5.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.51, 2.44] |
| 5.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.70, 3.58] |
| 6 Headache relief at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 6 Headache relief at 2 hours ‐ effect of formulation. | ||||
| 6.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.43, 1.89] |
| 6.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.38, 1.95] |
| 7 Relief of associated symptoms at 2 hours Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 7 Relief of associated symptoms at 2 hours. | ||||
| 7.1 Nausea | 4 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.10, 1.44] |
| 7.2 Vomiting | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.94, 1.34] |
| 7.3 Photophobia | 5 | 1274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.29, 1.69] |
| 7.4 Phonophobia | 5 | 1217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.27, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [1.59, 4.55] |
| Analysis 2.1  Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 1 Pain free at 2 hours. | ||||
| 2 Headache relief at 2 hours Show forest plot | 3 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.78, 2.60] |
| Analysis 2.2  Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 2 Headache relief at 2 hours. | ||||
| 3 24‐hour sustained headache relief Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.39, 3.41] |
| Analysis 2.3  Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 3 24‐hour sustained headache relief. | ||||
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 4 Relief of associated symptoms at 2 hours. | ||||
| 4.1 Nausea | 2 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.53 [4.20, 13.50] |
| 4.2 Vomiting | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.14 [2.30, 113.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 1 Pain free at 2 hours. | ||||
| 1.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.03] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 2 Headache relief at 2 hours. | ||||
| 2.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.11] |
| 3 Headache relief at 1 hour Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 3 Headache relief at 1 hour. | ||||
| 3.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.26, 1.99] |
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 4 Relief of associated symptoms at 2 hours. | ||||
| 4.1 Photophobia | 2 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.04] |
| 4.2 Phonophobia | 2 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 1 Pain free at 2 hours. | ||||
| 1.1 Sumatriptan 100 mg | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.87] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 2 Headache relief at 2 hours. | ||||
| 2.1 Sumartiptan 100 mg | 2 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.10] |
| 3 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 3 Relief of associated symptoms at 2 hours. | ||||
| 3.1 Nausea | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 3.2 Vomiting | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.59 [1.43, 78.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 7 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.02, 1.55] |
| Analysis 5.1  Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 1 Any adverse event within 24 hours. | ||||
| 1.1 Aspirin alone | 5 | 1892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.00, 1.68] |
| 1.2 Aspirin + metoclopramide | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.82, 1.67] |
| 2 Use of rescue medication Show forest plot | 8 | 2922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.63, 0.72] |
| Analysis 5.2  Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 2 Use of rescue medication. | ||||
| 2.1 Aspirin 100 mg alone | 5 | 1881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.73] |
| 2.2 Aspirin 900 mg + metoclopramide 10 mg | 3 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 1 Any adverse event within 24 hours. | ||||
| 1.1 Aspirin versus sumatriptan 50 mg | 2 | 730 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 1.2 Aspirin+met versus sumatriptan 100 mg | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.84] |
| 2 Use of rescue medication Show forest plot | 4 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.01, 1.28] |
| Analysis 6.2  Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 2 Use of rescue medication. | ||||
| 2.1 Aspirin versus sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 2.2 Aspirin+met versus sumatriptan 100 mg | 2 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.39] |
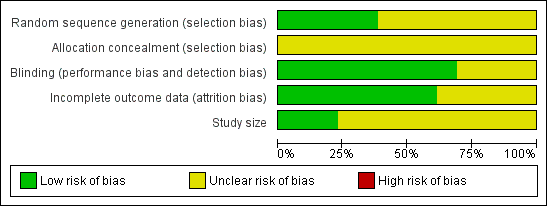
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.1 Pain free at 2 hours.

L'Abbé plot showing pain‐free at 2 h response in individual studies. Each circle represents one study, with size on the inset scale.

Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.2 Headache relief at 2 hours.

L'Abbé plot showing headache response at 2 h in individual studies. Each circle represents one study, with size on the inset scale.

Response rates for aspirin 900 mg plus metoclopramide 10 mg in consecutive attacks, reported in five studies (from left:Tfelt‐Hansen 1995; Chabriat 1994; Thomson 1992; Le Jeunne 1998; Geraud 2002)

Forest plot of comparison: 5 Aspirin ± metoclopramide versus placebo, outcome: 5.2 Use of rescue medication.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 1 Pain free at 2 hours.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 3 Headache relief at 1 hour.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 4 24‐hour sustained headache relief.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 5 Pain free at 2 hours ‐ effect of formulation.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 6 Headache relief at 2 hours ‐ effect of formulation.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 7 Relief of associated symptoms at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 1 Pain free at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 3 24‐hour sustained headache relief.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 4 Relief of associated symptoms at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 1 Pain free at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 2 Headache relief at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 3 Headache relief at 1 hour.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 4 Relief of associated symptoms at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 1 Pain free at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 2 Headache relief at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 3 Relief of associated symptoms at 2 hours.

Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 1 Any adverse event within 24 hours.

Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 2 Use of rescue medication.
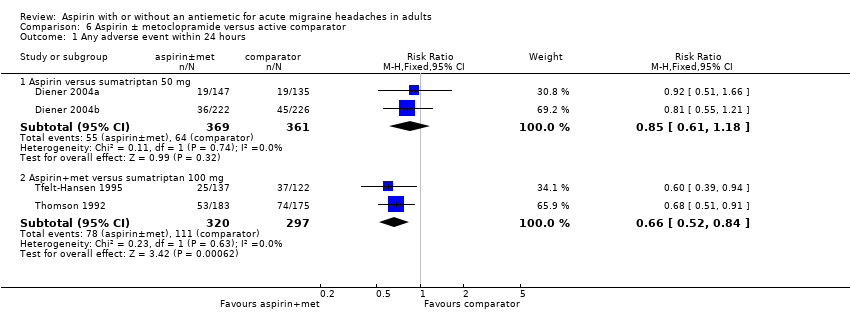
Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 1 Any adverse event within 24 hours.

Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 2 Use of rescue medication.
| Aspirin 900 mg or 1000 mg compared with placebo for migraine headache | ||||||
| Patient or population: migraine headache ‐ moderate or severe pain Settings: community Intervention: aspirin 900 mg or 1000 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | NNT or NNTH and/or | No of Participants | Quality of the evidence | Comments |
| Pain‐free at 2 h | 240 in 1000 | 110 in 1000 | NNT 8.1 (6.4 to 11) | 6 studies, 2027 participants 357 events | Moderate1 | Standard tablet and soluble formulations |
| Headache relief at 2 h | 520 in 1000 | 320 in 1000 | NNT 4.9 (4.1 to 6.2) | 6 studies, 2027 participants 848 events | Moderate1 | Standard tablet and soluble formulations |
| Sustained pain‐free at 24 h | No data |
|
|
|
|
|
| Sustained headache relief at 24 h | 390 in 1000 | 240 in 1000 | NNT 6.6 (4.9 to 10) | 3 studies, 1142 participants 361 events | Moderate1 | Standard tablet and soluble formulations |
| At least one AE | 120 in 1000 | 90 in 1000 | NNH 34 (18 to 340) | 5 studies, 1892 participants 206 events | Low | Standard tablet and soluble formulations |
| Serious AE | Insufficient data | |||||
| GRADE Working Group grades of evidence | ||||||
| 1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.70, 2.55] |
| 2 Headache relief at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.48, 1.83] |
| 3 Headache relief at 1 hour Show forest plot | 4 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.72, 2.63] |
| 4 24‐hour sustained headache relief Show forest plot | 3 | 1142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.37, 1.95] |
| 5 Pain free at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.51, 2.44] |
| 5.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.70, 3.58] |
| 6 Headache relief at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.43, 1.89] |
| 6.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.38, 1.95] |
| 7 Relief of associated symptoms at 2 hours Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Nausea | 4 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.10, 1.44] |
| 7.2 Vomiting | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.94, 1.34] |
| 7.3 Photophobia | 5 | 1274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.29, 1.69] |
| 7.4 Phonophobia | 5 | 1217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.27, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [1.59, 4.55] |
| 2 Headache relief at 2 hours Show forest plot | 3 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.78, 2.60] |
| 3 24‐hour sustained headache relief Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.39, 3.41] |
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 2 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.53 [4.20, 13.50] |
| 4.2 Vomiting | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.14 [2.30, 113.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.03] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.11] |
| 3 Headache relief at 1 hour Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.26, 1.99] |
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Photophobia | 2 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.04] |
| 4.2 Phonophobia | 2 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sumatriptan 100 mg | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.87] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sumartiptan 100 mg | 2 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.10] |
| 3 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 3.2 Vomiting | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.59 [1.43, 78.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 7 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.02, 1.55] |
| 1.1 Aspirin alone | 5 | 1892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.00, 1.68] |
| 1.2 Aspirin + metoclopramide | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.82, 1.67] |
| 2 Use of rescue medication Show forest plot | 8 | 2922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.63, 0.72] |
| 2.1 Aspirin 100 mg alone | 5 | 1881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.73] |
| 2.2 Aspirin 900 mg + metoclopramide 10 mg | 3 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aspirin versus sumatriptan 50 mg | 2 | 730 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 1.2 Aspirin+met versus sumatriptan 100 mg | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.84] |
| 2 Use of rescue medication Show forest plot | 4 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.01, 1.28] |
| 2.1 Aspirin versus sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 2.2 Aspirin+met versus sumatriptan 100 mg | 2 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.39] |

