Aspirina con o sin antiemético para la migraña aguda en adultos
Appendices
Appendix 1. Definitions
All terms relating to primary efficacy outcomes are defined according to the effect of the treatment on headache pain, measured using a four‐point pain intensity scale (ranging from 0 to 3 or none, mild, moderate, and severe).
• Baseline pain intensity ‐ level of pain participant must be experiencing in order to receive study medication, either 1 (mild pain) or 2/3 (moderate or severe pain).
• Pain‐free at 2 hours ‐ number of participants with a pain intensity of 0 (none) at 2 hours after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
• Headache relief at 2 hours ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at 2 hours after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
• 24‐hour sustained headache relief ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at 2 hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
• 24‐hour sustained pain‐free ‐ number of participants with a pain intensity of 0 (none) at 2 hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication expressed as a fraction of the treated participants with the appropriate baseline pain.
• Use of rescue medication ‐ number of participants requiring the use of additional medication to treat either recurrence of headache or an inadequate response to study medication, provided that the additional medication is not, or does not include, the study drug.
• Relief of associated symptoms ‐ number of participants with an absence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at 2 hours after administration of study medication, expressed as a fraction of the treated participants for whom the symptom was present at baseline.
• Relief of functional disability ‐ reduction in the level of functional disability, measured using a four‐point scale, from moderate or severe disability (grade 2/3) at baseline to mild or none (grade 1/0) at 2 hours after administration of study medication, expressed as a fraction of the treated participants with moderate or severe functional disability at baseline.
Appendix 2. Search strategy for MEDLINE (via Ovid)
-
Aspirin/
-
(aspirin OR acetylsalicylic acid OR ASA OR Migramax OR Migpriv OR Migrafin OR Migravess).mp
-
1 OR 2
-
Headache/ OR exp Headache Disorders/
-
exp Migraine Disorders/
-
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
-
4 OR 5 OR 6
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
OR/8‐15
-
3 AND 7 AND 16
Appendix 3. Search strategy for EMBASE (via Ovid)
-
Acetylsalicylic acid/
-
(aspirin OR acetylsalicylic acid OR ASA OR Migramax OR Migpriv OR Migrafin OR Migravess).mp.
-
1 OR 2
-
exp Headache and facial pain
-
exp migraine
-
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
-
4 OR 5 OR 6
-
clinical trials.sh.
-
controlled clinical trials.sh.
-
randomized controlled trial.sh.
-
double‐blind procedure.sh.
-
(clin* adj25 trial*).ab.
-
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab.
-
placebo*.ab.
-
random*.ab.
-
OR/8‐15
-
3 AND 7 AND 16
Appendix 4. Search strategy for CENTRAL
-
MeSH descriptor Aspirin
-
(aspirin OR acetylsalicylic acid OR ASA OR Migramax OR Migpriv OR Migrafin OR Migravess):ti,ab,kw.
-
1 OR 2
-
MeSH descriptor Headache/ OR MeSH descriptor Headache Disorders explode all trees
-
MeSH descriptor Migraine Disorders explode all trees
-
(headach* OR migrain* OR cephalgi* OR cephalalgi*):ti,ab,kw.
-
4 OR 5 OR 6
-
Randomized controlled trial:pt
-
MESH descriptor Double‐blind Method
-
random*:ti,ab,kw.
-
OR/8‐10
-
3 AND 7 AND 11
-
Limit 12 to Clinical Trials (CENTRAL)
Appendix 5. Summary of outcomes: efficacy
| Study ID | Treatment | HR 1 h | HR 2 h | PF 2 h | 24 h SHR | 24 h SPF | Use of rescue medication |
| Boureau 1994 | (1) Aspirin 1000 mg, n = 198 (2) Paracetamol 400 mg + codeine 25 mg, n = 198 (3) Placebo, n = 198 | No data | (1) 104/198 (2) 98/198 (3) 59/198 | (1) 36/198 (2) 44/198 (3) 22/198 | No data | No data | (1) 110/198 (2) 100/198 (3) 148/198 |
| Chabriat 1994 | (1) LAS 1650 mg (= 900 mg aspirin) + metoclopramide 10 mg, n = 126 (2) Placebo, n = 124 | No data | 1st attack (1) 74/126 (2) 36/124 | No usable data ‐ denominator unclear | No usable data ‐ total attacks | No data | Total attacks (1) 111/237 (2) 162/238 |
| Diener 2004a | (1) Effervescent aspirin 1000 mg, n = 147 (2) Sumatriptan 50 mg, n = 135 (3) Placebo, n = 153 | (1) 35/146 (2) 31/135 (3) 23/152 | (1) 72/146 (2) 66/135 (3) 50/152 | (1) 37/146 (2) 33/135 (3) 22/152 | (1) 48/146 (2) 47/135 (3) 33/152 | No data | (1) 62/146 (2) 53/135 (3) 98/152 |
| Diener 2004b | (1) Effervescent aspirin 1000 mg, n = 222 (2) Ibuprofen 400 mg, n = 212 (3) Sumatriptan 50 mg, n = 226 (4) Placebo, n = 222 | (1) 76/221 (2) 65/211 (3) 54/224 (4) 25/222 | (1) 116/221 (2) 127/211 (3) 125/224 (4) 68/222 | (1) 60/221 (2) 70/221 (3) 83/224 (4) 28/222 | (1) 93/221 (2) 103/211 (3) 96/224 (4) 56/222 | No data | (1) 99/221 (2) 87/211 (3) 92/224 (4) 147/222 |
| Geraud 2002 | (1) Aspirin 900 mg + metoclopramide 10 mg, n = 340 (2) Zolmitriptan 2.5 mg, n = 326 | No data | 1st attack (1) 226/340 (2) 197/326 | No usable data ‐ average over 3 attacks | No usable data ‐ denominator unclear | No data | Overall use: (1) 255/949 (2) 229/909 |
| Henry 1995 | (1) Effervescent aspirin 900 mg + metoclopramide 10 mg, n = 152 (2) Placebo, n = 151 | No data | (1) 69/127* (2) 34/131* | (1) 18/127 (2) 7/131 | No usable data ‐ denominator unclear | No data | (1) 66/152 (2) 91/151 |
| Lange 2000 | (1) Effervescent acetylsalicylic acid 1000 mg, n = 169 (2) Placebo, n = 174 | No data | (1) 93/169 (2) 64/174 | (1) 49/169 (2) 29/174 | No data | No data | (1) 65/169 (2) 100/174 |
| Le Jeunne 1998 | (1) calcium carbasalate (= 900 mg aspirin) plus metoclopramide 10 mg, n = 134 | No data | 1st attack (1) 73/134 (2) 48/132 | 1st attack (1) 27/134 (2) 11/132 | 1st attack (1) 61/73 (2) 44/48 | No data | 1st attack (1) 49/134 (2) 61/131 |
| Lipton 2005 | (1) Aspirin 1000 mg, n = 205 (2) Placebo, n = 204 | (1) 68/201 (2) 36/200 | (1) 105/201 (2) 68/200 | (1) 40/201 (2) 12/200 | (1) 82/201 (2) 49/200 | No data | (1) 68/201 (2) 104/200 |
| MacGregor 2002 | (1) Mouth‐dispersible aspirin 900 mg, n = 73 (2) Placebo, n = 73 | (1) 30/73 (2) 15/73 | (1) 35/73 (2) 14/73 | (1) 10/73 (2) 4/73 | No usable data ‐ recurrence less with aspirin | No usable data | No usable data ‐ less with aspirin |
| Tfelt‐Hansen 1995 | (1) LAS 1620 mg (= 900 mg aspirin) + metoclopramide 10 mg, n = 137 (2) Sumatriptan 100 mg, n = 122 (3) Placebo, n = 126 | No data | 1st attack (1) 76/133 (2) 63/119 (3) 30/124 | 1st attack (1) 29/135 (2) 36/122 (3) 10/126 | 1st attack (1) 49/133 (2) 39/119 (3) 21/124 | No data | 1st attack (1) 74/137 (2) 77/122 (3) 102/126 |
| Thomson 1992 | (1) Aspirin 900 mg + metoclopramide 10 mg, n = 183 (2) Sumatriptan 100 mg, n = 175 | No data | 1st attack, in pts with mod/sev pain (1) 62/138 (2) 74/133 | 1st attack (1) 19/138 (2) 35/133 | No usable data ‐ data for 48 h | No data | 1st attack (1) 102/183 (2) 59/175 |
| Titus 2001 | (1) LAS 1620 mg (= 900 mg aspirin) + metoclopramide 10 mg, n = 125 (2) Ergotamine 2 mg + caffeine 200 mg, n = 121 | No data | No usable data | No usable data | No usable data | No usable data | No usable data |
| * 25 aspirin + metoclopramide and 20 placebo participants asleep HR ‐ headache relief; LAS ‐ lysine acetylsalicylate; PF ‐ pain‐free; SHR ‐ sustained headache relief; SPF ‐ sustained pain‐free | |||||||
Appendix 6. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | Any AE | Specific AEs | Serious AEs | AE withdrawal | Other withdrawals/exclusions |
| Boureau 1994 | (1) Aspirin 1000 mg, n = 198 (2) Paracetamol 400 mg + codeine 25 mg, n = 198 (3) Placebo, n = 198 | (1) 29/198 (2) 36/198 (3) 27/198 | None | None | 12 pts excl from analyses because of serious protocol deviations | |
| Chabriat 1994 | (1) LAS 1650 mg (= 900 mg aspirin) + metoclopramide 10 mg, n = 126 (2) Placebo, n = 124 | No usable data | "Minor and transient AE reported" (no numbers): | One patient reported epigastric pain one month after one dose of LAS‐MCP and was found to have a gastric ulcer. | None | None |
| Diener 2004a | (1) Effervescent aspirin 1000 mg, n = 147 (2) Sumatriptan 50 mg, n = 135 (3) Placebo, n = 153 | (1) 19/147 | Gastrointestinal: (1) 5/147 (2) 7/135 (3) 7/153 | None reported | None reported | 2 pts excl from efficacy ‐ did not return diary |
| Diener 2004b | (1) Effervescent aspirin 1000 mg, n = 222 (2) Ibuprofen 400 mg, n = 212 (3) Sumatriptan 50 mg, n = 226 (4) Placebo, n = 222 | (1) 36/222 (2) 26/212 (3) 45/226 (4) 32/222 | No data | (1) 1/222 (renal colic) (2) 1/212 (perforated duodenal ulcer) (3) 0/226 (4) 0/222 | Possibly 2 serious AEs, but unconfirmed | 1 treated pt excluded from analysis ‐ did not return diary |
| Geraud 2002 | (1) Aspirin 900 mg + metoclopramide 10 mg, n = 340 (2) Zolmitriptan 2.5 mg, n = 326 | (1) 99/340 (2) 133/326 | Most common: (1) Abdominal pain (5.0%), somnolence (5.0%), asthenia (4.9%) (2) vertigo (6.7%), somnolence (5.5%), paraesthesia (4.3%) | (1) 5/340 (2) 6/326 | Over 3 attacks: (1) 5/340 (2) 3/326 | Details provided, but numbers are for entire study period and most due to failure to treat 3 attacks. |
| Henry 1995 | (1) Effervescent aspirin 900 mg + metoclopramide 10 mg, n = 152 (2) Placebo, n = 151 | (1) 31/152 (2) 28/151 | Vomiting: Stomach ache: Somnolence: | No data | None reported | None reported, but 45 pts were asleep and did not contribute to the 2 h efficacy evaluation |
| Lange 2000 | (1) Effervescent acetylsalicylic acid 1000 mg, n = 169 (2) Placebo, n = 174 | (1) 14/169 (2) 5/174 | Most frequent body systems: (1) whole (5), digestive (3), nervous (3), respiratory (3) (2) whole (3) | None | None reported | None reported |
| Le Jeunne 1998 | (1) calcium carbasalate (= 900 mg aspirin) plus metoclopramide 10 mg, n = 134 | Over 2 attacks: (1) 30/136 (2) 42/132 | Any GI AE: (1) 9/136, (2) 28/132 Somnolence: (1) 6/136, (2) 5/132 Abdominal pain: (1) 5/136, (2) 12/132 Nausea: (1) 5/136, (2) 11/132 | (1) 1/136 (pulmonary embolism) (2) 0/132 | (1) 1/136 (pulmonary embolism) (2) 1/132 (back pain) | 2 pts did not return diary cards |
| Lipton 2005 | (1) Aspirin 1000 mg, n = 205 (2) Placebo, n = 204 | (1) 18/205 (2) 10/204 | Nausea: (1) 7/205 (2) 2/204 All other AE ≤1% | (1) 0/205 (2) 1/204 (perforated appendix) | Possibly one serious AE, but not confirmed | 4 pts in each group treated non‐migraine headaches, so excluded from efficacy analysis |
| MacGregor 2002 | (1) Mouth‐dispersible aspirin 900 mg, n = 73 (2) Placebo, n = 73 | No usable data | Gastrointestinal: (1) 8/73 (dyspepsia, nausea, vomiting) (2) 4/73 (abdominal pain, nausea, vomiting) | (1) 1/73 (headache) (2) 1/73 (endometriosis) | (1) 4/73 (nausea, tinnitus, coughing, taste perversion) (2) 0/73 | 28 pts originally randomised were not included in analyses: insufficient attacks (6), unable to complete diary (7), withdrew consent (4), lost to follow‐up (4) other (5) |
| Tfelt‐Hansen 1995 | (1) LAS 1620 mg (= 900 mg aspirin) + metoclopramide 10 mg, n = 137 (2) Sumatriptan 100 mg, n = 122 (3) Placebo, n = 126 | Over 3 attacks:(1) 25/137 (2) 37/122 (3) 18/126 | Most common over 3 attacks: (1) somnolence, abdominal pain, nausea + vomiting (2) nausea + vomiting, fatigue, constriction of throat/chest pain, paraesthesia, somnolence, abdominal pain (3) nausea + vomiting, fatigue | 1 sumatriptan pt had acute AF requiring hospital admission | Over 3 attacks: (1) 1/138 (2) 4/125 (3) 2/126 | 32 pts originally randomised did not have any attacks and 4 took treatment but did not complete diaries |
| Thomson 1992 | (1) Aspirin 900 mg + metoclopramide 10 mg, n = 183 (2) Sumatriptan 100 mg, n = 175 | Over 3 attacks: (1) 53/183 (2) 74/175 | Most common over 3 attacks: Nausea and/or vomiting: | No data | (1) 0/183 (2) 5/175 (headache + faintness + vomiting, scalp tingling + heaviness in chest + globus + prolonged aura, stomach pain, dyspnoea + heaviness in extremities, worsened headache + nausea | 3 pts excl from efficacy because did not return diary cards 87 pts excl from efficacy because had mild or no pain at baseline. |
| Titus 2001 | (1) LAS 1620 mg (= 900 mg aspirin) + metoclopramide 10 mg, n = 125 (2) Ergotamine 2 mg + caffeine 200 mg, n = 121 | Over multiple attacks and with possible multiple dosing: (1) 21/125 (2) 28/121 | Most common over multiple attacks: (1) somnolence, diarrhoea, dyspepsia, nausea (2) abdominal pain, malaise, anxiety | None | (1) 1/125 (sinusitis) (2) 0/121 | Lost to follow‐up, non‐cooperation, other: (1) 8/125 (2) 6/121 |
| pts ‐ participants | ||||||
Appendix 7. Other outcomes
Repeat dosing for a single attack
Studies frequently reported use of rescue medication (usually as a different medicine from that under test). Two reported use of a second (repeat) dose of the medicine under test for treating the same attack. This is a potentially useful strategy for nonprescription medicines. A second dose of study medication after 2 hours was used by just over half of each treatment group in Geraud 2002, either because pain was not relieved or because it returned. Headache relief at 2 hours following the second dose occurred in a similar proportion to that following the first dose, with no difference between aspirin plus metoclopramide and zolmitriptan. In the other study permitting a second or third dose of study medication to treat a single attack, just under half of each treatment group (aspirin plus metoclopramide and ergotamine plus caffeine) used a second dose, but no data were reported for our primary efficacy outcomes (Titus 2001).
Multiple attacks
Response to therapy after a single migraine attack is useful knowledge, but migraineurs will suffer many attacks, and knowledge is needed about consistency of response. Few studies gave useful information on response in multiple attacks. However, five studies provided data for headache relief at 2 hours separately for two (Chabriat 1994; Le Jeunne 1998; Tfelt‐Hansen 1995) or three (Geraud 2002; Thomson 1992) consecutive attacks treated with the same medication. Response rates either increased compared with the first attack by up to 9%, or decreased by up to 11%. There was no consistent pattern of change either within a treatment group (Figure 6) or between treatments (aspirin plus metoclopramide and control). All changes are within those that might be expected by the play of chance.

Response rates for aspirin 900 mg plus metoclopramide 10 mg in consecutive attacks, reported in five studies (from left:Tfelt‐Hansen 1995; Chabriat 1994; Thomson 1992; Le Jeunne 1998; Geraud 2002)
Use of rescue medication
All studies asked participants whose symptoms were not adequately controlled to wait for 2 hours before taking any additional medication in order to give the test medication enough time to have an effect. Use of rescue or 'escape' medication (usually a different analgesic) after that time and up to 24 hours after dosing was reported in all studies and is a measure of treatment failure (lack of efficacy). In the study allowing multiple dosing for a single attack (Titus 2001), use of a second dose of study medication was interpreted as use of rescue medication for this analysis (Figure 7).

Forest plot of comparison: 5 Aspirin ± metoclopramide versus placebo, outcome: 5.2 Use of rescue medication.
| Summary of results: Use of rescue medication | ||||||
| Placebo comparators | Studies | Attacks treated | Treatment (%) | Comparator (%) | Relative risk (95% CI) | NNTp (95% CI) |
| Aspirin (± metoclopramide) versus placebo (Analysis 5.2) | 8 | 2922 | 44 | 65 | 0.68 (0.63 to 0.73) | 4.8 (4.1 to 5.7) |
| Aspirin 1000 mg versus placebo | 5 | 1881 | 42 | 63 | 0.67 (0.61 to 0.73) | 4.8 (3.9 to 6.0) |
| Aspirin 900 mg plus metoclopramide 10 mg versus placebo | 3 | 1041 | 48 | 69 | 0.69 (0.62 to 0.77) | 4.7 (3.7 to 6.5) |
| Active comparators | Studies | Attacks treated | Treatment (%) | Comparator (%) | Relative risk (95% CI) | NNH (95% CI) |
| Aspirin (± metoclopramide) versus sumatriptan 50 mg or 100 mg (Analysis 6.2) | 4 | 1340 | 49 | 43 | 1.1 (1.01 to 1.3) | 17 (8.8 to 140) |
| Aspirin 1000 mg versus sumatriptan 50 mg | 2 | 726 | 44 | 40 | 1.1 (0.92 to 1.3) | Not calculated |
| Aspirin 900 mg plus metoclopramide 10 mg versus sumatriptan 100 mg | 2 | 614 | 55 | 46 | 1.2 (1.01 to 1.4) | 11 (6.0 to 120) |
Relief of headache‐associated symptoms
In general, relief of headache‐associated symptoms (defined as a symptom reduction from moderate or severe to mild or none) was inconsistently reported. Of the eight studies that reported dichotomous data for symptom relief and comparing aspirin with placebo (Boureau 1994; Chabriat 1994; Diener 2004a; Diener 2004b; Lange 2000; Lipton 2005; MacGregor 2002; Tfelt‐Hansen 1995), only one study provided data for all four symptoms of interest (Lange 2000). The 900 mg aspirin dose used in one study (MacGregor 2002) was assumed to have the same efficacy as 1000 mg aspirin. Although two studies with an aspirin plus metoclopramide treatment arm provided data for relief of nausea and vomiting (Chabriat 1994; Tfelt‐Hansen 1995), there were no data available for the effects of this combination treatment on relief of photophobia and phonophobia.
Two studies (Tfelt‐Hansen 1995; Thomson 1992) provided data on relief of nausea and of vomiting for aspirin plus metoclopramide versus sumatriptan 100 mg (Analysis 4.3), and two (Diener 2004a; Diener 2004b) provided data on relief of photophobia and of phonophobia versus sumatriptan 50 mg (Analysis 3.4). Five studies compared aspirin to other active migraine treatments for relief of headache‐associated symptoms (Boureau 1994; Diener 2004b; Geraud 2002; Le Jeunne 1998; Titus 2001), but there were insufficient data for analysis. In this update, a small correction has been made to the placebo denominator for photophobia in Lange 2000; the result is not significantly changed.
Aspirin alone significantly relieved all symptoms except vomiting compared with placebo (Analysis 1.7), while aspirin plus metoclopramide significantly relieved both nausea and vomiting compared to placebo (Analysis 2.4). Subgroup analysis showed a statistically significant difference in favour of aspirin plus metoclopramide over aspirin alone for relief of nausea at two hours (z = 5.595, P < 0.00006), and relief of vomiting at two hours (z = 3.131, P = 0.002). Aspirin plus metoclopramide relieved more vomiting, but not nausea, compared to sumatriptan 100 mg (Analysis 4.3), while aspirin alone was not significantly different from sumatriptan 50 mg for relief of photophobia or phonophobia (Analysis 3.4).
| Summary of results: Relief of associated symptoms 2 hours after taking study medication | ||||||
| Intervention | Studies | Attacks with symptom present | Treatment (%) | Placebo (%) | Relative risk (95% CI) | NNT |
| Nausea | ||||||
| Aspirin 1000 mg versus placebo | 4 | 878 | 56 | 44 | 1.3 (1.1 to 1.4) | 9.0 (5.6 to 22) |
| Aspirin 900 mg plus metoclopramide 10 mg versus placebo | 2 | 417 | 45 | 6 | 7.5 (4.2 to 14) | 2.6 (2.1 to 3.1) |
| Aspirin 900 mg plus metoclopramide 10 mg versus sumatriptan 100 mg | 2 | 410 | 35 | 31 | 1.1 (0.83 to 1.5) | Not calculated |
| Vomiting | ||||||
| Aspirin 1000 mg versus placebo | 3 | 139 | 73 | 66 | 1.1 (0.94 to 1.3) | Not calculated |
| Aspirin 900 mg plus metoclopramide 10 mg versus placebo | 2 | 59 | 46 | 0 | 17 (2.3 to 120) | 2.1 (1.5 to 3.7) [12 events] |
| Aspirin 900 mg plus metoclopramide 10 mg versus sumatriptan 100 mg | 2 | 67 | 33 | 0 | 11 (1.4 to 78) | 3.3 (2.1 to 7.4) [11 events] |
| Photophobia | ||||||
| Aspirin 1000 mg versus placebo | 5 | 1274 | 47 | 31 | 1.5 (1.3 to 1.7) | 6.6 (4.9 to 10) |
| Aspirin 1000 mg versus sumatriptan 50 mg | 2 | 575 | 60 | 66 | 0.91 (0.80 to 1.03) | Not calculated |
| Phonophobia | ||||||
| Aspirin 1000 mg | 5 | 1217 | 49 | 34 | 1.4 (1.3 to 1.7) | 6.6 (4.9 to 10) |
| Aspirin 1000 mg versus sumatriptan 50 mg | 2 | 540 | 63 | 65 | 0.98 (0.86 to 1.1) | Not calculated |
Functional disability
Only one study with 73 participants reported data on functional disability (MacGregor 2002). More individuals with moderate or severe disability reported improvement following treatment with aspirin (22/53) than with placebo (3/61).
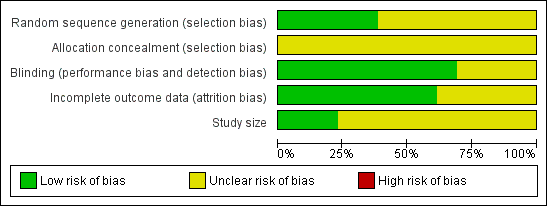
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.1 Pain free at 2 hours.

L'Abbé plot showing pain‐free at 2 h response in individual studies. Each circle represents one study, with size on the inset scale.

Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.2 Headache relief at 2 hours.

L'Abbé plot showing headache response at 2 h in individual studies. Each circle represents one study, with size on the inset scale.

Response rates for aspirin 900 mg plus metoclopramide 10 mg in consecutive attacks, reported in five studies (from left:Tfelt‐Hansen 1995; Chabriat 1994; Thomson 1992; Le Jeunne 1998; Geraud 2002)

Forest plot of comparison: 5 Aspirin ± metoclopramide versus placebo, outcome: 5.2 Use of rescue medication.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 1 Pain free at 2 hours.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 3 Headache relief at 1 hour.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 4 24‐hour sustained headache relief.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 5 Pain free at 2 hours ‐ effect of formulation.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 6 Headache relief at 2 hours ‐ effect of formulation.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 7 Relief of associated symptoms at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 1 Pain free at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 3 24‐hour sustained headache relief.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 4 Relief of associated symptoms at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 1 Pain free at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 2 Headache relief at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 3 Headache relief at 1 hour.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 4 Relief of associated symptoms at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 1 Pain free at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 2 Headache relief at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 3 Relief of associated symptoms at 2 hours.

Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 1 Any adverse event within 24 hours.

Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 2 Use of rescue medication.
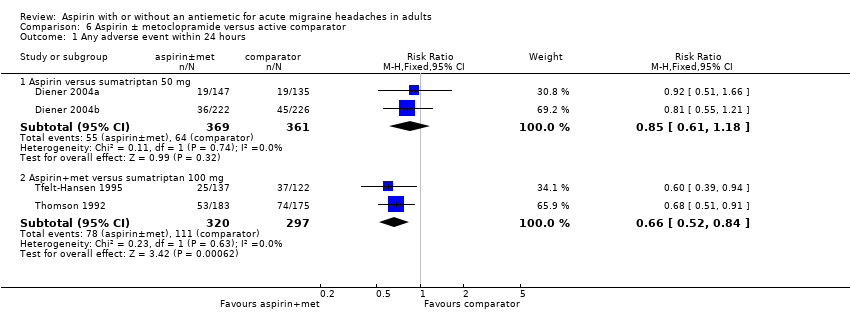
Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 1 Any adverse event within 24 hours.

Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 2 Use of rescue medication.
| Aspirin 900 mg or 1000 mg compared with placebo for migraine headache | ||||||
| Patient or population: migraine headache ‐ moderate or severe pain Settings: community Intervention: aspirin 900 mg or 1000 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | NNT or NNTH and/or | No of Participants | Quality of the evidence | Comments |
| Pain‐free at 2 h | 240 in 1000 | 110 in 1000 | NNT 8.1 (6.4 to 11) | 6 studies, 2027 participants 357 events | Moderate1 | Standard tablet and soluble formulations |
| Headache relief at 2 h | 520 in 1000 | 320 in 1000 | NNT 4.9 (4.1 to 6.2) | 6 studies, 2027 participants 848 events | Moderate1 | Standard tablet and soluble formulations |
| Sustained pain‐free at 24 h | No data |
|
|
|
|
|
| Sustained headache relief at 24 h | 390 in 1000 | 240 in 1000 | NNT 6.6 (4.9 to 10) | 3 studies, 1142 participants 361 events | Moderate1 | Standard tablet and soluble formulations |
| At least one AE | 120 in 1000 | 90 in 1000 | NNH 34 (18 to 340) | 5 studies, 1892 participants 206 events | Low | Standard tablet and soluble formulations |
| Serious AE | Insufficient data | |||||
| GRADE Working Group grades of evidence | ||||||
| 1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.70, 2.55] |
| 2 Headache relief at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.48, 1.83] |
| 3 Headache relief at 1 hour Show forest plot | 4 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.72, 2.63] |
| 4 24‐hour sustained headache relief Show forest plot | 3 | 1142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.37, 1.95] |
| 5 Pain free at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.51, 2.44] |
| 5.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.70, 3.58] |
| 6 Headache relief at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.43, 1.89] |
| 6.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.38, 1.95] |
| 7 Relief of associated symptoms at 2 hours Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Nausea | 4 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.10, 1.44] |
| 7.2 Vomiting | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.94, 1.34] |
| 7.3 Photophobia | 5 | 1274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.29, 1.69] |
| 7.4 Phonophobia | 5 | 1217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.27, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [1.59, 4.55] |
| 2 Headache relief at 2 hours Show forest plot | 3 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.78, 2.60] |
| 3 24‐hour sustained headache relief Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.39, 3.41] |
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 2 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.53 [4.20, 13.50] |
| 4.2 Vomiting | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.14 [2.30, 113.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.03] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.11] |
| 3 Headache relief at 1 hour Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.26, 1.99] |
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Photophobia | 2 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.04] |
| 4.2 Phonophobia | 2 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sumatriptan 100 mg | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.87] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sumartiptan 100 mg | 2 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.10] |
| 3 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 3.2 Vomiting | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.59 [1.43, 78.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 7 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.02, 1.55] |
| 1.1 Aspirin alone | 5 | 1892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.00, 1.68] |
| 1.2 Aspirin + metoclopramide | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.82, 1.67] |
| 2 Use of rescue medication Show forest plot | 8 | 2922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.63, 0.72] |
| 2.1 Aspirin 100 mg alone | 5 | 1881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.73] |
| 2.2 Aspirin 900 mg + metoclopramide 10 mg | 3 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aspirin versus sumatriptan 50 mg | 2 | 730 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 1.2 Aspirin+met versus sumatriptan 100 mg | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.84] |
| 2 Use of rescue medication Show forest plot | 4 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.01, 1.28] |
| 2.1 Aspirin versus sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 2.2 Aspirin+met versus sumatriptan 100 mg | 2 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.39] |

