Aspirina con o sin antiemético para la migraña aguda en adultos
Resumen
Antecedentes
Ésta es una versión actualizada de la revisión Cochrane original publicada en el número 4, 2010 (Kirthi 2010). La migraña es un trastorno común e invalidante y una carga para el individuo, los servicios sanitarios y la sociedad. Muchos enfermos eligen no buscar ayuda profesional o no pueden hacerlo y dependen de los analgésicos sin prescripción. El tratamiento con un antiemético debe ayudar a reducir las náuseas y los vómitos habitualmente asociados con la migraña.
Objetivos
Determinar la eficacia y la tolerabilidad de la aspirina, sola o en combinación con un antiemético, comparado con placebo y otras intervenciones activas en el tratamiento de las migrañas agudas en adultos.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, EMBASE, la Oxford Pain Relief Database, ClinicalTrials.gov, y en listas de referencias de los estudios hasta el 10 de marzo de 2010 para la revisión original y hasta el 31 de enero de 2013 para la actualización.
Criterios de selección
Se incluyeron los estudios aleatorizados, doble ciego, controlados con placebo o tratamiento activo, o ambos, que utilizaron aspirina para el tratamiento de un episodio de migraña, con al menos diez participantes por brazo de tratamiento.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos. Los números de participantes que lograron cada resultado se utilizaron para calcular el riesgo relativo y los números necesarios a tratar (NNT) o dañar (NND) en comparación con placebo u otro tratamiento activo.
Resultados principales
No se encontraron más estudios para esta actualización. Trece estudios (4222 participantes) compararon aspirina de 900 mg o 1000 mg, sola o en combinación con metoclopramida de 10 mg, con placebo u otros comparadores activos, principalmente sumatriptán de 50 mg o 100 mg. En todos los resultados de eficacia todos los tratamientos activos fueron superiores a placebo, con NNT de 8,1; 4,9 y 6,6 para los resultados sin dolor a las dos horas, alivio de la cefalea a las dos horas y alivio de la cefalea a las 24 horas con aspirina sola versus placebo y de 8,8; 3,3 y 6,2 con aspirina más metoclopramida versus placebo. El sumatriptán de 50 mg no fue diferente de la aspirina sola para los resultados sin dolor y alivio de la cefalea a las dos horas, mientras que el sumatriptán de 100 mg fue mejor que la combinación de aspirina más metoclopramida para el resultado sin dolor a las dos horas, pero no para el alivio de la cefalea; no hubo datos para el alivio del dolor a las 24 horas.
Los eventos adversos fueron fundamentalmente leves y transitorios y ocurrieron ligeramente con más frecuencia con aspirina que con placebo.
La metoclopramida adicional redujo significativamente las náuseas (p < 0,00006) y los vómitos (p = 0,002) en comparación con la aspirina sola.
Conclusiones de los autores
No se encontraron estudios nuevos desde la última versión de esta revisión. La aspirina de 1000 mg es un tratamiento efectivo para las migrañas agudas, similar al sumatriptán de 50 mg o 100 mg. El agregado de metoclopramida de 10 mg mejora el alivio de las náuseas y los vómitos. Los eventos adversos fundamentalmente fueron leves y transitorios, y ocurrieron ligeramente con más frecuencia con aspirina que con placebo, aunque fueron menos comunes que con sumatriptán de 100 mg.
PICO
Resumen en términos sencillos
Aspirina con o sin antiemético para la migraña aguda en adultos
Esta es una versión actualizada de la revisión Cochrane original publicada en el Número 4, 2010 (Kirthi 2010); no se encontraron estudios nuevos. Una dosis oral única de 1000 mg de aspirina alivió el dolor de moderado o intenso a ningún dolor a las dos horas en aproximadamente uno de cuatro pacientes (24%) que recibieron aspirina, en comparación con cerca de uno de diez (11%) que recibieron placebo. El dolor se redujo de moderado o intenso a ningún dolor peor que el dolor leve a las dos horas en cerca de uno de dos pacientes (52%) que recibieron aspirina, en comparación con cerca de uno de tres (32%) que recibieron placebo. De los que tuvieron un alivio efectivo de la cefalea a las dos horas, más pacientes presentaron un alivio mantenido durante 24 horas con aspirina en comparación con placebo. El agregado de 10 mg del antiemético metoclopramida aumentó de manera significativa el alivio de las náuseas y los vómitos en comparación con aspirina sola, aunque se observaron pocas diferencias en cuanto al dolor.
El sumatriptán oral de 100 mg fue mejor que la aspirina más metoclopramida para la respuesta sin dolor a las dos horas, aunque por otro lado no hubo diferencias importantes entre la aspirina con o sin metoclopramida y el sumatriptán de 50 mg o 100 mg. Los eventos adversos con el uso a corto plazo fueron fundamentalmente leves y transitorios, y ocurrieron ligeramente con más frecuencia con aspirina que con placebo y con sumatriptán de 100 mg que con aspirina.
Authors' conclusions
Summary of findings
| Aspirin 900 mg or 1000 mg compared with placebo for migraine headache | ||||||
| Patient or population: migraine headache ‐ moderate or severe pain Settings: community Intervention: aspirin 900 mg or 1000 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | NNT or NNTH and/or | No of Participants | Quality of the evidence | Comments |
| Pain‐free at 2 h | 240 in 1000 | 110 in 1000 | NNT 8.1 (6.4 to 11) | 6 studies, 2027 participants 357 events | Moderate1 | Standard tablet and soluble formulations |
| Headache relief at 2 h | 520 in 1000 | 320 in 1000 | NNT 4.9 (4.1 to 6.2) | 6 studies, 2027 participants 848 events | Moderate1 | Standard tablet and soluble formulations |
| Sustained pain‐free at 24 h | No data |
|
|
|
|
|
| Sustained headache relief at 24 h | 390 in 1000 | 240 in 1000 | NNT 6.6 (4.9 to 10) | 3 studies, 1142 participants 361 events | Moderate1 | Standard tablet and soluble formulations |
| At least one AE | 120 in 1000 | 90 in 1000 | NNH 34 (18 to 340) | 5 studies, 1892 participants 206 events | Low | Standard tablet and soluble formulations |
| Serious AE | Insufficient data | |||||
| GRADE Working Group grades of evidence | ||||||
| 1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events | ||||||
Background
This is an updated version of the original Cochrane review published in Issue 4, 2010 (Kirthi 2010).
Description of the condition
Migraine is a common, disabling headache disorder, with considerable social and economic impact (Hazard 2009). Recent surveys found a one year prevalence of 15% for adults in European countries (Stovner 2010) and13% for all ages in the United States (US) (Victor 2010), 21% in Russia (Ayzenberg 2012) and 9% for adults in China (Yu 2012). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes (IHS 2004). Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity and associated with nausea or photophobia and phonophobia, or both. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of 5 to 20 minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases the headache may lack migrainus features or be absent altogether (IHS 2004).
A recent large prevalence study in the US found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers seek professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for offering or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen) and paracetamol with caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, functional health and well‐being is well documented (Buse 2011; Leonardi 2005; Vos 2012) A cross‐sectional survey of eight European Union (EU) countries (representing 55% of the adult population) has estimated an annual direct and indirect cost of migraine per person of €1222, and a total annual cost for the EU of €111 billion for adults aged 18 to 65 years (Linde 2012). Costs are substantially greater for the minority with chronic migraine compared with episodic migraine; they also vary between countries, probably due to differences in available therapies and they way they are delivered, and structural differences in healthcare systems (Bloudek 2012). In the US, the average annual direct cost per person has been estimated at $1757 for episodic migraine and $7750 for chronic migraine (Munakata 2009). Whatever the exact direct and indirect costs are for each country, it is clear that migraine presents a significant economic burden. Successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also has the potential to reduce the need for healthcare resources and increase economic productivity. Migraine is ranked in the top 10 disorders for global years lived with disability (Vos 2012).
Description of the intervention
Medicines derived from willow bark, which is rich in salicylate, have been used for centuries for treating pain, fever and inflammation. In the mid‐19th century, chemists first synthesised acetylsalicylic acid, and by the end of the century, Bayer had patented and was selling the drug, which they called aspirin, around the world.
Aspirin is used to treat mild to moderate pain, including migraine headache pain; inflammatory conditions such as rheumatoid arthritis; and, in low doses, it is used as an antiplatelet agent in cardiovascular disease. It is a potent gastrointestinal irritant, and may cause discomfort, ulcers and bleeding. It may aIso cause tinnitus at high doses, and it is no longer used in children and adolescents, in whom it may cause Reye's syndrome (swelling of the brain that may lead to coma and death). Its use as an analgesic and antipyretic agent has declined, largely due to these adverse events, as newer products have become available. However, in some countries it may be the only drug readily available, and for some conditions, such as migraine, some individuals report it to be an effective and reliable treatment.
In order to establish whether aspirin is an effective analgesic at a specified dose in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once analgesic efficacy is established in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine attack early while pain is mild, and using a low dose initially, with a second dose if response is inadequate.
How the intervention might work
Aspirin irreversibly inhibits cyclo‐oxygenase enzymes, which are needed for prostaglandin and thromboxane synthesis. Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in mediating inflammatory and nociceptive processes. Suppression of prostaglandin synthesis is believed to underlie the analgesic effects of aspirin (Vane 1971).
The efficacy of oral medications is reduced in many migraineurs because of impaired gastrointestinal motility, which is associated with nausea, and because of non‐absorption of the drug due to vomiting (Volans 1974). The addition of an antiemetic may improve outcomes by alleviating the often incapacitating symptoms of nausea and vomiting, and (at least potentially) by enhancing the bioavailability of the co‐administered analgesic. In particular, prokinetic antiemetics such as metoclopramide, which stimulate gastric emptying, may improve outcomes by increasing absorption of the analgesic (in this case, aspirin; Ross‐Lee 1983; Volans 1975). It has been claimed that treatment with metoclopramide alone can reduce pain in severe migraine attacks (Colman 2004; Salazar‐Tortolero 2008), but this claim requires further investigation because it is based on studies involving few participants, and metoclopramide has not been shown to be an analgesic in classical pain studies. The present review sought to determine whether treatment of acute migraine attacks with aspirin plus an antiemetic is in any way superior to treatment with aspirin alone.
Why it is important to do this review
Population surveys show that aspirin is frequently used to treat migraine headaches, but we could find no systematic review of the efficacy of this intervention in adults. It is important to know where this widely available and inexpensive drug fits in the range of therapeutic options for migraine therapy. For many migraineurs, non‐prescription therapies offer convenience and may be the only therapies available or affordable. Aspirin is included in the World Health Organization (WHO) essential medicines list (WHO 2011).
This review is one of a series examining the efficacy of OTC treatments for migraine, including ibuprofen (Rabbie 2013), paracetamol (acetaminophen; Derry 2013a), and diclofenac (Derry 2013b), as well as oral sumatriptan (Derry 2012b), which is available without prescription in some countries.
Objectives
The objective of this review is to determine the efficacy and tolerability of aspirin, alone or in combination with an antiemetic, compared to placebo and other active interventions in the treatment of acute migraine headaches in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐controlled or active‐controlled studies using aspirin to treat a migraine headache episode. Studies had to have a minimum of 10 participants per treatment arm and report dichotomous data for at least one of the outcomes specified. We accepted studies reporting treatment of consecutive headache episodes if outcomes for the first, or each, episode were reported separately. We accepted cross‐over studies if there was adequate (at least 24 hours) washout between treatments.
Types of participants
Studies included adults (at least 18 years of age) with migraine. We used the definition of migraine specified by the IHS (IHS 1988; IHS 2004), although other definitions were considered if they conformed in general to IHS diagnostic criteria. There were no restrictions on migraine frequency, duration or type (with or without aura). We accepted studies that included participants taking stable prophylactic therapy to reduce the frequency of migraine attacks. If reported, details on any prophylactic therapy prescribed or allowed are provided in the Characteristics of included studies table.
Types of interventions
We included studies using a single dose of aspirin to treat a migraine headache episode when pain was of moderate to severe intensity, or investigated different dosing strategies or timing, or both, of the first dose in relation to headache intensity. There were no restrictions on dose or route of administration, provided the medication was self‐administered.
Included studies could use either aspirin alone, or aspirin plus an antiemetic. The antiemetic had to be taken either combined with aspirin in a single formulation, or separately not more than 30 minutes before aspirin, and had to be self‐administered.
A placebo comparator is essential to demonstrate that aspirin is effective in this condition. We considered active‐controlled trials without a placebo as secondary evidence. We excluded studies designed to demonstrate prophylactic efficacy in reducing the number or frequency of migraine attacks.
Types of outcome measures
In selecting the main outcome measures for this review, we considered scientific rigour, availability of data and patient preferences (Lipton 1999). Patients with acute migraine headaches have rated complete pain relief, no headache recurrence, rapid onset of pain relief, and no side effects as the four most important outcomes (Lipton 1999).
In view of these patient preferences, and in line with the guidelines for controlled trials of drugs in migraine issued by the IHS (IHS 2000), the main outcomes to be considered were:
Primary outcomes
-
Pain‐free at two hours, without the use of rescue medication (PF2).
-
Reduction in headache pain ('headache relief') at wo hours (HR2) ‐ pain reduced from moderate or severe to none or mild without the use of rescue medication.
Data for pain‐free and headache relief at earlier time points were collected when reported and relevant, in this case because an effervescent formulation for early relief of headache pain was used in some studies.
Secondary outcomes
-
Sustained pain‐free during 24 hours (SPF24) ‐ pain‐free within two hours, with no use of rescue medication or recurrence of moderate to severe pain within 24 hours.
-
Sustained pain reduction over 24 hours (SHR24) ‐ headache relief at two hours, sustained for 24 hours, with no use of rescue medication or a second dose of study medication.
-
Adverse events: participants with any adverse event during 24 hours postdose; serious adverse events; adverse events leading to withdrawal.
Pain intensity or pain relief had to be measured by the patient (not the investigator or care provider). Pain measures accepted for the primary outcomes were:
-
Pain intensity (PI): 4‐point categorical scale, with wording equivalent to none, mild, moderate and severe; or 100 mm visual analogue scale (VAS), where < 30 mm was considered equivalent to mild or no pain and ≥ 30 mm equivalent to moderate or severe pain (Collins 1997);
-
Pain relief (PR): 5‐point categorical scale, with wording equivalent to none, a little, some, a lot, complete; or 100 mm VAS, where < 30 mm was considered equivalent to none or a little, and ≥ 30 mm equivalent to some, a lot or complete.
We considered only data obtained directly from the patient.
Other outcomes
In the earlier review we reviewed a number of other secondary outcomes:
-
use of rescue medication.
-
relief of headache‐associated symptoms.
-
relief of functional disability.
These are not now reported in detail in Results but have been moved to an Appendix (see 'Differences between the protocol and the review').
Definitions of important terms, including all measured outcomes, are provided in Appendix 1.
Search methods for identification of studies
Electronic searches
The following databases were searched for the original review on 10 March 2010:
-
the Cochrane Central Register of Controlled Trials (CENTRAL);
-
MEDLINE (via Ovid);
-
EMBASE (via Ovid);
-
Oxford Pain Relief Database (Jadad 1996a).
For the update we searched:
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 12, 2012);
-
MEDLINE (via Ovid) from 1 January 2010 to 31 January 2013;
-
EMBASE (via Ovid) from 1 January 2010 to 31 January 2013.
See Appendix 2 for the search strategy for MEDLINE, Appendix 3 for the search strategy for EMBASE, and Appendix 4 for the search strategy for CENTRAL. There were no language restrictions.
Searching other resources
We searched reference lists of retrieved studies and review articles for additional studies, and for the update we searched www.Clinicaltrials.gov for information about both published and unpublished data, but no additional studies were identified. Grey literature and abstracts were not searched.
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed titles and abstracts of all studies identified by the electronic searches on screen, and excluded any that clearly did not satisfy inclusion criteria. We read full copies of the remaining studies to identify those suitable for inclusion. Disagreements were settled by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements were settled by discussion with a third review author. One author entered data for the original review into RevMan 5.0, and one author entered information for the update (RevMan 2012).
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996b) as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum. The scores for each study are reported in the Characteristics of included studies table.
Two authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study:
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). Studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number) were excluded.
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). Studies that did not conceal allocation (e.g. open list) were excluded.
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). Studies that were not double‐blind were excluded.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants provided no data without acceptable reason ‐ e.g. they were randomised but did not have a qualifying headache). Studies with high data loss were excluded.
-
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
We used relative risk (or 'risk ratio', RR) to establish statistical difference. We used numbers needed to treat (NNT) and pooled percentages as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
-
When significantly fewer adverse outcomes occur with aspirin than with control (placebo or active) we use the term the number needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occur with aspirin compared with control (placebo or active) we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
The unit of analysis was the individual patient.
Dealing with missing data
The most likely source of missing data was in cross‐over studies; we planned to use only first‐period data where possible, but where that was not provided, we treated the results as if they were parallel group results. Where there were substantial missing data in any study, we would comment on this and perform sensitivity analyses to investigate their effect.
For all outcomes we carried out analyses, as far as possible, on a modified intention‐to‐treat basis, i.e. we included all participants who were randomised and received an intervention. Where sufficient information was reported, we re‐included missing data in the analyses we undertook. We would exclude data from outcomes where results from ≥ 10% of participants are missing with no acceptable reason provided or apparent.
Assessment of heterogeneity
We assessed heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in results of individual studies. (L'Abbé 1987). Where data could be pooled, we report the I2 statistic.
Assessment of reporting biases
We assessed publication bias by examining the number of participants in trials with zero effect (relative risk of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as an NNT of ≥ 8 for pain‐free at two hours, and NNT ≥6 for headache relief at two hours.
Data synthesis
We analysed studies using a single dose of aspirin in established pain of at least moderate intensity separately from studies in which medication was taken before pain was well established or in which a second dose of medication was permitted.
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). We calculated relative risk of benefit or harm with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp and NNH with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). A statistically significant difference from control is assumed when the 95% CI of the relative risk of benefit or harm did not include the number one.
We used the z test to determine significant differences between NNT, NNTp or NNH for different groups in subgroup and sensitivity analyses (Tramèr 1997).
We describe data from comparisons and outcomes with only one study or fewer than 200 participants in the text and summary tables where appropriate for information and comparison, but they are not analysed quantitatively.
Subgroup analysis and investigation of heterogeneity
Issues for potential subgroup analysis were dose, monotherapy versus combination with an antiemetic, formulation and route of administration. For combined treatment with an antiemetic, we planned to compare different antiemetics if there were sufficient data.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more), and for migraine type (with aura versus without aura). A minimum of two studies and 200 participants had to be available for any sensitivity analysis.
Results
Description of studies
Included studies
New searches in January 2013 did not identify any additional studies.
Thirteen studies fulfilled the inclusion criteria for this review (Boureau 1994; Chabriat 1994; Diener 2004a; Diener 2004b; Geraud 2002; Henry 1995; Lange 2000; Le Jeunne 1998; Lipton 2005; MacGregor 2002; Tfelt‐Hansen 1995; Thomson 1992; Titus 2001), with a total of 5261 treated migraine attacks (4222 participants) providing data. Boureau 1994; Diener 2004b; MacGregor 2002 together provided information on treatment of 1039 more migraine attacks than participants. Details of the included studies are provided in the Characteristics of included studies table.
All included studies recruited participants between 18 and 65 years of age (mean ages ranging from 37 to 44 years), meeting IHS criteria for migraine with or without aura (IHS 1988; IHS 2004). All participants had a history of migraine symptoms for at least 12 months, with between one and six attacks per month of moderate to severe intensity, prior to the study period. One study (Thomson 1992) excluded participants needing prophylactic treatment. Four studies specified that any prophylactic treatment had to have been stable for at least two (Boureau 1994) or three (Chabriat 1994; Le Jeunne 1998; Lipton 2005) months before the study period, while the remainder did not mention prophylaxis.
Three studies excluded participants who vomited either at least 20% of the time during migraine attacks (Lange 2000; Lipton 2005) or during the majority of attacks (MacGregor 2002). Another study excluded participants whose migraine headaches were never accompanied by nausea or vomiting (Chabriat 1994). One study excluded data from migraine attacks with aura (Henry 1995), whilst another study used only data from migraine attacks without aura that also featured all three symptoms of nausea, photophobia and phonophobia (Diener 2004a). Five studies excluded participants who also experienced other types of headache (Boureau 1994; Diener 2004b; Geraud 2002; Henry 1995; Titus 2001).
Five studies had only a placebo comparator (Chabriat 1994; Henry 1995; Lange 2000; Lipton 2005; MacGregor 2002), four had only an active comparator (Geraud 2002; Le Jeunne 1998; Thomson 1992; Titus 2001), and four had both placebo and active comparators (Boureau 1994; Diener 2004a; Diener 2004b; Tfelt‐Hansen 1995).
All treatments were administered orally, and when the headache was of moderate or severe intensity, except in Boureau 1994, where up to 15% of participants had "slight" headache at baseline. No studies specifically investigated early treatment of attacks while pain intensity was still mild. Aspirin 1000 mg was given either as a tablet or an effervescent solution to 940 participants in five studies (Boureau 1994; Diener 2004a; Diener 2004b; Lange 2000; Lipton 2005). In one study, aspirin was given to 73 participants as a 900 mg mouth‐dispersible dose (MacGregor 2002). In seven studies, aspirin equivalent to 900 mg was given either as the lysine salt, calcium carbasalate (a soluble complex of aspirin) or an effervescent solution, in combination with metoclopramide 10 mg, to 1186 participants (Chabriat 1994; Geraud 2002; Henry 1995; Le Jeunne 1998; Tfelt‐Hansen 1995; Thomson 1992; Titus 2001). We did not identify any studies in which aspirin was combined with an antiemetic other than metoclopramide.
Sumatriptan 50 mg was given to 361 participants in two studies (Diener 2004a; Diener 2004b), and sumatriptan 100 mg to 294 participants in another two studies (Tfelt‐Hansen 1995; Thomson 1992). Zolmitriptan 2.5 mg was given to 326 participants in one study (Geraud 2002). Four studies compared aspirin treatment with non‐triptan medications: one gave acetaminophen 400 mg plus codeine 25 mg to 198 participants (Boureau 1994); one gave ibuprofen 400 mg to 212 participants (Diener 2004b); one gave ergotamine 1 mg plus 100 mg caffeine to 132 participants (Le Jeunne 1998); and the other gave ergotamine 2 mg plus caffeine 200 mg to 115 participants (Titus 2001). A total of 1424 participants received placebo.
Some studies were inconsistent in the denominators reported and, for instance, reported on one or two patients fewer than the intention‐to‐treat population for some outcomes, but not for others, without giving a reason. As the denominators were always within a few patients of the intention‐to‐treat population, we used the denominators given.
Three studies (Boureau 1994; Diener 2004b; MacGregor 2002) used a cross‐over design in which participants treated consecutive headaches with different study medications. Boureau 1994 did not specify a washout period between attacks, while Diener 2004b and MacGregor 2002 specified a minimum of 48 hours between qualifying attacks. The remaining studies used a parallel‐group design, in which participants received only one type of medication. In five studies (Diener 2004a; Henry 1995; Lange 2000; Lipton 2005; Titus 2001) participants treated only one attack, while in the remaining five studies they treated more than one attack with the same medication. In Chabriat 1994, Le Jeunne 1998 and Tfelt‐Hansen 1995 two attacks were treated, while in Geraud 2002 and Thomson 1992 three attacks were treated; some outcomes were reported for each attack separately, while for other outcomes attacks were combined. We have used data for the first attack only, where these data were reported separately, for efficacy outcomes to avoid problems of double counting participants and repeated measures for the same individuals; for use of rescue medication and adverse event data, we have accepted data from multiple attacks in the absence of first‐attack data in order to be inclusive and provide conservative estimates. In Geraud 2002, a second dose of study medication was permitted if there was an inadequate response to the first; data for the first dose in the first attack were available for one primary outcome. In Titus 2001 one attack was treated with up to three doses of the same medication if an adequate response was not obtained; data for our primary outcomes were not available for the first dose only.
Excluded studies
Six studies were excluded after reading the full report (Chabriat 1993; Diener 2005; Limmroth 1999; Nebe 1995; Tfelt‐Hansen 1980; Tfelt‐Hansen 1984). Reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
All studies were randomised and double‐blind, and all reported on withdrawals and dropouts, thus minimising bias. Four scored 5 of 5 (Diener 2004a; Diener 2004b; Geraud 2002; Titus 2001), six scored 4 of 5 (Boureau 1994; Le Jeunne 1998; Lipton 2005; MacGregor 2002; Tfelt‐Hansen 1995; Thomson 1992) and three scored 3 of 5 (Chabriat 1993; Henry 1995; Lange 2000) on the Oxford Quality Score. Points were lost because of failure to adequately describe the methods of randomisation and blinding. Details are provided in the Characteristics of included studies table.
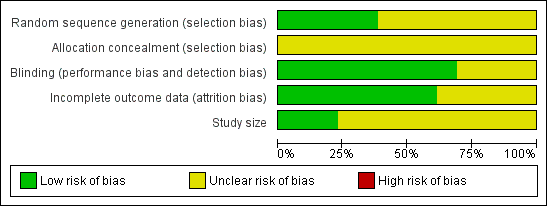
A risk of bias table was completed for randomisation, allocation concealment, blinding, incomplete outcome data, and study size. No study described the method of allocation concealment, and only three (Diener 2004b; Geraud 2002; Lipton 2005) had more than 200 attacks per treatment arm, but none were at high risk of bias (Figure 1).
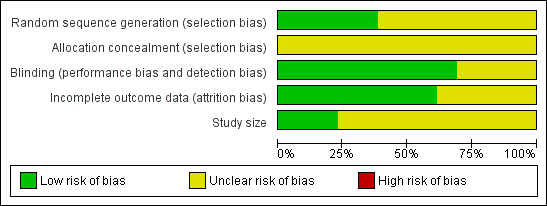
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
See: Summary of findings for the main comparison
Aspirin doses of 900 mg and 1000 mg were considered sufficiently similar to combine for analysis. All included studies that provided data for analysis reported outcomes using the standard 4‐point categorical pain intensity scale (none, mild, moderate, severe).
Details of outcomes in individual studies are provided in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals). Results for pain‐free and headache relief responses are summarised in Summary of results A.
Pain‐free at two hours
Aspirin 900 mg or 1000 mg versus placebo
Six studies (2027 participants) provided data; three used a 1000 mg effervescent formulation of aspirin (Diener 2004a; Diener 2004b; Lange 2000), two used a 1000 mg oral tablet formulation (Boureau 1994; Lipton 2005) and one used a 900 mg mouth‐dispersible dose (MacGregor 2002).
-
The proportion of participants pain‐free at two hours with aspirin 1000 mg was 24% (240/1008; range 14% to 29%).
-
The proportion of participants pain‐free at two hours with placebo was 11% (117/1019; range 5% to 17%).
-
The relative benefit of treatment compared with placebo was 2.1 (95% CI 1.7 to 2.6) (Analysis 1.1; Figure 2); the NNT was 8.1 (6.4 to 11).
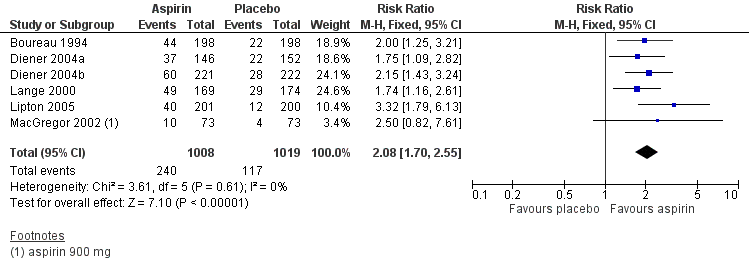
Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.1 Pain free at 2 hours.
Aspirin 900 mg plus metoclopramide 10 mg versus placebo
Two studies (519 participants) provided data for the 900 mg oral tablet formulation in combination with oral metoclopramide 10 mg versus placebo (Henry 1995; Tfelt‐Hansen 1995).
-
The proportion of participants pain‐free at two hours with aspirin 900 mg plus metoclopramide 10 mg was 18% (47/262; range 14% to 21%).
-
The proportion of participants pain‐free at two hours with placebo was 7% (17/257; range 5% to 8%).
-
The relative benefit of treatment compared with placebo was 2.7 (1.6 to 4.6) (Analysis 2.1); the NNT was 8.8 (5.9 to 17).
Subgroup analysis comparing studies using aspirin alone and studies using aspirin plus metoclopramide gave z = 0.3051, P = 0.76. There was no significant difference between treatments for this outcome.
A L'Abbé plot for this outcome shows a high degree of clinical homogeneity in these studies of aspirin ± metoclopramide versus placebo (Figure 3).
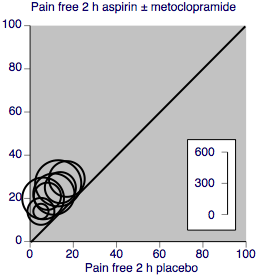
L'Abbé plot showing pain‐free at 2 h response in individual studies. Each circle represents one study, with size on the inset scale.
Aspirin 1000 mg versus active comparator
Two studies (726 participants) compared effervescent aspirin 1000 mg with sumatriptan 50 mg (Diener 2004a; Diener 2004b).
-
The proportion of participants pain‐free at two hours with aspirin 1000 mg was 26% (97/367).
-
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 32% (116/359).
-
The relative benefit of aspirin compared with sumatriptan was 0.82 (0.65 to 1.03) (Analysis 3.1). There was no significant difference between treatments.
One study (Boureau 1994) compared 1000 mg aspirin (tablet) with paracetamol 400 mg plus codeine 25 mg, while another (Diener 2004b) compared 1000 mg aspirin (tablet) with ibuprofen 400 mg. For neither were there sufficient data for analysis (Appendix 5).
Aspirin 900 mg plus metoclopramide 10 mg versus active comparator
Two studies (528 participants) compared aspirin 900 mg (tablet or lysine equivalent) plus metoclopramide 10 mg with sumatriptan 100 mg (Tfelt‐Hansen 1995; Thomson 1992).
-
The proportion of participants pain‐free at two hours with aspirin 900 mg or 1000 mg plus metoclopramide 10 mg was 18% (48/273).
-
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 28% (71/255).
-
The relative benefit of aspirin plus metoclopramide compared with sumatriptan was 0.63 (0.45 to 0.87) (Analysis 4.1); the NNT for sumatriptan compared with aspirin plus metoclopramide was 9.8 (5.8 to 32).
One study (Le Jeunne 1998) compared aspirin 900 mg (as calcium carbasalate) plus metoclopramide 10 mg with ergotamine 1 mg plus caffeine 100 mg (266 participants). There were insufficient data for analysis (Appendix 5).
Headache relief at two hours
Aspirin 900 or 1000 mg versus placebo
Six studies (2027 participants) in which individuals were treated with aspirin alone provided data. Three studies used an effervescent formulation (Diener 2004a; Diener 2004b; Lange 2000), whilst the other three studies used a mouth‐dispersible (MacGregor 2002) or oral tablet formulation (Boureau 1994; Lipton 2005).
-
The proportion of participants experiencing headache relief at two hours with aspirin 900 mg or 1000 mg was 52% (525/1008; range 48% to 55%).
-
The proportion of participants experiencing headache relief at two hours with placebo was 32% (323/1019; range 19% to 37%).
-
The relative benefit of treatment compared with placebo was 1.6 (1.5 to 1.8) (Analysis 1.2; Figure 4); the NNT was 4.9 (4.1 to 6.2).
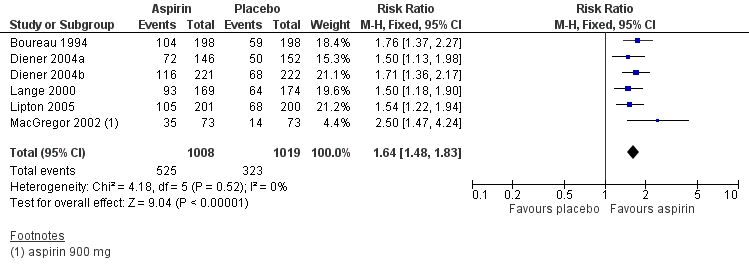
Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.2 Headache relief at 2 hours.
Aspirin 900 mg plus metoclopramide 10 mg versus placebo
Three studies (765 participants) provided data. Two studies used a lysine equivalent formulation of aspirin (Chabriat 1994; Tfelt‐Hansen 1995), whilst the other study used an effervescent formulation (Henry 1995).
-
The proportion of participants experiencing headache relief at two hours with aspirin 900 mg plus metoclopramide 10 mg was 57% (219/386; range 54% to 59%).
-
The proportion of participants experiencing headache relief at two hours with placebo was 26% (100/379; range 24% to 29%).
-
The relative benefit of treatment compared with placebo was 2.2 (1.8 to 2.6) (Analysis 2.2); the NNT was 3.3 (2.7 to 4.2).
Subgroup analysis comparing studies using aspirin alone and studies using aspirin plus metoclopramide gave z = 2.48, P = 0.0131, showing that aspirin plus metoclopramide was significantly more effective than aspirin alone at achieving headache relief at two hours.
A L'Abbé plot for this outcome shows a high degree of clinical homogeneity in these studies of aspirin ± metoclopramide versus placebo (Figure 5).
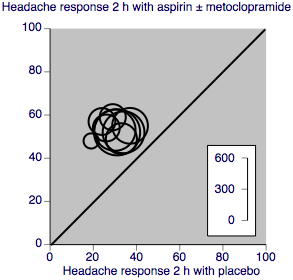
L'Abbé plot showing headache response at 2 h in individual studies. Each circle represents one study, with size on the inset scale.
Aspirin 1000 mg versus active comparator
Two studies (726 participants) compared effervescent aspirin 1000 mg with sumatriptan 50 mg (Diener 2004a; Diener 2004b).
-
The proportion of participants experiencing headache relief at two hours with aspirin 1000 mg was 51% (188/367).
-
The proportion of participants experiencing headache relief at two hours with sumatriptan 50 mg was 53% (191/359).
-
The relative benefit of aspirin compared with sumatriptan was 0.96 (0.84 to 1.1) (Analysis 3.2). There was no significant difference between treatments.
One study (Boureau 1994) compared 1000 mg aspirin (tablet) with paracetamol 400 mg plus codeine 25 mg, and another (Diener 2004b) compared 1000 mg aspirin (tablet) with ibuprofen 400 mg. In both cases there were insufficient data for analysis (Appendix 5).
Aspirin 900 mg plus metoclopramide 10 mg versus active comparator
Two studies (523 participants) compared aspirin 900 mg plus metoclopramide 10 mg with sumatriptan 100 mg (Tfelt‐Hansen 1995; Thomson 1992). One used the lysine equivalent and the other a standard oral tablet.
-
The proportion of participants experiencing headache relief at two hours with aspirin 900 mg plus metoclopramide 10 mg was 51% (138/271).
-
The proportion of participants experiencing headache relief at two hours with sumatriptan 100 mg was 54% (137/252).
-
The relative benefit of treatment compared with placebo was 0.93 (0.79 to 1.1) (Analysis 4.2). There was no significant difference between treatments.
One study each compared aspirin 900 mg plus metoclopramide 10 mg with zolmitriptan 25 mg (Geraud 2002) and ergotamine 1 mg plus caffeine 100 mg (Le Jeunne 1998). There were insufficient data for analysis with either comparator (Appendix 5). Titus 2001 compared aspirin 900 mg plus metoclopramide 10 mg with ergotamine 2 mg plus caffeine 200 mg, but reported no usable data.
Headache relief at one hour
Aspirin 900 or 1000 mg versus placebo
Four studies (1288 participants) comparing aspirin 900 mg or 1000 mg with placebo provided data. Two used an effervescent formulation (Diener 2004a; Diener 2004b), one a mouth dispersible formulation (MacGregor 2002) and one a tablet (Lipton 2005).
-
The proportion of participants experiencing headache relief at one hour with aspirin 900 mg or 1000 mg was 33% (209/641; range 24% to 41%.
-
The proportion of participants experiencing headache relief at one hour with placebo was 15% (99/647; range 11% to 21%).
-
The relative benefit of treatment compared with placebo was 2.1 (1.7 to 2.6) (Analysis 1.3); the NNT was 5.8 (4.3 to 8.0).
Removing the study using the tablet formulation (Lipton 2005) did not significantly change the result (RR 2.3 (1.7 to 3.0); NNT 5.6 (3.4 to 5.4)).
Aspirin 1000 mg versus active comparator
Two studies (726 participants) comparing aspirin 1000 mg with sumatriptan 50 mg provided data (Diener 2004a; Diener 2004b). Both used effervescent aspirin and oral sumatriptan.
-
The proportion of participants experiencing headache relief at 1 hour with aspirin 1000 mg was 38% (138/367; range 34% to 42%).
-
The proportion of participants experiencing headache relief at 1 hour with sumatriptan 50 mg was 24% (85/359; range 23% to 24%).
-
The relative benefit of treatment compared with sumatriptan 50 mg was 1.6 (1.3 to 2.0) (Analysis 3.3); the NNT was 7.2 (4.9 to 14).
One study (Diener 2004b) compared effervescent aspirin 1000 mg with ibuprofen 400 mg (432 participants). There were insufficient data for analysis.
Aspirin plus metoclopramide versus placebo or versus active comparator
No studies using aspirin plus metoclopramide reported on headache relief at one hour.
Sustained pain‐free at 24 hours
None of the studies provided data on the proportion of participants who were pain‐free at two hours and remained pain‐free for 24 hours.
Sustained headache relief at 24 hours
Aspirin 1000 mg versus placebo
Three studies (1142 participants) provided data for this outcome. Two used an effervescent formulation (Diener 2004a; Diener 2004b) and one a tablet (Lipton 2005).
-
The proportion of participants with 24‐hour sustained relief with aspirin 1000 mg was 39% (223/568; range 33% to 42%).
-
The proportion of participants with 24‐hour sustained relief with placebo was 24% (138/574; range 22% to 25%).
-
The relative benefit of treatment compared with placebo was 1.6 (1.4 to 2.0) (Analysis 1.4); the NNT was 6.6 (4.9 to 10).
Removing the study using the tablet formulation (Lipton 2005) did not significantly change the result (RR 1.6 (1.3 to 2.0); NNT 6.8 (4.7 to 12)).
Aspirin 900 mg plus metoclopramide 10 mg versus placebo
One study (257 participants) reported this outcome (Tfelt‐Hansen 1995). Because there were more than 200 participants, for information and comparison the results are given in Summary of results A; there was a significant benefit over placebo. Subgroup analysis comparing studies using aspirin alone and studies using aspirin plus metoclopramide gave z = 0.7789, P = 0.43, indicating no benefit with metoclopramide for this outcome.
| Summary of results A: Pain‐free and headache relief | ||||||
| Studies | Attacks treated | Treatment (%) | Placebo or comparator (%) | NNT/NNTp (95% CI) | P for difference | |
| Pain‐free at 2 hours | ||||||
| Aspirin 900 or 1000 mg versus placebo | 6 | 2027 | 24 | 11 | 8.1 (6.4 to 11) | z = 0.3051 P = 0.76 |
| Aspirin 900 mg plus metoclopramide 10 mg versus placebo | 2 | 519 | 18 | 7 | 8.8 (5.9 to 17) | |
| Aspirin 1000 mg versus sumatriptan 50 mg | 2 | 726 | 26 | 32 | Not calculated | |
| Aspirin 900 mg + metoclopramide 10 mg versus sumatriptan 100 mg | 2 | 528 | 18 | 28 | 9.8 (5.8 to 32) | |
| Headache relief at 2 hours | ||||||
| Aspirin 900 or 1000 mg versus placebo | 6 | 2027 | 52 | 32 | 4.9 (4.1 to 6.2) | z = 2.4847 P = 0.013 |
| Aspirin 900 mg plus metoclopramide 10 mg versus placebo | 3 | 765 | 57 | 26 | 3.3 (2.7 to 4.2) | |
| Aspirin 1000 mg versus sumatriptan 50 mg | 2 | 522 | 51 | 53 | Not calculated | |
| Aspirin 900 mg + metoclopramide 10 mg versus sumatriptan 100 mg | 2 | 523 | 51 | 54 | Not calculated | |
| Headache relief at 1 hour | ||||||
| Aspirin 900 or 1000 mg versus placebo | 4 | 1288 | 33 | 15 | 5.8 (4.6 to 7.9) | |
| Aspirin 1000 mg versus sumatriptan 50 mg | 2 | 726 | 38 | 24 | 7.2 (4.9 to 14) | |
| Sustained headache relief at 24 hours | ||||||
| Aspirin 1000 mg versus placebo | 3 | 1142 | 39 | 24 | 6.6 (4.9 to 10) | z = 0.7789 P = 0.43 |
| Aspirin 900 mg plus metoclopramide 10 mg versus placebo | 1 | 257 | 37 | 17 | Not calculated | |
Subgroup analyses
Dose, route of administration and choice of antiemetic drug
No subgroup analysis was possible for dose, route of administration or choice of antiemetic drug, since all studies used aspirin 900 mg or 1000 mg, all medication was administered orally, and the only antiemetic used was metoclopramide 10 mg.
Formulation: soluble versus tablet
For both pain‐free (Analysis 1.5) and headache relief at two hours (Analysis 1.6) with aspirin alone versus placebo, there was no difference between soluble formulations (effervescent and mouth soluble) and tablets. For headache relief at one hour (Analysis 1.3), removing the single study using the tablet formulation did not significantly change the result. There were insufficient data to investigate the effect of formulation for other outcomes.
Monotherapy versus combination with an antiemetic
Results for aspirin alone compared with aspirin plus antiemetic (metoclopramide) are dealt with in the main analysis above.
Sensitivity analyses
Sensitivity analysis according to methodological quality was not possible because all studies scored ≥ 3/5 on the Oxford Quality Score. Because one study (Boureau 1994) had no information on washout we repeated the analyses without this study. Omitting it made no difference to the results.
Adverse events
For studies that treated more than one attack with a single medication, results for adverse events were usually presented for all treated attacks. These data have been included in the adverse event analyses in order to be more inclusive and conservative (Appendix 6).
Any adverse event
All studies reported on the number of participants experiencing any adverse events after treatment; however, one did not report data for each treatment group separately (MacGregor 2002). Most studies appeared to collect data using spontaneous reports in diary cards. Studies did not specify whether adverse event data continued to be collected after any rescue medication was taken; it seems likely that they were. Treatments were generally described as well tolerated, with most adverse events being of mild or moderate severity and self‐limiting.
Results for participants experiencing any adverse event are summarised in Summary of results B.
Five studies with 1892 participants provided data on the number of participants experiencing adverse events for aspirin 1000 mg versus placebo (Boureau 1994; Diener 2004a; Diener 2004b; Lange 2000; Lipton 2005), and two studies with 566 participants for aspirin 900 mg plus metoclopramide versus placebo (Henry 1995; Tfelt‐Hansen 1995). Overall, adverse events occurred in 14% (172/1230) of aspirin (± metoclopramide)‐treated participants, and in 11% (136/1228) of placebo‐treated participants, giving an RR of 1.3 (1.02 to 1.6) (Analysis 5.1). The NNH was 34 (18 to 340).
Two studies (730 participants) provided data for aspirin 1000 mg versus sumatriptan 50 mg (Diener 2004a; Diener 2004b); 15% (55/369) of aspirin‐treated participants and 18% (64/361) of sumatriptan‐treated participants experienced adverse events. There was no significant difference between the treatments. Two studies (617 participants) reported data for aspirin 900 mg plus metoclopramide 10 mg compared with sumatriptan 100 mg (Tfelt‐Hansen 1995; Thomson 1992); 24% (78/320) of aspirin‐treated participants and 37% (111/297) of sumatriptan‐treated participants experienced adverse events, giving an RR of 0.66 (0.52 to 0.84) (Analysis 6.1). The NNTp was 8.4 (5.3 to 21).
One study presented adverse events data for aspirin 1000 mg versus ibuprofen 400 mg (Diener 2004b), and another reported for aspirin 1000 mg versus acetaminophen 400 mg with codeine 25 mg (Boureau 1994). Two studies reported adverse event data for aspirin 900 mg plus metoclopramide 10 mg compared with ergotamine 1 mg plus caffeine 100 mg (Le Jeunne 1998), or ergotamine 2 mg plus caffeine 200 mg (Titus 2001). There were insufficient data for analysis of this outcome for these active comparators (Appendix 6).
Overall, single doses of aspirin, with or without metoclopramide, did not cause significantly more or fewer adverse events in these studies than did placebo or comparator treatments, with the exception of sumatriptan 100 mg, where for every eight individuals treated with sumatriptan, one would experience adverse events who would not have done with aspirin plus metoclopramide.
| Summary of results B: Number of participants with adverse events within 24 hours of taking study medication | ||||||
| More or no more adverse events with treatment than comparator | Studies | Participants | Aspirin (%) | Comparator (%) | Relative risk (95% CI) | NNH (95% CI) |
| Aspirin ± metoclopramide versus placebo | 7 | 2458 | 14 | 11 | 1.3 (1.02 to 1.6) | 34 (18 to 340) |
| Aspirin 1000 mg versus placebo | 5 | 1892 | 12 | 9 | 1.3 (1.00 to 1.7) | Not calculated |
| Aspirin 900 mg with metoclopramide 10 mg versus placebo | 2 | 566 | 19 | 17 | 1.2 (0.82 to 1.7) | Not calculated |
| Fewer adverse events with treatment than comparator | Studies | Participants | Aspirin (%) | Comparator (%) | Relative risk (95% CI) | NNTp (95% CI) |
| Aspirin 1000 mg versus sumatriptan 50 mg | 2 | 730 | 15 | 18 | 0.85 (0.61 to 1.2) | Not calculated |
| Aspirin 900 mg plus metoclopramide 10 mg versus sumatriptan 100 mg | 2 | 642 | 24 | 36 | 0.66 (0.52 to 0.84) | 8.4 (5.3 to 21) |
Specific adverse events
Detailed adverse event reporting was inconsistent. Some studies did not report any details of individual adverse events; others reported all adverse events for each treatment group; while others reported those occurring in, say, ≥ 2% of participants, or reported events for a specific body system. The body systems most frequently affected were the digestive system and nervous system. Individual studies were underpowered to detect differences between treatment groups, and inconsistent reporting prevented pooling of data (Appendix 6).
Serious adverse events
Serious adverse events were uncommon and were reported in only five studies. In one study a case of phlebitis following use of aspirin plus metoclopramide was considered to be drug‐related, whilst another four events with aspirin plus metoclopramide and six with zolmitriptan were considered unrelated (Geraud 2002). In another study renal colic was reported in one participant after treating a migraine attack with aspirin, with no suspected causal relationship, but a perforated duodenal ulcer following use of ibuprofen was thought to be drug‐related (Diener 2004b). In Lipton 2005, one participant had a perforated appendix following placebo treatment, and no causal relationship to the study medication was suspected. Acute atrial fibrillation requiring hospital admission was reported in one participant, and prolonged palpitations in another, after treatment with sumatriptan 100 mg (Tfelt‐Hansen 1995). MacGregor 2002 reported two serious adverse events, considered unrelated to study medication: headache following treatment with aspirin and endometriosis following placebo. It is possible that these events were "severe" rather than "serious" (Appendix 6).
Three studies that provided data for adverse events did not explicitly state whether any serious adverse events had occurred (Boureau 1994; Diener 2004a; Henry 1995).
Withdrawals
Withdrawals due to adverse events were reported in six studies (Appendix 6). Geraud 2002 reported five withdrawals following aspirin plus metoclopramide (diarrhoea, palpitations plus asthenia, anxiety plus dry mouth, phlebitis) and three following zolmitriptan (dizziness, somnolence, vasodilation). MacGregor 2002 reported four withdrawals following aspirin (nausea, tinnitus, coughing, taste perversion) and none with placebo. Le Jeunne 1998 reported one withdrawal due to pulmonary embolism following aspirin plus metoclopramide, and one due to back pain following placebo. Tfelt‐Hansen 1995 reported one withdrawal following aspirin plus metoclopramide, four following sumatriptan, and one following placebo, with no details given. Thomson 1992 reported no withdrawals following aspirin, and five following sumatriptan (headache, faintness and vomiting; scalp tingling, heaviness in the chest, globus and prolonged aura; stomach pain; dyspnoea and heaviness; worsened headache and nausea). Titus 2001 reported one participant treated with aspirin plus metoclopramide who withdrew because of sinusitis.
Withdrawals for other reasons and exclusions for protocol violations or missing data were generally well reported, although it was not always clear whether they occurred before or after taking rescue medication. The numbers of withdrawals were not likely to affect estimates of efficacy or harm. No statistical analysis of withdrawals was carried out.
Discussion
Summary of main results
This review included 13 randomised, double‐blind, controlled studies, with 4222 participants treating 5261 migraine headaches of moderate to severe intensity with either aspirin alone or aspirin plus metoclopramide. Nine of the studies were placebo‐controlled; eight included an active comparator (sumatriptan, zolmitriptan, ibuprofen, paracetamol plus codeine, and ergotamine plus caffeine). No new studies were identified in searches carried out for the update in January 2013.
For the IHS preferred outcome of pain‐free at two hours, both aspirin 900 mg or 1000 mg alone and aspirin 900 mg plus metoclopramide 10 mg were better than placebo, with NNTs of 8 to 9, and with no significant difference between active treatments. Only around one in four or one in five individuals treated with aspirin achieved this outcome. Sumatriptan 100 mg was significantly better than aspirin plus metoclopramide for this outcome (NNT = 10), but sumatriptan 50 mg was not different from aspirin alone. For headache relief at two hours, aspirin plus metoclopramide was significantly better than aspirin alone, with NNTs versus placebo of 3.3 and 4.9, respectively (P = 0.013). Around half of individuals treated achieved this outcome. There were no differences between aspirin alone and sumatriptan 50 mg, or aspirin plus metoclopramide and sumatriptan 100 mg. Headache relief at one hour with aspirin alone versus placebo was not significantly different from headache relief at two hours (NNTs of 5.8 (4.6 to 7.9) and 4.9 (4.1 to 6.2), respectively). For sustained headache relief at 24 hours, aspirin alone was better than placebo (NNT = 6.6), as was the combination of aspirin plus metoclopramide (NNT = 6.2).
Using a more soluble formulation of aspirin did not appear to give a substantial benefit over standard tablets for any of the primary outcomes. One would expect that a soluble formulation would be more rapidly absorbed and provide more rapid relief, but this effect (if present) might be missed if the first assessments are not made until one or two hours after dosing. In some circumstances, early, rapid pain relief is accompanied by better overall pain relief, but there was no indication of this in these studies.
Overall, slightly more participants experienced adverse events with either aspirin alone or aspirin plus metoclopramide than with placebo, but the difference barely reached statistical significance. There were slightly more participants experiencing adverse events with sumatriptan than with aspirin, but the difference was statistically significant only for sumatriptan 100 mg versus aspirin plus metoclopramide (NNH = 8.4). Most adverse events were described as mild or moderate, and transient; the digestive and nervous systems were most commonly affected. There were few serious adverse events, and most were not thought to be causally related to study medication, although one event of phlebitis was attributed to aspirin.
There were very limited data for aspirin with or without metoclopramide compared with other active comparators; there was no evidence for substantial differences between these interventions and aspirin for the main efficacy outcomes in these studies. We did not include in this review studies where aspirin was used in combination with another analgesic.
Additional analyses (Appendix 7) show that fewer participants treated with either aspirin alone or aspirin plus metoclopramide than with placebo needed rescue medication (NNTp = 4.8). There was no difference for aspirin alone versus sumatriptan 50 mg for this outcome, but for aspirin plus metoclopramide versus sumatriptan 100 mg, the difference just reached statistical significance in favour of sumatriptan 100 mg (NNH = 11).
Both aspirin alone and aspirin plus metoclopramide were better than placebo for alleviation of headache‐associated symptoms, although there were too few vomiting events for reliable analysis, and no data for photophobia and phonophobia following aspirin plus metoclopramide. The combination therapy was significantly better than aspirin alone for relief of nausea (P < 0.00006) and vomiting (P = 0.002, but based on few data), as might be expected for an antiemetic.
Overall completeness and applicability of evidence
Included participants all had a diagnosis of migraine according to IHS criteria, with attacks occurring at a frequency of one to six per month and of moderate to severe intensity. Studies did not specifically recruit participants who usually used OTC medications, although Lipton 2005 excluded those previously unresponsive to them. Participants requiring prophylaxis were either excluded or were able to continue with stable prophylaxis. The population studied is therefore not likely to be greatly biased towards milder or OTC‐responsive individuals, or likely to exclude those with particularly difficult‐to‐treat headaches. Overall, there may be over‐selection of individuals with more severe or difficult headaches than the general population since participants were recruited through headache clinics. Those with very frequent migraine attacks would be excluded, and this could include those whose headaches were regularly initially relieved, but then returned.
Generally the studies reported on most of the outcomes we considered important (Lipton 1999; IHS 2000), although some presented data in ways that prevented pooling (for example, no first‐attack data, but rather the sum, mean or range for ≥ 2 attacks). In general, the amount of missing data was small and unlikely to affect the results.
The amount of information for active comparators was small, so that even for sumatriptan conclusions about relative efficacy and harm must be cautious.
Individual studies are underpowered to determine differences between treatments for adverse events, and even pooling studies may not provide adequate numbers of events to demonstrate differences or allow confidence in the size of the effect. Single‐dose studies are certainly unlikely to reveal rare, but potentially serious, adverse events. In these studies the number of participants experiencing any adverse event was probably a little higher with aspirin than with placebo, although these results may be confounded by recording of adverse events after taking rescue medication (which may disproportionately increase rates in the placebo group), and probably a little higher with larger doses of sumatriptan than with aspirin.
In the studies reviewed, participants with any contraindication to a study medication were excluded, so that the populations studied may differ from the general public who choose to self‐medicate with OTC aspirin. In addition, some studies used buffered formulations of aspirin that may cause less irritation in the stomach than standard OTC aspirin.
We found no studies specifically investigating the early use of aspirin, alone or in combination with an antiemetic, while headache intensity was still mild. In clinical practice most migraine sufferers do not wait until the headache becomes moderate or severe, and there is some evidence from studies with triptans that treating early, or when pain intensity is still mild, is better (Gendolla 2008).
Quality of the evidence
Included studies were of good methodological quality and validity. None adequately described the method of allocation concealment, but this may reflect the limitation of space in published articles rather than any flaw in methodology. Migraine was diagnosed using standard, validated criteria, and outcomes measured were generally those recommended by the IHS as being of clinical relevance, although not all studies reported all the outcomes we sought.
Single‐dose studies of a medication, or studies examining a single dose taken a few times, do not capture all adverse events that may occur with longer term use. While short‐term use of aspirin probably does not pose a large problem (Steiner 2009), the potential for gastrointestinal harm with long‐term use is well documented (Derry 2000).
Potential biases in the review process
The main area for concern is the small numbers of actual events used to calculate some results combined with the modest effect size of aspirin (Moore 1998).
In the five studies treating more than one attack with the same intervention (Chabriat 1994; Geraud 2002; Le Jeunne 1998; Tfelt‐Hansen 1995; Thomson 1992) we have used only data from the first attack for the primary outcomes. For cross‐over studies we have used data from each phase, which may introduce unknown biases (Elbourne 2002).
There were substantial amounts of information in comparisons of aspirin alone compared to placebo for the primary outcomes of pain‐free and headache relief at two hours. We consider that NNTs of 8 for the outcome pain‐free at two hours (13% benefit over comparator), and 6 for headache relief at two hours (17% benefit over comparator), are working limits of clinical utility in this condition. For pain‐free at two hours aspirin is already on the limit of utility, so even a small body of unidentified evidence showing no effect would affect interpretation of the results for this outcome. For headache relief at two hours we calculate that there would need to be at least 455 participants in unidentified studies showing no effect of aspirin over placebo to increase the NNT to the limit of utility (Moore 2008). We feel that it is unlikely this amount of null data exists.
Agreements and disagreements with other studies or reviews
This review is in broad agreement with earlier reviews comparing aspirin with placebo for acute migraine headaches (Diener 2006; Lampl 2007; Oldman 2002), but includes more studies. Other reviews comparing aspirin with triptans also agree that aspirin has similar efficacy to oral sumatriptan (Mett 2008; Tfelt‐Hansen 2008). Our findings are consistent with guidelines from the European Federation of Neurological Societies on drug treatment of acute migraine headaches (Evers 2009) and a summary of evidence‐based recommendations for self‐medication of migraine (Haag 2011).
A review of adverse events associated with single doses of aspirin in trials for migraine or tension‐type headache or postoperative dental pain suggests that any small increases in gastrointestinal adverse events compared with placebo are not great enough to drive choice of drug therapy (Steiner 2009). We also found a small increase in mostly mild and transient adverse events with aspirin, suggesting good tolerability among the participants recruited into these studies.
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.1 Pain free at 2 hours.

L'Abbé plot showing pain‐free at 2 h response in individual studies. Each circle represents one study, with size on the inset scale.

Forest plot of comparison: 1 Aspirin 900 mg or 1000 mg versus placebo, outcome: 1.2 Headache relief at 2 hours.

L'Abbé plot showing headache response at 2 h in individual studies. Each circle represents one study, with size on the inset scale.

Response rates for aspirin 900 mg plus metoclopramide 10 mg in consecutive attacks, reported in five studies (from left:Tfelt‐Hansen 1995; Chabriat 1994; Thomson 1992; Le Jeunne 1998; Geraud 2002)

Forest plot of comparison: 5 Aspirin ± metoclopramide versus placebo, outcome: 5.2 Use of rescue medication.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 1 Pain free at 2 hours.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 3 Headache relief at 1 hour.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 4 24‐hour sustained headache relief.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 5 Pain free at 2 hours ‐ effect of formulation.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 6 Headache relief at 2 hours ‐ effect of formulation.

Comparison 1 Aspirin 900 mg or 1000 mg versus placebo, Outcome 7 Relief of associated symptoms at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 1 Pain free at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 3 24‐hour sustained headache relief.

Comparison 2 Aspirin 900 mg plus metoclopramide 10 mg versus placebo, Outcome 4 Relief of associated symptoms at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 1 Pain free at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 2 Headache relief at 2 hours.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 3 Headache relief at 1 hour.

Comparison 3 Aspirin 900 mg or 1000 mg versus active comparator, Outcome 4 Relief of associated symptoms at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 1 Pain free at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 2 Headache relief at 2 hours.

Comparison 4 Aspirin 900 mg plus metoclopramide 10 mg versus active comparator, Outcome 3 Relief of associated symptoms at 2 hours.

Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 1 Any adverse event within 24 hours.

Comparison 5 Aspirin ± metoclopramide versus placebo, Outcome 2 Use of rescue medication.
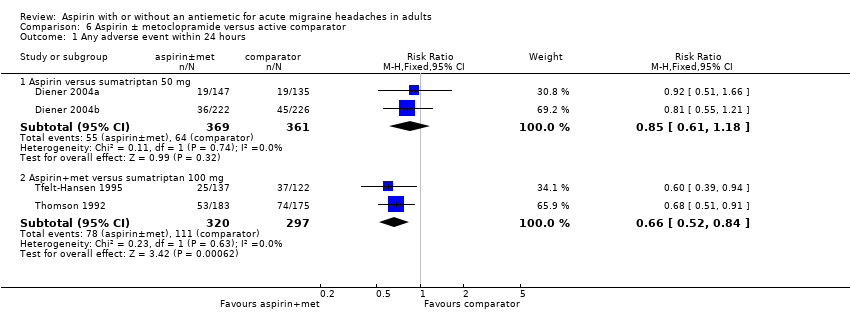
Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 1 Any adverse event within 24 hours.

Comparison 6 Aspirin ± metoclopramide versus active comparator, Outcome 2 Use of rescue medication.
| Aspirin 900 mg or 1000 mg compared with placebo for migraine headache | ||||||
| Patient or population: migraine headache ‐ moderate or severe pain Settings: community Intervention: aspirin 900 mg or 1000 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | NNT or NNTH and/or | No of Participants | Quality of the evidence | Comments |
| Pain‐free at 2 h | 240 in 1000 | 110 in 1000 | NNT 8.1 (6.4 to 11) | 6 studies, 2027 participants 357 events | Moderate1 | Standard tablet and soluble formulations |
| Headache relief at 2 h | 520 in 1000 | 320 in 1000 | NNT 4.9 (4.1 to 6.2) | 6 studies, 2027 participants 848 events | Moderate1 | Standard tablet and soluble formulations |
| Sustained pain‐free at 24 h | No data |
|
|
|
|
|
| Sustained headache relief at 24 h | 390 in 1000 | 240 in 1000 | NNT 6.6 (4.9 to 10) | 3 studies, 1142 participants 361 events | Moderate1 | Standard tablet and soluble formulations |
| At least one AE | 120 in 1000 | 90 in 1000 | NNH 34 (18 to 340) | 5 studies, 1892 participants 206 events | Low | Standard tablet and soluble formulations |
| Serious AE | Insufficient data | |||||
| GRADE Working Group grades of evidence | ||||||
| 1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.70, 2.55] |
| 2 Headache relief at 2 hours Show forest plot | 6 | 2027 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.48, 1.83] |
| 3 Headache relief at 1 hour Show forest plot | 4 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.72, 2.63] |
| 4 24‐hour sustained headache relief Show forest plot | 3 | 1142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.37, 1.95] |
| 5 Pain free at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.51, 2.44] |
| 5.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.70, 3.58] |
| 6 Headache relief at 2 hours ‐ effect of formulation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Soluble | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.43, 1.89] |
| 6.2 Tablet | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.38, 1.95] |
| 7 Relief of associated symptoms at 2 hours Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Nausea | 4 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.10, 1.44] |
| 7.2 Vomiting | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.94, 1.34] |
| 7.3 Photophobia | 5 | 1274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.29, 1.69] |
| 7.4 Phonophobia | 5 | 1217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.27, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [1.59, 4.55] |
| 2 Headache relief at 2 hours Show forest plot | 3 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.78, 2.60] |
| 3 24‐hour sustained headache relief Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.39, 3.41] |
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 2 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.53 [4.20, 13.50] |
| 4.2 Vomiting | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.14 [2.30, 113.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.03] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.11] |
| 3 Headache relief at 1 hour Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.26, 1.99] |
| 4 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Photophobia | 2 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.04] |
| 4.2 Phonophobia | 2 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain free at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sumatriptan 100 mg | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.87] |
| 2 Headache relief at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sumartiptan 100 mg | 2 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.10] |
| 3 Relief of associated symptoms at 2 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 3.2 Vomiting | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.59 [1.43, 78.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 7 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.02, 1.55] |
| 1.1 Aspirin alone | 5 | 1892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.00, 1.68] |
| 1.2 Aspirin + metoclopramide | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.82, 1.67] |
| 2 Use of rescue medication Show forest plot | 8 | 2922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.63, 0.72] |
| 2.1 Aspirin 100 mg alone | 5 | 1881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.73] |
| 2.2 Aspirin 900 mg + metoclopramide 10 mg | 3 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event within 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aspirin versus sumatriptan 50 mg | 2 | 730 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 1.2 Aspirin+met versus sumatriptan 100 mg | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.84] |
| 2 Use of rescue medication Show forest plot | 4 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.01, 1.28] |
| 2.1 Aspirin versus sumatriptan 50 mg | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 2.2 Aspirin+met versus sumatriptan 100 mg | 2 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.39] |

