Ibuprofen s ili bez lijekova protiv povraćanja za akutnu migrenu u odraslih osoba
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose Medication taken when migraine of moderate or severe intensity Assessments at 0, 0.5, 1, 1.5, 2, 3, 4, 5, and 6 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication (of participant's choice) | |
| Participants | Migraine with/without aura (IHS 1988) of at least moderate severity. History: 0.5 to 6 episodes/month in the year before study entry Excluded participants with > 50% episodes requiring bedrest or > 20% including vomiting Prophylactic medication continued unchanged, if stable N = 660 M 104, F 556 Mean age 39 years History of aura: 27% | |
| Interventions | Ibuprofen 200 mg, n = 216 Ibuprofen 400 mg, n = 223 Placebo, n = 221 | |
| Outcomes | Pain‐free at 2 hours Headache relief at 1 and 2 hours Use of rescue medication Presence of nausea, vomiting, photophobia, phonophobia Functional disability Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization code" |
| Allocation concealment (selection bias) | Low risk | "unopened treatment‐blinding tear‐off portion of winged label was affixed to the patient's case report form" |
| Blinding (performance bias and detection bias) | Low risk | All participants "received a blister card containing two tablets that were identical in colour, size, and shape" |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Low risk | > 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind (double‐dummy), placebo‐controlled, three‐period, cross‐over. Single oral dose of each treatment for each of three migraine attacks, with at least 48 hours between consecutive treatments Medication taken within 6 hours of onset, when migraine of moderate or severe intensity, and not improving Assessments at 0, 0.5, 1, 1.5, 2, and 24 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication (of participant's choice ‐ 12 hours if triptan or ergot) | |
| Participants | Migraine with or without aura (IHS 1988). History: 1 to 6 attacks/month in previous year N = 312 (cross‐over trial, 882 attacks) M 59, F 253 Mean age 38 years History of aura: 21% | |
| Interventions | Ibuprofen 400 mg, n = 212 ASA 2 x 500 mg, n = 222 Sumatriptan 50 mg, n = 226 Placebo, n = 222 | |
| Outcomes | Pain‐free at 2 hours Headache relief at 1 and 2 hours Use of rescue medication Presence of vomiting, photophobia, phonophobia Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predetermined randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "double‐dummy" method with "matching placebo" for each treatment |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Low risk | > 200 participants per treatment group |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose of ibuprofen with or without intravenous metoclopramide Assessments at 0, 0.5 and 1 hour If pain not controlled, participants asked to wait 1 hour before taking rescue medication | |
| Participants | Migraine (predates, but consistent with IHS criteria), presenting at hospital emergency department N = 40 No information on mean age, sex of population Median baseline pain ≥ 8/10 History of aura: not reported | |
| Interventions | Ibuprofen 600 mg (oral) + placebo (IV), n = 10 Placebo (oral) + placebo (IV), n = 10 Ibuprofen 600 mg (oral) + metoclopramide 1 mg IV, n = 10 Placebo (oral) + metoclopramide 1 mg IV, n = 10 | |
| Outcomes | Use of rescue medication at 1 hour | |
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "identically‐appearing placebo" |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | High risk | < 50 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind (double‐dummy), placebo‐controlled, parallel‐group. Single oral dose Medication taken when migraine of moderate or severe intensity Assessments at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, and 4 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication (of participant's choice) | |
| Participants | Migraine with and without aura (IHS 1988). History: attack at least once every 2 months during past year. Untreated attacks ≥ moderate severity N = 1559 M 306, F 1249 Mean age 38 years History of aura: 21% | |
| Interventions | Ibuprofen 2 x 200 mg, n = 669 Paracetamol + aspirin + caffeine 2 x 250/250/65 mg, n = 669 Placebo, n = 221 | |
| Outcomes | Pain‐free at 2 hours Presence of nausea, vomiting, photophobia, phonophobia Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Low risk | > 200 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose Medication taken when migraine of moderate or severe intensity Assessments at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, and 24 hours Rescue medication allowed, but no details reported | |
| Participants | Migraine with/without aura (IHS 1988). At least 12‐month history of migraine with/without aura, average frequency of 0.5 to 8 attacks/month in the previous year. Untreated attacks ≥ moderate severity. Previous experience of some relief from OTC analgesics Excluded participants with headaches that were usually severely disabling or incapacitating, or ≥ 20% accompanied by vomiting N = 729 M 179, F 550 Mean age 37 years (35 participants were 12 to 19 years) History of aura: 12% | |
| Interventions | Ibuprofen liquigel 200 mg, n = 198 Ibuprofen liquigel 400 mg, n = 191 Ibuprofen liquigel 600 mg, n = 198 Placebo, n = 142 | |
| Outcomes | Pain‐free at 2 hours Headache relief at 1 and 2 hours 24‐hour sustained relief Use of rescue medication Presence of nausea, photophobia, phonophobia Functional disability Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose/attack (≥ 2 attacks treated) Medication taken when migraine of moderate or severe intensity Assessments at 0, 2, and 24 hours If moderate or severe headache persisted after 2 hours, rescue medication allowed (sumatriptan 100 mg or piroxicam 20 mg) | |
| Participants | Migraine with and without aura (IHS 1988). History: at least 12‐month history of migraine with/without aura, no more than 6 attacks/month. Untreated attacks ≥ moderate severity Excluded participants with headaches usually needing bedrest, or ≥ 20% accompanied by vomiting N = 124 (101 analysed) M 18, F 83 Mean age 32 years History of aura: not reported | |
| Interventions | Ibuprofen 400 mg, n = 35 Rofecoxib 25 mg, n = 33 Placebo, n = 33 | |
| Outcomes | Headache relief at 2 hours 24‐hour sustained relief Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB1, W1. Total 4 Note exclusions >10% lost to follow‐up Note: headache relief not specifically defined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number tables" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation done by one investigator and responses evaluated by the other, but no details about method of concealment |
| Blinding (performance bias and detection bias) | Unclear risk | Tablets had identical packets, but were not identical in appearance |
| Incomplete outcome data (attrition bias) | Unclear risk | 18.5% lost to follow‐up without adequate explanation |
| Study size | High risk | < 50 participants per treatment group |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group. Single oral dose/attack (≥ 2 attacks treated) Medication taken when migraine of moderate or severe intensity Assessments at 0, 2, and 24 hours If moderate or severe headache persisted after 2 hours, rescue medication allowed (piroxicam 20 mg) | |
| Participants | Migraine (IHS 1988). History: ≤ 8 attacks/month. Untreated attacks ≥ moderate severity Excluded participants with headaches associated with recurrent vomiting N = 155 analysed M 59, F 106 Mean age 30 years History of aura: not reported | |
| Interventions | Ibuprofen 400 mg, n = 52 Rizatriptan 10 mg, n = 53 Placebo, n = 50 | |
| Outcomes | Pain‐free at 2 hours Headache relief at 2 hours 24‐hour sustained relief Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB1, W1 Note: headache relief not specifically defined, and may be reduction from moderate or severe by two grades (Kalita 2009), which is not quite the same as reduction to mild or none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation done by one investigator and responses evaluated by the other, but no details about method of concealment |
| Blinding (performance bias and detection bias) | Unclear risk | Medication "provided in identical packets" |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs described |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | DB, cross‐over, double‐dummy trial Two centre, randomised, double‐blind, placebo‐controlled, cross‐over. Single oral dose of each treatment for each of two migraine attacks ‐ time between consecutive treated attacks not specified Medication taken when pain was ≥ 60 mm Assessments at 0, 0.25, 0.5, 0.75, 1, 2, 4, and 6 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Migraine headache (IHS 1988). History: ≥ 2 months, without aura, 2 to 6 headache episodes/month N = 34 (29 analysed for efficacy) M 8, F 26 Mean age 34 years | |
| Interventions | Ibuprofen arginine 400 mg, n = 34 Placebo, n = 34 | |
| Outcomes | Headache relief at 1 and 2 hours Use of rescue medication Adverse events Withdrawas | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "identical sachets" |
| Incomplete outcome data (attrition bias) | Unclear risk | Completer analysis for efficacy, but adequate reasons given |
| Study size | High risk | < 50 participants per treatment group |
| Methods | Multicentre, randomised, double‐blind, triple‐dummy, placebo‐ and active‐controlled, parallel‐group. Single oral dose, and extension phase Medication taken when migraine of moderate or severe intensity, and not resolving spontaneously. Assessments at 0, 2, and 24 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication | |
| Participants | Migraine with and without aura (IHS 1988). History: 1 to 8 migraine attacks/month in the 6 months before enrolment N = 783 M 108, F 675 Mean age 40 years History of aura: 12% 32 participants took medication but were excluded from efficacy analyses ‐ probably due to protocol violations or lack of post‐baseline data. | |
| Interventions | Ibuprofen 400 mg, n = 199 (189 analysed for efficacy) Rofecoxib 25 mg, n = 194 (187 analysed for efficacy) Rofecoxib 50 mg, n = 196 (188 analysed for efficacy) Placebo, n = 194 (187 analysed for efficacy) | |
| Outcomes | Pain‐free at 2 hours Headache relief at 2 hours Use of rescue medication Presence of nausea, vomiting, photophobia, phonophobia Functional disability Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) | Low risk | Placebo tablets visually matched the three active treatments |
| Incomplete outcome data (attrition bias) | Unclear risk | ≤ 5% drop‐outs in each group, with no reasons given |
| Study size | Unclear risk | 50 to 200 participants per treatment group |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| 33% of ibuprofen and 7% of placebo treatment arms had mild headaches | |
| Probably mostly the same population as in Misra 2007. Primary analysis according to presence or not of allodynic symptoms | |
| No usable data | |
| Mixed tension‐type and migraine headaches | |
| No usable data |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.36, 2.81] |
| Analysis 1.1  Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 1 Pain‐free at 2 hours. | ||||
| 2 Headache relief at 2 hours Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.17, 1.61] |
| Analysis 1.2  Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 2 Headache relief at 2 hours. | ||||
| 3 Headache relief at 1 hour Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.15, 1.83] |
| Analysis 1.3  Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 3 Headache relief at 1 hour. | ||||
| 4 Any adverse event within 24 hours Show forest plot | 2 | 780 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| Analysis 1.4  Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 4 Any adverse event within 24 hours. | ||||
| 5 Participants using rescue medication Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.58, 0.86] |
| Analysis 1.5  Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 5 Participants using rescue medication. | ||||
| 6 Relief of associated symptoms at 2 h Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 6 Relief of associated symptoms at 2 h. | ||||
| 6.1 Nausea | 2 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.67] |
| 6.2 Photophobia | 2 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.05, 1.85] |
| 6.3 Phonophobia | 2 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.08, 1.82] |
| 7 Relief of functional disability at 2 hours Show forest plot | 2 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.18, 1.66] |
| Analysis 1.7  Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 7 Relief of functional disability at 2 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 6 | 2575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.60, 2.28] |
| Analysis 2.1  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 1 Pain‐free at 2 hours. | ||||
| 2 Headache relief at 2 hours Show forest plot | 7 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.92, 2.45] |
| Analysis 2.2  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 2 Headache relief at 2 hours. | ||||
| 2.1 Standard formulation | 5 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [2.12, 2.91] |
| 2.2 "Soluble" formulation | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.32, 1.92] |
| 3 Headache relief at 1 hour Show forest plot | 4 | 1269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.54, 2.30] |
| Analysis 2.3  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 3 Headache relief at 1 hour. | ||||
| 3.1 Standard formulation | 2 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.34, 2.27] |
| 3.2 "Soluble" formulation | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.55, 2.89] |
| 4 Sustained headache relief over 24 hours Show forest plot | 4 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.76, 2.69] |
| Analysis 2.4  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 4 Sustained headache relief over 24 hours. | ||||
| 5 Any adverse event within 24 hours Show forest plot | 7 | 2656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| Analysis 2.5  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 5 Any adverse event within 24 hours. | ||||
| 6 Specific adverse events Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 6 Specific adverse events. | ||||
| 6.1 Nausea | 7 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.54, 1.00] |
| 6.2 Abdominal pain | 6 | 2230 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.12, 4.96] |
| 6.3 Dizziness | 3 | 1615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| 6.4 Somnolence | 4 | 1717 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.79, 8.17] |
| 7 Participants using rescue medication Show forest plot | 7 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.74] |
| Analysis 2.7  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 7 Participants using rescue medication. | ||||
| 8 Relief of associated symptoms at 2 h Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 8 Relief of associated symptoms at 2 h. | ||||
| 8.1 Nausea | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.27, 1.86] |
| 8.2 Vomiting | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.21, 1.92] |
| 8.3 Photophobia | 4 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.29, 1.77] |
| 8.4 Phonophobia | 4 | 1261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.39, 1.90] |
| 9 Relief of functional disability at 2 hours Show forest plot | 3 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.38, 1.89] |
| Analysis 2.9  Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 9 Relief of functional disability at 2 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache relief at 2 hours Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.17] |
| Analysis 3.1  Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 1 Headache relief at 2 hours. | ||||
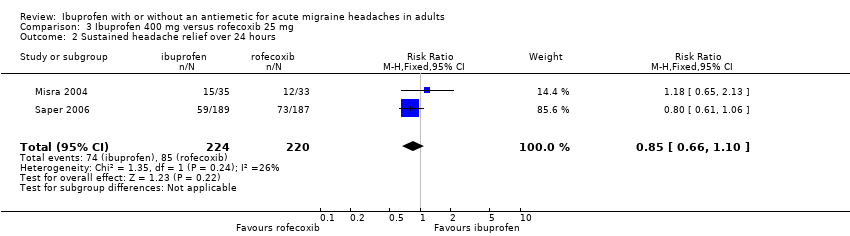
| 2 Sustained headache relief over 24 hours Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.10] |
| Analysis 3.2  Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 2 Sustained headache relief over 24 hours. | ||||
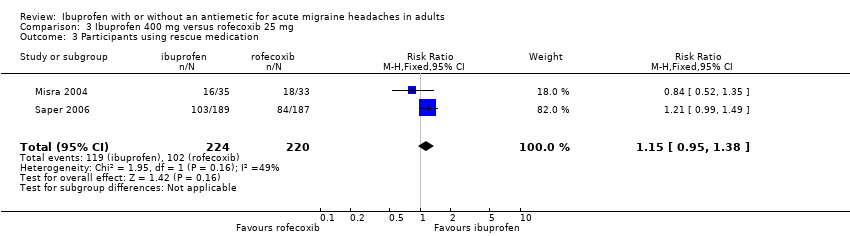
| 3 Participants using rescue medication Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.95, 1.38] |
| Analysis 3.3  Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 3 Participants using rescue medication. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.37, 3.51] |
| Analysis 4.1  Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 1 Pain‐free at 2 hours. | ||||
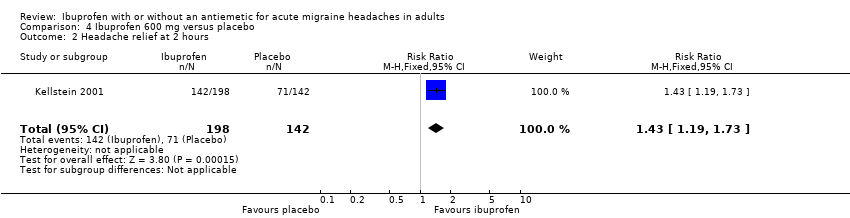
| 2 Headache relief at 2 hours Show forest plot | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.19, 1.73] |
| Analysis 4.2  Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 2 Headache relief at 2 hours. | ||||

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 2 Ibuprofen 400 mg versus placebo, outcome: 2.1 Pain‐free at 2 hours.

L'Abbé plot showing 2‐hour pain‐free response for ibuprofen versus placebo. Size of circle is proportional to size of study. Cream ‐ 200 mg; Yellow ‐ 400 mg; Brown ‐ 600 mg ibuprofen

Forest plot of comparison: 2 Ibuprofen 400 mg versus placebo, outcome: 2.2 Headache relief at 2 hours.

L'Abbé plot showing 2‐hour headache relief for ibuprofen versus placebo. Size of circle is proportional to size of study. Cream ‐ 200 mg; Yellow ‐ 400 mg; Brown ‐ 600 mg ibuprofen

L'Abbé plot showing 1‐hour headache relief for ibuprofen versus placebo. Size of circle is proportional to size of study. Cream ‐ 200 mg; Yellow ‐ 400 mg; Brown ‐ 600 mg ibuprofen

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 1 Pain‐free at 2 hours.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 3 Headache relief at 1 hour.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 4 Any adverse event within 24 hours.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 5 Participants using rescue medication.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 6 Relief of associated symptoms at 2 h.
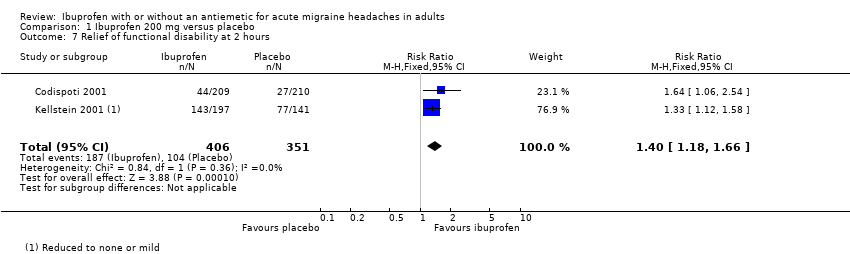
Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 7 Relief of functional disability at 2 hours.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 1 Pain‐free at 2 hours.
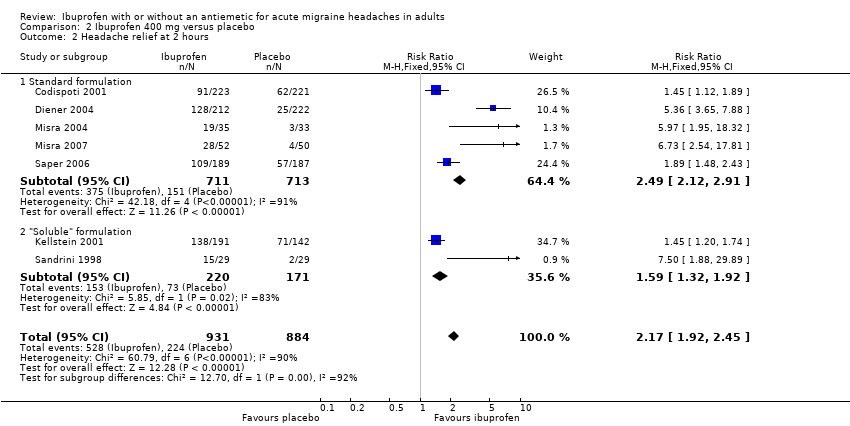
Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 3 Headache relief at 1 hour.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 4 Sustained headache relief over 24 hours.
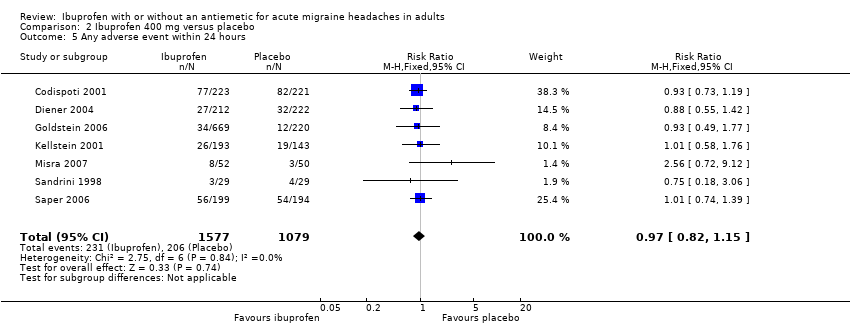
Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 5 Any adverse event within 24 hours.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 6 Specific adverse events.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 7 Participants using rescue medication.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 8 Relief of associated symptoms at 2 h.
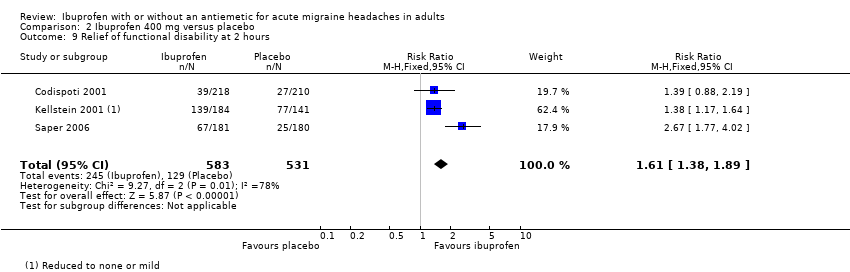
Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 9 Relief of functional disability at 2 hours.

Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 1 Headache relief at 2 hours.
Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 2 Sustained headache relief over 24 hours.
Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 3 Participants using rescue medication.

Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 1 Pain‐free at 2 hours.
Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 2 Headache relief at 2 hours.
| Ibuprofen 400 mg compared with placebo for migraine headache | ||||||
| Patient or population: adults with migraine headache ‐ moderate or severe pain Settings: community Intervention: ibuprofen 400 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | NNT or NNTH and/or | No of studies, attacks, events | Quality of the evidence | Comments |
| Pain free response at 2 h | 260 in 1000 | 120 in 1000 | NNT 7.2 (5.9 to 9.2) | 6 studies, 2575 attacks, 529 events | Moderate1 | |
| Headache relief at 2 h | 250 in 1000 | 570 in 1000 | NNT 3.2 (2.8 to 3.7) | 7 studies, 1815 attacks, 752 events | Moderate1 | |
| Sustained pain‐free at 24 h | no data | |||||
| Sustained headache relief at 24 h | 190 in 1000 | 450 in 1000 | NNT 4.0 (3.2 to 5.2) | 4 studies, 879 attacks, 288 events | Moderate1 | |
| At least one AE | 180 in 1000 | 150 in 1000 | NNH 26 (15 to 100) | 8 studies, 2722 attacks, 441 events | Moderate1 | |
| Serious AE | insufficient data | |||||
| CI: Confidence interval; NNT: number needed to treat; NNH: number needed to harm | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.36, 2.81] |
| 2 Headache relief at 2 hours Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.17, 1.61] |
| 3 Headache relief at 1 hour Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.15, 1.83] |
| 4 Any adverse event within 24 hours Show forest plot | 2 | 780 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| 5 Participants using rescue medication Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.58, 0.86] |
| 6 Relief of associated symptoms at 2 h Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 2 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.67] |
| 6.2 Photophobia | 2 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.05, 1.85] |
| 6.3 Phonophobia | 2 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.08, 1.82] |
| 7 Relief of functional disability at 2 hours Show forest plot | 2 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.18, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 6 | 2575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.60, 2.28] |
| 2 Headache relief at 2 hours Show forest plot | 7 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.92, 2.45] |
| 2.1 Standard formulation | 5 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [2.12, 2.91] |
| 2.2 "Soluble" formulation | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.32, 1.92] |
| 3 Headache relief at 1 hour Show forest plot | 4 | 1269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.54, 2.30] |
| 3.1 Standard formulation | 2 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.34, 2.27] |
| 3.2 "Soluble" formulation | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.55, 2.89] |
| 4 Sustained headache relief over 24 hours Show forest plot | 4 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.76, 2.69] |
| 5 Any adverse event within 24 hours Show forest plot | 7 | 2656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 6 Specific adverse events Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 7 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.54, 1.00] |
| 6.2 Abdominal pain | 6 | 2230 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.12, 4.96] |
| 6.3 Dizziness | 3 | 1615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| 6.4 Somnolence | 4 | 1717 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.79, 8.17] |
| 7 Participants using rescue medication Show forest plot | 7 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.74] |
| 8 Relief of associated symptoms at 2 h Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Nausea | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.27, 1.86] |
| 8.2 Vomiting | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.21, 1.92] |
| 8.3 Photophobia | 4 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.29, 1.77] |
| 8.4 Phonophobia | 4 | 1261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.39, 1.90] |
| 9 Relief of functional disability at 2 hours Show forest plot | 3 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.38, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache relief at 2 hours Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.17] |
| 2 Sustained headache relief over 24 hours Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.10] |
| 3 Participants using rescue medication Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.95, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.37, 3.51] |
| 2 Headache relief at 2 hours Show forest plot | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.19, 1.73] |

