Ibuprofen s ili bez lijekova protiv povraćanja za akutnu migrenu u odraslih osoba
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008039.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 30 abril 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All authors were involved with planning and writing the protocol. For the full review, RR and SD carried out searches, selected studies for inclusion and performed data extraction and analysis. RAM was involved with analysis. HJM acted as arbitrator. All authors were involved with writing.
For the update, SD carried out searches. RAM and SD updated the review. All authors read and approved the update.
Sources of support
Internal sources
-
Oxford Pain Research Trust, UK.
General institutional support
External sources
-
Lifting The Burden: the Global Campaign against Headache, UK.
Funding for administrative costs associated with editorial and peer review
Declarations of interest
RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM and SD have received research support from charities, government and industry sources at various times. RR has no such interests to declare. The Oxford Pain Research Trust, the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme provided support for the original review. The Oxford Pain Research Trust provided support for the update. None had any input into the review at any stage.
Acknowledgements
Henry McQuay was an author on the original review.
We received financial support for the original review from the NHS Cochrane Collaboration Programme Grant Scheme and NIHR Biomedical Research Centre Programme. Lifting The Burden: the Global Campaign against Headache provided support for the editorial process.
The Oxford Pain Research Trust provided institutional support for this update, and editorial support was funded by the International Headache Society.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Apr 30 | Ibuprofen with or without an antiemetic for acute migraine headaches in adults | Review | Roy Rabbie, Sheena Derry, R Andrew Moore | |
| 2010 Oct 06 | Ibuprofen with or without an antiemetic for acute migraine headaches in adults | Review | Roy Rabbie, Sheena Derry, R Andrew Moore, Henry J McQuay | |
| 2009 Oct 07 | Ibuprofen with or without an antiemetic for acute migraine in adults | Protocol | Roy Rabbie, Sheena Derry, R Andrew Moore, Henry J McQuay | |
Differences between protocol and review
For the original review we included an outcome that was not specified in the protocol. Use of rescue medication was reported by the majority of studies and provides a measure of efficacy from the point of view of the patient. In taking rescue medication the patient is indicating that the efficacy of the medication is not adequate and that they need alternative analgesia. They are effectively withdrawing due to lack of efficacy, where efficacy is defined by their preparedness to carry on without additional analgesia, rather than a predefined outcome such as headache relief at two hours. We believe this is useful additional information relevant to clinical practice.
The protocol stated that "Studies reporting treatment of consecutive headache episodes will be accepted if outcomes for the first, or each, episode were reported separately". Two studies (Misra 2004; Misra 2007) treated two or more attacks with single doses of the same study medication and reported results as numbers of participants with various responses. It is not clear how the data for multiple attacks were combined. We have included the data from these studies on the assumption that an individual's response was consistent across attacks, given that a sensitivity analysis was to be done excluding these studies on the grounds of potentially unreliable blinding.
For the update, after discussion with headache specialists and editorial staff and in line with Cochrane recommendations, we decided to limit our outcomes for acute migraine headache reviews in order to focus attention on the most important outcomes and to make them more readable for both clinicians and patients. For the majority of interventions we now include 2‐hour pain‐free and headache relief (PF2 and HR2) as primary outcomes, and 24‐hour sustained pain‐free and headache relief (SPF24 and SHR24) and adverse events as secondary outcomes. In this update we have moved results for use of rescue medication and relief of headache‐associated symptoms and functional disability to Appendix 7.
In the update we have expanded the Risk of bias table; this review uses the new criteria for analysis. We have also included an assessment of publication bias, which was not included in the protocol or original review. This assessment is now being added routinely to all our reviews as a measure of reliability and robustness of the results.
Notes
A restricted search in September 2017 did not identify any potentially relevant studies. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 2 Ibuprofen 400 mg versus placebo, outcome: 2.1 Pain‐free at 2 hours.

L'Abbé plot showing 2‐hour pain‐free response for ibuprofen versus placebo. Size of circle is proportional to size of study. Cream ‐ 200 mg; Yellow ‐ 400 mg; Brown ‐ 600 mg ibuprofen

Forest plot of comparison: 2 Ibuprofen 400 mg versus placebo, outcome: 2.2 Headache relief at 2 hours.

L'Abbé plot showing 2‐hour headache relief for ibuprofen versus placebo. Size of circle is proportional to size of study. Cream ‐ 200 mg; Yellow ‐ 400 mg; Brown ‐ 600 mg ibuprofen

L'Abbé plot showing 1‐hour headache relief for ibuprofen versus placebo. Size of circle is proportional to size of study. Cream ‐ 200 mg; Yellow ‐ 400 mg; Brown ‐ 600 mg ibuprofen

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 1 Pain‐free at 2 hours.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 3 Headache relief at 1 hour.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 4 Any adverse event within 24 hours.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 5 Participants using rescue medication.

Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 6 Relief of associated symptoms at 2 h.
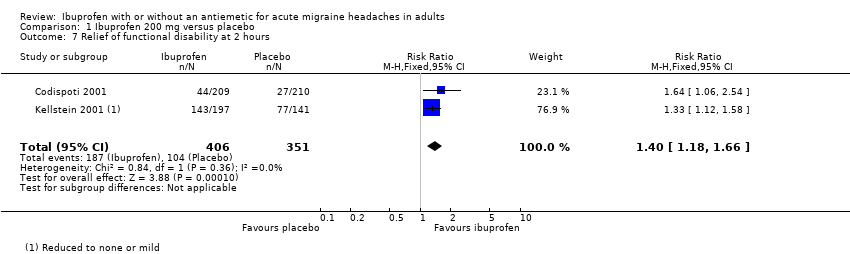
Comparison 1 Ibuprofen 200 mg versus placebo, Outcome 7 Relief of functional disability at 2 hours.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 1 Pain‐free at 2 hours.
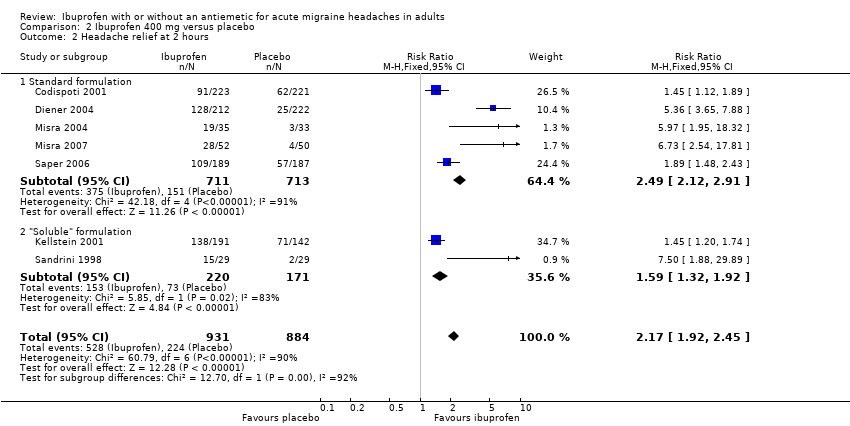
Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 2 Headache relief at 2 hours.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 3 Headache relief at 1 hour.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 4 Sustained headache relief over 24 hours.
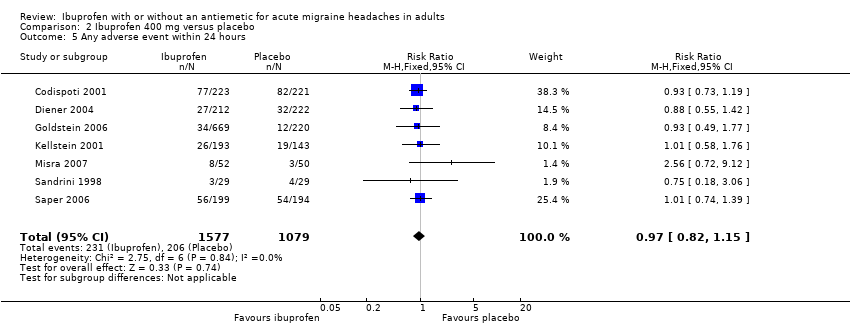
Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 5 Any adverse event within 24 hours.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 6 Specific adverse events.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 7 Participants using rescue medication.

Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 8 Relief of associated symptoms at 2 h.
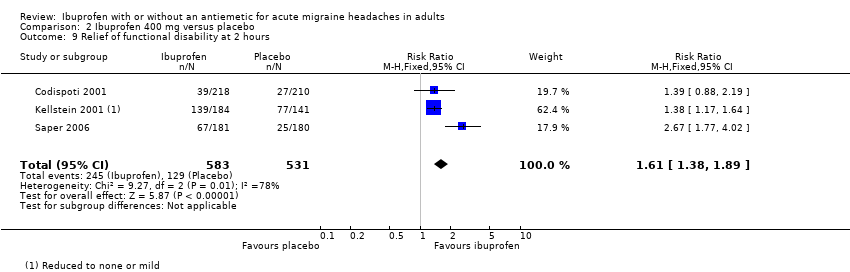
Comparison 2 Ibuprofen 400 mg versus placebo, Outcome 9 Relief of functional disability at 2 hours.

Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 1 Headache relief at 2 hours.
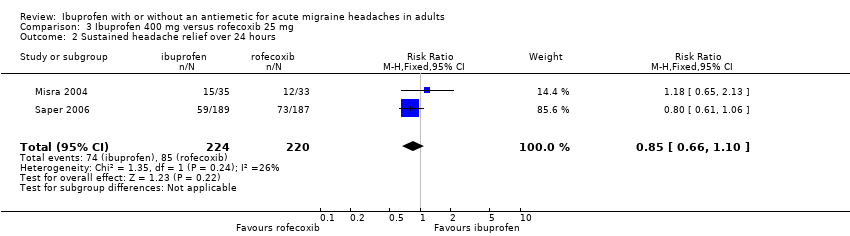
Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 2 Sustained headache relief over 24 hours.
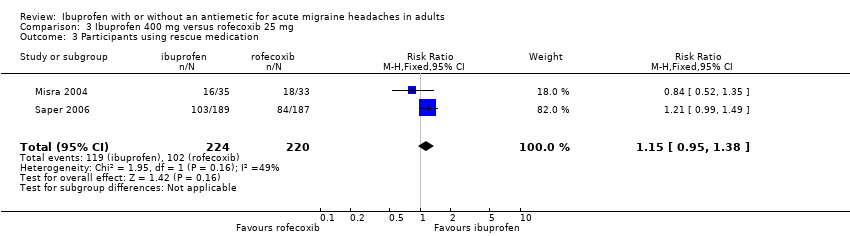
Comparison 3 Ibuprofen 400 mg versus rofecoxib 25 mg, Outcome 3 Participants using rescue medication.

Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 1 Pain‐free at 2 hours.
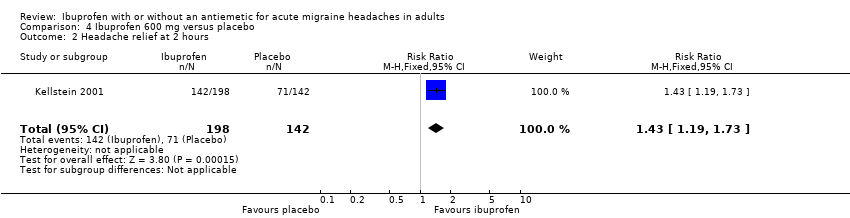
Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 2 Headache relief at 2 hours.
| Ibuprofen 400 mg compared with placebo for migraine headache | ||||||
| Patient or population: adults with migraine headache ‐ moderate or severe pain Settings: community Intervention: ibuprofen 400 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | NNT or NNTH and/or | No of studies, attacks, events | Quality of the evidence | Comments |
| Pain free response at 2 h | 260 in 1000 | 120 in 1000 | NNT 7.2 (5.9 to 9.2) | 6 studies, 2575 attacks, 529 events | Moderate1 | |
| Headache relief at 2 h | 250 in 1000 | 570 in 1000 | NNT 3.2 (2.8 to 3.7) | 7 studies, 1815 attacks, 752 events | Moderate1 | |
| Sustained pain‐free at 24 h | no data | |||||
| Sustained headache relief at 24 h | 190 in 1000 | 450 in 1000 | NNT 4.0 (3.2 to 5.2) | 4 studies, 879 attacks, 288 events | Moderate1 | |
| At least one AE | 180 in 1000 | 150 in 1000 | NNH 26 (15 to 100) | 8 studies, 2722 attacks, 441 events | Moderate1 | |
| Serious AE | insufficient data | |||||
| CI: Confidence interval; NNT: number needed to treat; NNH: number needed to harm | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.36, 2.81] |
| 2 Headache relief at 2 hours Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.17, 1.61] |
| 3 Headache relief at 1 hour Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.15, 1.83] |
| 4 Any adverse event within 24 hours Show forest plot | 2 | 780 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| 5 Participants using rescue medication Show forest plot | 2 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.58, 0.86] |
| 6 Relief of associated symptoms at 2 h Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 2 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.67] |
| 6.2 Photophobia | 2 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.05, 1.85] |
| 6.3 Phonophobia | 2 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.08, 1.82] |
| 7 Relief of functional disability at 2 hours Show forest plot | 2 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.18, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 6 | 2575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.60, 2.28] |
| 2 Headache relief at 2 hours Show forest plot | 7 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.92, 2.45] |
| 2.1 Standard formulation | 5 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [2.12, 2.91] |
| 2.2 "Soluble" formulation | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.32, 1.92] |
| 3 Headache relief at 1 hour Show forest plot | 4 | 1269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.54, 2.30] |
| 3.1 Standard formulation | 2 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.34, 2.27] |
| 3.2 "Soluble" formulation | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.55, 2.89] |
| 4 Sustained headache relief over 24 hours Show forest plot | 4 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.76, 2.69] |
| 5 Any adverse event within 24 hours Show forest plot | 7 | 2656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 6 Specific adverse events Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 7 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.54, 1.00] |
| 6.2 Abdominal pain | 6 | 2230 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.12, 4.96] |
| 6.3 Dizziness | 3 | 1615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| 6.4 Somnolence | 4 | 1717 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.79, 8.17] |
| 7 Participants using rescue medication Show forest plot | 7 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.74] |
| 8 Relief of associated symptoms at 2 h Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Nausea | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.27, 1.86] |
| 8.2 Vomiting | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.21, 1.92] |
| 8.3 Photophobia | 4 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.29, 1.77] |
| 8.4 Phonophobia | 4 | 1261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.39, 1.90] |
| 9 Relief of functional disability at 2 hours Show forest plot | 3 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.38, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache relief at 2 hours Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.17] |
| 2 Sustained headache relief over 24 hours Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.10] |
| 3 Participants using rescue medication Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.95, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain‐free at 2 hours Show forest plot | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.37, 3.51] |
| 2 Headache relief at 2 hours Show forest plot | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.19, 1.73] |

