Rheum officinale (una medicina tradicional china) para la nefropatía crónica
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether other risks of bias exist |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Baseline balance; no other bias detected |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Baseline balance, we did not find any other bias |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ESKD definition: SCr up to 707 µmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but the published reports included primary outcomes for this review |
| Other bias | Unclear risk | Insufficient information to assess whether other important risks of bias exist |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | No outcome data were available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess risk of bias |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all specified outcomes were reported |
| Other bias | Unclear risk | Insufficient information to assess if risk of bias exists |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | No outcomes data were available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete outcome data may exist |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete outcome data may exist |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified outcomes have been reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
BUN ‐ blood urea nitrogen; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; DKD ‐ diabetic kidney disease; ESKD ‐ end‐stage kidney disease; GSHPx ‐ glutathione peroxidase; HD ‐ haemodialysis; HDL ‐ high density lipoprotein; hs‐CPR ‐ high sensitivity C‐reactive protein; IL‐6 ‐ interleukin‐6; LDL ‐ low‐density lipoprotein; MDA ‐ malonaldehyde; NS ‐ not stated; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; TG ‐ triglyceride; TNF‐alpha: tumour necrosis factor‐alpha; VLDL ‐ very low density lipoprotein
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not RCT | |
| Rheum officinale was a component of compounded Chinese medicinal herbal treatment. It was not possible to determine which herb was the active treatment | |
| Not RCT | |
| Not RCT | |
| Rheum officinale was a component of compounded Chinese medicinal herbal treatment. It was not possible to determine which herb was the active treatment | |
| Not RCT | |
| Rheum officinale was a component of compounded Chinese medicinal herbal treatment. It was not possible to determine which herb was the active treatment | |
| Review article | |
| Review article | |
| Rheum officinale plus other drugs versus Rheum officinale | |
| Review article | |
| Rheum officinale plus other drugs versus Rheum officinale | |
| Rheum officinale plus other drugs versus Rheum officinale | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Rheum officinale was a component of compounded Chinese medicinal herbal treatment. It was not possible to determine which herb was the active treatment | |
| Rheum officinale was a component of compounded Chinese medicinal herbal treatment. It was not possible to determine which herb was the active treatment | |
| Rheum officinale was a component of compounded Chinese medicinal herbal treatment. It was not possible to determine which herb was the active treatment | |
| Comparison of Rheum officinale administration | |
| Error in randomisation method | |
| Rheum officinale plus other drugs versus Rheum officinale | |
| Rheum officinale was a component of compounded Chinese medicinal herbal treatment. It was not possible to determine which herb was the active treatment | |
| Not RCT | |
| Not RCT | |
| Not RCT |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
|
| Outcomes |
|
| Notes | We were unable to contact the author to obtain more detailed information |
NS ‐ not stated; RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of ESKD Show forest plot | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.28, 1.00] |
| Analysis 1.1  Comparison 1 Rheum officinale versus no treatment, Outcome 1 Incidence of ESKD. | ||||
| 2 CrCl Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Rheum officinale versus no treatment, Outcome 2 CrCl. | ||||
| 3 SCr Show forest plot | 4 | 196 | Mean Difference (IV, Random, 95% CI) | ‐87.49 [‐139.25, ‐35.72] |
| Analysis 1.3  Comparison 1 Rheum officinale versus no treatment, Outcome 3 SCr. | ||||
| 4 Proteinuria Show forest plot | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.02, 0.31] |
| Analysis 1.4  Comparison 1 Rheum officinale versus no treatment, Outcome 4 Proteinuria. | ||||
| 5 BUN Show forest plot | 4 | 256 | Mean Difference (IV, Random, 95% CI) | ‐10.83 [‐19.45, ‐2.21] |
| Analysis 1.5  Comparison 1 Rheum officinale versus no treatment, Outcome 5 BUN. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CrCl Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Rheum officinale versus captopril, Outcome 1 CrCl. | ||||
| 2 BUN Show forest plot | 2 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐2.21, 1.65] |
| Analysis 2.2  Comparison 2 Rheum officinale versus captopril, Outcome 2 BUN. | ||||
| 3 Working capacity Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Rheum officinale versus captopril, Outcome 3 Working capacity. | ||||
| 3.1 Part‐time working capacity | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Full‐time working capacity | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
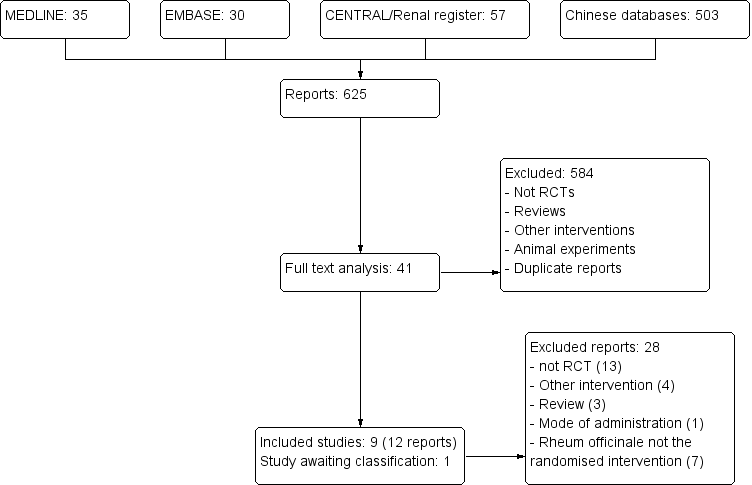
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
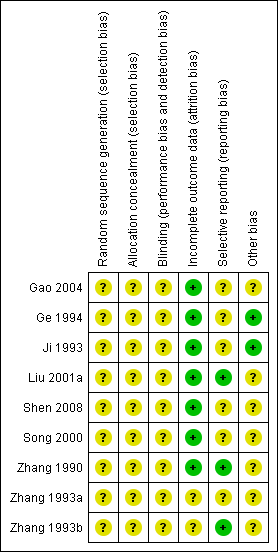
Methodological quality summary: review authors' judgements about each methodological quality item for each included study

Comparison 1 Rheum officinale versus no treatment, Outcome 1 Incidence of ESKD.
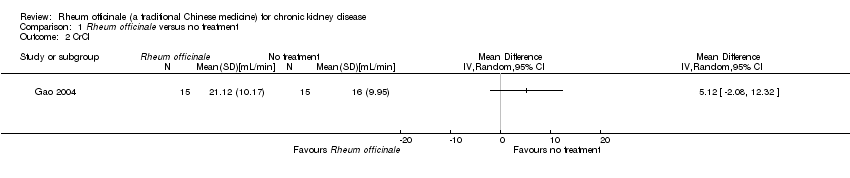
Comparison 1 Rheum officinale versus no treatment, Outcome 2 CrCl.
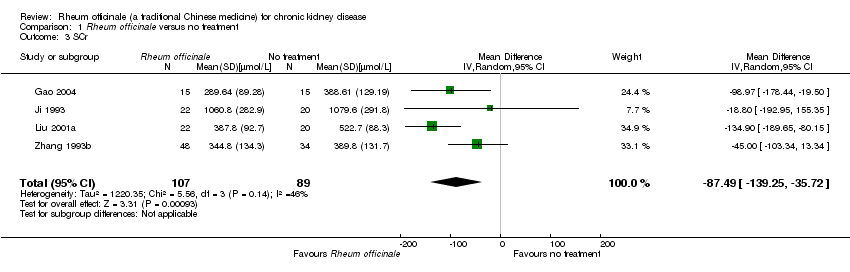
Comparison 1 Rheum officinale versus no treatment, Outcome 3 SCr.

Comparison 1 Rheum officinale versus no treatment, Outcome 4 Proteinuria.

Comparison 1 Rheum officinale versus no treatment, Outcome 5 BUN.

Comparison 2 Rheum officinale versus captopril, Outcome 1 CrCl.

Comparison 2 Rheum officinale versus captopril, Outcome 2 BUN.

Comparison 2 Rheum officinale versus captopril, Outcome 3 Working capacity.
| Rheum officinale versus no treatment for chronic kidney disease | ||||||
| Patient or population: Patients with chronic kidney disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rheum officinale versus no treatment | |||||
| Incidence of ESKD | Study population | RR 0.53 | 124 | ⊕⊕⊝⊝ | ||
| 222 per 1000 | 118 per 1000 | |||||
| Medium risk population | ||||||
| 378 per 1000 | 200 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Potential limitations are likely to reduce confidence in the estimate of effect | ||||||
| Rheum officinale versus captopril for chronic kidney disease | ||||||
| Patient or population: Patients chronic kidney disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rheum officinale versus captopril | |||||
| Full time working capacity | Study population | RR 1.02 | 20 | ⊕⊕⊝⊝ | ||
| 444 per 1000 | 453 per 1000 | |||||
| Medium risk population | ||||||
| 444 per 1000 | 453 per 1000 | |||||
| Part‐time working capacity | Study population | RR 1.64 | 20 | ⊕⊕⊝⊝ | ||
| 222 per 1000 | 364 per 1000 | |||||
| Medium risk population | ||||||
| 222 per 1000 | 364 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most information from studies at low or unclear risk of bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of ESKD Show forest plot | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.28, 1.00] |
| 2 CrCl Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 SCr Show forest plot | 4 | 196 | Mean Difference (IV, Random, 95% CI) | ‐87.49 [‐139.25, ‐35.72] |
| 4 Proteinuria Show forest plot | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.02, 0.31] |
| 5 BUN Show forest plot | 4 | 256 | Mean Difference (IV, Random, 95% CI) | ‐10.83 [‐19.45, ‐2.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CrCl Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 BUN Show forest plot | 2 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐2.21, 1.65] |
| 3 Working capacity Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Part‐time working capacity | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Full‐time working capacity | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

