Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007892.pub6Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 julio 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
David Haas is the guarantor for the review. Drs. Haas, Morgan, and Contreras developed the original protocol, data extraction sheet, and preparation of results and final original report and previous updates. Ms. Enders was added for this update and all four authors contributed to study selection, data extraction, and preparation of results and final report for this update.
Sources of support
Internal sources
-
Indiana University School of Medicine, Indianapolis, USA.
External sources
-
No sources of support supplied
Declarations of interest
David Haas is the Principal Investigator for a randomized trial included in this review (Haas 2010). He has no financial conflicts of interest to disclose.
Sarah Morgan is also an investigator in the Haas 2010 trial. She has no financial conflicts of interest to disclose.
Trial authors for Haas 2010 were not involved in assessing trial quality or extracting data from the Haas 2010 study. This task was carried out by Karenrose Contreras and a third party (Dr Jon Hathaway, MD, PhD).
Karenrose Contreras has no financial conflicts of interest to disclose.
Savannah Enders has no financial conflicts of interest to disclose.
Acknowledgements
The authors thank Dr Jon Hathaway for his independent assessment of trial quality and data extraction for the Haas 2010 study (original version of the review) and Erika Ota for preparing the 'Summary of findings' table for the previous version of this review (Haas 2014b). At that time, Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization.
The 'Summary of findings' table in this update was prepared by the current review team using GradePro software.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We wish to thank Dr Ida Envall of Stockholm, who brought a trial to our attention that had been missed in our search methodology after the 2014 update (Haas 2014b).
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Apr 26 | Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections | Review | David M Haas, Sarah Morgan, Karenrose Contreras, Savannah Kimball | |
| 2018 Jul 17 | Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections | Review | David M Haas, Sarah Morgan, Karenrose Contreras, Savannah Enders | |
| 2014 Dec 21 | Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections | Review | David M Haas, Sarah Morgan, Karenrose Contreras | |
| 2014 Sep 09 | Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections | Review | David M Haas, Sarah Morgan, Karenrose Contreras | |
| 2013 Jan 31 | Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections | Review | David M Haas, Sarah Morgan, Karenrose Contreras | |
| 2010 Mar 17 | Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections | Review | David M Haas, Sarah Morgan Al Darei, Karenrose Contreras | |
| 2009 Jul 08 | Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections | Protocol | David M Haas, Sarah Al Darei, Karenrose Contreras | |
Differences between protocol and review
Three of the planned subgroup analyses were unable to be performed as they were not reported in the trials.
In the 2017 update, we added an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports.
A new co‐author (Savannah Enders) has joined the review team for this update.
We have edited the list of outcomes for use in GRADE. We have edited, postpartum endometritis, postoperative wound infection and postoperative fever to include definitions as per the list of outcomes in the main methods/types of outcomes. We have also added 'Composite wound complications or endometritis' to our list of outcomes for use in GRADE.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Intravaginal;
- Anti-Infective Agents, Local [*administration & dosage];
- Benzalkonium Compounds [administration & dosage];
- Cesarean Section [*adverse effects];
- Chlorhexidine [administration & dosage];
- Disinfection [*methods];
- Endometritis [*prevention & control];
- Fever [prevention & control];
- Povidone-Iodine [administration & dosage];
- Preoperative Care [*methods];
- Randomized Controlled Trials as Topic;
- Surgical Wound Infection [*prevention & control];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO
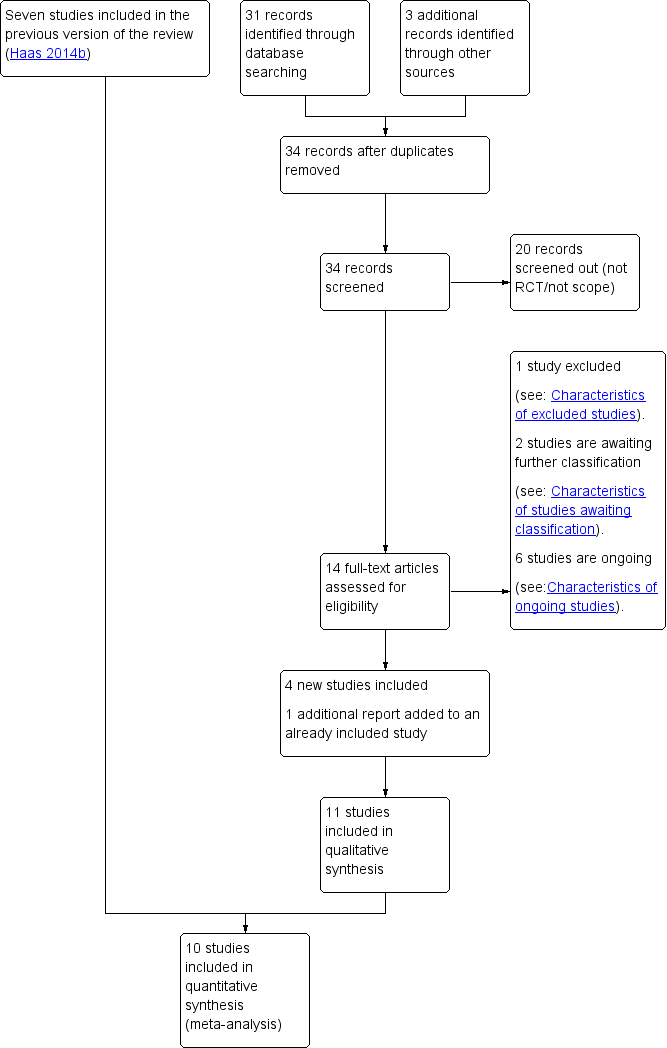
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 1 Post‐cesarean endometritis.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 2 Postoperative fever.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 3 Postoperative wound infection.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 4 Composite wound complication.
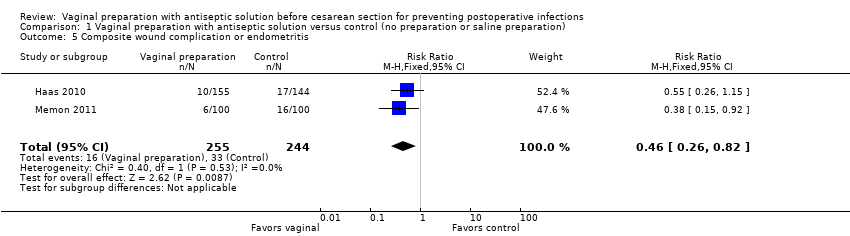
Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 5 Composite wound complication or endometritis.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 1 Post‐cesarean endometritis.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 2 Postoperative fever.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 3 Postoperative wound infection.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 4 Composite wound complication.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 5 Composite wound complication or endometritis.
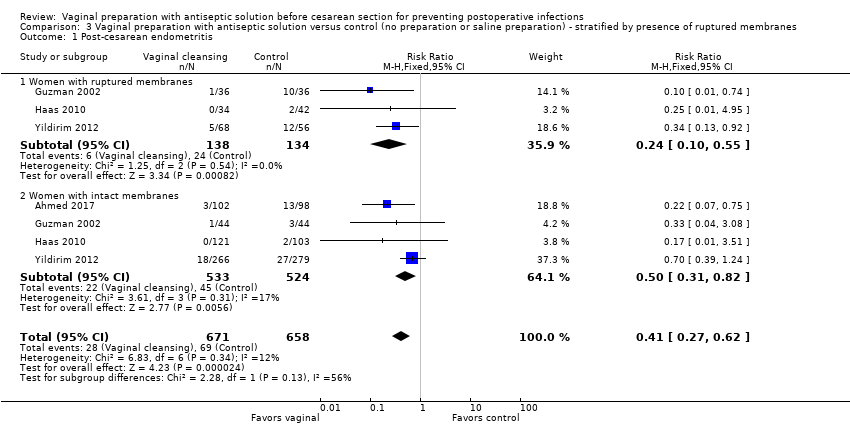
Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 1 Post‐cesarean endometritis.
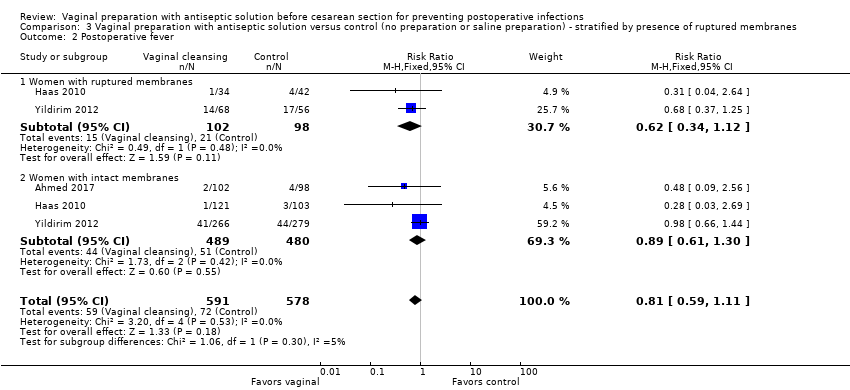
Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 2 Postoperative fever.

Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 3 Postoperative wound infection.
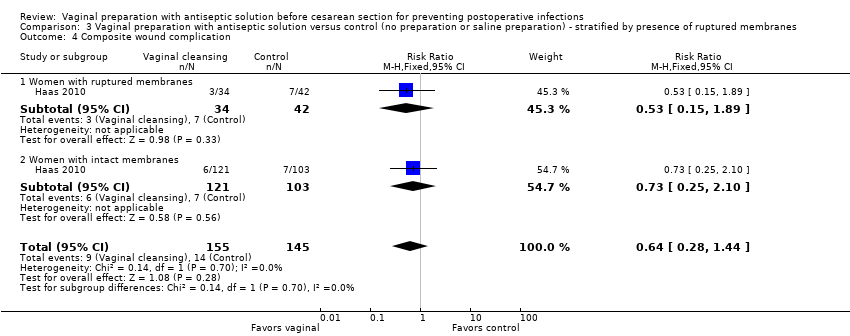
Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 4 Composite wound complication.

Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 5 Composite wound complication or endometritis.
| Vaginal preparation with antiseptic solution compared to control (no preparation or saline preparation) for preventing postoperative infections | ||||||
| Patient or population: pregnant women who were about to receive a cesarean delivery. This included women receiving elective, laboring, or urgent cesareans | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control | Risk with vaginal preparation | |||||
| Post‐cesarean endometritis | Study population | Average RR 0.36 | 3283 | ⊕⊕⊕⊝ | ||
| 86 per 1000 | 31 per 1000 | |||||
| Postoperative fever | Study population | RR 0.87 | 3109 | ⊕⊕⊕⊝ | ||
| 125 per 1000 | 109 per 1000 | |||||
| Postoperative wound infection | Study population | RR 0.74 | 2839 | ⊕⊕⊕⊝ | ||
| 36 per 1000 | 27 per 1000 | |||||
| Composite wound complication or endometritis | Study population | RR 0.46 | 499 | ⊕⊕⊕⊝ | ||
| 135 per 1000 | 62 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Over 40% of included studies had some design limitations. 2 Wide confidence intervals in included studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 10 | 3283 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.63] |
| 1.1 Iodine‐based solution | 8 | 3069 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.69] |
| 1.2 Chlorhexidine‐based solution | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.75] |
| 2 Postoperative fever Show forest plot | 8 | 3109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| 2.1 Iodine‐based solution | 7 | 2909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| 2.2 Chlorhexidine‐based solution | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.09, 2.56] |
| 3 Postoperative wound infection Show forest plot | 8 | 2839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.11] |
| 3.1 Iodine‐based solution | 7 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.19] |
| 3.2 Chlorhexidine‐based solution | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.82] |
| 4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.37, 1.07] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.32, 1.06] |
| 1.1 Women in labor | 4 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.19, 0.89] |
| 1.2 Women not in labor | 4 | 886 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.84] |
| 2 Postoperative fever Show forest plot | 3 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.57, 1.03] |
| 2.1 Women in labor | 3 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.96] |
| 2.2 Women not in labor | 2 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.49] |
| 3 Postoperative wound infection Show forest plot | 3 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.08] |
| 3.1 Women in labor | 3 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.24] |
| 3.2 Women not in labor | 2 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.27, 1.57] |
| 4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.09] |
| 4.1 Women in labor | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.61] |
| 4.2 Women not in labor | 2 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.16] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.85] |
| 5.1 Women in labor | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.87] |
| 5.2 Women not in labor | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 4 | 1329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.27, 0.62] |
| 1.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 1.2 Women with intact membranes | 4 | 1057 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.82] |
| 2 Postoperative fever Show forest plot | 3 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.11] |
| 2.1 Women with ruptured membranes | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.12] |
| 2.2 Women with intact membranes | 3 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.30] |
| 3 Postoperative wound infection Show forest plot | 4 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.43, 1.30] |
| 3.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.16, 6.70] |
| 3.2 Women with intact membranes | 4 | 1057 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.28] |
| 4 Composite wound complication Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.44] |
| 4.1 Women with ruptured membranes | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.89] |
| 4.2 Women with intact membranes | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.10] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.85] |
| 5.1 Women with ruptured membranes | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.13] |
| 5.2 Women with intact membranes | 2 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |

