Tratamiento con células madre para la cardiopatía isquémica crónica y la insuficiencia cardíaca congestiva
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Type of study: Parallel RCT Study setting: Leicester, UK | |
| Participants | Description: Chronic IHD (aged 18 to 80 years; presence of at least 1 chronic, irreversible myocardial scar; elective cardiac surgery). Number of diseased vessels: n/r (multivessel). | |
| Interventions | Intervention arms: BMSC‐IM or BMSC‐IC Type of stem cells: Mononuclear cells administered intramyocardially (IM) or intracoronarily (IC) G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration; no placebo or sham procedure) Co‐intervention: CABG | |
| Outcomes | Primary outcome: Improvement in contractile function of treated scar areas at 6 months. Global left ventricular functions at 6 months (infarct size, global end‐diastolic volume and end‐systolic volume, and improvement in stroke volume and LVEF). Additional outcomes: Postoperative complications, troponin I levels within 24 hours of surgery, and clinical evaluation (assessment of functional status and adverse events). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was administered; participants and clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians treating the participants during the postoperative period and the investigators performing the examinations and interpreting the results were blind to which group participants had been assigned. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 controls (2/21) were excluded from analysis of mortality and morbidity (1x withdrawal before surgery, 1x deemed unsuitable for follow‐up). 12 participants were lost to follow‐up (4 IM, 4 IC, 3 controls), and 2 died within 30 days. MRI was performed in 33 participants, of which 4 were “not suitable for accurate analysis”. However, MRI results were only reported for 25 participants; this discrepancy was unexplained. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00560742) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Cross‐over RCT Study setting: Frankfurt, Germany | |
| Participants | Description: Chronic IHD (aged 18 to 80 years; MI at least 3 months previously; well‐demarcated region of left ventricular dysfunction and a patent infarct‐related artery). Number of diseased vessels: BMSC: 1 (n = 7), 2 (n = 13), 3 (n = 8); CPC: 1 (n = 7), 2 (n = 4), 3 (n = 12); Controls: 1 (n = 2), 2 (n = 9), 3 (n = 12). | |
| Interventions | Intervention arm: BMSC or CPC G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration; no placebo or sham procedure) Co‐intervention: Standard medical therapy; PCI (in 27% of participants) | |
| Outcomes | Primary outcome: Change in global left ventricular function (measured by quantitative left ventricular angiography).
Outcome assessment points: Baseline and 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computerised simple random allocation with known N. No blockwise randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was administered; participants and clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quantitative analysis of angiograms and MRI images was performed by an investigator who was blinded to the individual participant's treatment. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis of mortality and morbidity. 14 participants (4x cell therapy, 5x CPC, 5x controls) were excluded from MRI/angiography and functional status at follow‐up, with reasons given for all exclusions. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00289822) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Langen, Germany | |
| Participants | Description: Chronic ischaemic HF (aged 18 to 80 years; anterior MI occurring 3 months or more prior to inclusion and stable chronic postinfarction HF defined by LVEF less than 50% or symptoms of NYHA class II or greater; a patent vessel supplying the target region). Age distribution in each arm: BMSC: 65 (12) (LD), 58 (11) (HD); Controls: 60 (10) (LD), 63 (10) (HD). Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC Dose of stem cells: 123 (69) x 106 (HD), 150 (77) x 106 (LD). G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; 10 mL of X‐VIVO 10 medium, including 2 mL of the participant's own serum). Co‐intervention: Shockwave (HD or LD) | |
| Outcomes | Primary outcome: Improvement in global LVEF on quantitative LV angiography at 4 months' follow‐up.
Outcome assessment points: Baseline, 4 months, and mean 45.7 (17) months (clinical outcomes only). Method(s) of outcome measurement: LV angiography; MRI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a simple random allocation using a computer list with known N. No blockwise randomisation was performed. |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were generated at the cell‐processing centre for the entire study cohort. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was reported as double‐blind. All participants underwent BM aspiration, and the control group received a placebo. Participants were blinded; blinding of clinicians was not specifically reported. |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators were blinded for the intracoronary infusion of the study medication. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity. All participants were included in angiography analyses on an intention‐to‐treat basis. MRI was performed in a subset of participants (15 cell therapy and 12 controls). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00326989) were reported. |
| Other bias | High risk | Supported by an unrestricted grant from t2cure GmbH. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Belgium, Serbia, Switzerland | |
| Participants | Description: IHD (aged 18 to 75 years; stable HF population with a history of MI; baseline LVEF 15% to 40%; ischaemic event at least 2 months prior to recruitment; optimally managed and revascularised). Age distribution in each arm: BMSC: 55.3 (SE 10.4) years; Controls: 58.7 (SE 8.2) years Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: mean 733 x 106 (range 605 x 1168 x 106) cells G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration; no placebo or sham procedure) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcome: Changes in LVEF at 6 months Note: Main study publication reports primary endpoint as feasibility and safety at 2‐year follow‐up (Bartunek 2012).
Note: Main study publication reports secondary endpoints as including cardiac structure and function (Bartunek 2012). Outcome assessment points: Baseline, 6 months, and 2 years (clinical outcomes only). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted through a site‐independent, centralised process after exclusion of participants that did not meet the inclusion criteria. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was not fully described, but randomisation was conducted in a site‐independent manner through a centralised process. |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was administered; neither participants nor clinicians were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | An independent core laboratory masked to study arm assignment and chronology of clinical evaluation provided data analysis. |
| Incomplete outcome data (attrition bias) | High risk | 11 participants in the cell therapy group were excluded from analysis (2x clinical inclusion criteira not met; 2x BM inclusion criteria not met; 5x cell release inclusion criteria not met; 2x cell growth inclusion criteria not met). In primary analyses described in the paper, these participants were analysed as part of the control group (although they are excluded from analysis in the results of this review). All other randomised participants were included in all analyses. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol (NCT00810238) defined the primary outcome as change in LVEF at 6 months, whereas the study publication defined the primary outcome as feasibility and safety at 2 years. Follow‐up of exercise capacity and quality of life at 1 and 2 years was also defined as an outcome according to the trial protocol but was not reported. All other outcomes described in the trial protocol were reported in results. |
| Other bias | High risk | This study received commercial funding from Cardio3 BioSciences, although the authors reported that they had no relationships relevant to the contents of the paper to disclose. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: China | |
| Participants | Description: Severe ischaemic HF (isolated, chronic, total, or subtotal occluded LAD due to previous anterior wall infarction untreated by either thrombolysis or primary PCI; reversible perfusion defect detectable by SPECT; LVEF < 40%). Age distribution in each arm: BMSC: 59.3 ± 6.8 years; Controls: 57.8 ± 7.2 years. Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC Summary of stem cell isolation and type and route of delivery: 60 mL of autologous bone marrow were aspirated under local anaesthesia from the ilium of all participants during the morning of the 8th day after the PCI procedure and then cultured for 7 days. BM mesenchymal stem cells were harvested and washed 3 to 4 times with heparinised saline. 2 hours before transplantation, the stem cell suspension was mixed with heparin, filtered, and prepared for implantation. Cell viability was > 92%. G‐CSF details: No G‐CSF administered. Comparator arm: Control (no placebo). Co‐intervention: PCI | |
| Outcomes | Primary outcome: None reported. Reversible defects, metabolic equivalents, exercise, LVEF, NYHA, mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was used; neither participants nor clinicians were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Low risk | 2 cell therapy participants and 1 control were excluded from all analyses due to failed PCI. All remaining participants were included in the analysis of mortality and morbidity; all surviving participants were included in the analysis of LVEF, NYHA class, and exercise tolerance at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Leipzig, Germany | |
| Participants | Description: Chronic total artery occlusion with clinical signs of myocardial ischaemia and local wall motion abnormalities. Number of diseased vessels: BMSC: 1 (n = 8), 2 (n = 4), 3 (n = 2); Control arm: 1 (n = 6), 2 (n = 5), 3 (n = 3). | |
| Interventions | Intervention arm: G‐CSF + BMSC G‐CSF details: 300 mg of G‐CSF administered for 4 days to all participants. Comparator arm: G‐CSF + placebo (BM aspiration; cell‐free serum solution). Co‐intervention: PCI | |
| Outcomes | Primary outcomes: LV function Assessment of coronary endothelial function, myocardial viability (number of myocardial segments with hibernation), regional wall motion, LV mass (myocardial mass; infarct size). Clinical outcomes, restenosis, coronary endothelium function, myocardial viability, number of hibernating segments in myocardium. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | G‐CSF was administered and blood was taken from all participants. Control participants received a placebo. Participants and clinicians were blinded to treatment. |
| Blinding of outcome assessment (detection bias) | Low risk | Image analysis assessors remained blinded after the results at 3 months' follow‐up. Other assessors were blinded to 3 months only. |
| Incomplete outcome data (attrition bias) | Low risk | 1 cell therapy participant and 2 controls were excluded from all analyses (1x reocclusion of target vessel, 2x withdrawal of consent). MRI was performed in 23 participants (12 cell therapy, 11 controls) at 3 months and 22 participants (12 cell therapy and 10 controls) at 15 months. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: London, UK | |
| Participants | Description: Advanced HF (NYHA class II‐IV; optimal medical therapy and device therapy with no further treatment options). Age distribution in each arm: n/r Number of diseased vessels: n/r | |
| Interventions | Intervention arm: G‐CSF + BMSC Dose of stem cells: n/r G‐CSF details: 10 ug/kg/day for 5 days Comparator arm: G‐CSF + placebo (BM aspiration; 10 mL autologous serum) Co‐intervention: standard medical therapy | |
| Outcomes | Primary outcomes: Change in global LVEF from baseline (12 months) Change in quality of life (6 and 12 months), NT‐proBNP (6 months); major adverse cardiac events (12 months), change in NYHA class (12 months), change in CCS class (12 months). Outcome assessment points: Baseline, 6 and 12 months. | |
| Notes | Outcome data for this trial were obtained directly from the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were enrolled in a 1:1 computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants received G‐CSF, underwent bone marrow aspiration, and received a placebo infusion. Blinding of clinicians was not specifically reported, but the trial was described as "double‐blind". |
| Blinding of outcome assessment (detection bias) | Low risk | The endpoints of NYHA and CCS classifications were measured by an investigator blinded to the participant's treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | Information for all outcomes requested from the authors was provided. |
| Other bias | High risk | Partially sponsored by Chugai Pharma UK and the Cordis Corporation. The primary investigator of this trial is an author of this Cochrane review. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: London, UK | |
| Participants | Description: Advanced HF (NYHA class II‐IV; optimal medical therapy and device therapy with no further treatment options). Age distribution in each arm: n/r Number of diseased vessels: n/r | |
| Interventions | Intervention arm: G‐CSF + BMSC Dose of stem cells: n/r G‐CSF details: 10 ug/kg/day for 5 days Comparator arm: G‐CSF + placebo (BM aspiration; 2 mL autologous serum) Co‐intervention: standard medical therapy | |
| Outcomes | Primary outcomes: Change in global LVEF from baseline (12 months) Change in quality of life (6 and 12 months), NT‐proBNP (6 months); major adverse cardiac events (12 months), change in NYHA class (12 months), change in CCS class (12 months). Outcome assessment points: Baseline, 6 and 12 months. | |
| Notes | Outcome data for this trial were obtained directly from the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were enrolled in a 1:1 computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants received G‐CSF, underwent bone marrow aspiration, and received a placebo infusion. Blinding of clinicians was not specifically reported, but the trial was described as "double‐blind". |
| Blinding of outcome assessment (detection bias) | Low risk | The endpoints of NYHA and CCS classifications were measured by an investigator blinded to the participant's treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | Information for all outcomes requested from the authors was provided. |
| Other bias | High risk | Partially sponsored by Chugai Pharma UK and the Cordis Corporation. The primary investigator of this trial is an author of this Cochrane review. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Florida, USA | |
| Participants | Description: Chronic MI and LV dysfunction (aged 21 to 90 years; ischaemic cardiomyopathy with LV dysfunction resulting from chronic MI, as documented by confirmed coronary artery disease with a corresponding area of myocardial akinesis, dyskinesis, or severe hypokinesis and had LVEF < 50% within 6 months of screening while taking maximally tolerated doses of beta‐adrenergic blocking and angiotensin‐converting enzyme or angiotensin II receptor blocking drugs and not during or recently after an ischaemic event). Age distribution in each arm: BM‐MSC: 57.1 (10.6) years; Controls: 60.0 (12.0) years Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: n/r Timing of stem cell procedure: 4 to 6 weeks from BM aspiration to cell administration. G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; vehicle placebo) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Incidence of treatment‐emergent serious adverse events (defined as composite of death, non‐fatal MI, stroke, hospitalisation for worsening HF, cardiac perforation, pericardial tamponade, ventricular arrhythmias > 15 sec, or with haemodynamic compromise or atrial fibrillation) at 1 month Secondary outcomes:
Additional outcomes: Infarct size, regional wall motion at the sites of study agent injection, global LV size and function, exercise peak O2 consumption, NYHA class, quality of life measured at 3 and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An electronic data entry system was used for randomisation. Participants were randomised to the MSC or BMMNC group, and further randomised within groups to cell therapy or placebo. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised (unblinded) to the MSC or BMMNC group. Participants were further randomised (blinded) within groups to cell therapy or placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM harvest, and control participants received a placebo. Neither participants nor clinicians were aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Preparation and administration of the study product was blinded to investigators outside the cell‐processing laboratory. |
| Incomplete outcome data (attrition bias) | Unclear risk | 3 cell therapy participants were excluded from the analysis of mortality and morbidity (2x withdrew consent, 1x cell‐processing failure). MRI analysis included 16 cell therapy participants and 17 controls (BMSC and MSC control groups combined); missing data were unexplained. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the trial protocol (NCT00768066) were reported; some additional outcomes were reported in the publication of results. |
| Other bias | High risk | Received partial funding from BioCardia. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Florida, USA | |
| Participants | Description: Chronic MI and LV dysfunction (aged 21 to 90 years; ischaemic cardiomyopathy with LV dysfunction resulting from chronic MI, as documented by confirmed coronary artery disease with a corresponding area of myocardial akinesis, dyskinesis, or severe hypokinesis and had LVEF < 50% within 6 months of screening while taking maximally tolerated doses of beta‐adrenergic blocking and angiotensin‐converting enzyme or angiotensin II receptor blocking drugs and not during or recently after an ischaemic event). Age distribution in each arm: BMSC: 61.1 (8.4) years; Controls: 61.3 (9.0) years Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: n/r G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; vehicle placebo) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Incidence of treatment‐emergent serious adverse events (defined as composite of death, non‐fatal MI, stroke, hospitalisation for worsening HF, cardiac perforation, pericardial tamponade, ventricular arrhythmias > 15 sec, or with haemodynamic compromise or atrial fibrillation) at 1 month Secondary outcomes:
Additional outcomes: Infarct size, regional wall motion at the sites of study agent injection, global LV size and function, exercise peak O2 consumption, NYHA class, quality of life measured at 3 and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An electronic data entry system was used for randomisation. Participants were randomised to the MSC or BMMNC group, and further randomised within groups to cell therapy or placebo. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised (unblinded) to the MSC or BMMNC group. Participants were further randomised (blinded) within groups to cell therapy or placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM harvest, and control participants received a placebo. Neither participants nor clinicians were aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Preparation and administration of the study product was blinded to investigators outside the cell‐processing laboratory. |
| Incomplete outcome data (attrition bias) | Unclear risk | 3 cell therapy participants were excluded from the analysis of mortality and morbidity (2x withdrew consent, 1x ineligible before BM aspiration). MRI analysis included 16 cell therapy participants and 17 controls (BMSC and MSC control groups combined); missing data were unexplained. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the trial protocol (NCT00768066) were reported; some additional outcomes were reported in the publication of results. |
| Other bias | High risk | Received partial funding from BioCardia. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Hasselt, Belgium | |
| Participants | Description: Elective CABG surgery; transmural MI on ECG and akinesia or dyskinesia in part of the left ventricle as shown by angiography. Number of diseased vessels: BMSC: 1 (n = 0), 2 (n = 2), 3 (n = 8); Controls: 1 (n = 1), 2 (n = 2), 3 (n = 7). | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; heparinised saline) Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Global LVEF change and regional wall‐thickening changes in the infarct area. Changes in metabolic activity measured by thallium scintigraphy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (1:1) was carried out using sequentially numbered, sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | Low risk | Both treatment groups underwent BM aspiration: the BM group had bone marrow isolated the day before surgery from the iliac crest, and the control group had bone marrow aspirated from the sternum during the operation. Controls received a placebo. Both participants and the surgeon were unaware of whether cells or only saline was injected. |
| Blinding of outcome assessment (detection bias) | Low risk | Cardiac MR images were analysed by an investigator blinded to treatment assignment. For thallium scintigraphy, 2 investigators independently analysed data and were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity. 3 participants (1x cell therapy and 1x control) were excluded from MRI analysis (2x death, 1x acute psychiatric illness). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Frankfurt/Main, Germany | |
| Participants | Description: Coronary artery disease (aged > 18 years; previous MI at least 3 months prior to cell therapy and well demarcated LV regional wall motion abnormality; receiving constant state‐of‐the‐art pharmacotherapy for at least 3 months prior to enrolment). Age distribution in each arm: BMSC: 53.4 ± 12.3 years; Controls: 58.8 ± 7.3 years. Number of diseased vessels: BMSC: 1 (n = 10), 2 (n = 6), 3 (n = 6); Controls: 1 (n = 4), 2 (n = 2), 3 (n = 4). | |
| Interventions | Intervention arm: G‐CSF + BMSC G‐CSF details: 5 ug/kg/day (first 12 participants) or 10 ug/kg/day (20 participants) for 5 days. Comparator arm: G‐CSF + control (no BM aspiration, no placebo) Co‐intervention: Standard medical therapy; PCI (in 33% of participants) | |
| Outcomes | Primary outcomes: Safety and efficacy. Global and regional LV function and volumes after 3 months, determined by both LV angiography and MRI. Clinical parameters like functional NYHA class, cardiopulmonary exercise testing, and NT‐proBNP serum levels were obtained during a 5‐year follow‐up period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of clinicians and participants was not specifically reported, but no placebo was administered to the control group. |
| Blinding of outcome assessment (detection bias) | Low risk | MRI independent observers were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | All randomised participants were included in the analysis of mortality and morbidity, although 1 participant randomised to (but who did not receive) cell therapy was analysed in the control group. Angiography was carried out at follow‐up in 26 participants (21 cell therapy, 5 controls). MRI was performed in a subset of participants (9 cell therapy, 4 controls). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Beijing, China | |
| Participants | Description: Chronic HF (aged 18 to 75 years; at least 3 months since last MI; severe ischaemic cardiomyopathy with LVEF < 30% by MRI and suitable for CABG; no evidence of surviving myocardium in the infarct area, as shown by SPECT and LV angiography; without LV aneurysm or valvular diseases requiring surgical intervention). Age distribution in each arm: BMSC: 56.6 ± 9.7 years; Controls: 58.3 ± 8.9 years. Number of diseased vessels: BMSC: 3; Controls: 3. | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; mixture of 8 mL of saline solution and 2 mL of the participant's own serum) Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Changes in LVEF from baseline to 6 months' follow‐up. None reported. Additional outcomes: LVEDV index (MRI; echocardiograpy); LVESV index (MRI); wall motion score index (echocardiography); perfusion score (SPECT), 6‐min walking test, and BNP value. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation table was generated by statistical software. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM harvest, and control participants received a placebo. The study processes were blinded to surgeons and participants. |
| Blinding of outcome assessment (detection bias) | Low risk | The study processes were blinded to investigators who were responsible for participant assessments. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis of mortality and morbidity; 1 control participant did not attend follow‐up at 6 months. MRI at 12 months included 25 participants in each group. Echocardiography at 12 months included 42 participants (24 cell therapy and 18 controls); missing data were explained. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00395811) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Spain | |
| Participants | Description: Refractory angina (CCS class II‐IV; optimal medical therapy; not suitable for surgical/percutaneous revascularisation; and with reversible perfusion defect measured by SPECT). Number of diseased vessels: n/r | |
| Interventions | Intervention arm: G‐CSF; BMSC Dose of stem cells: 20 to 30 × 106 cells G‐CSF details: 5 μg/kg per 12 hours for 4 days Comparator arm: Control (BM aspiration; sham procedure but no placebo administered) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Major adverse cardiac and cerebrovascular events, defined as cardiovascular death, non‐fatal MI, ischaemic stroke, need for revascularisation, or procedure‐related complications (pericardial effusion/cardiac tamponade, vascular complications, and sustained ventricular arrhythmias) at 6, 12, and 24 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A centralised telephone randomisation was performed using a computer‐generated code before the index procedure. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using a centralised telephone procedure. |
| Blinding of participants and personnel (performance bias) | Low risk | Both groups were treated with G‐CSF, underwent an apheresis and electromechanical mapping; however, transendocardial injections were not performed in the control group but were simulated to keep the participant and all the investigators except the 2 operators who performed the injections blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | A blinded investigator analysed angiograms with the use of a computer‐based system. All the analyses were centralised in an independent core laboratory blinded to the randomisation. All investigators except 2 operators who performed the injections were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis of all outcomes on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00694642) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: USA Length of follow‐up: 6 months | |
| Participants | Description: Chronic refractory angina (aged > 21 years; CCS class III–IV; optimal medical therapy; not suitable for revascularisation; ischaemia on nuclear perfusion imaging, to complete at least 1 minute but no more than 6 mins of a standard Bruce protocol, and to experience angina/angina equivalent during the baseline exercise test). Age distribution in each arm: Mean 62.4 (range 48 to 84 years) for all groups. Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: G‐CSF, BMSC at low dose, medium dose, or high dose. G‐CSF details: G‐CSF was given to all participants at 5 μg/kg for 4 to 5 days. Comparator arm: G‐CSF; placebo (BM aspiration; saline (0.9% sodium chloride) with 5% autologous plasma). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were established by the study statistician. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants were administered G‐CSF prior to treatment and had CD34+ cells collected. Controls received a placebo solution in a syringe that was identical for all treatment arms. |
| Blinding of outcome assessment (detection bias) | Low risk | Randomisation codes were only revealed to the stem cell laboratory technician responsible for separating the cells into aliquots or preparing the placebo material. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | High risk | Partially funded by a grant from Baxter Healthcare. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: USA | |
| Participants | Description: Chronic refractory angina (aged 21 to 80 years; CCS class III–IV; optimum medical management; not suitable for revascularisation; SPECT imaging to document the presence of reversible ischaemia; patients required to walk a minimum of 3 mins but no longer than 10 mins on a modified Bruce protocol exercise tolerance test and had to experience angina or their angina equivalent during exercise testing. Age distribution in each arm: BMSC‐LD: 61.3 (9.1) years; BMSC‐HD: 59.8 ± 9.2 years; Controls: 61.8 ± 8.5 years. Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: G‐CSF, BMSC at low dose or high dose. G‐CSF details: G‐CSF was given to all participants at 5 μg/kg for 4 to 5 days. Comparator arm: G‐CSF; placebo (BM aspiration; saline (0.9% sodium chloride) with 5% autologous plasma). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Angina frequency 6 months after treatment. None reported in study protocol. Additional outcomes: Efficacy endpoints including exercise tolerance testing; use of antianginal medication; CCS functional class; health‐related QOL (Seattle Angina Questionnaire, SF‐36, Dyspnea Questionnaire, EQ‐5D); combined rate of MACE, SPECT, cardiac MRI (in a substudy). Safety endpoints including adverse event reporting, chest X‐ray, and echocardiology and laboratory screening. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 3 treatment groups via a telephone call‐in and an interactive voice‐response system. |
| Allocation concealment (selection bias) | Low risk | The cell‐processing laboratory at each centre was responsible for making the randomisation call and preparing the CD34+ cells or control injection accordingly. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants were administered G‐CSF prior to treatment and had CD34+ cells collected. Controls received a placebo solution in a syringe that was identical for both treatment arms. |
| Blinding of outcome assessment (detection bias) | Low risk | An independent committee conducted the analysis. All study personnel remained blinded until the end of the study. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in analyses at follow‐up on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00300053) were reported. |
| Other bias | High risk | Baxter Healthcare sponsored the study and was responsible for the conduct of the investigation. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Copenhagen, Denmark | |
| Participants | Description: Severe ischaemic HF (aged 30 to 80 years; optimal medical therapy with no change in medication for 2 months; no revascularisation options, LVEF < 45%; NYHA class II‐III). Age distribution in each arm: BMSC: 66.1 (7.7) years; Controls: 64.2 (10.6) years Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: mean 77.5 (67.9) x 10e6 G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; PBS mixed with a drop of the participant’s blood to maintain blinded appearance of placebo solution). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcome: Changes in LVESV at 6 months' follow‐up Clinical improvements at 6 and 12 months Note: the main study publication reports secondary outcomes as LVEDV, LVEF, SV, cardiac output, LV myocardial mass, wall thickness, wall thickening, scar volume, NYHA class, CCS class, 6MWT, weekly angina attacks and weekly use of nitroglycerine, biomarkers, the Seattle Angina Questionnaire and Kansas City Cardiomyopathy Questionnaire, and safety (Mathiasen 2015). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were enrolled in a 2:1 computer‐generated randomisation list blocks of 6. |
| Allocation concealment (selection bias) | Low risk | The randomisation list was generated by a person unrelated to the study group. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial investigators, study nurses, and participants were blinded to treatment allocation. To maintain blinding, a drop of the participant's blood was mixed into the syringe containing MSC or placebo by the stem cell laboratory to make the MSC solution and placebo identical. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial investigators and experienced physicians who performed the MRI analyses were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in all analyses at follow‐up on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00644410) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: London, UK | |
| Participants | Description: Advanced HF (NYHA class II‐IV; optimal medical therapy and device therapy with no further treatment options). Age distribution in each arm: Mean 70 (10) years Number of diseased vessels: n/r | |
| Interventions | Intervention arm: G‐CSF + BMSC Dose of stem cells: Mean 8.6 (11.0) x 107 G‐CSF details: 10 ug/kg/day for 5 days Comparator arm: G‐CSF + placebo (BM aspiration; 10 mL autologous serum) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: None (change in global LVEF from baseline (12 months) is a primary outcome in the main REGENERATE‐IHD trial but not included in the pilot study) Change in quality of life (6 and 12 months); NT‐proBNP (6 months); major adverse cardiac events (12 months); change in NYHA class (12 months) Outcome assessment points: Baseline and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were enrolled in a 1:1 computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants received G‐CSF, underwent bone marrow aspiration, and received a placebo infusion. Blinding of clinicians was not specifically reported, but the trial was described as "double‐blind". |
| Blinding of outcome assessment (detection bias) | Low risk | The endpoints of NYHA and CCS classifications were measured by an investigator blinded to the participant's treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Unclear risk | This is a pilot study report that only reports 6‐month follow‐up of the secondary outcomes described in the protocol (NCT00747708). |
| Other bias | High risk | Partially sponsored by Chugai Pharma UK and the Cordis Corporation. The primary investigator of this trial is an author of this Cochrane review. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: London, UK | |
| Participants | Description: Advanced HF (NYHA class II‐IV; optimal medical therapy and device therapy with no further treatment options). Number of diseased vessels: n/r | |
| Interventions | Intervention arm: G‐CSF + BMSC Dose of stem cells: Mean 5.2 (5.3) x 107 G‐CSF details: 10 ug/kg/day for 5 days Comparator arm: G‐CSF + placebo (BM aspiration; 2 mL autologous serum) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: None (change in global LVEF from baseline (12 months) is a primary outcome in the main REGENERATE‐IHD trial but not included in the pilot study) Change in quality of life (6 and 12 months); NT‐proBNP (6 months); major adverse cardiac events (12 months); change in NYHA class (12 months) Outcome assessment points: Baseline and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were enrolled in a 1:1 computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants received G‐CSF, underwent bone marrow aspiration, and received a placebo infusion. Blinding of clinicians was not specifically reported, but the trial was described as "double‐blind". |
| Blinding of outcome assessment (detection bias) | Low risk | The endpoints of NYHA and CCS classifications were measured by an investigator blinded to the participant's treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Unclear risk | This is a pilot study report that only reports 6‐month follow‐up of the secondary outcomes described in the protocol (NCT00747708). |
| Other bias | High risk | Partially sponsored by Chugai Pharma UK and the Cordis Corporation. The primary investigator of this trial is an author of this Cochrane review. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Berlin, Germany | |
| Participants | Description: Chronic IHD (indication for CABG surgery; reduced global LVEF by transthoracic echocardiography at rest (LVEF ≤ 35); presence of akinetic or hypokinetic and hypoperfused LV myocardium on MRI for defining the target area). Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: Median 5.1 (IQR 3.0 to 9.1) x 106 CD133+ cells G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; isotonic sodium chloride solution containing 10% autologous serum) Co‐intervention: CABG | |
| Outcomes | Primary outcomes: LVEF at rest, measured 6 months' postoperatively by MRI.
Post‐hoc outcome: Infarct scar size. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted in the cell preparation facility. Group allocation was performed according to a predefined non‐block‐wise 1:1 randomisation plan. |
| Allocation concealment (selection bias) | Low risk | The randomisation plan was accessible only to the external cell processing team that prepared the cell product or placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent bone marrow aspiration. Syringes were prepared with either cells or placebo solution, and an ID number was added so that participants, the surgical team, and all investigators were unaware of treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Syringes were prepared with either cells or placebo solution, and an ID number was added so that participants, the surgical team, and all investigators were unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity (3x cell therapy and 1x control were excluded from exercise testing). In MRI analysis, the number of withdrawals was low (treatment: 2/30 vs control: 4/30), and reasons for exclusion were given. 4 participants (3 cell therapy, 1 control) did not undergo exercise tests. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00462774) were reported. One post‐hoc outcome was clearly defined as such. |
| Other bias | High risk | Supported in part by Miltenyi Biotec and the German Bundesministerium fur Bildung und Forshcung. Two authors received lecture fees from Miltenyi Biotec. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Rosario, Argentina | |
| Participants | Description: Ischaemic HF (LVEF < 35% by echocardiography and multiplanar cardiac catheterisation; NYHA class III or IV; requiring revascularisation, undergoing off‐pump CABG). Age distribution in each arm: BMSC arm: 64.8 ± 7.1 years old; Control arm: 63.6 ± 5.2 years old (pilot study data). Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration, no placebo) Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A person who did not participate in the trial had the choice of picking a coloured ball (red = BMSC arm; blue = control arm). |
| Allocation concealment (selection bias) | Unclear risk | The person undertaking the randomisation procedure did not participate in the trial. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Clinicians were not blinded. The authors report that participants were blinded, although the control group did not undergo bone marrow aspiration and no placebo was used. |
| Blinding of outcome assessment (detection bias) | Low risk | The reviewers of imaging studies (cardiologists) were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Utah and California, USA; Rostock and Berlin, Germany; and Grugoan, India | |
| Participants | Description: CHF (aged > 18 years; LVEF < 40% by contrast echocardiography, NYHA class III or IV, stable with standard medical therapy for at least 1 month before screening, and a life expectancy of 6 months or longer). Age distribution in each arm: BMSC: 58.5 (12.7) years; Controls: 52.7 (8.5) years. Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: Mean 3.7 (0.9) x 109 nucleated cells. G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration; no placebo) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Number of participants with adverse events as a measure of safety and tolerability (12 months) To assess the effect of the infusion of bone marrow nucleated cells on the clinical course of angina as measured by QOL questionnaire, MLHFQ, NYHA and CCS classification, and SPECT (12 months) Assess the effect of the infusion of bone marrow nucleated cells on the clinical course of HF (12 months) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Electronic randomisation" was performed. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was used; neither participants nor clinicians were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | All imaging and follow‐up information was blinded to the reviewers. |
| Incomplete outcome data (attrition bias) | Low risk | With the exception of 2 early withdrawals with reasons clearly defined, all randomised participants were included in the analysis of mortality and morbidity on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT01299324) were reported. |
| Other bias | High risk | Commercially sponsored study (funded by Harvest Technologies). No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Helsinki, Finland | |
| Participants | Description: Ischaemic HF (aged 18 to 75 years; managed by optimal medical care; undergoing CABG; LVEF between 15% and 45%; NYHA class II‐IV HF symptoms). Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: Median 8.4 (range 5.2 to 13.5) x 108 cells G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; vehicle medium) Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Change in LVEF after 1‐year follow‐up measured by MRI (12 months) Changes in any other cardiac parameters as measured by echocardiography, MRI, or PET ischaemia area (6 months; 1 year) Change in plasma concentrations of proBNP (6 months; 1 year) Primary hospitalisation or days stayed in hospital Correlation between pericardial fluid growth factor concentrations and left ventricular function improvement (up to 1 year) Correlation between autologous cardiac stem cell quality and left ventricular function improvement (6 months; 1 year) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before examination, numbered randomisation envelopes were sealed by stem cell laboratory personnel blinded to other participants. After delivery of the BM harvest to the stem cell laboratory, randomisation of each participant was done at the time of operation. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes were prepared by the stem cell laboratory before examinations. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent bone marrow aspiration, and the control group received a placebo. Syringes containing cell therapy or placebo were masked using a non‐transparent tape. |
| Blinding of outcome assessment (detection bias) | Low risk | 1 investigator analysed all MRI imaging data in a random order. Areas of scar and ischaemic myocardium were assessed by 2 study‐blind, experienced nuclear medicine physicians. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity. The number of withdrawals from MRI analysis was low (treatment: 2/20 vs control: 2/19), and reasons for withdrawals were reported in detail. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the trial protocol (NCT00418418) were reported, although results were reported for 12 months only and not at 6 months as specified in the trial protocol. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Texas and Minneapolis, USA | |
| Participants | Description: Ischaemic HF (functional class III or IV (angina) and/or HF symptoms (NYHA) on maximal medical therapy; chronic CAD with a reversible perfusion defect ≥ 7% (SPECT); LVEF < 40%; MVO2 < 21 mL/kg/min; ineligible for percutaneous or surgical revascularisation). Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration; sham procedure performed but no placebo administered). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Safety of cell injections at 3 time points: i) early safety (periprocedural and up to 2 weeks); ii) 3 months; and iii) 6 months: major adverse events (hospitalisation, arrhythmia, exacerbation of CHF, acute coronary syndrome, MI, stroke, or death).
Outcome assessment points: Baseline, 3 and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation tables were prepared by the statistical department. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | Low risk | Clinicians were not blinded, but participants received a simulated mock injection procedure (although it was unclear whether BM aspiration was undertaken in control group). |
| Blinding of outcome assessment (detection bias) | Low risk | Efficacy studies were read by an independent, blinded investigator. Blinding was maintained until the end of the assessment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00203203) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: USA | |
| Participants | Description: Chronic IHD (aged > 18 years; clinically stable coronary artery disease, LVEF ≤ 45%, limiting angina (CCS class II‐IV) and/or CHF (NYHA class II‐III), a perfusion defect by SPECT, and no revascularisation options while receiving guideline‐based medical therapy). Age distribution in each arm: BMSC: 63.95 ± 10.90 years; Controls: 62.32 ± 8.25 years. Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; cell‐free suspension in the same volume). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcome assessment points: Baseline and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated and used variable block sizes of 6 or 9, randomly selected and stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was masked to all but 1 designated cell‐processing team member at each centre not involved in participant care. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM aspiration, and the control group received a placebo injection. All caregivers and participants were masked to treatment. |
| Blinding of outcome assessment (detection bias) | Low risk | The study was described as “double‐blind”. Major adverse clinical events were assessed by 2 independent cardiologists not affiliated with any clinical site and masked to treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity outcomes. Reasons for loss to follow‐up and withdrawals from echocardiography and SPECT analysis were given, with similar attrition rates in both treatment arms. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00824005) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Texas, USA | |
| Participants | Description: Advanced ischaemic HF (CCS class II‐IV angina or NYHA class II‐III HF; optimal medical therapy, LVEF < 45% by echocardiography; presence of a reversible perfusion defect on SPECT, ineligible for percutaneous or surgical revascularisation). Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; 5% albumin). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Safety (combined early and late adverse events) (baseline, 6 months) Secondary outcomes:
Outcome assessment points: Baseline and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomised sequence was used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Control participants underwent an identical bone marrow harvest procedure, including insertion of the needle, except that BM was not aspirated. Control participants received transendocardial injections of placebo solution instead of cell preparation. All personnel involved were blinded. Personnel involved in the harvesting procedure acted independently of the study team, thus maintaining blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was described as “double‐blind”. Two blinded, independent echocardiologists reviewed the echocardiograms, and the average of the 2 readings was reported. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (NCT00314366) were reported. |
| Other bias | High risk | This work was supported solely by Aldagen Inc, Durham, NC. No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Russia | |
| Participants | Description: Chronic MI and end‐stage chronic HF (history of MI > 12 months before enrolment and fixed perfusion defect on technetium‐99m tetrofosmin SPECT; clinical symptoms of HF; ineligible for revascularisation; LVEF < 35% as determined by 2‐dimensional echocardiography). Number of diseased vessels: BMSC: 1 (n = 2), 2 (n = 1), 3 (n = 52); Controls: 1 (n = 3), 2 (n = 3), 3 (n = 48). | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration or placebo administration reported). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Efficacy of the intramyocardial injection of autologous bone marrow mononuclear cells, measured by change in myocardial perfusion defects at rest and under pharmacological stress. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using an electronic system. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was administered; participants and clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | SPECT imaging was carried out though consensus by 2 readers blinded to the type of study and clinical data. Other blinding was not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes (other than deaths prior to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Jakarta (Indonesia) and Hong Kong (China) | |
| Participants | Description: Advanced ischaemic HF (NYHA class III or IV; HF refractory to conventional medical therapy not eligible for conventional percutaneous or surgical revascularisation; existence of 1 or 2 coronary territories of viable, ischaemic myocardium as documented by dipyridamole single‐photon emission computed tomographic perfusion study; and LVEF < 40% measured by transthoracic echocardiogram). Age distribution in each arm: BMSC: 58 (5.9) years; Controls: 60 (5.6) years Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: n/r G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; PBS with 10% autologous plasma). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Change in LVEF from baseline to 6 months' follow‐up measured by MRI Secondary outcomes: Changes in exercise duration and MVO2 (treadmill modified Bruce protocol) (baseline and 6 months) Note: main study publication reports secondary endpoints as changes in NYHA classification, LVESV, LV infarct and peri‐infarct ischaemic volume, and exercise performance (6MWT) (Santoso 2014). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated using a randomisation table; randomisation was constrained, stratified by study centre. |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted via a system of sealed and numbered envelopes provided to each investigation centre. |
| Blinding of participants and personnel (performance bias) | Low risk | Bone marrow cells were harvested from all participants. The control group received an identical final suspension but without cells. After randomisation, participants were blinded to the study processes. |
| Blinding of outcome assessment (detection bias) | Low risk | After randomisation, investigators who were responsible for participant assessment were blinded to study processes. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the trial protocol (NCT01150175) were reported; additional outcomes were reported in the publication of results. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Belgrade, Serbia | |
| Participants | Description: IHD (aged 35 to 72 years; scheduled for CABG surgery due to LAD occlusion or multivessel coronary disease; previous MI older than 30 days; established diagnosis of ischaemic cardiomyopathy with LVEF < 40% and in the NYHA III‐IV functional class, full medical treatment for HF). Number of diseased vessels: n/r Statistically significant baseline imbalances between the groups? Significantly higher hypercholesterolaemia in BMSC group than in control group. | |
| Interventions | Intervention arm: BMSC Dose of stem cells: Mean 70.7 (32.4) x 106 cells G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration, no placebo). Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Postoperative functional capacity and cardiac‐related mortality in the median follow‐up of 5 years. Cardiovascular mortality, NYHA, 6MWT, LVEF, perfusion defect; BNP levels. Method(s) of outcome measurement: SPECT, echocardiography, 6MWT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label trial; no blinding was carried out. |
| Blinding of outcome assessment (detection bias) | High risk | The heart team consisting of an interventional cardiologist/radiologist, heart surgeon, and clinical cardiologist evaluated coronary angiography and all clinical and imaging data and made decisions on coronary revascularisation and stem cell implantation. This was an open‐label trial; no blinding was reported. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Hong Kong (China) and Newcastle (Australia) | |
| Participants | Description: Refractory angina (CCS class III or IV; no conventional percutaneous or surgical revascularisation option; ability to complete > 3 min but < 10 min of treadmill exercise using modified Bruce protocol, and 1 or 2 coronary territories of viable, ischaemic myocardium as documented by dipyridamole SPECT perfusion study). Age distribution in each arm: BMSC: 65.2 ± 8.3 years; Controls: 68.9 ± 6.3 years. Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; 8 to 12 injections of 0.1 mL of phosphate buffered saline with 10% autologous serum). Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Change from baseline in total exercise time on a modified Bruce protocol at 6 months' follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a randomisation table and was constrained, stratified by the study centre. |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered envelopes were provided by the study centre (centralised) to each investigational centre. |
| Blinding of participants and personnel (performance bias) | Low risk | All randomised participants underwent BM aspiration, and the control group received a placebo injection. After randomisation, the study processes were blinded to participants. No details were given of the blinding of clinicians. |
| Blinding of outcome assessment (detection bias) | Low risk | After randomisation, the study processes were blinded to study co‐ordinators and investigators responsible for participants' assessment. Blinding was maintained until the end of the study. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Germany | |
| Participants | Description: IHD (aged 18 to 80 years; documented MI at least 3 months previously; clear‐cut demarcated region of left ventricular dysfunction with an open infarct‐related coronary artery at the time of stem cell therapy). Age distribution in each arm: BMSC: 62 ± 10 years old; Controls: 60 ± 9 years old. Number of diseased vessels: BMSC: 1.5 ± 0.5; Controls: 2.0 ± 0.6. | |
| Interventions | Intervention arm: BMSC. G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration; no placebo administered). Co‐intervention: PCI | |
| Outcomes | Primary outcomes: Change in global EF as well as the size of infarcted area measured by left ventriculography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants in the control group did not undergo BM aspiration, and no placebo was administered. Blinding was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | Haemodynamic investigations and laboratory results were obtained independently by 2 investigators. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of mortality and morbidity outcomes; 7 participants (5x cell therapy, 2x controls) were excluded from LVEF and functional capacity at follow‐up due to restenosis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Leiden, the Netherlands | |
| Participants | Description: Severe angina (CCS class II‐IV, myocardial ischaemia in at least 1 myocardial segment on Tc099m tetrofosmin SPECT, ineligible for CABG or PCI). Age distribution in each arm: BMSC: 64 ± 8 years; Controls: 62 ± 9 years. Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; sodium chloride 0.9% with 0.5% human serum albumin). Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Change in myocardial perfusion (SPECT) at 3 months' follow‐up relative to baseline.
Outcome assessment points: Baseline and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using sequentially numbered, sealed envelopes provided by the Department of Medical Statistics and Bioinformatics. A block size of 4 was used without further stratification. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM aspiration, and the control group received a placebo injection; participants were unaware of group assignment. A blinded syringe with either cell suspension or placebo was used. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, study co‐ordinators, and investigators involved in participant assessments were unaware of group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial protocol (ISRCTN58194927) were reported. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Beijing, China | |
| Participants | Description: Angina (no AMI in the month prior to transplantation). Age distribution in each arm: BMSC: 60.6 years; Controls: 60 years. Sex (% male) in each arm: BMSC: 56.25%; Controls: 63.25%. Number of diseased vessels: Not reported. | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Control (no BM aspiration, no placebo administered). Co‐intervention: PCI | |
| Outcomes | Primary outcomes: Not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of clinicians and participants was not reported. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: China | |
| Participants | Description: Intractable angina (aged > 30 years; diffuse triple‐vessel disease; CCS class III or IV; optimal medical therapy and considered ineligible for revascularisation, no ischaemia or nuclear perfusion imaging according to the Bruce protocol; angina experienced during baseline exercise test). Number of diseased vessels: 3 | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; saline + human serum albumin) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: Safety (mortality and morbidities). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM aspiration, and the control group received a placebo injection. Participants were unaware of the treatment received. Blinding of clinicians was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | All researchers were unaware of the treatments. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Conference abstract Study setting: Guangzhou, China | |
| Participants | Description: Chronic IHD (impaired LV function: LVEF < 35%). Number of diseased vessels: n/r | |
| Interventions | Intervention arm: BMSC Dose of stem cells: n/r G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration not reported; no further details) Co‐intervention: Standard medical therapy | |
| Outcomes | Primary outcomes: LVEF measured by cMRI. 5‐min walk test; NYHA, regional wall motion, scar mass, LVESV, LVEDV. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial was reported as "double‐blind", but no details of blinding were reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial was reported as "double‐blind", but no details of blinding were reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No withdrawals were reported in this conference abstract. |
| Selective reporting (reporting bias) | High risk | All outcomes mentioned in the methods were reported in the results, although selective reporting would be considered likely in this conference abstract. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Type of publication: Full paper Study setting: Shenyang, China | |
| Participants | Description: Chronic MI (multivessel disease; admitted for elective OPCAB surgery at least 4 weeks after a cardiac infarction). Number of diseased vessels: n/r (multivessel) | |
| Interventions | Intervention arm: BMSC Dose of stem cells: mean 5.21 (0.44) x 108 cells G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration, saline solution) Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Incidence of emergent adverse events within the follow‐up period (6 months). LVEF, wall motion score index, LVEDV/LVESV, LVEDD/LVESD, arrhythmias, atrial fibrillation, non‐sustained ventricular tachycardia, ventricular premature beats, sustained ventricular tachycardia, troponin T levels. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM aspiration and received cells or a placebo injection. The surgeon performing the OPCAB and injections was unaware of whether the light‐resistant syringe contained saline or BMC. |
| Blinding of outcome assessment (detection bias) | Low risk | All preoperative baseline and follow‐up echocardiography was performed by an experienced cardiologist blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Shanghai, China | |
| Participants | Description: IHD (aged < 75 years; history of transmural MI and revascularisation plus stent implantation at least 6 months earlier; patent infarct‐related artery at the time of stem cell transplantation). Number of diseased vessels: BMSC: 1 (67%), 2 (29%), 3 (4%); Controls: 1 (70%), 2 (26%), 3 (4%). | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (0.9% sodium chloride containing heparin). Unclear whether BM aspiration was performed. Co‐intervention: Standard medical therapy, PCI (in 30% of participants) | |
| Outcomes | Primary outcomes: Improvement of LV function. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised, but the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM aspiration, and the control group received a placebo injection. Blinding of clinicians was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors (MRI, echocardiography, SPECT) were blinded to the assigned therapy. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
| Methods | Type of study: Parallel RCT Study setting: Shanghai, China | |
| Participants | Description: Ischaemic HF (aged 18 to 75 years; admitted for elective CABG; history of transmural old MI with akinesis or dyskinesis of the left ventricle shown by echocardiography; multivessel disease with a reversible perfusion defect detected by SPECT; LVEF less than 40%). Age distribution in each arm: BMSC: 60.3 ± 10.4 years; Controls: 59.1 ± 15.7 years. Number of diseased vessels: multivessel, 2 or more | |
| Interventions | Intervention arm: BMSC G‐CSF details: No G‐CSF administered. Comparator arm: Placebo (BM aspiration; saline). Co‐intervention: CABG | |
| Outcomes | Primary outcomes: Death, MI, and recurrence of HF. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated sequence of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants underwent BM aspiration, and the control group received a placebo injection. Blinding of clinicians was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | The results were analysed by 2 independent, experienced observers; investigators (echocardiography, SPECT) were blinded to the randomisation scheme. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analysis of all outcomes at follow‐up (other than deaths). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting. No prospectively registered or published trial protocol was identified. |
| Other bias | Low risk | No other sources of bias were reported or identified. |
6MWT: 6‐minute walk test
AEs: adverse events
ALDH: aldehyde dehydrogenase
AMI: acute myocardial infarction
AOI: area of interest
APC: allophycocyanin
BM: bone marrow
BMC: bone marrow cells
BMMNC: bone marrow mononuclear cells
BMSC: bone marrow stem cells
BNP: brain natriuretic peptide
CABG: coronary artery bypass grafting
CAD: coronary artery disease
CCS: Canadian Cardiovascular Society
CCTRN: Cardiovascular Cell Therapy Research Network
CFU‐GM: colony forming unit‐granulocyte macrophage
CHF: congestive heart failure
cMRI: cardiovascular magnetic resonance imaging
CPC: circulating progenitor cells
CT: computed tomography
DM: diabetes mellitus
ECG: electrocardiogram
EDTA: ethylenediaminetetraacetic acid
EF: ejection fraction
EMM: electromechanical mapping
EPC: endothelial progenitor cells
FACS: fluorescence‐activated cell sorting
FITC: fluorescein isothiocyanate
G‐CSF: granulocyte colony‐stimulating factor
GMP: good manufacturing practices
HF: heart failure
HTN: hypertension
IC: intracoronarily
IgG: immunoglobulin G
IHD: ischaemic heart disease
IM: intramyocardial
IQR: interquartile range
LAD: left anterior descending
LDL: low‐density lipoprotein
LIMA: left internal mammary artery
LV: left ventricular
LVEDD: left ventricular end‐diastolic diameter
LVEDV: left ventricular end‐diastolic volume
LVEF: left ventricular ejection fraction
LVESD: left ventricular end‐systolic diameter
LVESV: left ventricular end‐systolic volume
MACE: major adverse clinical events
MI: myocardial infarction
MLHFQ: Minnesota Living with Heart Failure Questionnaire
MNC: mononuclear cells
MRI: magnetic resonance imaging
MSC: mesenchymal stem cells
MVO2: myocardial oxygen consumption
NHLBI: National Heart, Lung, and Blood Institute
n/r: not reported
NTG: nitroglycerine
NT‐proBNP: N‐terminal pro b‐type natriuretic peptide
NYHA: New York Heart Association
OPCAB: off‐pump coronary artery bypass
PBS: phosphate‐buffered saline
PBSC: peripheral blood stem cell
PC: progenitor cells
PCI: percutaneous coronary intervention
PET: positron emission tomography
QOL: quality of life
RCT: randomised controlled trial
SD: standard deviation
SE: standard error
SF‐36: 13‐Item Short Form Health Survey
SPECT: single‐photon emission computed tomography
SRS: segmental resting score
SV: stroke volume
STEMI: ST elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
VEGF: vascular endothelial growth factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT of allogeneic mesenchymal precursor cells. | |
| Single‐arm trial of CD133+ in ischaemic cardiomyopathy, no control arm included. | |
| Single‐arm substudy of BMMNC in refractory angina and chronic myocardial ischaemia, no control arm included. | |
| Single‐arm trial of BMMNC in chronic ischaemia, no control arm included. | |
| Single‐arm trial of BMMNC in chronic myocardial infarction and severe left ventricular dysfunction, no control arm included. | |
| Review of imaging techniques for cardiac stem cell therapy. | |
| Single‐arm trial of BMSC in severe coronary artery disease, no control arm included. | |
| RCT of cardiac stem cells in ischaemic cardiomyopathy, no BMSC administered. | |
| RCT of peripheral blood stem cells in AMI. | |
| Randomised trial of early versus late administration of BMMNC in AMI. | |
| RCT of G‐CSF‐mobilised PBSC in heart failure; G‐CSF was not administered to the control group. | |
| Single‐arm trial of autologous MSC in end‐stage dilated cardiomyopathy. | |
| RCT of BMMNC in people with AMI. | |
| A follow‐on study of people with refractory angina and documented ischaemia who received bone marrow‐derived cells in 2 previous trials, no control arm included. | |
| Single‐arm trial of bone marrow cells in advanced ischaemic heart disease, no control arm included. | |
| Non‐randomised trial of single or repeated infusion of PBSC and G‐CSF compared with a control group in refractory ischaemic heart failure. | |
| A single‐arm trial of autologous MSC in stable coronary artery disease and refractory angina, no control arm included. | |
| A comparison of outcomes in diabetic/non‐diabetic patients with end‐stage heart failure who received BMMNC in a previous trial. | |
| RCT of G‐CSF‐mobilised PBSC in people with acute and old myocardial infarction; G‐CSF was not administered to the control group. | |
| RCT of G‐CSF‐mobilised PBSC in people with acute myocardial infarction. | |
| RCT of autologous MSC in people undergoing CABG; trial suspended due to low accrual and no control participants included. | |
| Non‐randomised trial of BMMNC in chronic coronary artery disease. | |
| RCT of autologous MSC in people undergoing CABG; study of cardiac enzyme outcomes measured within 24 to 48 hours of treatment, which are not relevant to this review. No further publications have been identified. | |
| RCT of CD34+ in end‐stage diffuse coronary artery disease comparing 2 cell doses, no control arm included. | |
| RCT of cardiosphere‐derived cells in AMI. | |
| A follow‐on single‐arm substudy of 3 previous cell therapy trials, no control group included. | |
| RCT of BMMNC in sub‐acute myocardial infarction (within 15 days). | |
| Although this study was described as randomised, the 7 participants in each treatment arm (14 in total) were matched by age and sex. | |
| Non‐randomised study of BMSC in AMI. | |
| Non‐randomised study of BMMSC for severe chronic heart failure. | |
| Study withdrawn prior to enrolment. | |
| Trial terminated (reason not given), no relevant publications identified. | |
| Trial terminated (reason not given), no relevant publications identified. | |
| Trial terminated due to pilot study resulting in changes to protocol and new study required. | |
| RCT of intramyocardial versus intracoronary administration of enriched CD133+ cells, no control arm included. | |
| Trial terminated after phase I due to slow recruitment. | |
| RCT of single versus repeated administration of BMMNC in chronic postinfarction HF, no control arm included. | |
| Trial terminated due to lack of recruitment, no relevant publications identified. | |
| Non‐randomised controlled trial of BMMNC in chronic ischaemic heart failure. | |
| Non‐randomised trial of BMMNC in AMI. | |
| Single‐arm trial of CD34+ cell in ischaemic cardiomyopathy. | |
| Randomised cross‐over trial of cardiac resynchronisation and BMMNC in ischaemic heart failure, no control arm included. | |
| Randomised trial of autologous versus allogeneic MSC in dilated cardiomyopathy. | |
| RCT of cardiosphere‐derived cells for heart regeneration after myocardial infarction; no bone marrow‐derived cells were administered. | |
| Non‐RCT of BMMNC in people with heart failure. | |
| Pre‐clinical animal study of BMMSC after AMI. | |
| Non‐randomised trial of CD133+ cells in chronic ischaemic heart disease. | |
| A single‐arm substudy of the POSEIDON (Prevention of Contrast Renal Injury with Different Hydration Strategies) trial, no control arm. | |
| A single‐arm trial of autologous human cardiac‐derived stem cells in ischaemic cardiomyopathy. | |
| A comparison of outcomes in people with ischaemic and non‐ischaemic heart failure who received CD34+ and MSC. | |
| A single‐arm trial of BMMNC in refractory angina. | |
| A single‐arm trial of autologous unfractionated bone marrow in refractory angina. | |
| A comparison of the effects of CD34+ cell therapy in ischaemic and non‐ischaemic HF, no control arm included. | |
| Non‐randomised trial of BMMNC in AMI. |
AMI: acute myocardial infarction
BMMNC: bone marrow mononuclear cells
BMMSC: bone marrow mesenchymal stem cells
BMSC: bone marrow stem cells
CABG: coronary artery bypass grafting
G‐CSF: granulocyte colony‐stimulating factor
MSC: mesenchymal stem cells
PBSC: peripheral blood stem cell
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Type of study: Parallel RCT Type of publication: Abstract Study setting: Tehran, Iran |
| Participants | Description: Ischaemic cardiomyopathy and low global ejection fraction (< 35%) Number of diseased vessels: n/r |
| Interventions | Intervention arm: CABG + intramyocardial mesenchymal/CD133+ stem cells Dose of cells: n/r Timing of stem cell procedure: Bone marrow was harvested from iliac crest 2 weeks prior to surgery, and purified expanded mesenchymal/CD133+ stem/progenitor cells were injected in the ischaemic border zone of the heart during CABG. Comparator arm: CABG only |
| Outcomes | LV function, wall motion score index, mortality, morbidities Outcome assessment points: Baseline, 6 months Method of measurement: Echocardiography |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: n/r Number of centres: n/r Length of follow‐up: 35 months Number (N) of participants randomised to each arm: n/r (total 27) |
| Participants | Description: Severe ischaemic HF undergoing CABG Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: n/r |
| Interventions | Intervention arm 1: CABG + BMMSC Intervention arm 2: CABG + recycling stem cells Type of cells: BMMNC Dose of cells: n/r Timing of stem cell procedure: Cells were injected in the border zone of the infarcted myocardium. Comparator arm: CABG |
| Outcomes | Morbidy, mortality, LVEF wall motion score index. Outcome assessment points: Baseline, 36 months Method of measurement: Echocardiography |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: Italy Number of centres: n/r Length of follow‐up: 12 months Number (N) of participants randomised to each arm: n/r (total 37 randomised 1:1) |
| Participants | Description: Ischaemic cardiomyopathy (acute transmural MI less than 6 months prior to admission and LVEF lower than 35%). Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: < 6 months prior to admission |
| Interventions | Intervention arm: CABG + BMMNC Type of cells: BMMNC Dose of cells: n/r Timing of stem cell procedure: Bone marrow mononuclear cells were isolated from bone marrow aspirates and injected intramyocardially during cardiac surgery (CABG). Comparator arm: CABG + placebo |
| Outcomes | Periprocedural adverse events, mortality, LVEF and LV volumes; flow cytometry measures Outcome assessment points: 6 and 12 months Method of measurement: Not reported. |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: Buenos Aires, Argentina Number of centres: n/r Length of follow‐up: 12 months Number (N) of participants randomised to each arm: n/r |
| Participants | Description: Chronic MI with patent infarct‐related artery, extensive necrosis, no development of ischaemia, and impaired ventricular function. Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: < 3 months |
| Interventions | Intervention arm: BMSC Type of cells CD34+ and CD133+ cells Dose of cells: 4 ‐ 10 x 106 Timing of stem cell procedure: Bone marrow aspiration, with filter and cell processing to obtain CD34+ and CD133+, and latter intracoronary injection of this preparation through the infarct‐related artery. Comparator arm: Control (optimal medical therapy) |
| Outcomes | Major cardiovascular events, rehospitalisation, target vessel revascularisation, LVEF, myocardial perfusion (summed rest score) Outcome assessment points: Baseline, 12 months Method of measurement: Radioisotopic ventriculography |
| Notes | Participants are from the chronic branch of the "RECUPERAR" study. To our knowledge, this trial has published in abstract form only with insufficient reporting of methodology to determine whether this substudy is randomised. Should further publications be identified confirming this, the study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: China Number of centres: n/r Length of follow‐up: 12 months Number (N) of participants randomised to each arm: n/r (110 in total) |
| Participants | Description: Ischaemic HF with LVEF < 45% Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: n/r |
| Interventions | Intervention arm: BMSC Type of cells: Mononuclear cells Dose of cells: 8.6 x 108 Timing of stem cell procedure: Cells or placebo were injected intraoperatively into the MI border area. Comparator arm: Placebo (no details) |
| Outcomes | Myocardial scar size, LVEF, cell viability, wall thickening Outcome assessment points: Baseline, 12 months Method of measurement: MRI, PET |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: Moscow, Russia Number of centres: n/r Length of follow‐up: 6 months Number (N) of participants randomised to each arm: n/r |
| Participants | Description: Chronic HF patients in NYHA class III‐IV (24 participants with ischaemic dilated cardiomyopathy and 26 participants with idiopathic dilated cardiomyopathy) Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: n/r |
| Interventions | Intervention arm: BMSC Type of cells: CD133+ Dose of cells: n/r Timing of stem cell procedure: CD133+ were obtained by CliniMACS technology of magnetic separation. Comparator arm: Placebo |
| Outcomes | LVEF, LV volumes, LV mass Outcome assessment points: Baseline, 6 months Method of measurement: SPECT |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: Beijing, China Number of centres: n/r Length of follow‐up: 12 months Number (N) of participants randomised to each arm: n/r |
| Participants | Description: Old MI Age distribution in each arm: total: 57.48 ± 7.98 years Number of diseased vessels: n/r Time from symptom onset to initial treatment: n/r |
| Interventions | Intervention arm: CABG + BMSC Type of cells: BMMNC Dose of cells: n/r Timing of stem cell procedure: No details of cell isolation or cell delivery method reported. Comparator arm: CABG + placebo |
| Outcomes | LVEF, LV volumes, cardiac output, cardiac index, cardiac mass, infarct size Outcome assessment points: Baseline, 12 months Method of measurement: MRI |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Controlled trial (unclear whether randomised) Type of publication: Abstract Source of funding: n/r Study setting: Yazd, Iran Number of centres: n/r Length of follow‐up: n/r Number (N) of participants randomised to each arm: n/r (15 recruited in total) |
| Participants | Description: Severe ischaemic cardiomyopathy requiring CABG Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: n/r |
| Interventions | Intervention arm: CABG + BMSC Type of cells: BMSC Dose of cells: median 5 x 107 (SD 1 x 106) cells Timing of stem cell procedure: No details given. Comparator arm: CABG + placebo |
| Outcomes | Periprocedural adverse events, angina frequency, LVEF, LV volumes, wall motion Outcome assessment points: n/r Method of measurement: n/r |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: Modena, Italy Number of centres: n/r Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 19x BMSC, 11x controls |
| Participants | Description: Ischaemic HF Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: n/r |
| Interventions | Intervention arm: LV restoration + BMSC Type of cells: BMMNC Dose of cells: 5 cm3 to 8 cm3 Timing of stem cell procedure: Mononuclear cells were derived from the sternal bone marrow and processed in a sterile mini‐lab before injection by direct visualisation into the infarcted areas of the myocardium before LV reconstruction. Comparator arm: LV restoration only |
| Outcomes | Mortality, rehospitalisation, change in LVEF diameter, change in LVEF and NYHA, change in infarcted area Outcome assessment points: Baseline, 6 and 12 months Method of measurement: Echocardiography, PET |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
| Methods | Type of study: Parallel RCT Type of publication: Abstract Source of funding: n/r Study setting: St Petersburg, Russia Number of centres: n/r Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 18x BMMSC, 38x BMMNC, 13x controls |
| Participants | Description: Non‐acute IHD Age distribution in each arm: n/r Number of diseased vessels: n/r Time from symptom onset to initial treatment: n/r |
| Interventions | Intervention arm: BMMSC or BMMNC Type of cells: BMMSC or BMMNC Dose of cells: 12 x 108 cells Timing of stem cell procedure: Mesenchymal stem cells were isolated from bone marrow and cultured in vitro. Bone marrow mononuclear cells were also isolated from bone marrow. Cells were delivered via the coronary artery. Comparator arm: Placebo |
| Outcomes | Angina episodes, nitroglycerine consumption, myocardial viability and perfusion, LVEF Outcome assessment points: Baseline, 6, 9, and 12 months Method of measurement: PET, SPECT, echocardiography |
| Notes | To our knowledge, this trial has published in abstract form only with insufficient data reporting for inclusion. Should further publications be identified, this study will be incorporated into future updates to this review. |
AMI: acute myocardial infarction
BMMNC: bone marrow mononuclear cells
BMMSC: bone marrow mesenchymal stem cells
BMSC: bone marrow stem cells
BNP: brain natriuretic peptide
CABG: coronary artery bypass grafting
CCS: Canadian Cardiovascular Society
CPC: circulating progenitor cells
EPC: endothelial progenitor cells
G‐CSF: granulocyte colony‐stimulating factor
HF: heart failure
IC: intracoronary
IM: intramyocardial
LV: left ventricular
LVEF: left ventricular ejection fraction
LVEDV: left ventricular end‐diastolic volume
LVESV: left ventricular end‐systolic volume
MACE: major adverse clinical events
MI: myocardial infarction
MRI: magnetic resonance imaging
MSC: mesenchymal stem cells
MVO2: myocardial oxygen consumption
NYHA: New York Heart Association
PCI: percutaneous coronary intervention
PET: positron emission tomography
RCT: randomised controlled trial
SD: standard deviation
SPECT: single‐photon emission computed tomography
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Injection of autologous bone marrow cells into damaged myocardium of no‐option patients with ischaemic heart failure: a randomised placebo controlled trial ‐ cell therapy for ischaemic heart failure |
| Methods | A randomised, double‐blind, cross‐over, placebo‐controlled trial |
| Participants | Ischaemic HF:
|
| Interventions | Intervention arm: Intracardiac administration of bone marrow mononuclear cells Comparator arm: Intracardiac administration of placebo |
| Outcomes | Primary outcomes:
Secondary outcomes: Safety (incidence of arrhythmias via Holter monitoring, inflammation and myocardial damage) |
| Starting date | July 2010 |
| Contact information | None identified. |
| Notes | Planned enrolment: 64 Estimated completion date: The status of this trial is "completed", but no publications have been identified. |
| Trial name or title | Bone‐marrow derived stem cell transplantation in patients undergoing left ventricular restoration surgery for dilated ischaemic end‐stage heart failure: a randomised blinded controlled trial (TransACT 2) |
| Methods | A double‐blind, randomised, placebo‐controlled trial |
| Participants | End‐stage HF:
|
| Interventions | Intervention arm: Surgical ventricular restoration and transplantation of autologous CD133+ Comparator arm: Surgical ventricular restoration and injection of placebo, i.e. autologous plasma |
| Outcomes | Primary outcome: Regional LV thickening of the 'affected' segments 6 months after surgery. Secondary outcomes:
|
| Starting date | August 2009 |
| Contact information | University of Bristol, Bristol Royal Infirmary. Contact: Mr R Ascione ([email protected]) |
| Notes | Planned enrolment: 40 Estimated completion date: The status of this trial is "completed", but no publications have been identified. |
| Trial name or title | A pilot study to evaluate the efficacy of combined transplantation of progenitor cells and coronary artery bypass grafting (TOPCABG) |
| Methods | RCT |
| Participants | Participants undergoing CABG |
| Interventions | Intervention arm: Stem cells (5 participants) Comparator arm: Heparinised saline (5 participants) |
| Outcomes | Primary outcome: To show improvements in myocardial function, regional wall motion, and myocardial perfusion. |
| Starting date | January 2004 |
| Contact information | Southampton University Hospitals NHS Trust, Level D, East Wing, Southampton General Hospital, Tremona Road, Southampton, UK SO16 6YD. Contact: Mr D Varghese ([email protected]) |
| Notes | Planned enrolment: 10 Estimated completion date: The status of this trial is "completed", but no publications have been identified. |
| Trial name or title | Bypass surgery with stem cell therapy in chronic ischemic cardiopathy |
| Methods | A phase II, parallel, randomised, single‐blind (participant) controlled study |
| Participants | IHD:
|
| Interventions | Intervention arm: Surgical revascularisation associated with autologous bone marrow‐derived stem cells injection in viable territories. Comparator arm: Surgical revascularisation alone. |
| Outcomes | Primary outcome: Evolution of left ventricular volumes and contractility. Secondary outcome: Functional status. |
| Starting date | May 2008 |
| Contact information | Departments of Cardiac Surgery, Cardiology and Radiology, University Hospital, Clermont‐Ferrand, France (Principal Investigator: Dr J Lipiecki. Contact: Patrick Lacarin (placarin@chu‐clermontferrand.fr) |
| Notes | Planned enrolment: 12 Estimated completion date: June 2011 The status of this trial is "completed", but no publications have been identified. |
| Trial name or title | Phase II combination stem cell therapy for the treatment of severe coronary ischemia (CI) |
| Methods | A phase II, randomised, placebo‐controlled, safety/efficacy study |
| Participants | Severe coronary ischaemia:
|
| Interventions | Enrolled individuals (60) will be divided into 2 treatment groups for the infusion of the cell/placebo product:
In turn, each treatment group will consist of 2 subgroups of individuals that will receive the infusion of 1 of the 2 doses established of the cell product:
For the cell product, proper aliquots of each cell type will be taken to fulfil the doses established for this protocol. The 2 aliquots will be mixed and resuspended to a final volume of 3 mL in the "final suspension medium", which consists of Dulbecco's Phosphate Buffered Saline, containing 5% HSA. For placebo, 3 mL of the "final suspension medium", which consists of Dulbecco's Phosphate Buffered Saline, containing 5% HSA will be transferred to a 5‐millilitre syringe. |
| Outcomes | Primary outcome: Safety as measured by laboratory assessments, ECG, and temperature (2 weeks). |
| Starting date | November 2008 |
| Contact information | TCA Cellular Therapy, Covington, LA, United States, 70433 (Principal Investigator: Dr Patrick Lacarin) |
| Notes | Planned enrolment: 60 Estimated completion date: November 2011 This study has suspended participant recruitment due to lack of funding. |
| Trial name or title | Intramyocardial delivery of autologous bone marrow |
| Methods | A phase II, parallel, randomised, double‐blind (participant, investigator), safety/efficacy study |
| Participants | Refractory angina:
Angiographic inclusion criteria:
|
| Interventions | Intervention arm: Direct intramyocardial percutaneous delivery of autologous total bone marrow‐derived total mononuclear cells or selected CD34+ bone marrow‐derived cells. Comparator arm: Not specified. |
| Outcomes | Primary outcome: Incidence of MACE, defined as a combined endpoint of death, acute MI (Q wave and non‐Q wave), revascularisation procedures (percutaneous or surgical), and periprocedural complications (i.e. left ventricular perforation with haemodynamic consequences requiring pericardiocentesis, and stroke) (1/3/6/12 months). Secondary outcomes:
|
| Starting date | January 2009 |
| Contact information | Antonio Colombo, Director of Invasive Cardiology Unit, IRCCS San Raffaele, Milan, Italy |
| Notes | Planned enrolment: 13 Estimated completion date: February 2012 This study has suspended participant recruitment due to lack of further funding support. |
| Trial name or title | Intramyocardial transplantation of bone marrow stem cells in addition to coronary artery bypass graft (CABG) surgery (PERFECT) |
| Methods | A phase III, randomised, parallel, double‐blind (participant, caregiver, investigator, outcomes assessor) efficacy study |
| Participants | Chronic ischaemic coronary artery disease:
|
| Interventions | Intervention arm: Intramyocardial injection of 5 mL CD133+ cells (0.5 ‐ 5 x 106 cells) suspended in physiological saline + 10% autologous serum intramyocardially during CABG surgery. Comparator arm: Intramyocardial injection of 5 mL of physiological saline + 10% autologous serum intramyocardially during CABG surgery. |
| Outcomes | Primary outcome: LVEF at rest, measured by MRI (6 months).
|
| Starting date | July 2009 |
| Contact information | University of Rostock, Germany, 18057 (Principal Investigator: Dr G Steinhoff ([email protected]‐rostock.de)) |
| Notes | Planned enrolment: 142 Estimated completion date: December 2013 The status of this trial is "terminated"; no further details are provided, and no publications have been identified. |
| Trial name or title | IMPACT‐CABG trial: IMPlantation of Autologous CD133+ sTem Cells in Patients Undergoing CABG (IMPACT‐CABG) |
| Methods | A phase II, parallel, randomised, double‐blind (participant, caregiver, investigator, outcomes assessor), placebo‐controlled safety/efficacy study |
| Participants | Myocardial infarct; HF:
|
| Interventions | Intervention arm: Autologous CD133+ stem cells (total 2 mL with 10 to 15 injections) injected into the myocardium. Comparator arm: Placebo solution containing plasma injected into the myocardium. |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | December 2009 |
| Contact information | Centre de recherche du CHUM (CRCHUM), Montreal, Quebec, Canada, H2W 1T8 (Principal Investigators: Dr N Noiseux, Dr S Mansour, Dr D‐C Roy). Contact: Nicolas Noiseux, MD, MSc, FRCSC, ([email protected]) |
| Notes | Planned enrolment: 20 Estimated completion date: July 2013 The recruitment status of this study is unknown because the information has not been recently verified. |
| Trial name or title | Prospective, controlled and randomized clinical trial on cardiac cell regeneration with laser and autologous bone marrow stem cells, in patients with coronary disease and refractory angina |
| Methods | A phase II, randomised, single‐blind (outcome assessor), parallel safety/efficacy study |
| Participants | Coronary disease and refractory angina:
|
| Interventions | Intervention arm: Transmyocardial revascularisation with Holmium YAG laser plus the participant's own stem cells extracted from bone marrow. Comparator arm: Transmyocardial revascularisation with Holmium YAG laser. |
| Outcomes | Primary outcome: NYHA classification for angina (12 months).
|
| Starting date | October 2010 |
| Contact information | Cardiovascular Surgery Service, Hospital Universitario de La Princesa, Madrid, Spain, 28006 (Principal Investigator: Dr GR Copa ([email protected])) |
| Notes | Planned enrolment: 20 Estimated completion date: October 2012 The recruitment status of this study is unknown because the information has not been recently verified. |
| Trial name or title | Cell therapy in patients with chronic ischemic heart disease undergoing cardiac surgery |
| Methods | A phase I/II, randomised, double‐blind (participant, caregiver), parallel safety/efficacy study |
| Participants | Severe, chronic ischaemic disease:
|
| Interventions | Intervention arm: Direct intramyocardial injection of autologous bone marrow mononuclear cells during CABG. Comparator arm: Between 10 and 15 placebo injections consisting of saline and 5% HSA during CABG. |
| Outcomes | Primary outcome: Major adverse cardiac events (6 months) Left ventricular function (global function, regional myocardial perfusion, and function assessed by magnetic resonance imaging and echocardiogram) (6 months) |
| Starting date | December 2010 |
| Contact information | Chinese PLA General Hospital, Beijing, China (Principal Investigator: Dr C Gao ([email protected]) and Dr L Zhang ([email protected])) |
| Notes | Planned enrolment: 60 Estimated completion date: June 2013 The recruitment status of this study is unknown because the information has not been recently verified. |
| Trial name or title | Intramyocardial Multiple Precision Injection of Bone Marrow Mononuclear Cells in Myocardial Ischemia (IMPI) |
| Methods | A phase I, parallel, randomised, double‐blind (participant, caregiver, investigator) safety/efficacy study |
| Participants | Ischaemic HF:
|
| Interventions | Intervention arm: Intramyocardial multiple precision injection of bone marrow mononuclear cells. Comparator arm: Intramyocardial multiple precision injection with placebo. |
| Outcomes | Primary outcome: Change in global LVEF and regional wall motion score index (6/12 months). |
| Starting date | May 2011 |
| Contact information | Almazov Federal Center of Heart, Blood and Endocrinology (Principal Investigator: Prof EV Shlyakhto). Contact: Prof DS Lebedev ([email protected]); Prof OM Moiseeva ([email protected]) |
| Notes | Planned enrolment: 30 Estimated completion date: May 2015 This study is marked as ongoing but is not currently recruiting participants. First identified publication in Russian (Shlyakhto 2013) confirmed by translator as early report of safety in 1 participant. |
| Trial name or title | IMPACT‐CABG trial: IMPlantation of Autologous CD133+ sTem Cells in Patients Undergoing Coronary Artery Bypass Grafting |
| Methods | A phase II, randomised, parallel, double‐blind (participant, caregiver, investigator, outcomes assessor) safety/efficacy study |
| Participants | Chronic ischaemic cardiomyopathy:
|
| Interventions | Intervention arm: Autologous CD133+ stem cells injected into the mycardium. Comparator arm: Placebo (saline solution containing autologous plasma without CD133+). |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | September 2011 |
| Contact information | Terrence M Yau, MD, Peter Munk Cardiac Center/University Health Network, Toronto, Ontario, Canada, M5G 2C4 |
| Notes | Planned enrolment: 20 Estimated completion date: October 2015 This study is marked as completed, but no publications have been identified. |
| Trial name or title | Efficacy and Safety of Targeted Intramyocardial Delivery of Auto CD34+ Stem Cells for Improving Exercise Capacity in Subjects With Refractory Angina (RENEW) |
| Methods | A phase III, randomised, parallel, double‐blind (participant, investigator) safety/efficacy study |
| Participants | Refractory angina and chronic myocardial ischaemia:
|
| Interventions | Intervention arm: Targeted intramyocardial delivery of 1 x 105 Auto‐CD34+ cells after G‐CSF mobilisation and apheresis. Comparator arm: Targeted intramyocardial delivery of placebo after G‐CSF mobilisation and apheresis. |
| Outcomes | Primary outcome: Change from baseline in total exercise time on exercise tolerance test using the modified Bruce protocol (12 months).
|
| Starting date | April 2012 |
| Contact information | Baxter Healthcare Corporation (Study Director: Dr A Nada). Contact: Lauren Davis, Clinical Project Manager ([email protected]) |
| Notes | Planned enrolment: 291 Estimated completion date: June 2016 This study is marked as completed, but no publications have been identified. |
| Trial name or title | Implantation of Peripheral Stem Cells in Patient With Ischemic Cardiomyopathy (ISCIC) |
| Methods | A phase I, randomised, parallel, open‐label safety/efficacy study |
| Participants | Ischaemic cardiomyopathy:
|
| Interventions | Intervention arm: Intramyocardial implantation of peripheral mononuclear cells with CD34+ stem cells in participant with ischaemic cardiomyopathy after preparatory course of shockwave therapy. Comparator arm: Cardiospec shockwave therapy only. |
| Outcomes | Primary outcomes: Change in global LVEF and regional wall motion score index (6/12 months). Incidence of MACE (6/12 months). |
| Starting date | January 2012 |
| Contact information | Odessa Regional Clinical Hospital, Odessa, Ukraine, 65025 (Principal Investigator: Prof II Karpenko ([email protected])) |
| Notes | Planned enrolment: 50 Estimated completion date: January 2016 The recruitment status of this study is unknown because the information has not been recently verified. |
| Trial name or title | Intracardiac CD133+ cells in patients with no‐option resistant angina (RegentVsel) |
| Methods | A phase II, randomised, parallel, double‐blind (participant, investigator) efficacy study |
| Participants | Stable angina:
|
| Interventions | Intervention arm: Intramyocardial injection (electromechanical mapping based) of autological CD133+ cells, isolated from bone marrow. Comparator arm: Intramyocardial injection (electromechanical mapping based) of placebo (0.9% sodium chloride plus 0.5% solution of participant's serum). |
| Outcomes | Primary outcome: Myocardial perfusion change (4 months).
|
| Starting date | June 2012 |
| Contact information | Samodzielny Publiczny Szpital Kliniczny nr 7 Śląskiego Uniwersytetu Medycznego w Katowicach Górnośląskie Centrum Medyczne im. prof. Leszka Gieca, Katowice‐Ochojec, Silesian, Poland, 40‐635 (Principal Investigator: Prof W Wojakowski ([email protected])) |
| Notes | Planned enrolment: 60 Estimated completion date: June 2014 This study is currently recruiting participants. |
| Trial name or title | Intracoronary autologous mesenchymal stem cells implantation in patients with ischemic dilated cardiomyopathy |
| Methods | A phase II, randomised, parallel, open‐label efficacy study |
| Participants | Ischaemic dilated cardiomyopathy:
|
| Interventions | Intervention arm: Intracoronary implantation of autologous bone marrow‐derived mesenchymal stem cells. Comparator arm: Control (optimal medical therapy). |
| Outcomes | Primary outcome: Change in LV ejection fraction as measured by echocardiogram and cardiac MRI after implantation (measured at 1, 3, 6, 9, and 12 months). Secondary outcomes:
Other outcomes:
|
| Starting date | July 2012 |
| Contact information | Oteh Maskon, MB Bch (Principal Investigator), UKM Medical Centre, Cheras, Kuala Lumpur, Malaysia, 56000 |
| Notes | Planned enrolment: 80 Estimated completion date: December 2015 This study is ongoing but not recruiting participants. |
| Trial name or title | Cell therapy in severe chronic ischemic heart disease (MiHeart) |
| Methods | A phase II/III, randomised, parallel, double‐blind (participant, investigator) safety/efficacy study |
| Participants | Chronic IHD:
|
| Interventions | Intervention arm: Intramyocardial injection of autologous bone marrow‐derived cells. Comparator arm: Saline injection. |
| Outcomes | Primary outcome: Increase in myocardial perfusion assessed by MRI (1/6/12 months).
|
| Starting date | January 2006 |
| Contact information | Heart Institute, Sao Paulo, SP, Brazil 05403‐000 (Principal Investigator: Prof LHW Gowdak). Contact: Meyrielli A Vieira ([email protected]); Prof LHW Gowdak ([email protected]) |
| Notes | Planned enrolment: 200 Estimated completion date: July 2013 The recruitment status of this study is unknown because the information has not been recently verified. |
| Trial name or title | Transplantation of autologous cardiac stem cells in ischemic heart failure |
| Methods | A phase II, randomised, parallel, double‐blind (participant, investigator) safety/efficacy study |
| Participants | Ischaemic HF:
|
| Interventions | Intervention arm: Autologous cardiac stem cell intracoronary injection. Comparator arm: Placebo (no details). |
| Outcomes | Primary outcomes: Rate of mortality, arrhythmia, and hospitalisation at 18 months. Secondary outcomes:
|
| Starting date | December 2013 |
| Contact information | Hoda Madani, MD (Principal Investigator), Royan Institute, Tehran, Iran. Contact: Nasser Aghdami, MD, PhD +982123562000 ext 504 ([email protected]) |
| Notes | Planned enrolment: 50 Estimated completion date: December 2017 This study is currently recruiting participants. |
| Trial name or title | Safety and efficacy of autologous cardiopoietic cells for treatment of ischemic heart failure (CHART‐1) |
| Methods | A phase III, randomised, parallel, double‐blind (participant, outcomes assessor) safety/efficacy study |
| Participants | Ischaemic HF:
|
| Interventions | Intervention arm: Injection of C3BS‐CQR‐1 cardiopoietic cells using the C‐Cath injection catheter. Comparator arm: Mimic injection procedure through insertion of a sham catheter. No injection actually performed. |
| Outcomes | Primary outcome: Efficacy between groups post‐index procedure: change between groups from baseline in a hierarchical composite outcome comprising, from most‐ to least‐severe outcome, days to death from any cause, number of worsening of HF events, change in score for the MLHFQ (10‐point deterioration, no meaningful change, 10‐point improvement), change in 6‐minute walk distance (40 m deterioration, no meaningful change, 40 m improvement), and change in LVESV (15 mL deterioration, no meaningful change, 15 mL improvement) and LVEF (4% absolute deterioration, no meaningful change, 4% absolute improvement) (39 weeks).
|
| Starting date | November 2012 |
| Contact information | Multicentre study: Cardio3 BioSciences (Study Chairs: Dr A Terzic, Mayo Clinic, Division of Cardiovascular Diseases, Rochester, MN, USA and Dr J Bartunek, OLV Ziekenhuiz Aalst, Belgium). Contact: Dr Christian Homsy ([email protected]) |
| Notes | Planned enrolment: 240 Estimated completion date: March 2017 This study is ongoing but not recruiting participants. |
| Trial name or title | Intracoronary infusion of mononuclear cells autologous bone marrow in patients with chronic coronary occlusion and ventricular dysfunction, previously revascularized |
| Methods | Open‐label randomised controlled trial |
| Participants | Chronic coronary artery occlusion:
|
| Interventions | Intervention arm: Bone marrow mononuclear cells by intra‐arterial administration. Comparator arm: Control (no placebo). |
| Outcomes | Primary outcome: Change in ejection fraction measured by MRI between inclusion and 6 months' follow‐up. Secondary outcomes:
|
| Starting date | November 2013 |
| Contact information | None identified. |
| Notes | Planned enrolment: 66 Estimated completion date: May 2017 This study is currently recruiting participants. |
| Trial name or title | Autologous bone marrow mononuclear cells in the combined treatment of coronary heart disease (TAMIS) |
| Methods | A phase III, randomised, parallel, single‐blind (investigator) efficacy study |
| Participants | Coronary heart disease:
|
| Interventions | Intervention arm 1: Bone marrow mononuclear cells (intramyocardial). Intervention arm 2: Bone marrow mononuclear cells (intramyocardial and intracoronary). Comparator arm: Placebo (intramyocardial administration of 0.9% sodium chloride 0.2 mL). |
| Outcomes | Primary outcome: All‐cause mortality associated with the progression of basic disease (60 months). Secondary outcomes: Quality of life (12 months). Other outcomes:
|
| Starting date | February 2013 |
| Contact information | Alexander S Nemkov, MD, PhD (Principal Investigator), First Pavlov State Medical University of St Petersburg, St Petersburg, Russia, 197089 ([email protected]) |
| Notes | Planned enrolment: 100 Estimated completion date: February 2018 This study is ongoing but not recruiting participants. |
| Trial name or title | Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART‐2) trial |
| Methods | A phase III, randomised, parallel, double‐blind (participant, caregiver, investigator, outcomes assessor) safety/efficacy study |
| Participants | Advanced chronic ischaemic HF:
|
| Interventions | Intervention arm: BM‐derived mesenchymal cardiopoietic cells (C3BR‐CQR‐1) using intramyocardial injection. Comparator arm: Control (standard of care with sham procedure). |
| Outcomes | Primary outcome: 6‐minute walk test at 39 weeks' postprocedure. Secondary outcomes: None reported. |
| Starting date | December 2014 |
| Contact information | Celyad (formerly named Cardio3 BioSciences) |
| Notes | Planned enrolment: 240 Estimated completion date: August 2018 This study is not yet open for participant recruitment. |
| Trial name or title | Safety & efficacy of intramyocardial injection of mesenchymal precursor cells on myocardial function in LVAD recipients |
| Methods | A phase II, randomised, parallel, double‐blind (participant, caregiver, investigator, outcomes assessor) safety/efficacy study |
| Participants | Left ventricular assist device recipients:
|
| Interventions | Intervention arm: Mensenchymal precursor cells (intramyocardial injection). Comparator arm: Placebo (50% Alpha‐Minimum Essential Medium/42.5% ProFreeze NAO Freeze Medium/7.5% DMSO) |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | July 2015 |
| Contact information | Michael Bowdish, MD (Principal Investigator), University of Southern California, Los Angeles, California, United States, 90033 ([email protected]); Joseph Woo, MD (Principal Investigator), Stanford University School of Medicine, Stanford, California, United States, 94305 ([email protected]) |
| Notes | Planned enrolment: 120 Estimated completion date: August 2016 This study is currently recruiting participants. |
| Trial name or title | CardiAMP Heart Failure Trial |
| Methods | A phase III, randomised, parallel, double‐blind (participant, outcome assessor) safety/efficacy study |
| Participants | Post‐MI HF:
|
| Interventions | Intervention arm: Autologous CardiAMP cell therapy. Comparator arm: Placement of an introducer and performance of LV gram only. |
| Outcomes | Primary outcome: Change in 6‐minute walk distance at 12 months from baseline. Secondary outcomes:
|
| Starting date | January 2016 |
| Contact information | Cheryl Wong Po Foo, BioCardia Inc (Email [email protected]). Principal Investigators: Carl Pepine, University of Florida and Amish Raval, University of Wisconsin |
| Notes | Planned enrolment: 250 Estimated completion date: April 2019 This study is not yet open for participant recruitment. |
| Trial name or title | Administration of mesenchymal stem cells in patients with chronic ischemic cardiomyopathy (MESAMI2) |
| Methods | A phase II, randomised, parallel, double‐blind (participant, investigator) efficacy study |
| Participants | Chronic ischaemic cardiomyopathy and left ventricular dysfunction:
|
| Interventions | Intervention arm: BMMSC (isolated and cultured during 17 ± 2 days by the French Blood Establishment; administered by intramyocardial injections of MSC using the electromechanical NOGA‐XP system). Comparator arm: Human albumin 4%. |
| Outcomes | Primary outcome: Change in VO2max (or peak VO2) before injection and at 3 months' postinjection. Secondary outcomes:
Other outcomes:
|
| Starting date | June 2015 |
| Contact information | Jerome Roncalli, MD, PhD (Principal Investigator), Cardiology Department of Rangueil Hospital, Toulouse, France, 31059 (roncalli.j@chu‐toulouse.fr) |
| Notes | Planned enrolment: 90 Estimated completion date: May 2017 This study is currently recruiting participants. |
| Trial name or title | Combination of mesenchymal and c‐kit+ cardiac stem cells as regenerative therapy for heart failure (CONCERT‐HF) |
| Methods | A phase II, randomised, parallel, double‐blind (participant, caregiver, investigator, outcomes assessor) safety/efficacy study |
| Participants | Ischaemic cardiomyopathy:
|
| Interventions | Intervention arm 1: 15 transendocardial injections of 0.4 mL BMMSC administered to the left ventricle via NOGA MyoStar injection catheter (single procedure) (target dose 150 million MSC). Intervention arm 2: 15 transendocardial injections of 0.4 mL cardiac stem cells (c‐kit+ cells) administered to the left ventricle via NOGA MyoStar injection catheter (single procedure) (target dose 5 million CSC). Intervention arm 3: Combo: 15 transendocardial injections of 0.4 mL MSC administered to the left ventricle via NOGA MyoStar injection catheter (single procedure) and 15 transendocardial injections of 0.4 mL CSC administered to the left ventricle via NOGA MyoStar injection catheter (single procedure) (target dose 150 million MSC and 5 million CSC). Comparator arm: 15 transendocardial injections of 0.4 mL placebo (Plasma‐Lyte A) administered to the left ventricle via NOGA MyoStar injection catheter (single procedure). |
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcomes:
|
| Starting date | October 2015 |
| Contact information | The University of Texas Health Science Center, Houston, TX, United States; National Heart, Lung, and Blood Institute. Contact: Rachel Vojvodic, MPH ([email protected]); Lemuel Moye, MD, PhD ([email protected]) |
| Notes | Planned enrolment: 144 Estimated completion date: February 2019 This study is currently recruiting participants. |
| Trial name or title | The Transendocardial Autologous Cells (hMSC or hMSC and hCSC) in Ischemic Heart Failure Trial (TAC‐HFT II) |
| Methods | A phase I/II, randomised, parallel, single‐blind (participant) safety/efficacy study |
| Participants | Chronic ischaemic left ventricular dysfunction and HF secondary to MI:
|
| Interventions | Treatment arm 1: Autologous hMSC: 40 million cells/mL delivered in 0.5 mL injection volumes times 10 injections for a total of 2 x 108 (200 million) hMSC. Treatment arm 2: Autologous hMSC PLUS autologous c‐kit hCSC: mixture of 39.8 million hMSC and 0.2 million c‐kit hCSC/mL delivered in 0.5 mL injection volumes times 10 injections for a total of 1.99 x 108 (199 million) hMSC and 1 million c‐kit hCSC. Comparator arm: Placebo (10 0.5 mL injections of phosphate‐buffered saline and 1% human serum albumin). |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | March 2020 |
| Contact information | ISCI/University of Miami Miller School of Medicine, Miami, Florida, United States, 33136. Principal Investigator: Joshua M Hare, MD. Contact: Darcy L DiFede ([email protected]) |
| Notes | Planned enrolment: 55 Estimated completion date: March 2030 This study is not yet open for participant recruitment. |
| Trial name or title | Therapy of Preconditioned Autologous BMMSCs for Patients With Ischemic Heart Disease (TPAABPIHD) |
| Methods | A phase I/II, randomised, parallel, double‐blind (participant, outcome assessor) safety/efficacy study |
| Participants | IHD:
|
| Interventions | Intervention arm 1: Autologous BMMSC with hypoxia pre‐condition and endothelial pre‐induction. Intervention arm 2: Autologous BMMSC without pre‐condition. Comparator: Standard therapy without autologous BMMSC infusion. |
| Outcomes | Primary outcome: LVEF at 12 months. Secondary outcomes: None reported. |
| Starting date | November 2015 |
| Contact information | Academy Military Medical Science, China; Sun Yat‐sen University. Contact: Xuetao Pei, MD, PhD ([email protected]); Junnian Zhou, PhD ([email protected]) |
| Notes | Planned enrolment: 200 Estimated completion date: December 2017 This study is not yet open for participant recruitment. |
ABM MNC: autologous bone marrow mononuclear cell
ACE: angiotensin converting enzyme
AEs: adverse events
ACC/AHA: American College of Cardiology/American Heart Association
AICD: automatic implantable cardioverter defibrillator
AMI: acute myocardial infarction
AOI:
ARB: angiotensin receptor blocker
AST: aspartate transaminase
BMAC: bone marrow aspirate concentrate
BMMNC: bone marrow mononuclear cells
BMMSC: bone marrow mesenchymal stem cells
BMSC: bone marrow stem cells
BNP: brain natriuretic peptide
CABG: coronary artery bypass grafting
CCS: Canadian Cardiovascular Society
CHF: congestive heart failure
CMR: cardiac magnetic resonance
cMRI: cardiovascular magnetic resonance imaging
CPC: circulating progenitor cells
CSC: cardiac stem cells
DMSO: dimethyl sulfoxide
ECG: electrocardiogram
EDTA: ethylene‐diamine‐tetraacetic acid
EF: ejection fraction
EPC: endothelial progenitor cells
FDA: US Food and Drug Administration
G‐CSF: granulocyte‐colony stimulating factor
HARP: harmonic phase
HcG: human chorionic gonadotrophin
hCSC: human cardiac stem cells
HF: heart failure
hMSC: human mesenchymal stem cells
HSA: human serum albumin
IC: intracoronary
IHD: ischaemic heart disease
IM: intramuscular
INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support
IV: intravenous
LV: left ventricular
LVAD: left ventricular assist device
LVEDD: left ventricular end‐diastolic diameter
LVEDV: left ventricular end‐diastolic volume
LVEF: left ventricular ejection fraction
LVESD: left ventricular end‐systolic diameter
LVESV: left ventricular end‐systolic volume
MACE: major adverse clinical events
MI: myocardial infarction
MLHFQ: Minnesota Living with Heart Failure Questionnaire
MRI: magnetic resonance imaging
MSC: mesenchymal stem cells
MUGA: multigated radionuclide angiography
MVO₂: myocardial oxygen consumption
NT‐proBNP: N‐terminal pro b‐type natriuretic peptide
NYHA: New York Heart Association
PCI: percutaneous coronary intervention
QOL: quality of life
PET: positron emission tomography
RCT: randomised controlled trial
SF‐36: 13‐Item Short Form Health Survey
SGOT: serum aspartic aminotransferase
SGPT: serum glutamate pyruvate transaminase
SPECT: single‐photon emission computed tomography
TE‐SAEs: treatment emergent serious adverse events
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 37 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 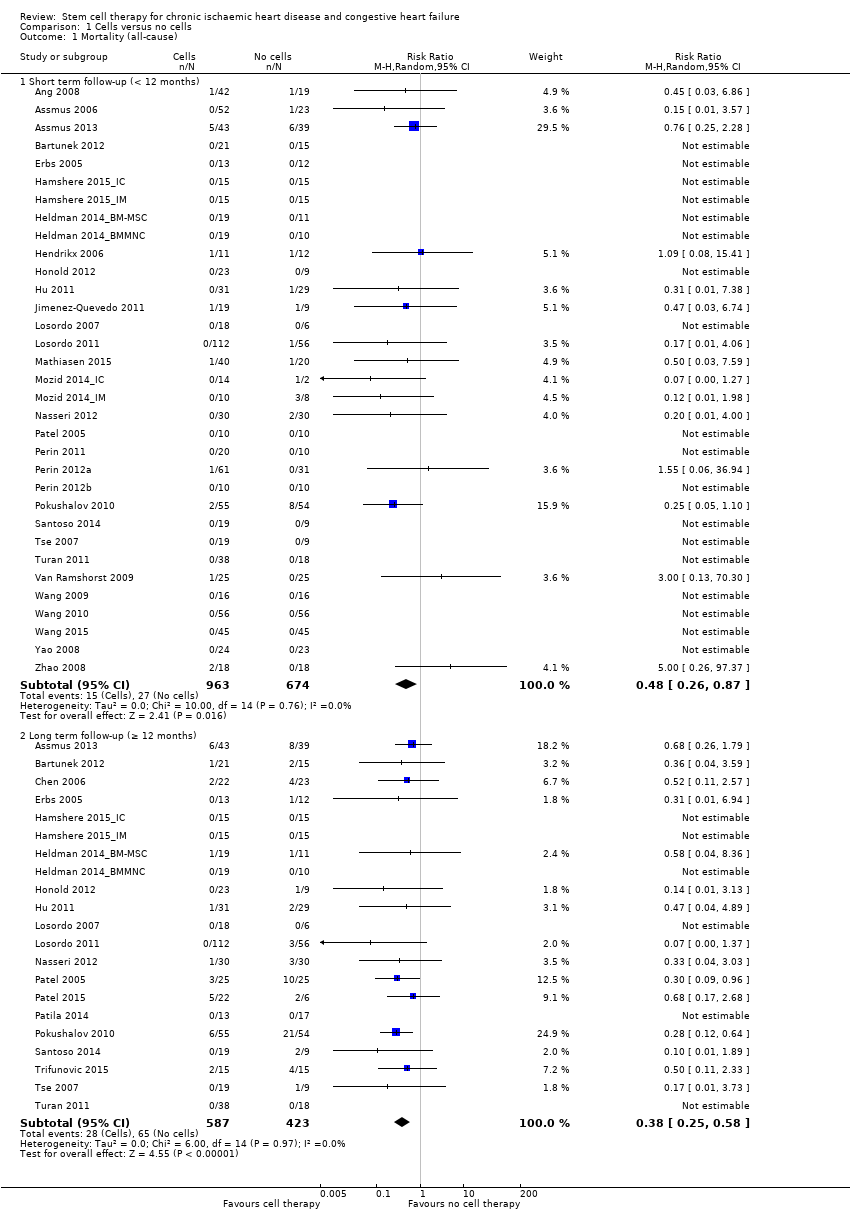 Comparison 1 Cells versus no cells, Outcome 1 Mortality (all‐cause). | ||||
| 1.1 Short term follow‐up (< 12 months) | 33 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.87] |
| 1.2 Long term follow‐up (≥ 12 months) | 21 | 1010 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.25, 0.58] |
| 2 Non‐fatal myocardial infarction Show forest plot | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Cells versus no cells, Outcome 2 Non‐fatal myocardial infarction. | ||||
| 2.1 Short term follow‐up (< 12 months) | 20 | 881 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.15] |
| 2.2 Long term follow‐up (≥ 12 months) | 9 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.17, 0.93] |
| 3 Rehospitalisation due to heart failure Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3 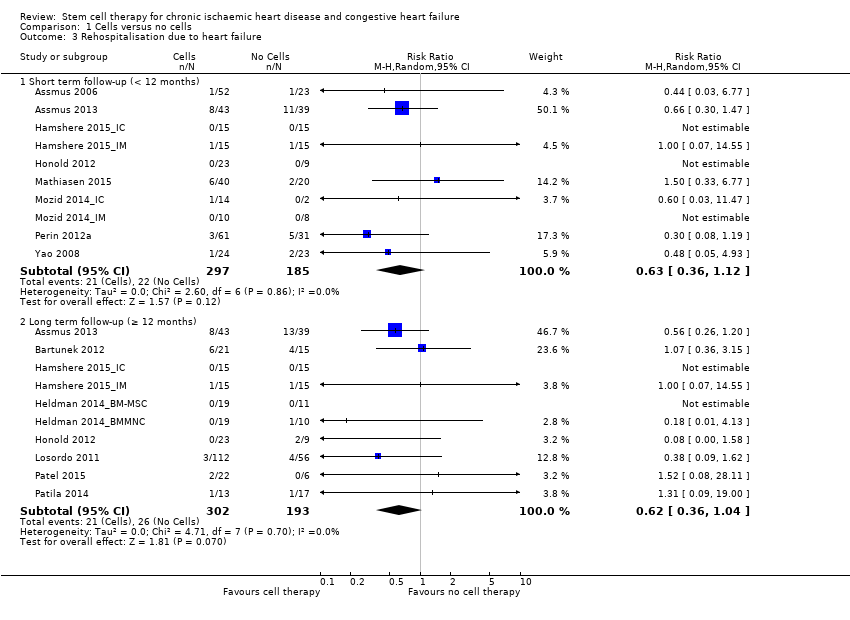 Comparison 1 Cells versus no cells, Outcome 3 Rehospitalisation due to heart failure. | ||||
| 3.1 Short term follow‐up (< 12 months) | 10 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.12] |
| 3.2 Long term follow‐up (≥ 12 months) | 10 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.36, 1.04] |
| 4 Arrhythmias Show forest plot | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Cells versus no cells, Outcome 4 Arrhythmias. | ||||
| 4.1 Short term follow‐up (< 12 months) | 22 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.33, 1.45] |
| 4.2 Long term follow‐up (≥ 12 months) | 7 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.22, 0.97] |
| 5 Composite MACE Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Cells versus no cells, Outcome 5 Composite MACE. | ||||
| 5.1 Short term follow‐up (< 12 months) | 8 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.42] |
| 5.2 Long term follow‐up (≥ 12 months) | 5 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.41, 1.12] |
| 6 MLHFQ: short term follow‐up (< 12 months) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Cells versus no cells, Outcome 6 MLHFQ: short term follow‐up (< 12 months). | ||||
| 6.1 Mean value at endpoint | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐29.52 [‐33.76, ‐25.27] |
| 6.2 Mean change from baseline | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐9.07 [‐22.09, 3.95] |
| 6.3 Combined | 4 | 197 | Mean Difference (IV, Random, 95% CI) | ‐18.96 [‐31.97, ‐5.94] |
| 7 MLHFQ: long term follow‐up (≥ 12 months) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7 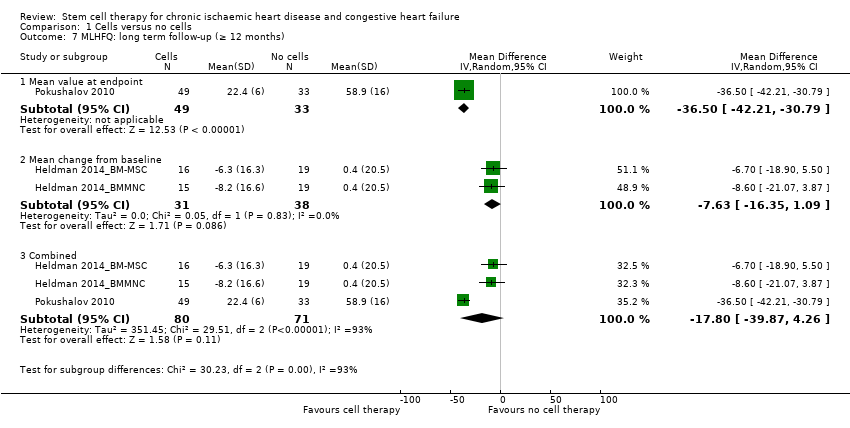 Comparison 1 Cells versus no cells, Outcome 7 MLHFQ: long term follow‐up (≥ 12 months). | ||||
| 7.1 Mean value at endpoint | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐36.5 [‐42.21, ‐30.79] |
| 7.2 Mean change from baseline | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐7.63 [‐16.35, 1.09] |
| 7.3 Combined | 3 | 151 | Mean Difference (IV, Random, 95% CI) | ‐17.80 [‐39.87, 4.26] |
| 8 Seattle Angina Questionnaire: short term follow‐up (< 12 months) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Cells versus no cells, Outcome 8 Seattle Angina Questionnaire: short term follow‐up (< 12 months). | ||||
| 8.1 Mean value at endpoint | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐3.21, 13.21] |
| 8.2 Mean change from baseline | 2 | 211 | Mean Difference (IV, Random, 95% CI) | 9.34 [2.62, 16.07] |
| 8.3 Combined | 2 | 211 | Mean Difference (IV, Random, 95% CI) | 9.34 [2.62, 16.07] |
| 9 Angina episodes per week: short term follow‐up (< 12 months) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.9 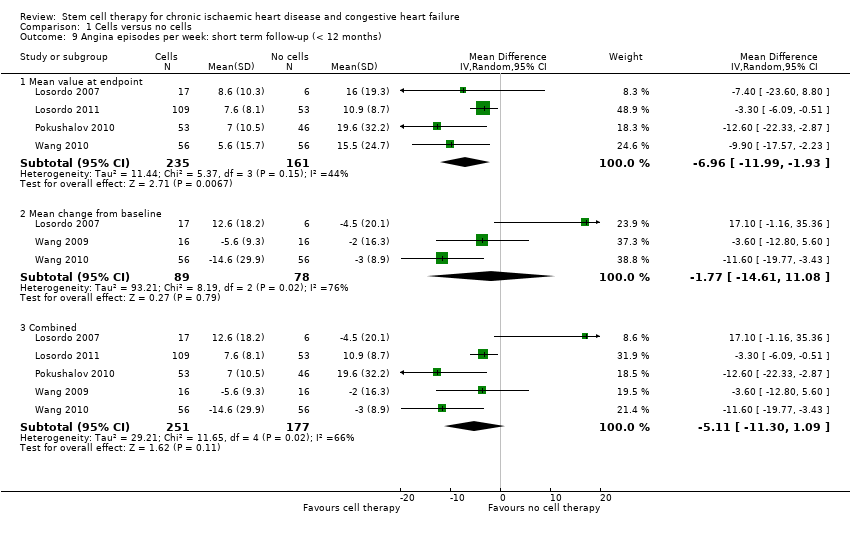 Comparison 1 Cells versus no cells, Outcome 9 Angina episodes per week: short term follow‐up (< 12 months). | ||||
| 9.1 Mean value at endpoint | 4 | 396 | Mean Difference (IV, Random, 95% CI) | ‐6.96 [‐11.99, ‐1.93] |
| 9.2 Mean change from baseline | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐14.61, 11.08] |
| 9.3 Combined | 5 | 428 | Mean Difference (IV, Random, 95% CI) | ‐5.11 [‐11.30, 1.09] |
| 10 NYHA classification: short‐term follow‐up (< 12 months) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.10 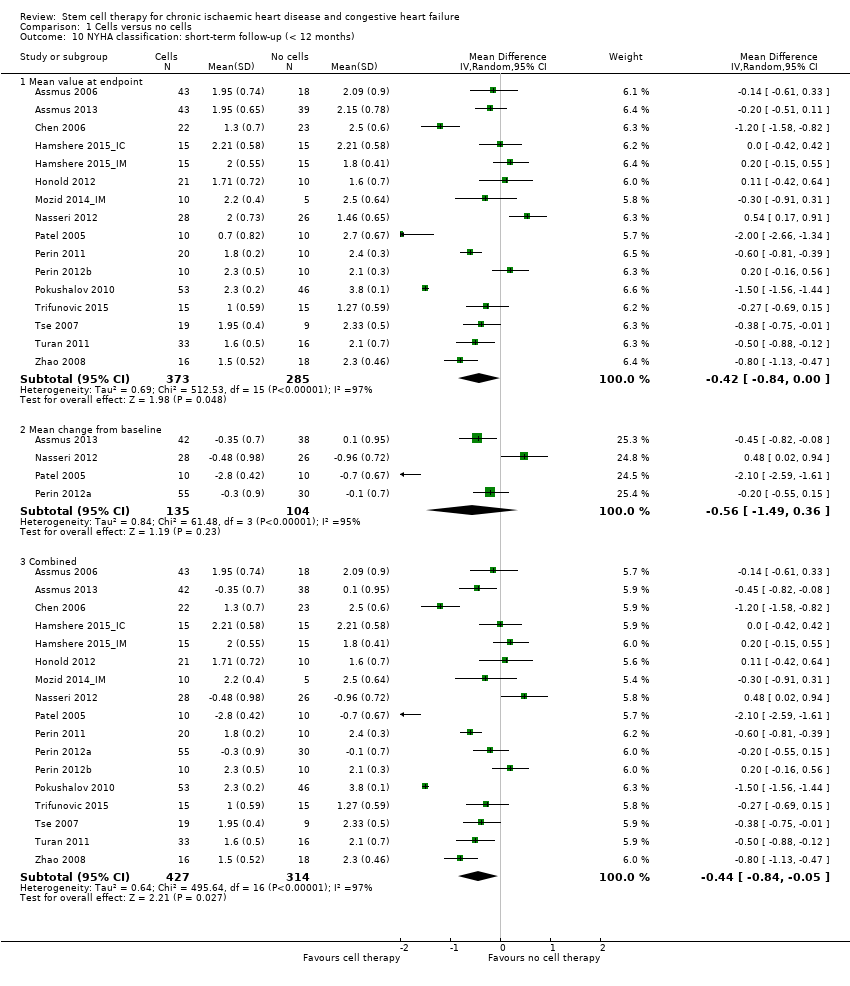 Comparison 1 Cells versus no cells, Outcome 10 NYHA classification: short‐term follow‐up (< 12 months). | ||||
| 10.1 Mean value at endpoint | 16 | 658 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.84, ‐0.00] |
| 10.2 Mean change from baseline | 4 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.49, 0.36] |
| 10.3 Combined | 17 | 741 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.84, ‐0.05] |
| 11 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Cells versus no cells, Outcome 11 NYHA classification: long term follow‐up (≥ 12 months). | ||||
| 11.1 Mean value at endpoint | 9 | 346 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.03, ‐0.10] |
| 11.2 Mean change from baseline | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐2.2 [‐2.70, ‐1.70] |
| 11.3 Combined | 9 | 346 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.23, ‐0.39] |
| 12 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Cells versus no cells, Outcome 12 CCS class: short term follow‐up (< 12 months). | ||||
| 12.1 Mean value at endpoint | 10 | 486 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.82, 0.18] |
| 12.2 Mean change from baseline | 6 | 318 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.40, 0.17] |
| 12.3 Combined | 13 | 608 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 13 CCS class: long term follow‐up (≥ 12 months) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.13 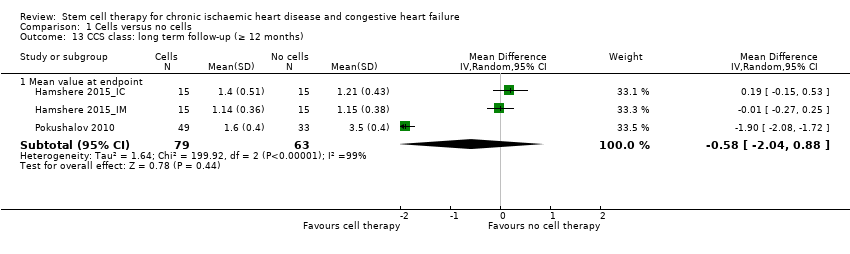 Comparison 1 Cells versus no cells, Outcome 13 CCS class: long term follow‐up (≥ 12 months). | ||||
| 13.1 Mean value at endpoint | 3 | 142 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐2.04, 0.88] |
| 14 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.14 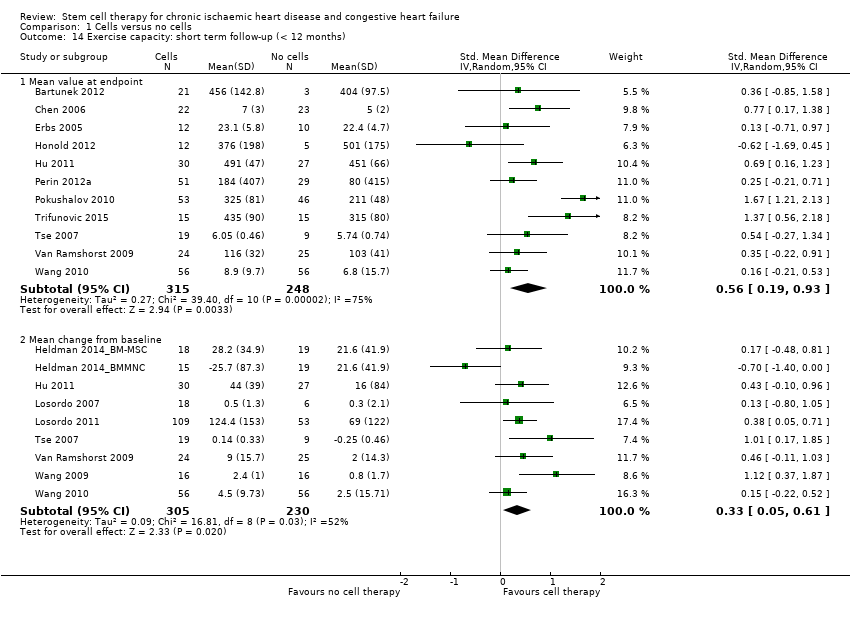 Comparison 1 Cells versus no cells, Outcome 14 Exercise capacity: short term follow‐up (< 12 months). | ||||
| 14.1 Mean value at endpoint | 11 | 563 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.93] |
| 14.2 Mean change from baseline | 9 | 535 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.05, 0.61] |
| 15 Exercise capacity: long term follow‐up (≥ 12 months) Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Cells versus no cells, Outcome 15 Exercise capacity: long term follow‐up (≥ 12 months). | ||||
| 15.1 Mean value at endpoint | 5 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 1.14 [0.04, 2.25] |
| 15.2 Mean change from baseline | 3 | 227 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.07, 0.62] |
| 16 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.16 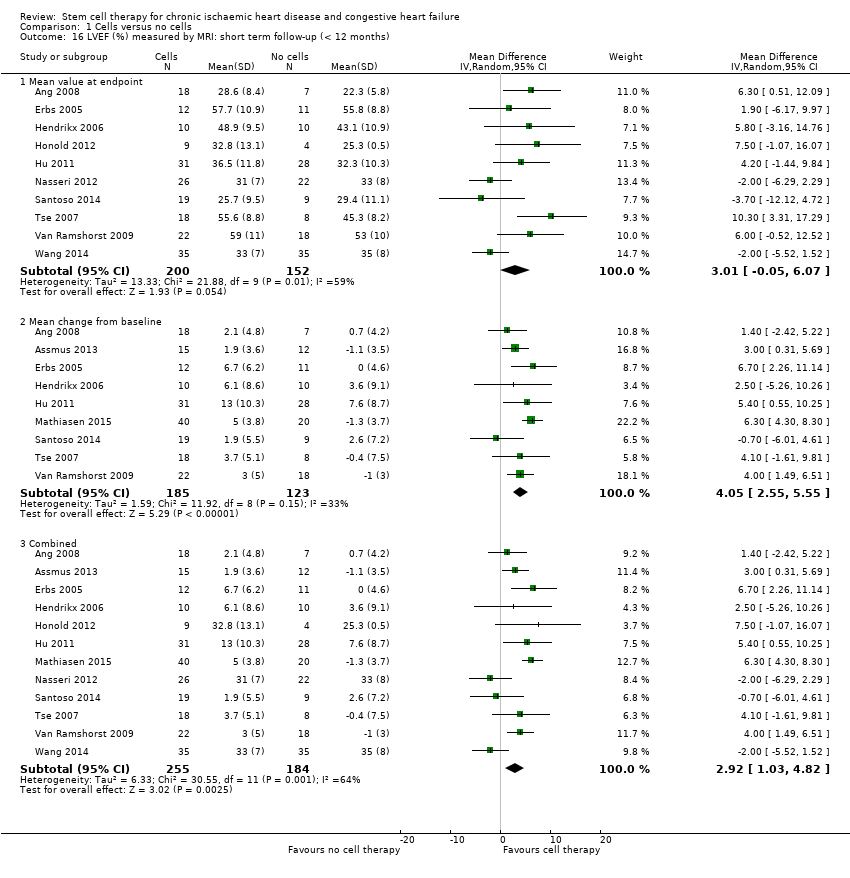 Comparison 1 Cells versus no cells, Outcome 16 LVEF (%) measured by MRI: short term follow‐up (< 12 months). | ||||
| 16.1 Mean value at endpoint | 10 | 352 | Mean Difference (IV, Random, 95% CI) | 3.01 [‐0.05, 6.07] |
| 16.2 Mean change from baseline | 9 | 308 | Mean Difference (IV, Random, 95% CI) | 4.05 [2.55, 5.55] |
| 16.3 Combined | 12 | 439 | Mean Difference (IV, Random, 95% CI) | 2.92 [1.03, 4.82] |
| 17 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Cells versus no cells, Outcome 17 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months). | ||||
| 17.1 Mean value at endpoint | 4 | 110 | Mean Difference (IV, Random, 95% CI) | 2.37 [‐1.54, 6.29] |
| 17.2 Mean change from baseline | 3 | 97 | Mean Difference (IV, Random, 95% CI) | 3.83 [‐0.42, 8.08] |
| 17.3 Combined | 4 | 110 | Mean Difference (IV, Random, 95% CI) | 4.38 [0.82, 7.93] |
| 18 LVEF (%) measured by echocardiography: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.18 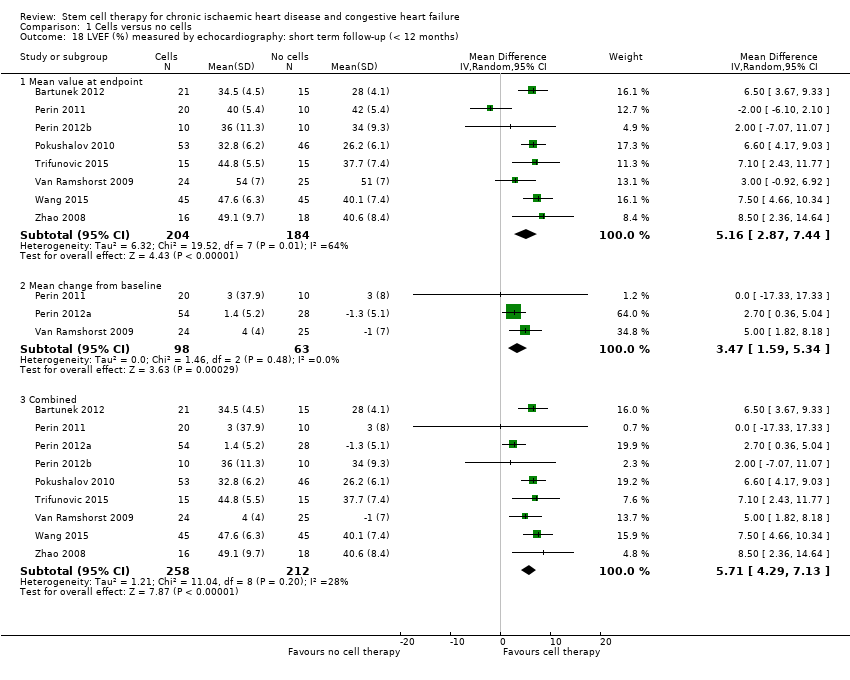 Comparison 1 Cells versus no cells, Outcome 18 LVEF (%) measured by echocardiography: short term follow‐up (< 12 months). | ||||
| 18.1 Mean value at endpoint | 8 | 388 | Mean Difference (IV, Random, 95% CI) | 5.16 [2.87, 7.44] |
| 18.2 Mean change from baseline | 3 | 161 | Mean Difference (IV, Random, 95% CI) | 3.47 [1.59, 5.34] |
| 18.3 Combined | 9 | 470 | Mean Difference (IV, Random, 95% CI) | 5.71 [4.29, 7.13] |
| 19 LVEF (%) measured by echocardiography: long term follow‐up (≥ 12 months) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.19 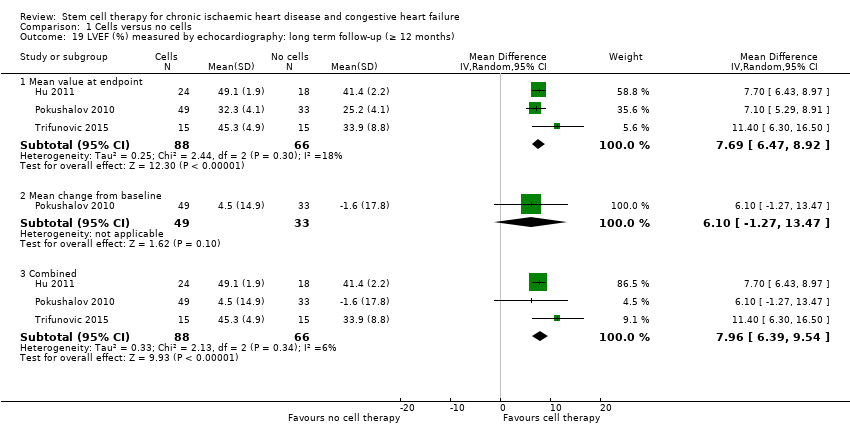 Comparison 1 Cells versus no cells, Outcome 19 LVEF (%) measured by echocardiography: long term follow‐up (≥ 12 months). | ||||
| 19.1 Mean value at endpoint | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 7.69 [6.47, 8.92] |
| 19.2 Mean change from baseline | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 6.1 [‐1.27, 13.47] |
| 19.3 Combined | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 7.96 [6.39, 9.54] |
| 20 LVEF (%) measured by SPECT: short term follow‐up (< 12 months) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 Cells versus no cells, Outcome 20 LVEF (%) measured by SPECT: short term follow‐up (< 12 months). | ||||
| 20.1 Mean value at endpoint | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 2.41 [‐2.65, 7.46] |
| 20.2 Mean change from baseline | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.3 [‐17.33, 12.73] |
| 20.3 Combined | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 5.22 [2.60, 7.85] |
| 21 LVEF (%) measured by SPECT: long term follow‐up (≥ 12 months) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.21 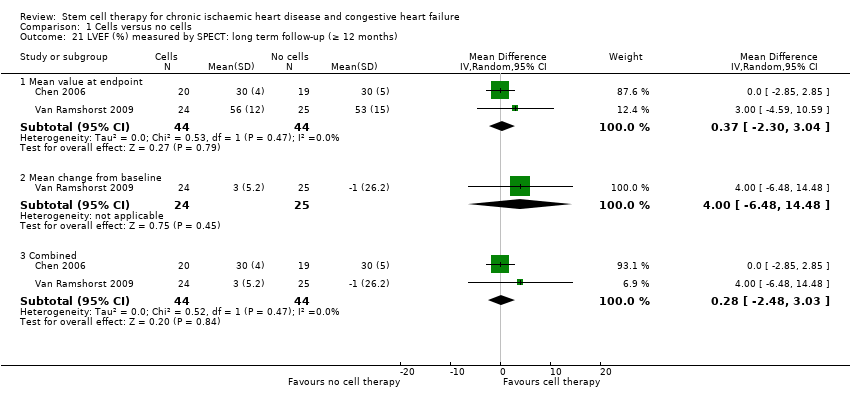 Comparison 1 Cells versus no cells, Outcome 21 LVEF (%) measured by SPECT: long term follow‐up (≥ 12 months). | ||||
| 21.1 Mean value at endpoint | 2 | 88 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐2.30, 3.04] |
| 21.2 Mean change from baseline | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐6.48, 14.48] |
| 21.3 Combined | 2 | 88 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐2.48, 3.03] |
| 22 LVEF (%) measured by LV angiography: short term follow‐up (< 12 months) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.22 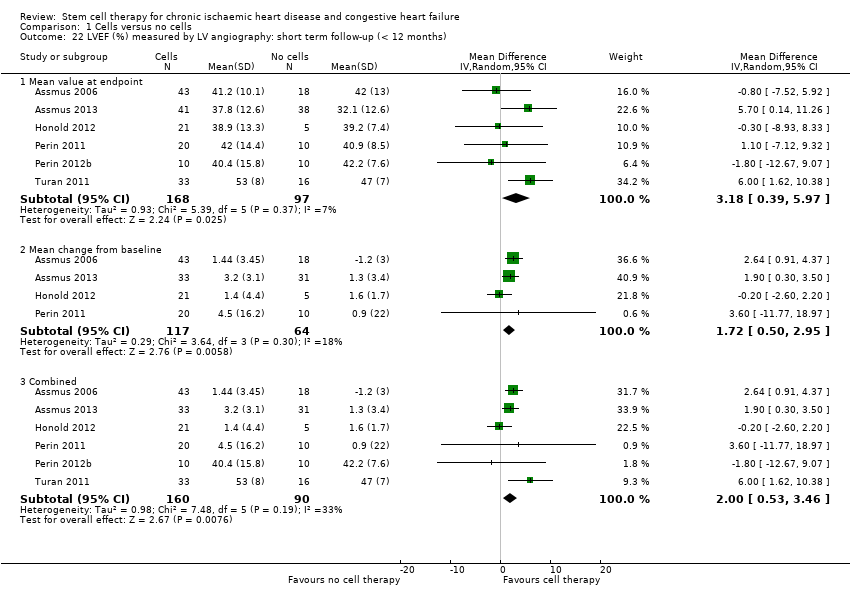 Comparison 1 Cells versus no cells, Outcome 22 LVEF (%) measured by LV angiography: short term follow‐up (< 12 months). | ||||
| 22.1 Mean value at endpoint | 6 | 265 | Mean Difference (IV, Random, 95% CI) | 3.18 [0.39, 5.97] |
| 22.2 Mean change from baseline | 4 | 181 | Mean Difference (IV, Random, 95% CI) | 1.72 [0.50, 2.95] |
| 22.3 Combined | 6 | 250 | Mean Difference (IV, Random, 95% CI) | 2.00 [0.53, 3.46] |
| 23 LVEF (%) measured by LV angiography: long term follow‐up (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.23  Comparison 1 Cells versus no cells, Outcome 23 LVEF (%) measured by LV angiography: long term follow‐up (≥ 12 months). | ||||
| 23.1 Mean value at endpoint | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.0 [0.81, 11.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Cell dose: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months). | ||||
| 1.1 < 107 cells | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.63] |
| 1.2 107 < 108 cells | 18 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.79] |
| 1.3 ≥ 108 cells | 8 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.35, 1.94] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 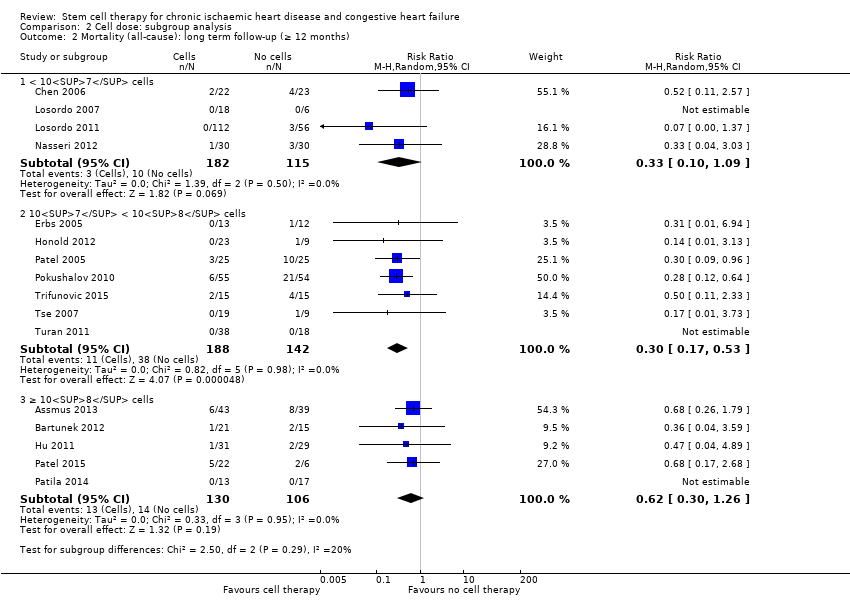 Comparison 2 Cell dose: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months). | ||||
| 2.1 < 107 cells | 4 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.10, 1.09] |
| 2.2 107 < 108 cells | 7 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.53] |
| 2.3 ≥ 108 cells | 5 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.30, 1.26] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Cell dose: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months). | ||||
| 3.1 < 107 cells | 4 | 149 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.94, 0.36] |
| 3.2 107 < 108 cells | 8 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.22, ‐0.08] |
| 3.3 ≥ 108 cells | 4 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.11] |
| 4 CCS class: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.4 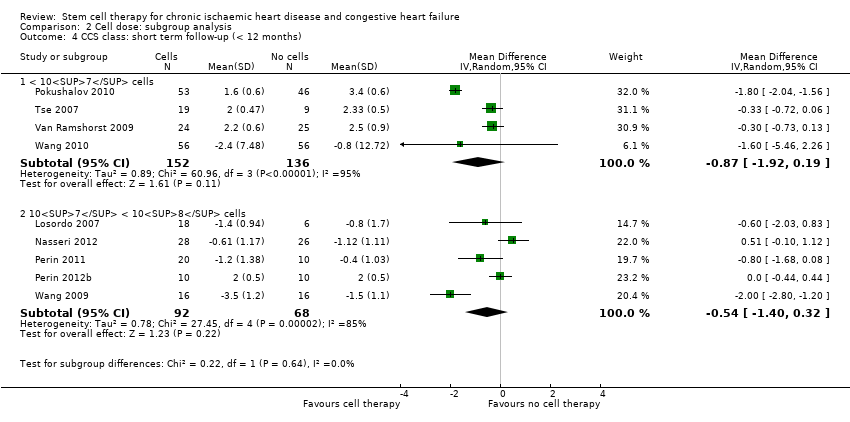 Comparison 2 Cell dose: subgroup analysis, Outcome 4 CCS class: short term follow‐up (< 12 months). | ||||
| 4.1 < 107 cells | 4 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.92, 0.19] |
| 4.2 107 < 108 cells | 5 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.40, 0.32] |
| 5 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.5 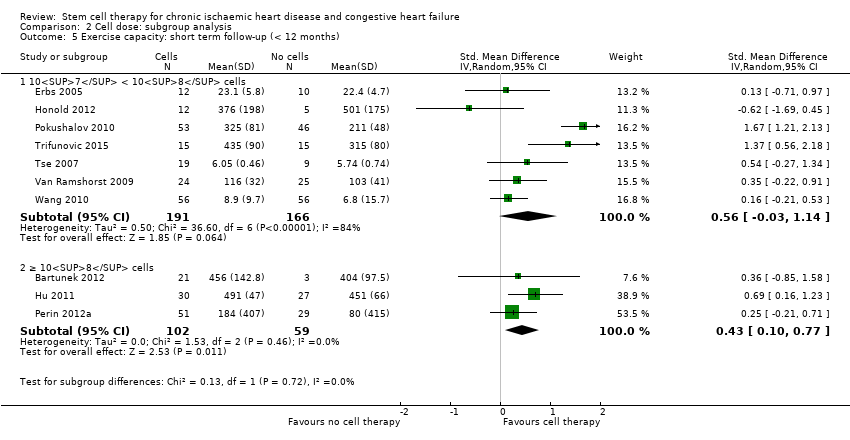 Comparison 2 Cell dose: subgroup analysis, Outcome 5 Exercise capacity: short term follow‐up (< 12 months). | ||||
| 5.1 107 < 108 cells | 7 | 357 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.03, 1.14] |
| 5.2 ≥ 108 cells | 3 | 161 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.10, 0.77] |
| 6 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Cell dose: subgroup analysis, Outcome 6 LVEF (%) measured by MRI: short term follow‐up (< 12 months). | ||||
| 6.1 107 < 108 cells | 7 | 199 | Mean Difference (IV, Random, 95% CI) | 5.23 [3.91, 6.54] |
| 6.2 ≥ 108 cells | 3 | 101 | Mean Difference (IV, Random, 95% CI) | 2.37 [‐0.92, 5.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months). | ||||
| 1.1 < 30% | 11 | 508 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.09, 0.59] |
| 1.2 30 ‐ 50% | 13 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.36, 2.11] |
| 1.3 > 50% | 4 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.35] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2 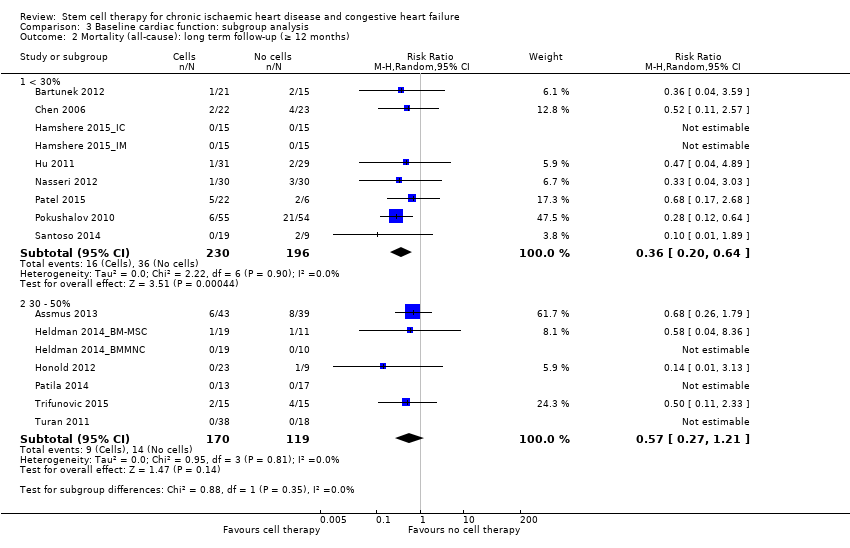 Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months). | ||||
| 2.1 < 30% | 9 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.64] |
| 2.2 30 ‐ 50% | 7 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.21] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months). | ||||
| 3.1 < 30% | 6 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.22, 0.43] |
| 3.2 30 ‐ 50% | 9 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.54, ‐0.10] |
| 4 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 4 NYHA classification: long term follow‐up (≥ 12 months). | ||||
| 4.1 < 30% | 5 | 202 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.28, ‐0.04] |
| 4.2 30 ‐ 50% | 4 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.72, ‐0.25] |
| 5 CCS class: short term follow‐up (< 12 months) Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 5 CCS class: short term follow‐up (< 12 months). | ||||
| 5.1 < 30% | 4 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.47, 0.97] |
| 5.2 30 ‐ 50% | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.31, 0.09] |
| 6 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 6 Exercise capacity: short term follow‐up (< 12 months). | ||||
| 6.1 < 30% | 4 | 225 | Std. Mean Difference (IV, Random, 95% CI) | 0.96 [0.37, 1.56] |
| 6.2 30 ‐ 50% | 3 | 127 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.57, 1.33] |
| 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.7 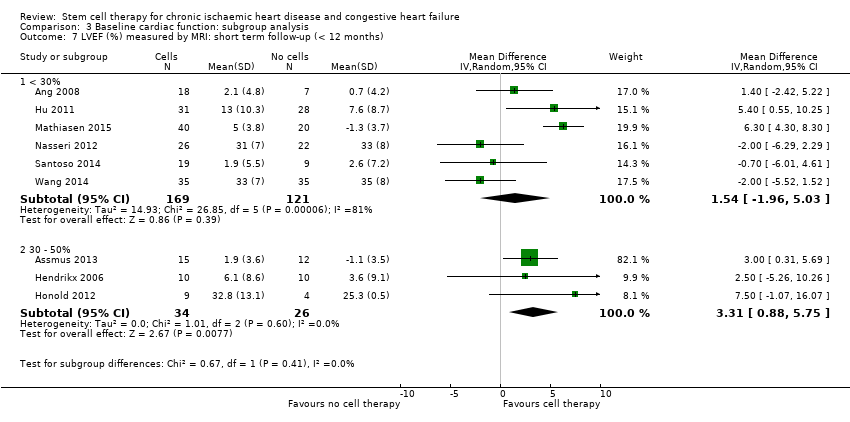 Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months). | ||||
| 7.1 < 30% | 6 | 290 | Mean Difference (IV, Random, 95% CI) | 1.54 [‐1.96, 5.03] |
| 7.2 30 ‐ 50% | 3 | 60 | Mean Difference (IV, Random, 95% CI) | 3.31 [0.88, 5.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Route of cell administration: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months). | ||||
| 1.1 Intramyocardial | 22 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.03] |
| 1.2 Intracoronary | 12 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.23] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Route of cell administration: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months). | ||||
| 2.1 Intramyocardial | 13 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.50] |
| 2.2 Intracoronary | 8 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.30, 1.09] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.3 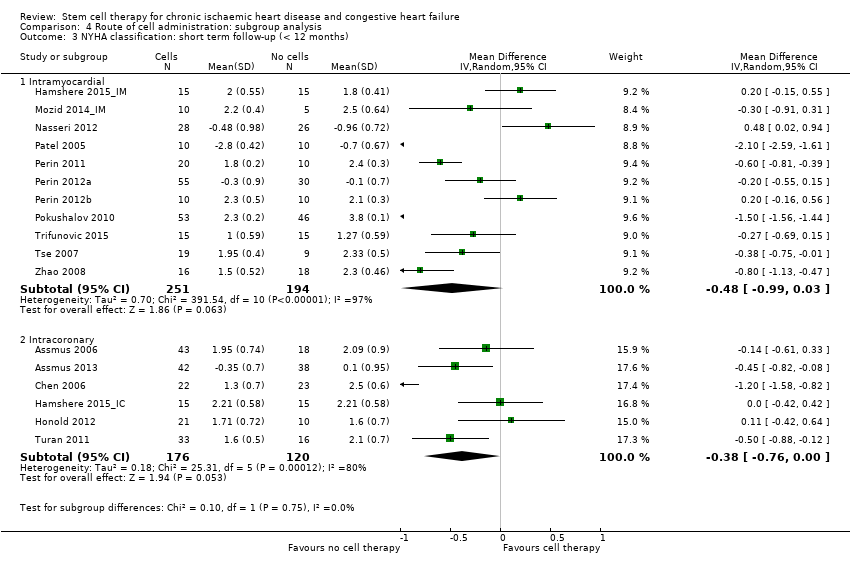 Comparison 4 Route of cell administration: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months). | ||||
| 3.1 Intramyocardial | 11 | 445 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.99, 0.03] |
| 3.2 Intracoronary | 6 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.76, 0.00] |
| 4 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Route of cell administration: subgroup analysis, Outcome 4 NYHA classification: long term follow‐up (≥ 12 months). | ||||
| 4.1 Intramyocardial | 4 | 181 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.76, ‐0.41] |
| 4.2 Intracoronary | 5 | 165 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.92, ‐0.30] |
| 5 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Route of cell administration: subgroup analysis, Outcome 5 CCS class: short term follow‐up (< 12 months). | ||||
| 5.1 Intramyocardial | 10 | 434 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.87, 0.22] |
| 5.2 Intracoronary | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.87, 0.86] |
| 6 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 Route of cell administration: subgroup analysis, Outcome 6 Exercise capacity: short term follow‐up (< 12 months). | ||||
| 6.1 Intramyocardial | 6 | 310 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [0.19, 1.36] |
| 6.2 Intracoronary | 5 | 253 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.06, 0.72] |
| 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.7  Comparison 4 Route of cell administration: subgroup analysis, Outcome 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months). | ||||
| 7.1 Intramyocardial | 8 | 309 | Mean Difference (IV, Random, 95% CI) | 2.18 [‐0.41, 4.77] |
| 7.2 Intracoronary | 5 | 137 | Mean Difference (IV, Random, 95% CI) | 3.72 [0.86, 6.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Cell type: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months). | ||||
| 1.1 Mononuclear cells | 20 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.28, 1.04] |
| 1.2 Circulating progenitor cells | 3 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.48] |
| 1.3 Haematopoietic progenitor cells | 8 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.05, 1.46] |
| 1.4 Mesenchymal stem cells | 3 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.03, 7.59] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.2 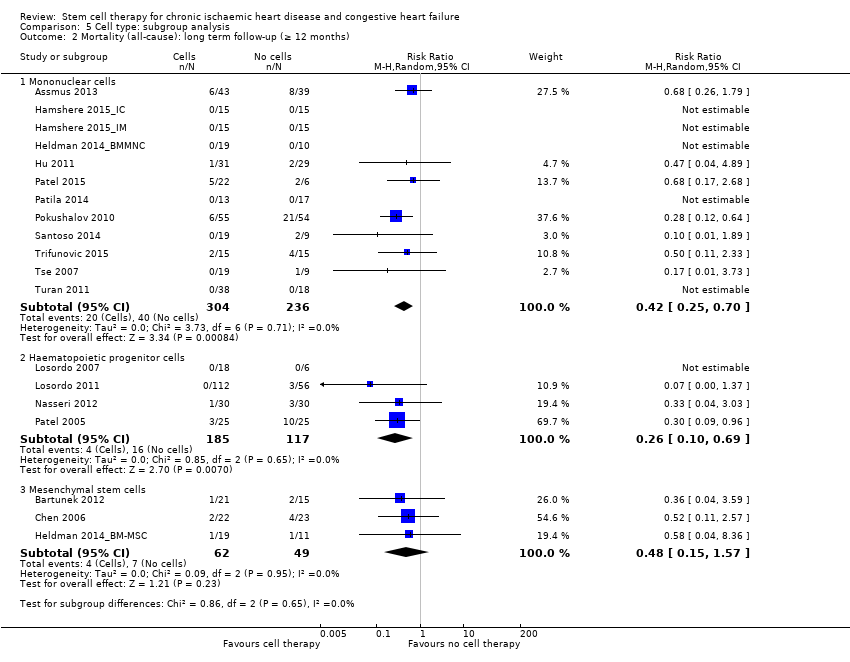 Comparison 5 Cell type: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months). | ||||
| 2.1 Mononuclear cells | 12 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.25, 0.70] |
| 2.2 Haematopoietic progenitor cells | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.10, 0.69] |
| 2.3 Mesenchymal stem cells | 3 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.57] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.3 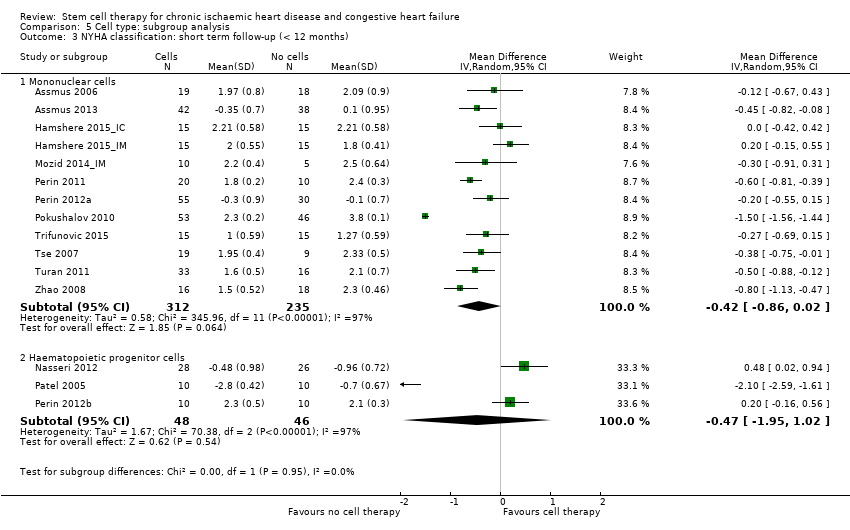 Comparison 5 Cell type: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months). | ||||
| 3.1 Mononuclear cells | 12 | 547 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.86, 0.02] |
| 3.2 Haematopoietic progenitor cells | 3 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.95, 1.02] |
| 4 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.4 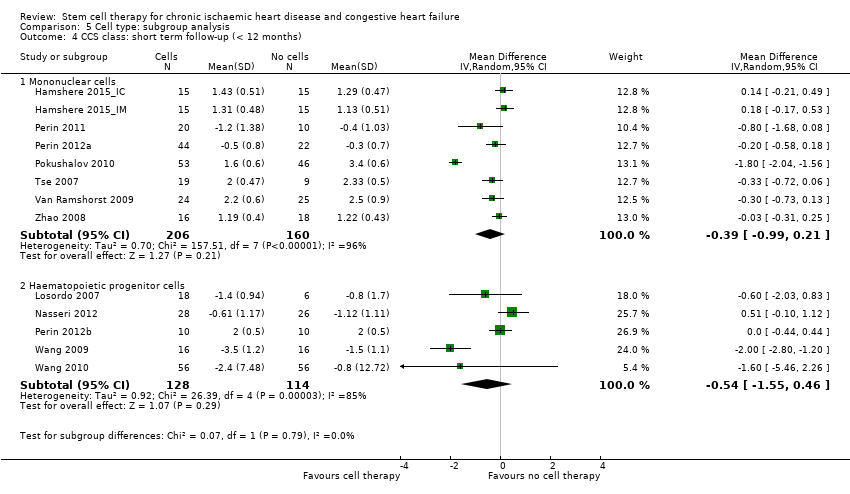 Comparison 5 Cell type: subgroup analysis, Outcome 4 CCS class: short term follow‐up (< 12 months). | ||||
| 4.1 Mononuclear cells | 8 | 366 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.99, 0.21] |
| 4.2 Haematopoietic progenitor cells | 5 | 242 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.55, 0.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Participant diagnosis: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months). | ||||
| 1.1 Chronic IHD | 11 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.26, 1.62] |
| 1.2 HF (secondary to IHD) | 15 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.14, 0.82] |
| 1.3 Refractory/intractable angina | 7 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.35] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.2 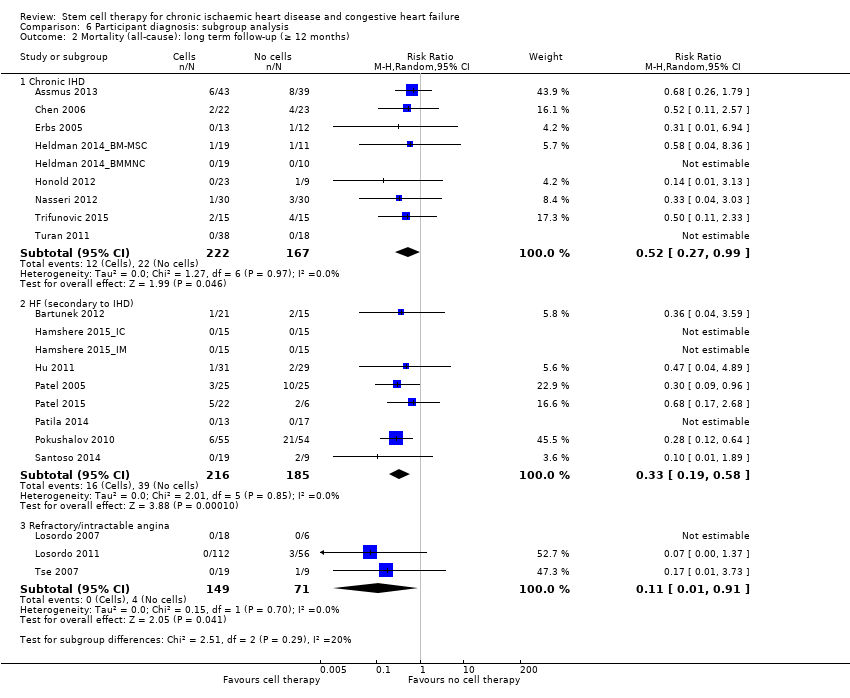 Comparison 6 Participant diagnosis: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months). | ||||
| 2.1 Chronic IHD | 9 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 0.99] |
| 2.2 HF (secondary to IHD) | 9 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.19, 0.58] |
| 2.3 Refractory/intractable angina | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.91] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Participant diagnosis: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months). | ||||
| 3.1 Chronic IHD | 6 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.78, ‐0.07] |
| 3.2 HF (secondary to IHD) | 10 | 417 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.02, 0.09] |
| 4 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.4 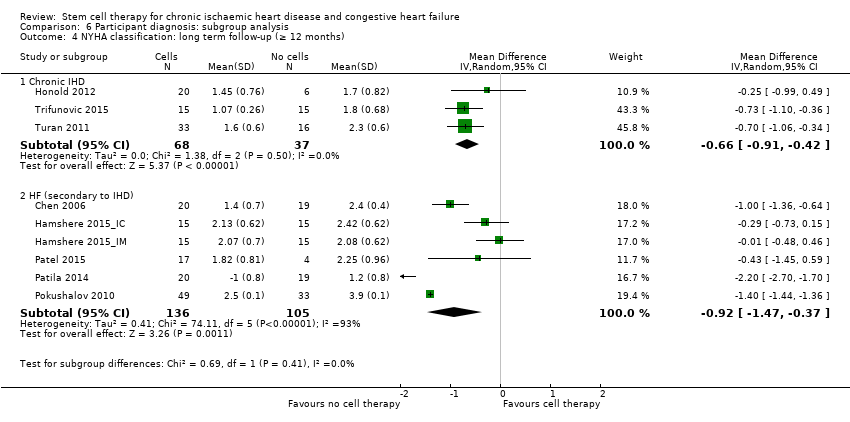 Comparison 6 Participant diagnosis: subgroup analysis, Outcome 4 NYHA classification: long term follow‐up (≥ 12 months). | ||||
| 4.1 Chronic IHD | 3 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.91, ‐0.42] |
| 4.2 HF (secondary to IHD) | 6 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.47, ‐0.37] |
| 5 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.5  Comparison 6 Participant diagnosis: subgroup analysis, Outcome 5 CCS class: short term follow‐up (< 12 months). | ||||
| 5.1 HF (secondary to IHD) | 8 | 363 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.90, 0.40] |
| 5.2 Refractory/intractable angina | 5 | 245 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.44, ‐0.11] |
| 6 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.6  Comparison 6 Participant diagnosis: subgroup analysis, Outcome 6 Exercise capacity: short term follow‐up (< 12 months). | ||||
| 6.1 Chronic IHD | 4 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.26, 1.22] |
| 6.2 HF (secondary to IHD) | 4 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.04, 1.53] |
| 6.3 Refractory/intractable angina | 3 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.03, 0.55] |
| 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.7 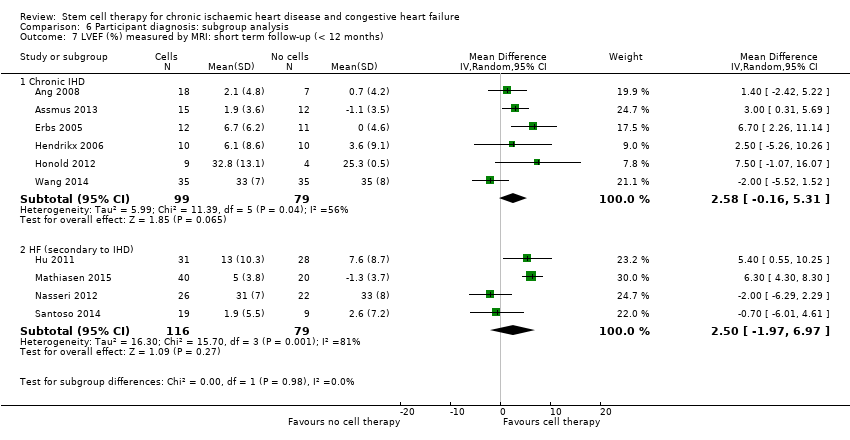 Comparison 6 Participant diagnosis: subgroup analysis, Outcome 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months). | ||||
| 7.1 Chronic IHD | 6 | 178 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐0.16, 5.31] |
| 7.2 HF (secondary to IHD) | 4 | 195 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐1.97, 6.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Co‐interventions: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months). | ||||
| 1.1 Co‐interventions | 8 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.32, 1.70] |
| 1.2 No co‐interventions | 25 | 1205 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.72] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.2 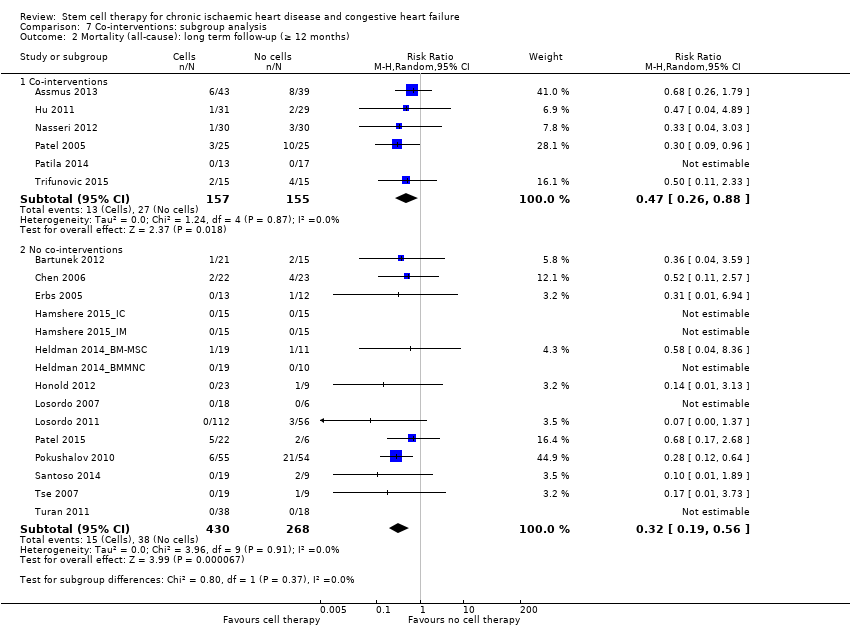 Comparison 7 Co‐interventions: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months). | ||||
| 2.1 Co‐interventions | 6 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.88] |
| 2.2 No co‐interventions | 15 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.19, 0.56] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.3  Comparison 7 Co‐interventions: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months). | ||||
| 3.1 Co‐interventions | 6 | 233 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.20, 0.05] |
| 3.2 No co‐interventions | 11 | 508 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.87, 0.13] |
| 4 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.4  Comparison 7 Co‐interventions: subgroup analysis, Outcome 4 LVEF (%) measured by MRI: short term follow‐up (< 12 months). | ||||
| 4.1 Co‐interventions | 5 | 179 | Mean Difference (IV, Random, 95% CI) | 2.01 [‐0.26, 4.29] |
| 4.2 No co‐interventions | 7 | 260 | Mean Difference (IV, Random, 95% CI) | 3.55 [0.82, 6.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.1 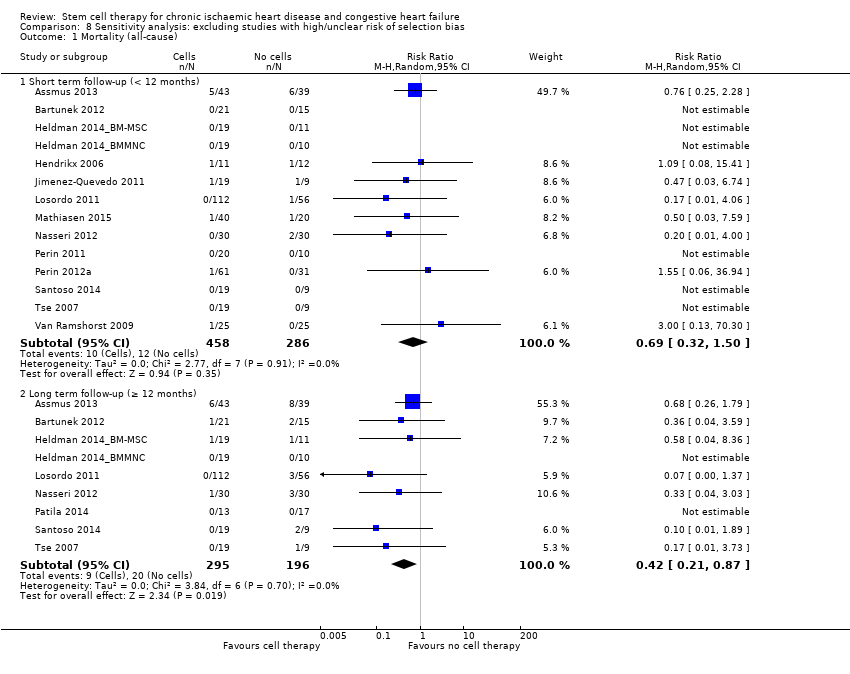 Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 1 Mortality (all‐cause). | ||||
| 1.1 Short term follow‐up (< 12 months) | 14 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.32, 1.50] |
| 1.2 Long term follow‐up (≥ 12 months) | 9 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.87] |
| 2 Non‐fatal myocardial infarction Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.2 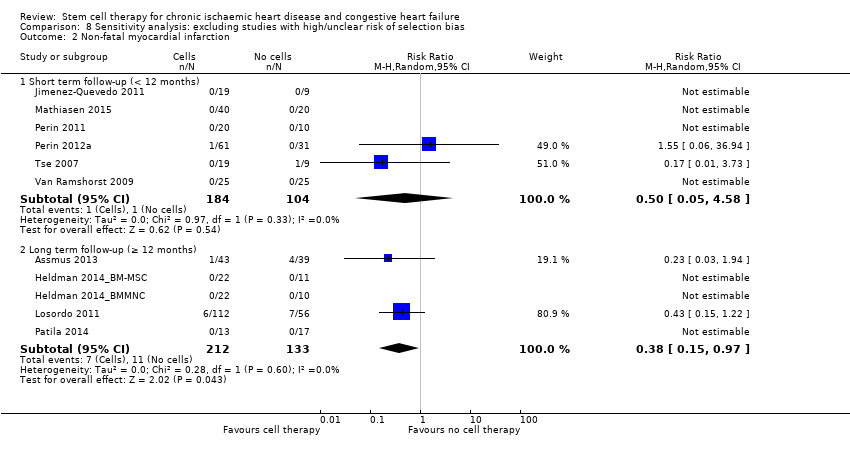 Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 2 Non‐fatal myocardial infarction. | ||||
| 2.1 Short term follow‐up (< 12 months) | 6 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.58] |
| 2.2 Long term follow‐up (≥ 12 months) | 5 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.97] |
| 3 Rehospitalisation due to heart failure Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.3  Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 3 Rehospitalisation due to heart failure. | ||||
| 3.1 Short term follow‐up (< 12 months) | 3 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.32, 1.32] |
| 3.2 Long term follow‐up (≥ 12 months) | 6 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 4 Arrhythmias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.4  Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 4 Arrhythmias. | ||||
| 4.1 Short term follow‐up (< 12 months) | 6 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.18, 3.21] |
| 4.2 Long term follow‐up (≥ 12 months) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 0.99] |
| 5 Composite MACE Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.5  Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 5 Composite MACE. | ||||
| 5.1 Short term follow‐up (< 12 months) | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Long term follow‐up (≥ 12 months) | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.08] |
| 6 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.6 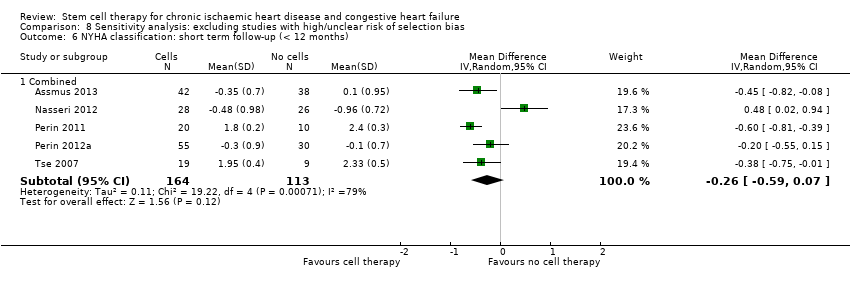 Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 6 NYHA classification: short term follow‐up (< 12 months). | ||||
| 6.1 Combined | 5 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 7 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.7  Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 7 NYHA classification: long term follow‐up (≥ 12 months). | ||||
| 7.1 Combined | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐2.2 [‐2.70, ‐1.70] |
| 8 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.8  Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 8 LVEF (%) measured by MRI: short term follow‐up (< 12 months). | ||||
| 8.1 Combined | 7 | 249 | Mean Difference (IV, Random, 95% CI) | 2.92 [0.67, 5.17] |
| 9 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.9  Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 9 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months). | ||||
| 9.1 Combined | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.70, 5.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 Sensitivity analysis: excluding studies with high/unclear risk of performance bias, Outcome 1 Mortality (all‐cause). | ||||
| 1.1 Short term follow‐up (< 12 months) | 25 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.29, 1.16] |
| 1.2 Long term follow‐up (≥ 12 months) | 13 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.21, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 32 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Sensitivity analysis: excluding studies with high/unclear risk of attrition bias, Outcome 1 Mortality (all‐cause). | ||||
| 1.1 Short term follow‐up (< 12 months) | 28 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 1.2 Long term follow‐up (≥ 12 months) | 17 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |

PRISMA flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
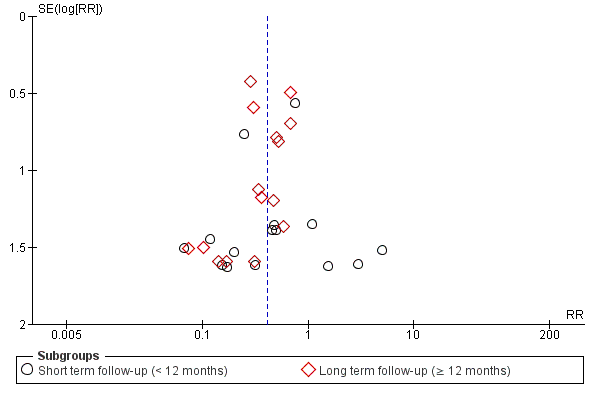
Funnel plot of comparison: 1 Stem cells versus no stem cells, outcome: 1.1 Mortality.
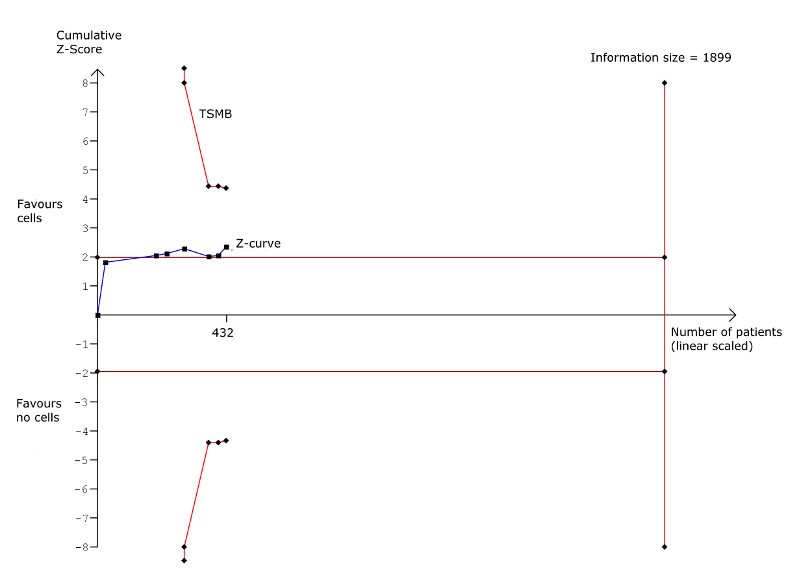
Trial sequential analysis: Mortality at long‐term follow‐up (≥ 12 months). TSMB = trial sequential monitoring boundary; horizontal red lines indicate conventional significance threshold.

Trial sequential analysis: Non‐fatal myocardial infarction at long‐term follow‐up (≥ 12 months). TSMB = trial sequential monitoring boundary; horizontal red lines indicate conventional significance threshold.

Trial sequential analysis: Rehospitalisation due to heart failure at long‐term follow‐up (≥ 12 months). TSMB = trial sequential monitoring boundary; horizontal red lines indicate conventional significance threshold.

Trial sequential analysis: Arrhythmias at long‐term follow‐up (≥ 12 months). TSMB = trial sequential monitoring boundary; horizontal red lines indicate conventional significance threshold.

Trial sequential analysis: Composite MACE at long‐term follow‐up (≥ 12 months). TSMB = trial sequential monitoring boundary; horizontal red lines indicate conventional significance threshold.

Trial sequential analysis: Left ventricular ejection fraction measured by MRI at long‐term follow‐up (≥ 12 months). TSMB = trial sequential monitoring boundary; horizontal red lines indicate conventional significance threshold.
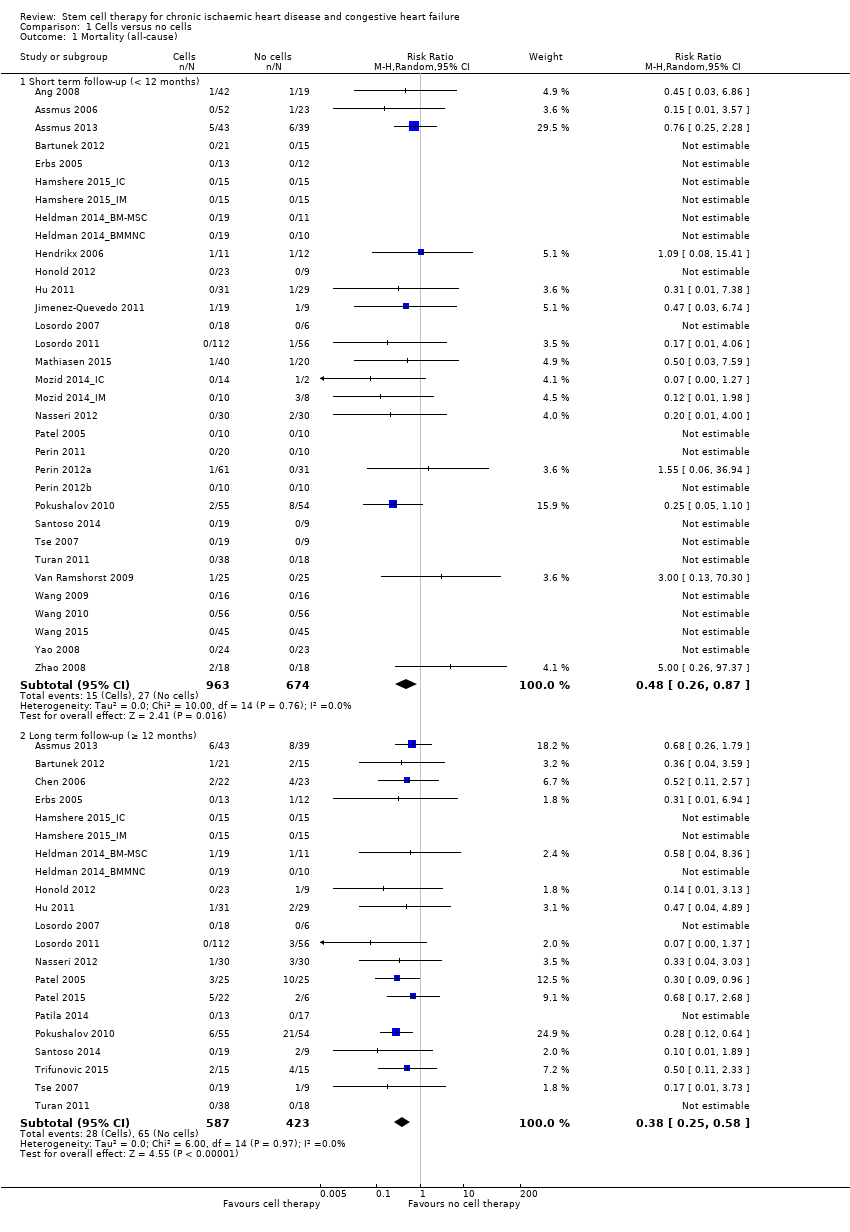
Comparison 1 Cells versus no cells, Outcome 1 Mortality (all‐cause).
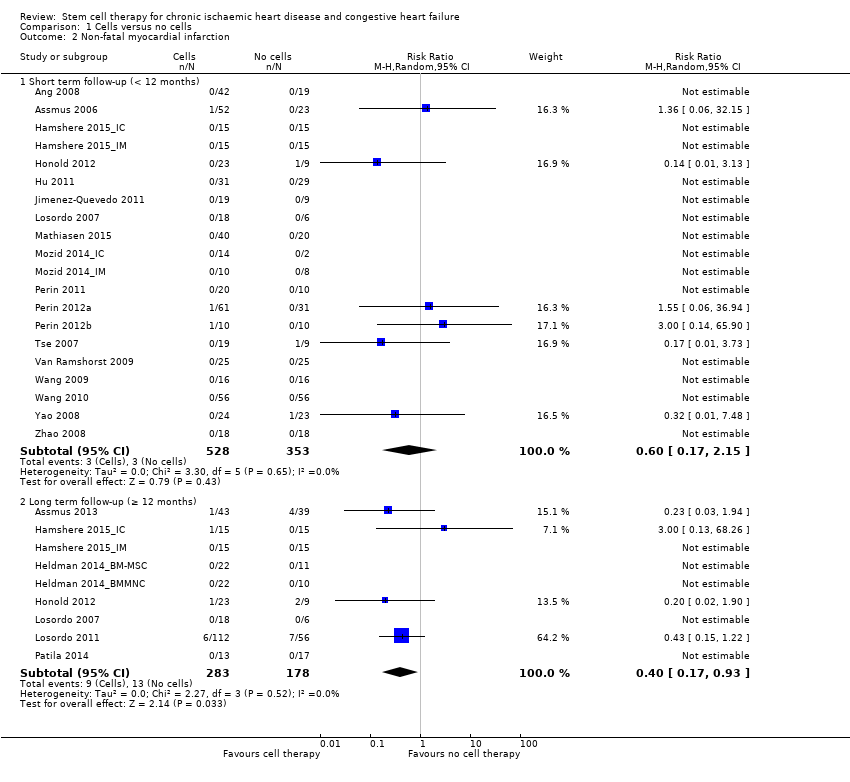
Comparison 1 Cells versus no cells, Outcome 2 Non‐fatal myocardial infarction.

Comparison 1 Cells versus no cells, Outcome 3 Rehospitalisation due to heart failure.

Comparison 1 Cells versus no cells, Outcome 4 Arrhythmias.

Comparison 1 Cells versus no cells, Outcome 5 Composite MACE.
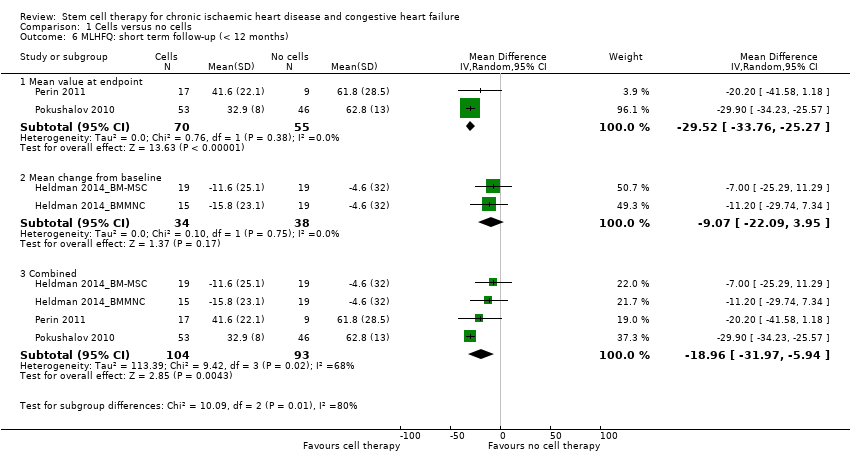
Comparison 1 Cells versus no cells, Outcome 6 MLHFQ: short term follow‐up (< 12 months).

Comparison 1 Cells versus no cells, Outcome 7 MLHFQ: long term follow‐up (≥ 12 months).

Comparison 1 Cells versus no cells, Outcome 8 Seattle Angina Questionnaire: short term follow‐up (< 12 months).

Comparison 1 Cells versus no cells, Outcome 9 Angina episodes per week: short term follow‐up (< 12 months).
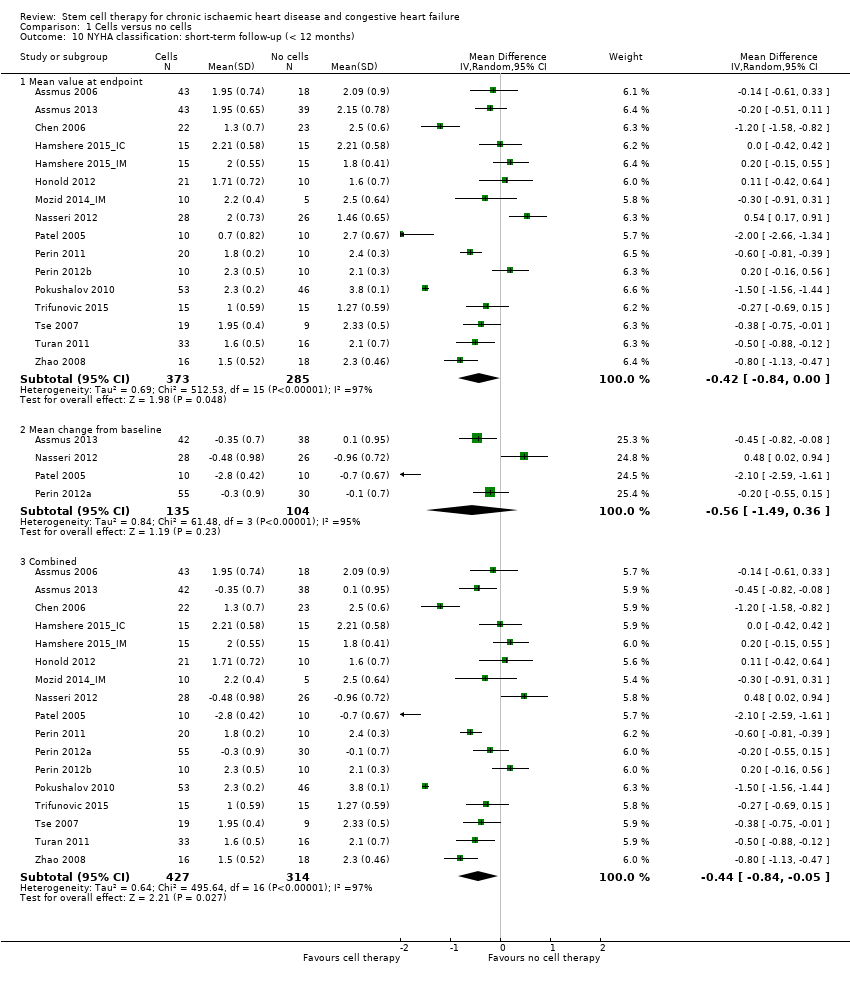
Comparison 1 Cells versus no cells, Outcome 10 NYHA classification: short‐term follow‐up (< 12 months).

Comparison 1 Cells versus no cells, Outcome 11 NYHA classification: long term follow‐up (≥ 12 months).
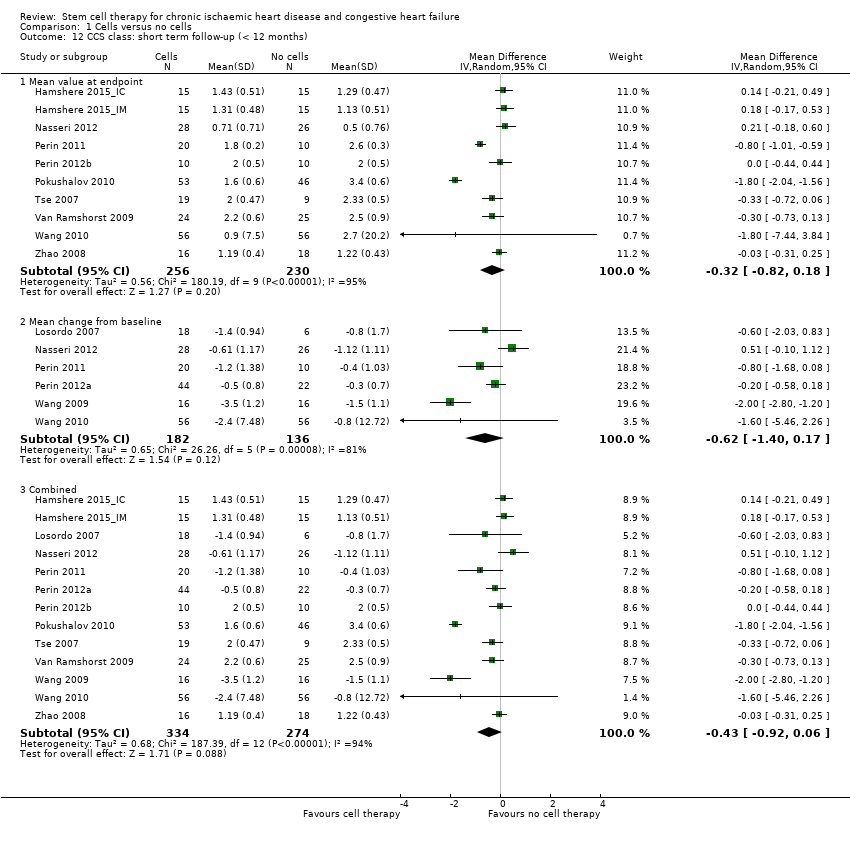
Comparison 1 Cells versus no cells, Outcome 12 CCS class: short term follow‐up (< 12 months).
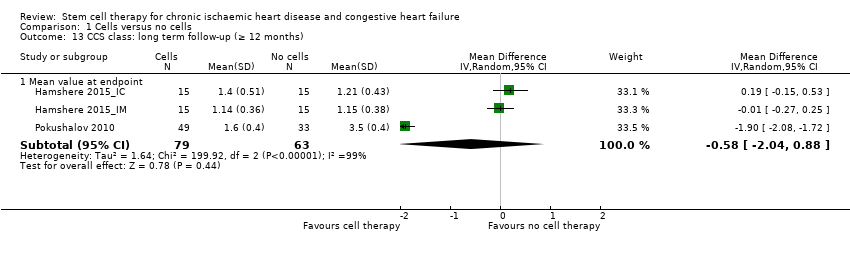
Comparison 1 Cells versus no cells, Outcome 13 CCS class: long term follow‐up (≥ 12 months).

Comparison 1 Cells versus no cells, Outcome 14 Exercise capacity: short term follow‐up (< 12 months).

Comparison 1 Cells versus no cells, Outcome 15 Exercise capacity: long term follow‐up (≥ 12 months).

Comparison 1 Cells versus no cells, Outcome 16 LVEF (%) measured by MRI: short term follow‐up (< 12 months).

Comparison 1 Cells versus no cells, Outcome 17 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months).

Comparison 1 Cells versus no cells, Outcome 18 LVEF (%) measured by echocardiography: short term follow‐up (< 12 months).
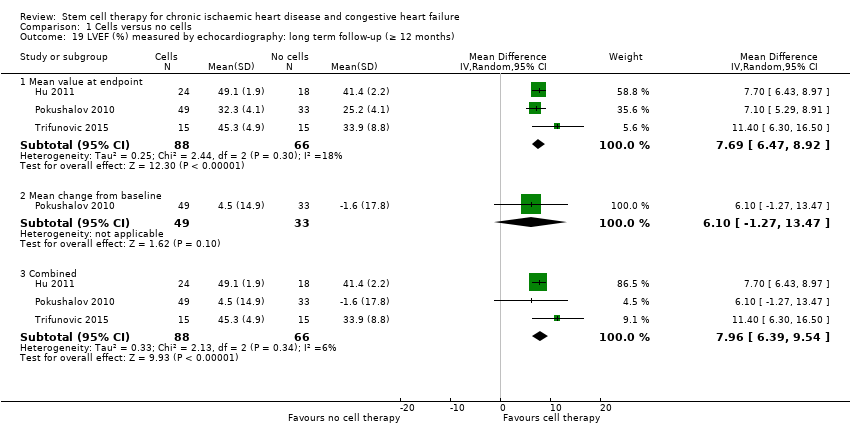
Comparison 1 Cells versus no cells, Outcome 19 LVEF (%) measured by echocardiography: long term follow‐up (≥ 12 months).

Comparison 1 Cells versus no cells, Outcome 20 LVEF (%) measured by SPECT: short term follow‐up (< 12 months).
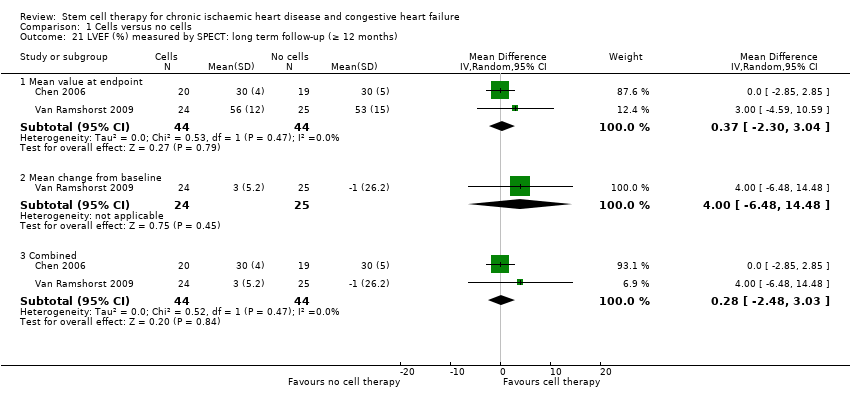
Comparison 1 Cells versus no cells, Outcome 21 LVEF (%) measured by SPECT: long term follow‐up (≥ 12 months).
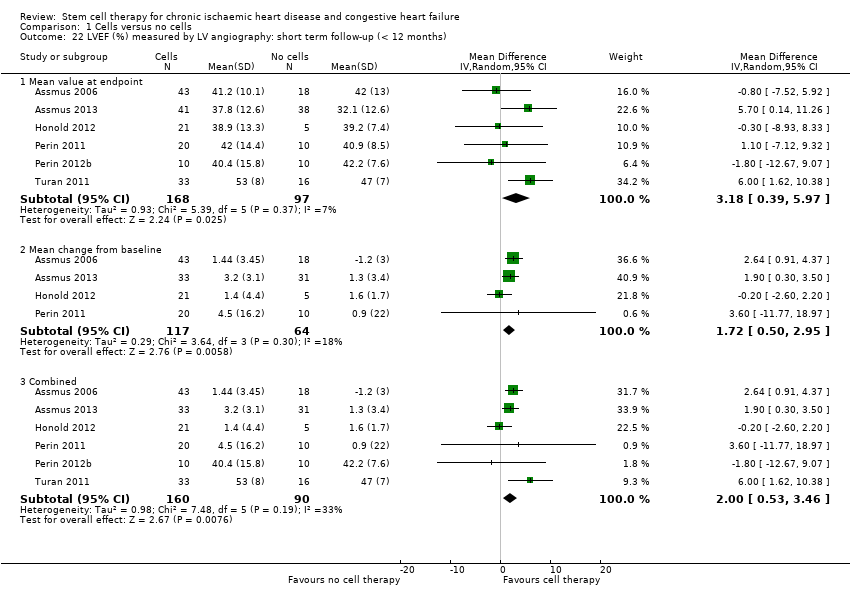
Comparison 1 Cells versus no cells, Outcome 22 LVEF (%) measured by LV angiography: short term follow‐up (< 12 months).
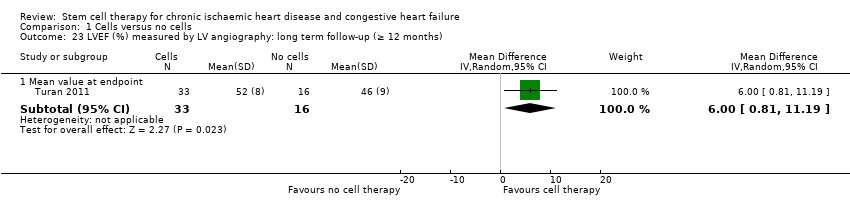
Comparison 1 Cells versus no cells, Outcome 23 LVEF (%) measured by LV angiography: long term follow‐up (≥ 12 months).
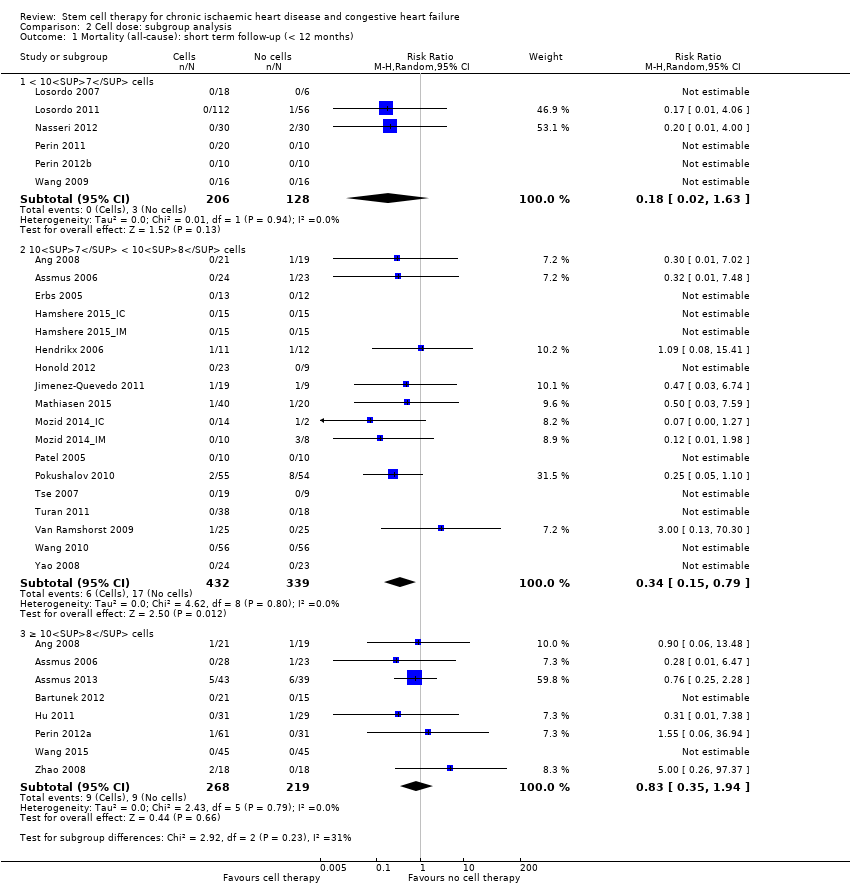
Comparison 2 Cell dose: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months).

Comparison 2 Cell dose: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months).

Comparison 2 Cell dose: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months).
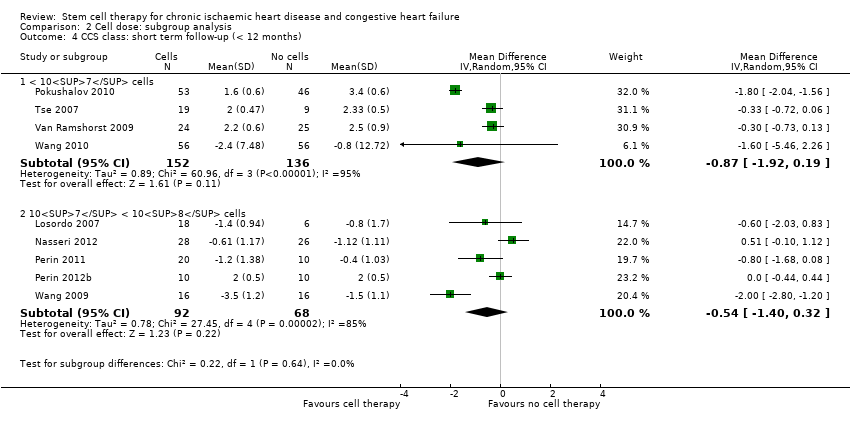
Comparison 2 Cell dose: subgroup analysis, Outcome 4 CCS class: short term follow‐up (< 12 months).

Comparison 2 Cell dose: subgroup analysis, Outcome 5 Exercise capacity: short term follow‐up (< 12 months).

Comparison 2 Cell dose: subgroup analysis, Outcome 6 LVEF (%) measured by MRI: short term follow‐up (< 12 months).

Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months).

Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months).

Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months).

Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 4 NYHA classification: long term follow‐up (≥ 12 months).

Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 5 CCS class: short term follow‐up (< 12 months).
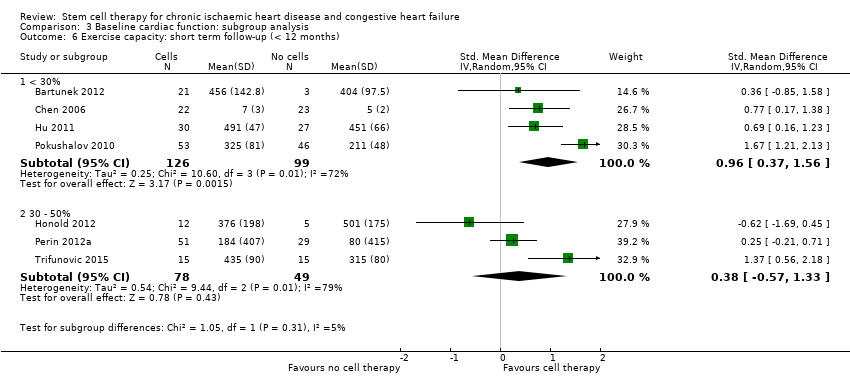
Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 6 Exercise capacity: short term follow‐up (< 12 months).

Comparison 3 Baseline cardiac function: subgroup analysis, Outcome 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months).
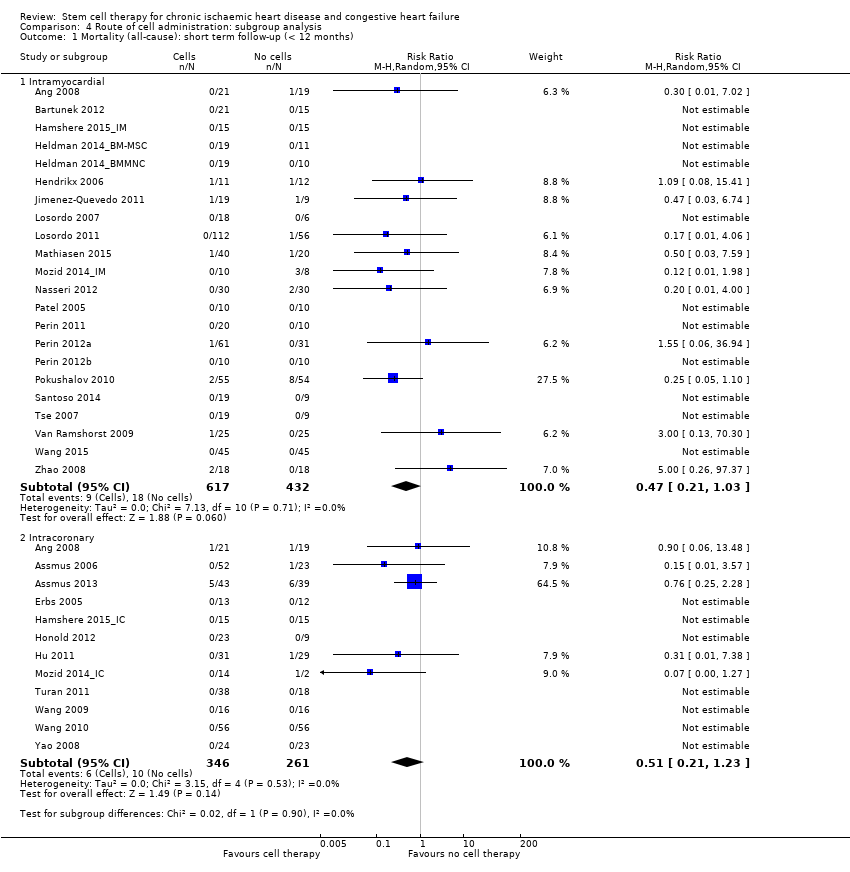
Comparison 4 Route of cell administration: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months).

Comparison 4 Route of cell administration: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months).
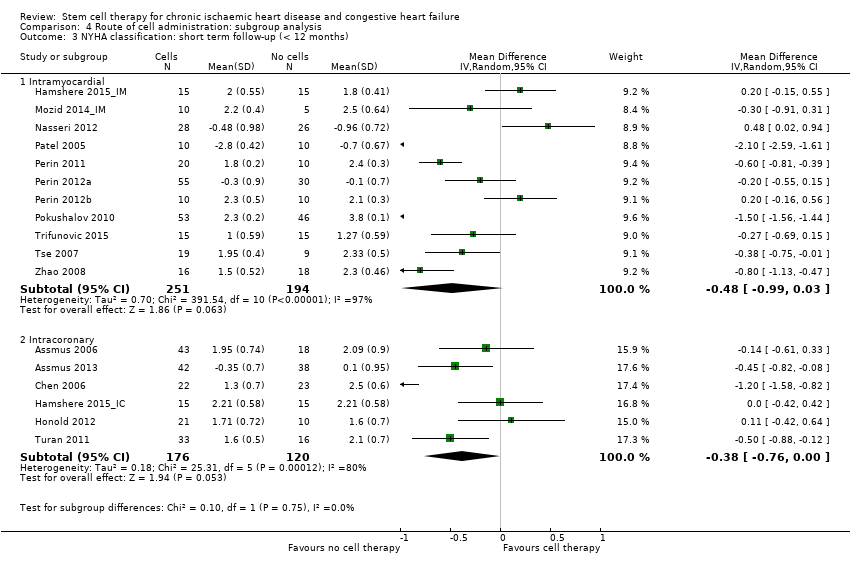
Comparison 4 Route of cell administration: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months).
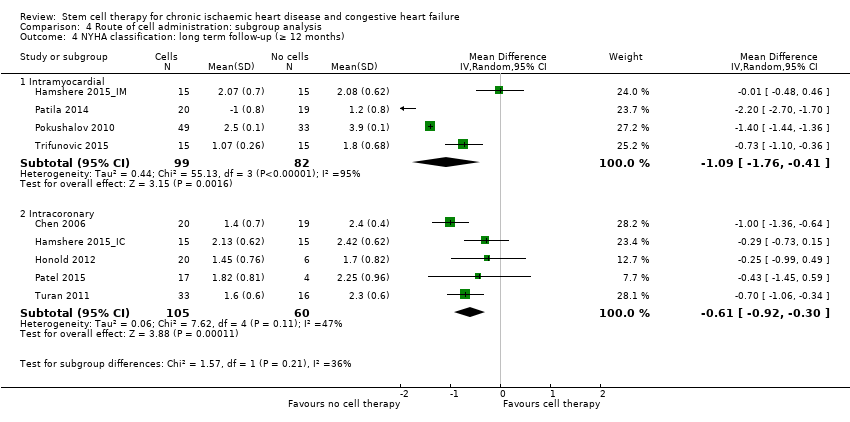
Comparison 4 Route of cell administration: subgroup analysis, Outcome 4 NYHA classification: long term follow‐up (≥ 12 months).

Comparison 4 Route of cell administration: subgroup analysis, Outcome 5 CCS class: short term follow‐up (< 12 months).
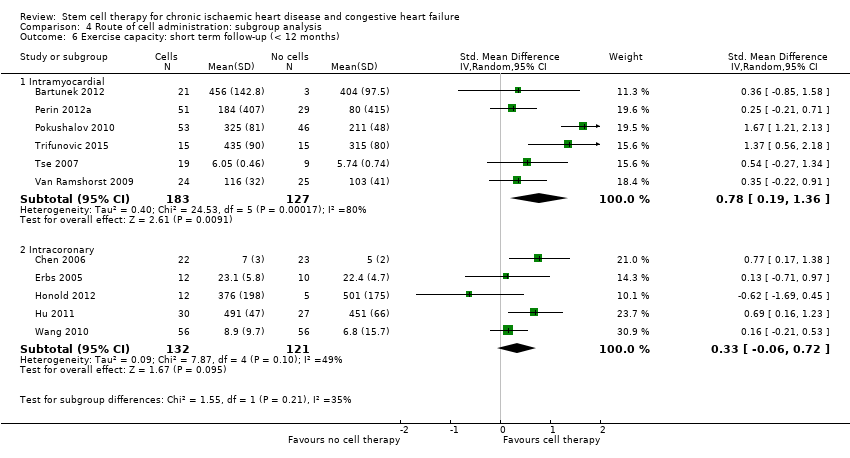
Comparison 4 Route of cell administration: subgroup analysis, Outcome 6 Exercise capacity: short term follow‐up (< 12 months).

Comparison 4 Route of cell administration: subgroup analysis, Outcome 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months).

Comparison 5 Cell type: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months).

Comparison 5 Cell type: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months).
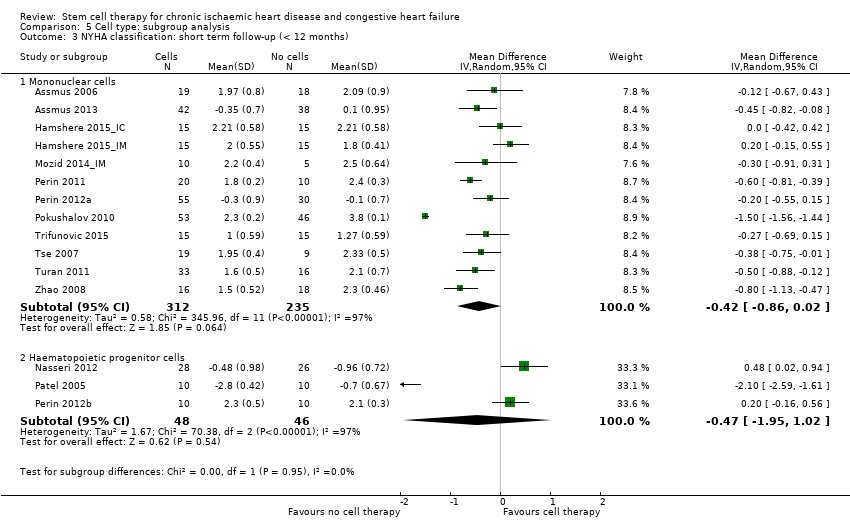
Comparison 5 Cell type: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months).
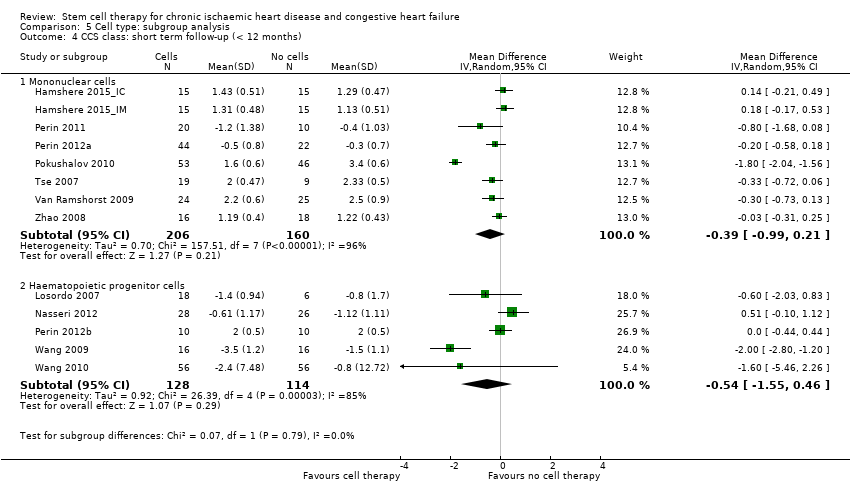
Comparison 5 Cell type: subgroup analysis, Outcome 4 CCS class: short term follow‐up (< 12 months).

Comparison 6 Participant diagnosis: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months).

Comparison 6 Participant diagnosis: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months).
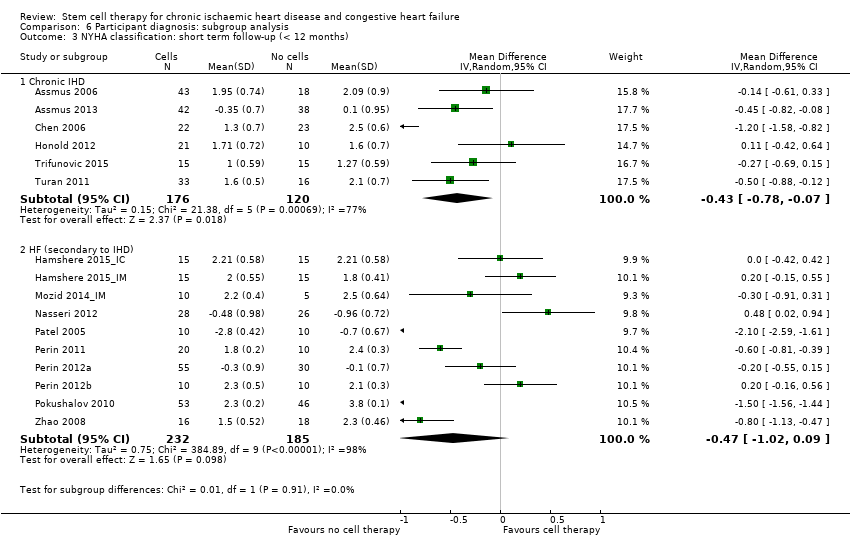
Comparison 6 Participant diagnosis: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months).

Comparison 6 Participant diagnosis: subgroup analysis, Outcome 4 NYHA classification: long term follow‐up (≥ 12 months).

Comparison 6 Participant diagnosis: subgroup analysis, Outcome 5 CCS class: short term follow‐up (< 12 months).
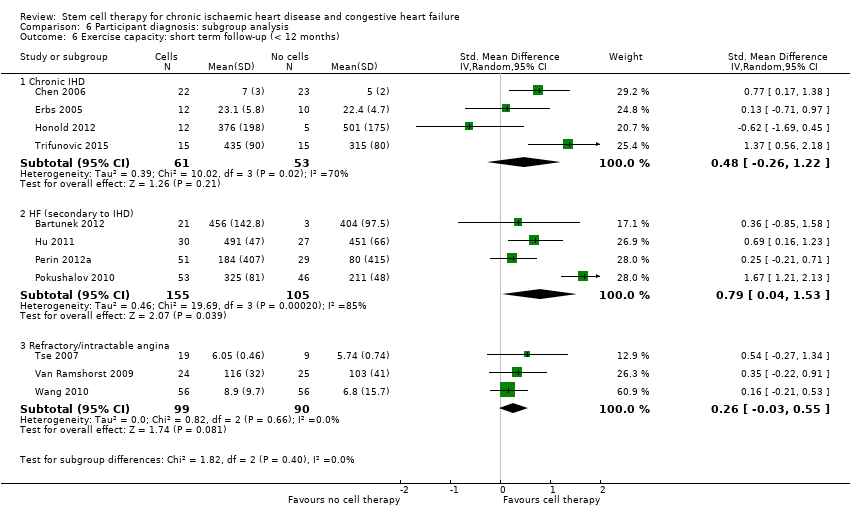
Comparison 6 Participant diagnosis: subgroup analysis, Outcome 6 Exercise capacity: short term follow‐up (< 12 months).
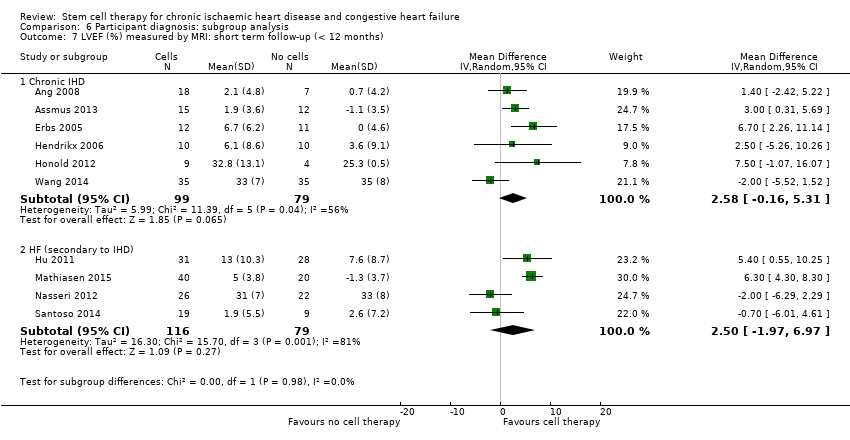
Comparison 6 Participant diagnosis: subgroup analysis, Outcome 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months).
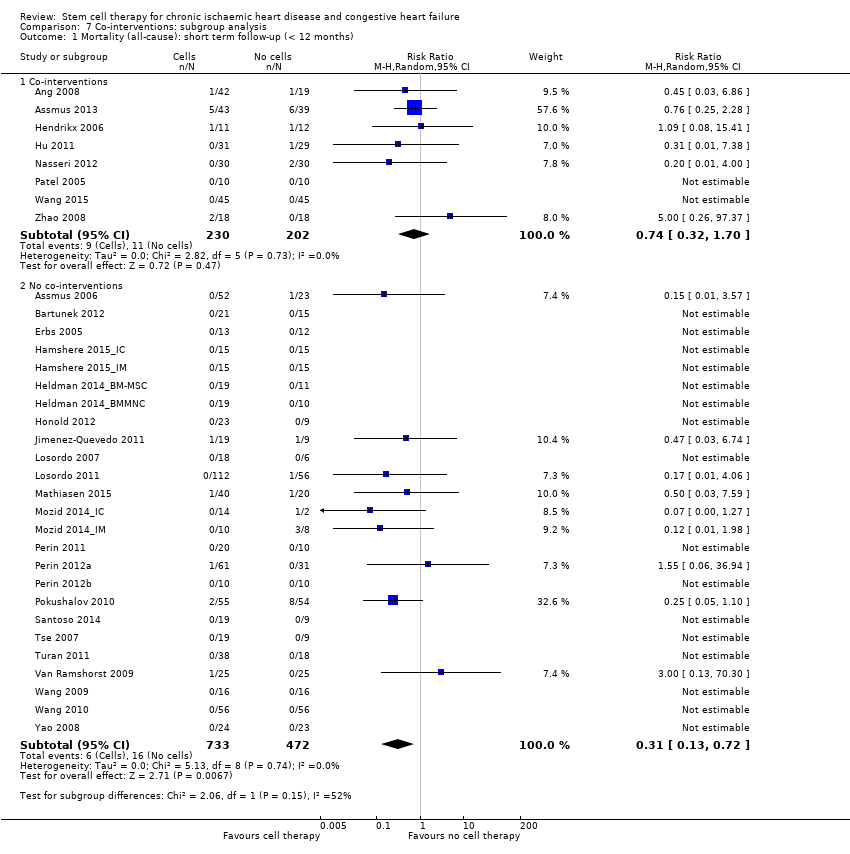
Comparison 7 Co‐interventions: subgroup analysis, Outcome 1 Mortality (all‐cause): short term follow‐up (< 12 months).

Comparison 7 Co‐interventions: subgroup analysis, Outcome 2 Mortality (all‐cause): long term follow‐up (≥ 12 months).

Comparison 7 Co‐interventions: subgroup analysis, Outcome 3 NYHA classification: short term follow‐up (< 12 months).
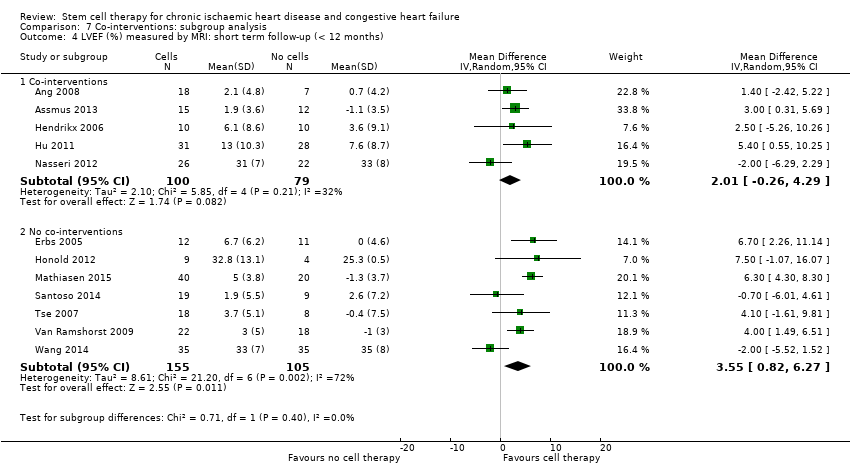
Comparison 7 Co‐interventions: subgroup analysis, Outcome 4 LVEF (%) measured by MRI: short term follow‐up (< 12 months).

Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 1 Mortality (all‐cause).
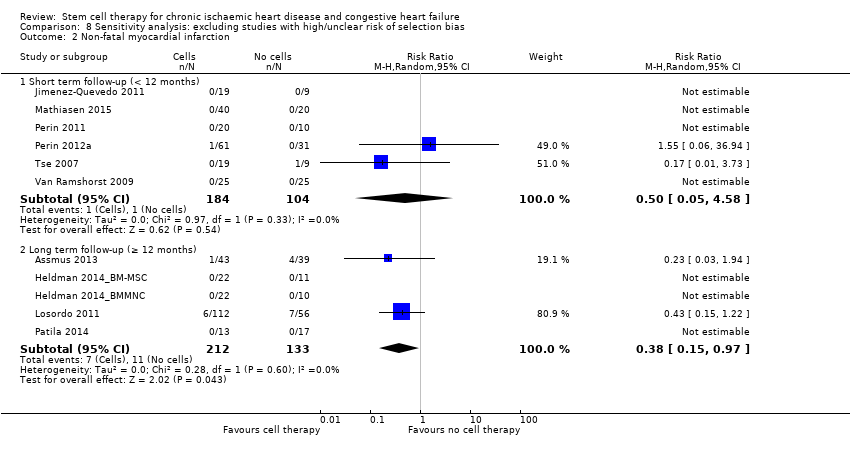
Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 2 Non‐fatal myocardial infarction.
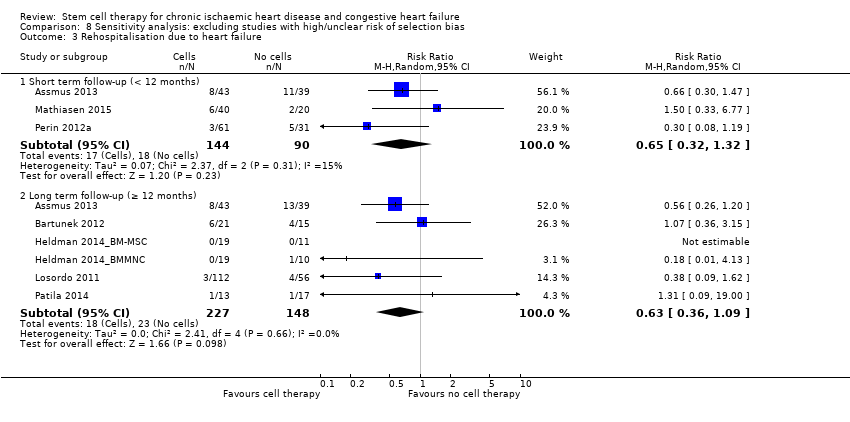
Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 3 Rehospitalisation due to heart failure.
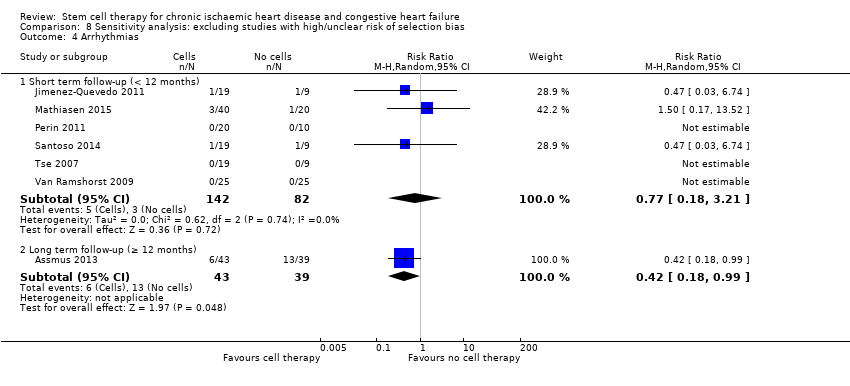
Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 4 Arrhythmias.

Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 5 Composite MACE.

Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 6 NYHA classification: short term follow‐up (< 12 months).
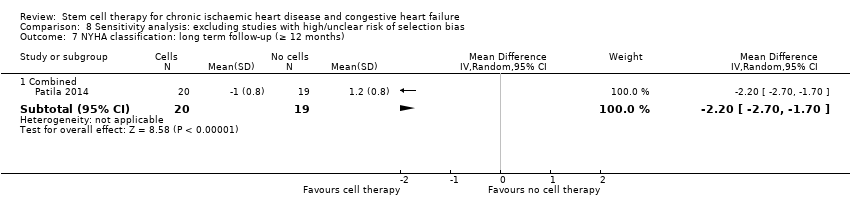
Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 7 NYHA classification: long term follow‐up (≥ 12 months).

Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 8 LVEF (%) measured by MRI: short term follow‐up (< 12 months).

Comparison 8 Sensitivity analysis: excluding studies with high/unclear risk of selection bias, Outcome 9 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months).

Comparison 9 Sensitivity analysis: excluding studies with high/unclear risk of performance bias, Outcome 1 Mortality (all‐cause).
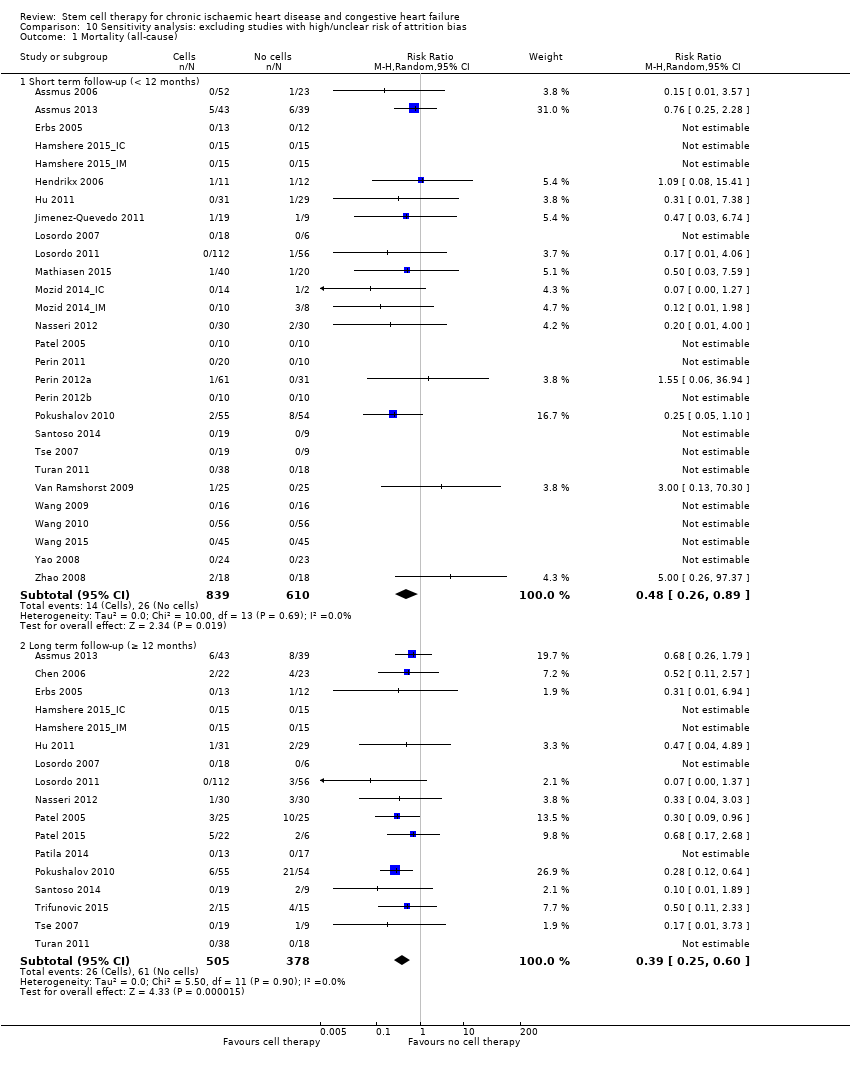
Comparison 10 Sensitivity analysis: excluding studies with high/unclear risk of attrition bias, Outcome 1 Mortality (all‐cause).
| Bone marrow‐derived cell therapy for people with chronic ischaemic heart disease and congestive heart failure | ||||||
| Patient or population: people with chronic ischaemic heart disease and congestive heart failure Comparison: no cell therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No cell therapy | Bone marrow‐derived cell therapy | |||||
| Mortality (all cause) Long‐term follow‐up (≥ 12 months) | 102 per 1000 | 43 per 1000 | RR 0.42 | 491 | ⊕⊕⊝⊝ | The required information size of 1899 participants to detect a RRR of 35% has not been reached. |
| Periprocedural adverse events | See comment | See comment | Not estimable | 1695 (34 studies) | See comment | Adverse events occurring during the mapping or cell/placebo injection procedure included ventricular tachycardia (7), ventricular fibrillation (1), atrial fibrillation (1), transient complete heart block (1), transient pulmonary oedema (3), thrombus on mapping catheter tip (1), visual disturbances (2), myocardial perforation (2), limited retrograde catheter‐related dissection of the abdominal aorta (1). |
| Non‐fatal myocardial infarction Long‐term follow‐up (≥ 12 months) | 83 per 1000 | 31 per 1000 | RR 0.38 | 345 | ⊕⊕⊝⊝ | The required information size of 2383 participants to detect a RRR of 35% has not been reached. |
| Rehospitalisation due to heart failure Long‐term follow‐up (≥ 12 months) | 155 per 1000 | 98 per 1000 | RR 0.63 | 375 | ⊕⊕⊝⊝ | The required information size of 1193 participants to detect a RRR of 35% has not been reached. |
| Arrhythmias Long‐term follow‐up (≥ 12 months) | 333 per 1000 | 140 per 1000 | RR 0.42 | 82 | ⊕⊕⊝⊝ | The required information size of 461 participants to detect a RRR of 35% has not been reached. |
| Composite MACE Long‐term follow‐up (≥ 12 months) | 350 per 1000 | 224 per 1000 | RR 0.64 | 141 | ⊕⊕⊝⊝ | The required information size of 431 participants to detect a RRR of 35% has not been reached. |
| LVEF (%) measured by MRI Long‐term follow‐up (≥ 12 months) | ‐ | The mean LVEF (%) measured by MRI in the intervention groups was 1.6 lower (8.7 lower to 5.5 higher). | ‐ | 25 | ⊕⊕⊝⊝ | The required information size of 322 participants to detect a mean difference of 4% has not been reached. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ¶Only studies with a low risk of selection bias are included. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Six trials received full or partial commercial funding, which could have resulted in a biased assessment of the intervention effect and were therefore deemed to have a high risk of bias. One trial was not blinded (high risk of performance bias) and had a high risk of attrition bias. | ||||||
| Study ID | Country of study | Patient population | Mean (SD) age of participants (years) | % Male | No. randomised participants receiving intervention | No. randomised participants receiving comparator | Mean duration of follow‐up |
| UK | CIHD (> 1 chronic myocardial scar; elective CABG) | BMMNC‐IM: 64.7 (8.7) BMMNC‐IC: 62.1 (8.7) Controls: 61.3 (8.3) | BMMNC‐IM: 71.4% BMMNC‐IC: 90.5% Controls: 90.0% | 42 (21 IM, 21 IC) | 21 | 6 months | |
| Germany | CIHD (MI > 3 months; LV dysfunction) | BMMNC: 59 (12) CPC: 54 (12) Controls: 61 (9) | BMMNC: 89% CPC: 79% Controls: 100% | 52 (28 MNC, 24 CPC) | 23 | 3 months | |
| Germany | CIHD (MI > 3 months; LVEF < 50%; NYHA class II or greater) | BMMNC‐LDSW: 65 (12) BMMNC‐HDSW: 58 (11) Controls‐LDSW: 60 (10) Controls‐HDSW: 63 (10) | BMMNC‐LDSW: 77% BMMNC‐HDSW: 86% Controls‐LDSW: 80% Controls‐HDSW: 90% | 43 (22 LDSW, 21 HDSW) | 39 (20 LDSW, 19 HDSW) | 45.7 (17) months | |
| Belgium/ Serbia/ Switzerland | HF (LVEF 15% to 40%; ischaemic event > 2 months) | BM‐MSC: 55.3 (SE 10.4) Controls: 58.7 (SE 8.2) | BM‐MSC: 90.5% Controls: 86.7% | 32 | 15 | 24 months | |
| China | CIHD (isolated, chronic LAD; LVEF < 40%) | BM‐MSC: 59.3 (6.8) Controls: 57.8 (7.2) | BM‐MSC: 88% Controls: 92% | 24 | 24 | 12 months | |
| Germany | CIHD (chronic total occlusion; myocardial ischaemia) | CPC: 63 (7) Controls: 61 (9) | CPC: 71% Controls: 86% | 14 | 14 | 15 months | |
| UK | HF (NYHA class II‐IV; no revascularisation options) | BMMNC: n/r Controls: n/r | BMMNC: n/r Controls: n/r | 15 | 15 | 12 months | |
| UK | HF (NYHA class II‐IV; no revascularisation options) | BMMNC: n/r Controls: n/r | BMMNC: n/r Controls: n/r | 15 | 15 | 12 months | |
| USA | CIHD (chronic MI; LV dysfunction) | BMMNC: 61.1 (8.4) Controls: 61.3 (9.0) | BMMNC: 89.5% Controls: 100% | 22 | 10 | 12 months | |
| USA | CIHD (chronic MI; LV dysfunction) | BM‐MSC: 57.1 (10.6) Controls: 60.0 (12.0) | BM‐MSC: 94.7% Controls: 90.9% | 22 | 11 | 12 months | |
| Belgium | CIHD (transmural MI; LV dysfunction; elective CABG) | BMMNC: 63.2 (8.5) Controls: 66.8 (9.2) | BMMNC: 100% Controls: 70% | 11 | 12 | 4 months | |
| Germany | CIHD (MI > 3 months; LV regional wall motion abnormality) | CPC: 53.4 (12.3) Controls: 58.8 (7.3) | CPC: 82% Controls: 100% | 23 | 10 | 60 months | |
| China | HF (MI > 3 months; LVEF < 30%; elective CABG) | BMMNC: 56.6 (9.7) Controls: 58.3 (8.9) | BMMNC: 88% Controls: 96% | 31 | 29 | 12 months | |
| Spain | Refractory angina (CCS class II‐IV) | CD133+: median 70.0 Controls: median 58.2 | CD133+: 78.9% Controls: 100% | 19 | 9 | 6 months | |
| USA | Refractory angina (CCS class III‐IV) | CD34+/controls pooled: 62.4 (range 48 to 84) | CD34+/controls pooled: 80% | 18 (6 LD, 6 MD 6, HD) | 6 | 6 months | |
| USA | Refractory angina (CCS class III‐IV) | CD34+/LD: 61.3 (9.1) CD34+/HD: 59.8 (9.2) Controls: 61.8 (8.5) | CD34+/LD: 83.6% CD34+/HD: 87.5% Controls: 89.3% | 112 (56 LD, 56 HD) | 56 | 12 months | |
| Denmark | HF (NYHA class II‐III; LVEF < 45%; no revascularisation options) | BM‐MSC: 66.1 (7.7) Controls: 64.2 (10.6) | BM‐MSC: 90% Controls: 70% | 40 | 20 | 6 months | |
| UK | HF (NYHA class II‐IV; no revascularisation options) | BMMNC/controls pooled (16 participants): 70 (10) | BMMNC/controls pooled (16 participants): 94% | 14 | 2 | 6 months | |
| UK | HF (NYHA class II‐IV; no revascularisation options) | BMMNC/controls pooled (18 participants): 64 (9) | BMMNC/controls pooled (18 participants): 100% | 10 | 8 | 6 months | |
| Germany | HF (LVEF < 35%; elective CABG) | CD133+: 61.9 (7.3) Controls: 62.7 (10.6) | CD133+: 93% Controls: 97% | 30 | 30 | 6 months | |
| Argentina | HF (LVEF < 35%; NYHA class III‐IV; elective CABG) | CD34+: 64.8 (7.1) Controls: 63.6 (5.2) | CD34+: 80% Controls: 80% | 25 | 25 | 10 years | |
| USA/Germany/India | HF (LVEF < 40%; NYHA class III‐IV) | BMAC: 58.5 (12.7) Controls: 52.7 (8.5) | BMAC: 91.7% Controls: 100% | 24 | 6 | 12 months | |
| Finland | HF (LVEF 15% to 40%; NYHA class II‐IV; elective CABG) | BMMNC: median 65 (range 57 to 73) Controls: median 64 (range 58 to 70) | BMMNC: 94.7% Controls: 95.0% | 20 | 19 | 12 months | |
| USA | HF (angina/HF symptoms; chronic CAD; LVEF < 40%; no revascularisation options) | BMMNC: 56.3 (8.6) Controls: 60.5 (6.4) | BMMNC: 50% Controls: 80% | 20 | 10 | 6 months | |
| USA | HF (CCS class II‐IV or NYHA class II‐III, or both; LVEF < 45%; no revascularisation options) | BMMNC: 64.0 (10.9) Controls: 62.3 (8.3) | BMMNC: 86.9% Controls: 93.7% | 61 | 31 | 6 months | |
| USA | HF (CCS class II‐IV or NYHA class II‐III, or both; LVEF < 45%; no revascularisation options) | ALDH+: 58.2 (6.1) Controls: 57.8 (5.5) | ALDH+: 90% Controls: 80% | 10 | 10 | 6 months | |
| Russia | HF (LVEF < 35%; no revascularisation options) | BMMNC: 61 (9) Controls: 62 (5) | BMMNC: 87% Controls: 85% | 55 | 54 | 12 months | |
| Indonesia/China | HF (NYHA class III‐IV; LVEF < 40%; no revascularisation options) | BMMNC: 58 (5.9) Controls: 60 (5.6) | BMMNC: 95% Controls: 100% | 19 | 9 | 6 months | |
| Serbia | CIHD (MI < 30 days; LVEF < 40%; NYHA class III‐IV; elective CABG) | BMMNC: 53.8 (10.1) Controls: 60.0 (6.8) | BMMNC: 93.3% Controls: 93.3% | 15 | 15 | Median 5 years (IQR 2.5 to 7.5) | |
| China/Australia | Refractory angina (CCS class III‐IV) | BMMNC: 65.2 (8.3) Controls: 68.9 (6.3) | BMMNC: 79% Controls: 88% | 19 | 9 | 6 months | |
| Germany | CIHD (MI > 3 months; LV dysfunction) | BMMNC: 62 (10) Controls: 60 (9) | BMMNC: 52.6% Controls: 55.6% | 38 | 18 | 12 months | |
| The Netherlands | Refractory angina (CCS class II‐IV) | BMMNC: 64 (8) Controls: 62 (9) | BMMNC: 92% Controls: 80% | 25 | 25 | 6 months | |
| China | Refractory angina (MI > 1 month) | CD34+: 60.6 (n/r) Controls: 60.0 (n/r) | CD34+: 56.3% Controls: 63.3% | 16 | 16 | 6 months | |
| China | Refractory angina (CCS class III‐IV) | CD34+: range 42 to 80 Controls: range 43 to 80 | CD34+: 51.8% Controls: 50.0% | 56 | 56 | 6 months | |
| China | CIHD (LVEF < 35%) | CD133+: n/r Controls: n/r | CD133+: n/r Controls: n/r | 35 | 35 | 6 months | |
| China | CIHD (multivessel disease; MI > 4 weeks; elective CABG) | BMMNC: 61.4 (7.5) Controls: 62.9 (6.9) | BMMNC: 82% Controls: 78% | 45 | 45 | 6 months | |
| China | CIHD (MI > 6 months) | BMMNC: 54.8 (11.5) Controls: 56.3 (7.9) | BMMNC: 96% Controls: 96% | 24 | 23 | 6 months | |
| China | HF (LVEF < 40%; elective CABG) | BMMNC: 60.3 (10.4) Controls: 59.1 (15.7) | BMMNC: 83.3% Controls: 83.3% | 18 | 18 | 6 months | |
| ALDH: aldehyde dehydrogenase | |||||||
| Study ID | Co‐intervention | Intervention given by: | Route of cell administration | Intervention cell type | How are cells obtained? | What were they resuspended in? | Dose administered? | Comparator arm (placebo or control) |
| CABG | Cardiothoracic surgeon | IC or IM | BMMNC | BM aspiration (**) | Autologous serum | IM: 84 (56) million cells IC: 115 (73) million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IC | BMMNC or CPC | BM aspiration (**) for BMMNC. Vein puncture, mononuclear cell isolation by gradient centrifugation and culture for 3 days for CPC | n/r | BMMNC: 205 (110) million cells CPC: 22 (11) million cells | No additional therapy (control) | |
| Shockwave | Cardiologist | IC | BMMNC | BM aspiration (**) | X‐VIVO 10 medium and autologous serum | HDSW: 123 (69) million cells LDSW: 150 (77) million cells | Placebo (10 mL X‐VIVO 10 medium and autologous serum) | |
| Standard medical therapy | Cardiologist | IC | BM‐MSC (cardiopoietic cells) | BM aspiration (**), culture for 6 days and exposure to cardiopoietic factors | Preservation solution (no details) | 733 (range 605 to 1168) million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IC | BM‐MSC | BM aspiration (**), culture for 7 days to select MSC | Heparinised saline | 5 million cells | No additional therapy (control) | |
| G‐CSF | Cardiologist | IC | CPC | G‐CSF infusion for 4 days prior to vein puncture, mononuclear cell isolation by gradient centrifugation and culture for 3 days for CPC | Saline and 10% autologous serum | 69 (14) million cells | Placebo (cell‐free serum solution) | |
| G‐CSF | Cardiologist | IC | BMMNC | G‐CSF infusion for 5 days and BM aspiration (**) | Autologous serum | n/r | Placebo (10 mL autologous serum) | |
| G‐CSF | Cardiologist | IM | BMMNC | G‐CSF infusion for 5 days and BM aspiration (**) | Autologous serum | n/r | Placebo (2 mL autologous serum) | |
| Standard medical therapy | Cardiologist | IM | BMMNC | BM aspiration (**) | n/r | n/r | Placebo (vehicle medium) | |
| Standard medical therapy | Cardiologist | IM | BM‐MSC | BM aspiration (**), culture to select MSC | n/r | n/r | Placebo (vehicle medium) | |
| CABG | Cardiothoracic surgeon | IM | BMMNC | BM aspiration (**) | Heparinised saline | 60 (31) million cells | Placebo (heparinised saline) | |
| G‐CSF | Cardiologist | IC | CPC | G‐CSF infusion for 5 days prior to vein puncture, mononuclear cell isolation by gradient centrifugation and culture for 4 days for CPC | n/r | 29 (12) million cells | No additional therapy (control) | |
| CABG | Cardiothoracic surgeon | IC | BMMNC | BM aspiration (**) | Saline solution and 20% autologous serum | 132 (107) million cells | Placebo (8 mL saline; 2 mL autologous serum) | |
| G‐CSF | Cardiologist | IM | CD133+ | G‐CSF infusion for 5 days prior to leukapheresis, mononuclear cell isolation by gradient centrifugation immunomagnetic selection to isolate CD133+ cells | Normal saline solution | 20 to 30 million cells | No additional therapy (control) | |
| G‐CSF | Cardiologist | IM | CD34+ | G‐CSF infusion for 5 days prior to leukapheresis, mononuclear cell isolation by gradient centrifugation immunomagnetic selection to isolate CD34+ cells | Saline solution and 5% autologous serum | LD: 0.05 million cells MD: 0.1 million cells HD: 0.5 million cells | Placebo (0.9% sodium chloride; 5% autologous plasma) | |
| G‐CSF | Cardiologist | IM | CD34+ | G‐CSF infusion for 5 days prior to leukapheresis, mononuclear cell isolation by gradient centrifugation immunomagnetic selection to isolate CD34+ cells | Saline solution and 5% autologous serum | LD: 0.1 million cells HD: 0.5 million cells | Placebo (0.9% sodium chloride; 5% autologous plasma) | |
| Standard medical therapy | Cardiologist | IM | BM‐MSC | BM aspiration (**), culture for 14 to 35 days to select MSC | Phosphate buffered saline with a drop of the participant’s blood | 77.5 (68) million cells | Placebo (phosphate buffered saline mixed with drop of participant’s blood) | |
| G‐CSF | Cardiologist | IC | BMMNC | G‐CSF infusion for 5 days and BM aspiration (**) | Autologous serum | 86 (110) million cells | Placebo (10 mL autologous serum) | |
| G‐CSF | Cardiologist | IM | BMMNC | G‐CSF infusion for 5 days and BM aspiration (**) | Autologous serum | 52 (53) million cells | Placebo (2 mL autologous serum) | |
| CABG | Cardiothoracic surgeon | IM | CD133+ | BM aspiration (**), immunomagnetic selection to isolate CD133+ cells | Sodium chloride and 10% autologous serum | Median 5.1 million cells | Placebo (isotonic saline solution; 10% autologous serum) | |
| CABG | Cardiothoracic surgeon | IM | CD34+ | BM aspiration (**), immunomagnetic selection to isolate CD34+ cells | Heparinised saline and autologous serum | Median 22 million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IC | BMAC | BM aspiration (**) and concentration | Autologous serum | 3700 (900) million cells | No additional therapy (control) | |
| CABG | Cardiothoracic surgeon | IM | BMMNC | BM aspiration (**) | Medium 199 containing albumin, heparin | Median 840 (range 52 to 135) million cells | Placebo (vehicle medium) | |
| Standard medical therapy | Cardiologist | IM | BMMNC | BM aspiration (**) | Saline containing 5% human serum albumin | 2 million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IM | BMMNC | BM aspiration (**) | Saline containing 5% human serum albumin | 100 million cells | Placebo (cell‐free suspension in same volume) | |
| Standard medical therapy | Cardiologist | IM | ALDH+ | BM aspiration (**) and cell sorting | Pharmaceutical grade human serum albumin | 2.4 (1.3) million cells | Placebo (5% pharmaceutical serum albumin) | |
| Standard medical therapy | Cardiologist | IM | BMMNC | BM aspiration (**) | Heparinised saline | 41 (16) million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IM | BMMNC | BM aspiration (**) | Phosphate buffered saline with 10% autologous plasma | n/r | Placebo (phosphate buffered saline; 10% autologous plasma) | |
| CABG | Cardiothoracic surgeon | IM | BMMNC | BM aspiration (**) | n/r | 70.7 (32.4) million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IM | BMMNC | BM aspiration (**) | Phosphate buffered saline with 10% autologous plasma | 15 million cells | Placebo (8 ‐ 12 x 0.1 mL phosphate buffered saline with 10% autologous serum) | |
| Standard medical therapy | Cardiologist | IC | BMMNC | BM aspiration (**) | n/r | 99 (25) million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IM | BMMNC | BM aspiration (**) | Phosphate buffered saline with 0.5% human serum albumin | 98 (6) million cells | Placebo (0.9% sodium chloride; 0.5% human serum albumin) | |
| Standard medical therapy | Cardiologist | IC | CD34+ | BM aspiration (**), immunomagnetic selection to isolate CD34+ cells | Normal saline | Range 1.0 to 6.1 million cells | No additional therapy (control) | |
| Standard medical therapy | Cardiologist | IC | CD34+ | BM aspiration (**), immunomagnetic selection to isolate CD34+ cells | Saline and human serum albumin | 56 (23) million cells | Placebo (saline; human serum albumin) | |
| Standard medical therapy | Cardiologist | IM | CD133+ | n/r | n/r | n/r | Placebo (n/r) | |
| CABG | Cardiothoracic surgeon | IM | BMMNC | BM aspiration (**) | Heparinised saline | 521 (44) million cells | Placebo (saline solution) | |
| Standard medical therapy | Cardiologist | IC | BMMNC | BM aspiration (**) | Heparinised saline | 72 million cells | Placebo (0.9% sodium chloride containing heparin) | |
| CABG | Cardiothoracic surgeon | IM | BMMNC | BM aspiration (**) | Heparinised saline | 659 (512) million cells | Placebo (saline) | |
| **BM aspiration ‐ bone marrow aspiration and isolation of bone marrow mononuclear cells by gradient centrifugation. ALDH: aldehyde dehydrogenase | ||||||||
| Study ID | Primary outcomes | Secondary outcomes | ||||||||||||||||||||
| All‐cause mortality | Non‐fatal MI | Hospital readmission for HF | Composite MACEa | Arrhythmias | NYHA class | CCS class | Angina frequency | Exercise tolerance | Quality of life | LVEFb | ||||||||||||
| ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | |
| FR | NR | PR* | NR | NR | NR | NR | NR | PR* | NR | PR | NR | PR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | NR | FR | NR | FR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | FR | NR | FR | FR | FR | NR | FR | NR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| PR* | FR | NR | NR | NR | FR | NR | NR | PR | PR | PR | NR | NR | NR | NR | NR | FR | NR | PR | NR | FR | NR | |
| NR | FR | NR | NR | NR | NR | NR | NR | PR* | NR | FR | FR | NR | NR | NR | NR | FR | FR | NR | NR | FR | FR | |
| PR* | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | NR | NR | FR | FR | |
| PR* | PR* | PR* | FR | PR* | PR* | PR* | FR | FR | FR | FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | PR | PR | |
| PR* | PR* | PR* | PR* | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | PR | PR | |
| PR* | PR* | NR | PR* | NR | FR | PR* | FR | NR | NR | NR | PR | NR | NR | NR | NR | FR | FR | FR | FR | NR | PR | |
| PR* | FR | NR | PR* | NR | PR* | PR* | FR | NR | NR | NR | PR | NR | NR | NR | NR | FR | FR | FR | FR | NR | PR | |
| FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| PR* | FR | FR | FR | PR* | FR | NR | NR | NR | NR | FR | FR | NR | NR | NR | NR | FR | FR | NR | NR | FR | FR | |
| FR | FR | PR* | NR | NR | NR | FR | NR | PR* | FR | NR | NR | NR | NR | NR | NR | FR | NR | NR | NR | FR | FR | |
| FR | NR | PR* | NR | NR | NR | PR | NR | FR | NR | NR | NR | PR | NR | PR | NR | PR | NR | PR | NR | PR | NR | |
| PR* | PR* | PR* | PR* | NR | NR | NR | NR | FR | FR | NR | NR | FR | NR | FR | NR | FR | NR | PR | NR | NR | NR | |
| FR | FR | NR | FR | NR | FR | NR | PR | NR | NR | NR | NR | PR | PR | FR | NR | FR | FR | FR | FR | NR | NR | |
| FR | NR | PR* | NR | FR | NR | NR | NR | FR | NR | PR | NR | PR | NR | PR | NR | PR | NR | PR | NR | FR | NR | |
| FR | NR | PR* | NR | FR | NR | FR | NR | PR* | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| FR | NR | PR* | NR | PR* | NR | FR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | FR | NR | NR | NR | PR | NR | PR | NR | FR | NR | |
| PR* | FR | NR | NR | NR | NR | NR | NR | PR* | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR | PR | |
| NR | FR | NR | NR | NR | FR | NR | NR | NR | PR* | NR | FR | NR | PR | NR | NR | NR | NR | NR | PR | PR | PR | |
| NR | PR* | NR | PR* | NR | FR | NR | NR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | PR | NR | FR | |
| PR* | NR | PR* | NR | NR | NR | NR | NR | PR* | NR | FR | NR | FR | NR | NR | NR | NR | NR | FR | NR | FR | NR | |
| FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | FR | NR | FR | NR | NR | NR | FR | NR | NR | NR | FR | NR | |
| PR* | NR | FR | NR | NR | NR | NR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | FR | NR | NR | NR | NR | NR | NR | PR* | PR* | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | |
| PR* | FR | NR | NR | NR | NR | NR | NR | FR | NR | PR | NR | NR | NR | NR | NR | PR | NR | NR | NR | FR | NR | |
| NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | NR | NR | NR | NR | FR | FR | NR | NR | FR | FR | |
| PR* | FR | FR | NR | NR | NR | NR | NR | PR* | NR | FR | NR | FR | NR | NR | NR | FR | NR | NR | NR | FR | NR | |
| PR* | PR* | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| FR | NR | PR* | NR | NR | NR | NR | NR | PR* | NR | NR | NR | FR | NR | NR | NR | FR | NR | FR | NR | FR | FR | |
| PR* | NR | PR* | NR | NR | NR | NR | NR | PR* | NR | NR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | |
| PR* | NR | PR* | NR | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | |
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR | NR | NR | NR | NR | NR | PR | NR | NR | NR | FR | NR | |
| PR* | NR | NR | NR | NR | NR | NR | NR | PR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| PR* | NR | FR | NR | FR | NR | NR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR | NR | |
| FR | NR | PR* | NR | NR | NR | NR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| Total (%) analysedc | 1637 (85.8) | 1010 (53.0) | 881 (46.2) | 461 (24.2) | 482 (25.3) | 495 (26.0) | 288 (15.1) | 201 (10.5) | 959 (50.3) | 363 (19.0) | 741 (38.9) | 346 (18.1) | 608 (31.9) | 142 (7.4) | 428 (22.4) | 82 (4.3)d | 535 (28.1) | 227 (11.9) | 197 (10.3)e | 151 (7.9)e | 439 (23.0)f | 110 (5.8)f |
| CCS: Canadian Cardiovascular Society; FR: full reporting, outcome included in analysis; HF: heart failure; LT: long‐term follow‐up (≥ 12 months); LVEF: left ventricular ejection fraction; MACE: major adverse clinical events; MI: myocardial infarction; NR: outcome not reported; NYHA: New York Heart Association; PR: partial reporting with insufficient information on outcome reported for inclusion in analysis; PR*: no incidence of outcome observed; ST: short‐term follow‐up (< 12 months) aComposite measure of mortality, reinfarction, or rehospitalisation for heart failure. | ||||||||||||||||||||||
| Study ID | Number of analysed participants | All‐cause mortality events | Non‐fatal MI events | Hospital readmission for HF | Composite MACEa | Arrhythmia events | |||||||||||
| Cells | No cells | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | |
| 42 | 19 | 1 | 1 | 6 mthsa | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 52 | 23 | 0 | 1 | 3 mths | 1 | 0 | 3 mths | 1 | 1 | 3 mths | 1 | 1 | 3 mths | 0 | 1 | 3 mths | |
| 43 | 39 | 6 | 8 | 45.7 (17) mths | 1 | 4 | 45.7 (17) mths | 8 | 13 | 45.7 (17) mths | 14 | 19 | 45.7 (17) mths | 6 | 13 | 45.7 (17) mths | |
| 21 | 15 | 1 | 2 | 24 mths | n/r | n/r | n/r | 6 | 4 | 24 mths | n/r | n/r | n/r | n/r | n/r | n/r | |
| 22 | 23 | 2 | 4 | 12 mths | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 13 | 12 | 0 | 1 | 15 mths | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 15 | 15 | 0 | 0 | 12 mths | 1 | 0 | 12 mths | 0 | 0 | 12 mths | 1 | 0 | 12 mths | 1 | 1 | 12 mths | |
| 15 | 15 | 0 | 0 | 12 mths | 0 | 0 | 12 mths | 1 | 1 | 12 mths | 1 | 1 | 12 mths | 0 | 1 | 12 mths | |
| 19 | 10 | 0 | 0 | 12 mths | 0 | 0 | 12 mths | 0 | 1 | 12 mths | 0 | 1 | 12 mths | n/r | n/r | n/r | |
| 19 | 11 | 1 | 1 | 12 mths | 0 | 0 | 12 mths | 0 | 0 | 12 mths | 1 | 1 | 12 mths | n/r | n/r | n/r | |
| 11 | 12 | 1 | 1 | 4 mths | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 23 | 9 | 0 | 1 | 60 mths | 1 | 2 | 60 mths | 0 | 2 | 60 mths | n/r | n/r | n/r | n/r | n/r | n/r | |
| 31 | 29 | 1 | 2 | 12 mths | 0 | 0 | 6 mths | n/r | n/r | n/r | 3 | 4 | 6 mths | 1 | 0 | 12 mths | |
| 19 | 9 | 1 | 1 | 6 mths | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 1 | 1 | 6 mths | |
| 18 | 6 | 0 | 0 | 12 mths | 0 | 0 | 12 mths | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 1 | 12 mths | |
| 112 | 56 | 0 | 3 | 12 mths | 6 | 7 | 12 mths | 3 | 4 | 12 mths | n/r | n/r | n/r | n/r | n/r | n/r | |
| 40 | 20 | 1 | 1 | 6 mths | 0 | 0 | 6 mths | 6 | 2 | 6 mths | n/r | n/r | n/r | 3 | 1 | 6 mths | |
| 14 | 2 | 0 | 1 | 6 mths | 0 | 0 | 6 mths | 1 | 0 | 6 mths | 1 | 1 | 6 mths | 0 | 0 | 6 mths | |
| 10 | 8 | 0 | 3 | 6 mths | 0 | 0 | 6 mths | 0 | 0 | 6 mths | 0 | 3 | 6 mths | 2 | 2 | 6 mths | |
| 30 | 30 | 1 | 3 | 34 mthsb | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 25 | 25 | 3 | 10 | 10 yrs | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 22 | 6 | 5 | 2 | 12 mths | n/r | n/r | n/r | 2 | 0 | 12 mths | n/r | n/r | n/r | 0 | 0 | 12 mths | |
| 13c | 17c | 0 | 0 | Median 60 mths | 0 | 0 | Median 60 mths | 1 | 1 | Median 60 mths | n/r | n/r | n/r | n/r | n/r | n/r | |
| 20 | 10 | 0 | 0 | 6 mths | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 61 | 31 | 1 | 0 | 6 mths | 1 | 0 | 6 mths | 3 | 5 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | |
| 10 | 10 | 0 | 0 | 6 mths | 1 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 3 | 2 | 6 mths | |
| 55 | 54 | 6 | 21 | 12 mths | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 12 mths | |
| 19 | 9 | 0 | 2 | 23 (8) mths | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | 1 | 1 | 6 mths | |
| 15 | 15 | 2 | 4 | Median 5 yrs | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 19 | 9 | 0 | 1 | 19 (9) mths | 0 | 1 | 3 mths | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 38 | 18 | 0 | 0 | 12 mths | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 25 | 25 | 1 | 0 | 6 mths | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 16 | 16 | 0 | 0 | 6 mths | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 56 | 56 | 0 | 0 | 6 mths | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 0 | 1 | 6 mthsd | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 45 | 45 | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 24 | 23 | 0 | 0 | 6 mths | 0 | 1 | 6 mths | 1 | 2 | 6 mths | n/r | n/r | n/r | 0 | 0 | 6 mths | |
| 18 | 18 | 2 | 0 | 6 mths | 0 | 0 | 6 mths | n/r | n/r | n/r | n/r | n/r | n/r | 1 | 0 | 6 mths | |
| HF: heart failure; MACE: major adverse clinical events; MI: myocardial infarction; n/r: not reported aAng 2008: participants followed up for six months; mortality reported as “death within 30 days of treatment”. | |||||||||||||||||
| Study ID | Periprocedural adverse events |
| 2 deaths (1 control, 1 intracoronary cell therapy) occurred within 30 days of treatment. Reasons were not given, but neither was considered to be related to cell therapy. | |
| In‐hospital events: MI occurred in 1 CPC participant and ventricular arrhythmia detected during monitoring in 1 control participant. | |
| n/r (only safety of shockwave procedure reported) | |
| In the cell therapy group, 1 participant had ventricular tachycardia during procedure which was resolved by cardioversion, and 1 participant had blurred vision after intervention (participant had pre‐existing ophthalmic migraines). Other reported adverse events (gastrointestinal, hepatobiliary, respiratory, thoracic, mediastinal, and peripheral vascular disorders) were not considered to be related to cell therapy. | |
| 3 participants in cell therapy group experienced a transient episode of pulmonary oedema during the injection of stem cells. No sustained arrhythmias were monitored during the procedure. | |
| 1 cell therapy and 1 control participant reported headache, and 1 control participant developed fever during G‐CSF stimulation. G‐CSF resulted in comparable increases in serum C‐reactive protein levels and blood leukocyte count in both CPC and control groups (returned to baseline values within 4 days after G‐CSF). Neither G‐CSF injection nor intracoronary transplantation of CPC caused any elevation in troponin T levels. | |
| n/r | |
| n/r | |
| No participant had significant postprocedural pericardial effusion. Small transient increases in CK‐MB and serum troponin I were observed. There were no treatment emergent serious adverse events among any of participants who received cell therapy. | |
| No participant had significant postprocedural pericardial effusion. Small transient increases in CK‐MB and serum troponin I were observed. There were no treatment emergent serious adverse events among any of participants who received cell therapy. | |
| 1 cell therapy participant died on postoperative day 7 from a perforated oesophageal ulcer complicated by mediastinitis. 1 control participant died on the 5th postoperative day from multiorgan failure secondary to low cardiac output syndrome. | |
| Mild cephalgies and episodes of mild to moderate bone and muscular pain were reported during 5‐day course of G‐CSF. No participant developed chest pain episodes or clinical signs of decompensated HF. No novel ischaemia‐related ECG changes were observed during G‐CSF treatment and after intracoronary CPC infusion. Troponin T levels remained unchanged. Moreover, no specific G‐CSF‐mediated severe complications occurred. Intracoronary infusions were successfully performed without any procedural complications. | |
| 2 participants (unclear which treatment arm) had neurological complications but recovered and were discharged. No participants had arrhythmia. | |
| G‐CSF treatment was well tolerated, all participants presented bone pain as the only symptom. After cell injection, none of the participants had a significant rise in creatine phosphokinase, symptoms, ECG changes, or echocardiographic abnormalities. | |
| 13 participants reported transient increase in angina frequency after administration of G‐CSF. There were no cardiac enzyme elevations, MIs, acute coronary syndromes, or deaths. 1 participant in the placebo group developed ventricular tachycardia during the mapping procedure. No arrhythmias were detected by implantable cardioverter defibrillator, LifeVest, or Holter monitoring in any participant during or after the injection procedure. | |
| Administration of G‐CSF was associated with bone pain (20.1%), angina (17.4%), CHF (2 participants), and 8 participants had troponin elevations consistent with non‐STEMI. In 1 participant a thrombus was observed on the mapping catheter tip as it was removed. 2 participants experienced an apparent myocardial perforation during the injection procedure (1 resulted in haemothorax, which was successfully treated; 1 resulted in cardiac tamponade; this participant died after unsuccessful pericardiocentesis procedure). Elevated troponin levels were observed in 28% of participants at some point during the mobilisation and injection period, all of which were minor and subclinical except for those mentioned above. | |
| 1 participant with a history of episodic ventricular tachycardia developed ventricular tachycardia during the NOGA mapping procedure. Another participant experienced double vision and dizziness during the injection procedure; cerebral‐CT afterwards was normal, but the incident was diagnosed as a minor stroke by the neurologist. 1 participant from the treatment group suffered a stroke 12 days after treatment. | |
| The most common side effects from G‑CSF were bone pain (22%) and low grade pyrexia (65%) (reported in all G‐CSF groups combined). Bleeding from the arterial access site did not differ significantly between the 2 intervention arms. All episodes were minor and resolved with conservative treatment within 24 h of the procedure. As expected, there were increases in troponin and creatine kinase levels postprocedure in both arms. | |
| The most common side effects from G‑CSF were bone pain (22%) and low grade pyrexia (65%) (reported in all G‐CSF groups combined). There were 3 cases of arrhythmia during the intramyocardial procedure that required treatment. Of these, 1 participant developed atrial fibrillation, which reverted to sinus rhythm within 24 h of the procedure. Another participant developed transient complete heart block periprocedure requiring temporary pacing only. The final participant suffered an episode of pulseless ventricular tachycardia following intramyocardial injection, which was successfully cardioverted with a single 200 J external defibrillation and remained haemodynamically stable afterwards. 1 participant died from suspected acute LV failure 6 days after discharge. Bleeding from the arterial access site did not differ significantly between the two intervention arms. All episodes were minor and resolved with conservative treatment within 24 h of the procedure. As expected, there were increases in troponin and creatine kinase levels postprocedure in both arms. | |
| 2 participants in the placebo group died early postoperatively: 1 died on day 8 after developing Candida sepsis following LV failure despite intra‐aortic balloon pump and catecholamine treatment and mechanical assist device implantation, and 1 died on day 22 (reason not given). | |
| 1 participant in the OPCAB plus stem cell therapy group had a haematoma at the bone marrow harvest site. There were no other adverse events in either group (i.e. neurologic, haematologic, vascular, death, or infection events). No participants had any postoperative arrhythmias. | |
| 5 participants who received BMAC experienced “non‐serious adverse events possibly related to the procedure”. Procedure‐related complications included haematomas at the catheterisation site and elevated serum creatinine levels. | |
| There were no differences between treatment groups in participants’ haemodynamics, arterial blood gases, systemic vein oxygen level, blood glucose, acid–base balance, lactate, haemoglobin, body temperature, and diuresis, as well as medications needed. Perioperative measures are reported in detail in Lehtinen 2014. | |
| No perforations or arrhythmias were associated with cell injection procedures. Postprocedural transient left bundle‐branch block (resolved in 24 h) was seen in 1 treated and 1 control participant. 1 treated participant had non‐significant pericardial effusion. No sustained ventricular arrhythmias were observed by Holter monitoring in any participant. Transient fever but no sepsis occurred in 1 control participant. | |
| 1 participant experienced a limited retrograde catheter‐related dissection of the abdominal aorta (withdrawn from study). 1 participant experienced recurrent ventricular tachycardia with hypotension (and received only a small volume of cell product). | |
| No major adverse clinical cardiac events were associated with the cell injection procedures, including no perforations. Electromechanical mapping–related ventricular tachycardia occurred in 2 control participants, and ventricular fibrillation occurred in 1 control participant. No deaths occurred, and HF was not exacerbated in any participant. Holter monitoring showed no sustained ventricular arrhythmia in any participant. | |
| No periprocedural complications occurred in participants who received cell therapy. 2‐dimensional echocardiography did not reveal postprocedural pericardial effusion. Creatine kinase activity and peak troponin T level remained unaltered. No new periprocedural arrhythmias were recorded during 24 h of consecutive electrocardiographic monitoring. An implantable cardioverter defibrillator was implanted to 2 participants with ventricular tachycardia prior to cell injections. | |
| There were no acute procedural‐related complications, including stroke, transient ischaemic attack, ECG changes, sustained ventricular or atrial arrhythmias, and elevation of CPK‐MB. There was also no echocardiographic evidence of pericardial effusion in any participant within the first 24 h of the procedure. | |
| The early postoperative course was uneventful in both groups with no significant differences between them with regard to adverse side effects during hospital stay. There were no significant differences in cardiac‐specific enzymes activities after the operation or the number of atrial fibrillation episodes or appearance of pericardial effusion between the groups. | |
| There were no acute procedure‐related complications, including stroke, transient ischaemic attack, ECG changes, sustained ventricular or atrial arrhythmias, elevation of CPK‐MB, or echocardiographic evidence of pericardial effusion within the first 24 h after the procedure. | |
| There was no inflammatory response or myocardial reaction (white blood cell count, C‐reactive protein, CK, troponin) after cell therapy. There were no immediate pre‐ or postprocedure adverse complications, new electrocardiographic changes, or significant elevations in CK or troponin, and no inflammatory response was observed in participants with bone marrow cell transplant. | |
| In the placebo group, a greater than 0.5‐centimetre pericardial effusion was detected on 2‐dimensional echocardiography in an asymptomatic participant 2 days after the injection procedure, and pericardiocentesis was subsequently performed. | |
| No periprocedural adverse events; cardiac proteins in normal range. | |
| No increase in angina frequency or usage of sublingual NTG was observed in participants of either group. There were no cardiac enzyme elevations, MIs, acute coronary syndromes, or deaths. No participants from either group developed ventricular tachycardia during the cell or saline infusion procedure. No arrhythmias were detected by Holter monitoring in any participant during or after the infusion process. | |
| n/r | |
| Predischarge arrhythmias were reported (as number of events) in both cell therapy and control participants. | |
| Intracoronary application of BMC was performed without any acute or long‐term side effects. There was no inflammatory response or myocardial reaction (i.e. white blood cell count, C‐reactive protein, and creatinine phosphokinase) after cell therapy. | |
| In the perioperative period, sporadic ventricular premature beats and self terminating bouts of rapid atrial fibrillation were observed in both groups. However, 2 participants developed VF, and 1 died in the BMMNC group: 1 participant developed VF on the 5th day postoperatively but was successfully resuscitated and VF well‐controlled, and the other developed refractory VF 5 hours' postoperatively with death on postoperative day 3. There were no ventricular arrhythmias in the control group. | |
| AMI: acute myocardial infarction | |
| Study ID | No. analysed participants | Performance assessment | Mean follow‐up | No. analysed participants | Quality of life assessment | Mean follow‐up | ||||
| Cells | No cells | ST | LT | Cells | No cells | ST | LT | |||
| 21 | 21 | NYHA class (SR)a | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 21 | 21 | CCS class (SR)b | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 43 | 18 | NYHA class (EP) | 3 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 43 | 39 | NYHA class (EP/MC) | 4 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 21 | 15 | NYHA class (SR)c | 6 mths | n/r | 21 | 15 | MLHFQ (SR)c | 6 mths | n/r | |
| 21 | 15 | 6MWT (distance) (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 22d | 23d | NYHA class (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 22d | 23d | ETT (METs) (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 12 | 10 | Bike test (max O2 update) (EP) | 3 mths | 15 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 15 | 15 | NYHA class (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 15 | 15 | CCS class (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 15 | 15 | NYHA class (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 15 | 15 | CCS class (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 17 | 16 | NYHA class (SR)e | n/r | 12 mths | 15 | 19 | MLHFQ (MC) | 6 mths | 12 mths | |
| 15f | 19f | 6MWT (distance) (MC) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 17 | 16 | NYHA class (SR)e | n/r | 12 mths | 19g | 19g | MLHFQ (MC) | 6 mths | 12 mths | |
| 18h | 19h | 6MWT (distance) (MC) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 21j | 10j | NYHA class (EP) | 3 mths | 60 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 12k | 5k | Bike test (sec) (EP) | 3 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 30 | 27 | 6MWT (distance) (EP/MC) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 19 | 9 | CCS class (median)m | 6 mths | n/r | n/r | n/r | SAQ (median)m | 6 mths | n/r | |
| 15 | 7 | ETT (time; METs) (median)m | 6 mths | n/r | 19 | 9 | Angina frequency (median)n | 6 mths | n/r | |
| 18 | 6 | CCS class (MC) | 6 mths | n/r | 18 | 6 | SAQ (SR)p | 6 mths | n/r | |
| 18 | 6 | ETT (time) (MC) | 6 mths | n/r | 17 | 6 | Angina frequency (EP/MC) | 6 mths | n/r | |
| 109q | 53q | CCS class (SR)r | 6 mths | 12 mths | 109q | 53q | SAQ (MC) | 6 mths | 12 mths | |
| 109q | 53q | ETT (time) (MC) | 6 mths | 12 mths | 109 | 53 | Angina frequency (EP) | 6 mths | n/r | |
| 40 | 40 | NYHA class (SR)s | 6 mths | n/r | 40 | 40 | KCCQ‐QOL (SR)s | 6 mths | n/r | |
| 40 | 40 | CCS class (SR)s | 6 mths | n/r | 40 | 40 | SAQ (SR)s | 6 mths | n/r | |
| 40 | 40 | 6MWT (SR)s | 6 mths | n/r | 40 | 40 | Angina frequency (SR)s | 6 mths | n/r | |
| 14 | 2 | NYHA class (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 14 | 2 | CCS class (SR) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 10 | 8 | NYHA class (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 10 | 8 | CCS class (SR) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 28 | 26 | NYHA class (EP/MC)t | 6 mths | n/r | 28 | 26 | MLHFQu | 6 mths | n/r | |
| 28 | 26 | 6MWTu | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 28 | 26 | CCS class (EP/MC)t | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 10 | 10 | NYHA class (EP/MC)t | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 17 | 4 | NYHA class (EP)t | n/r | 12 mths | 17 | 4 | MLHFQ (SR) | n/r | 12 mths | |
| 17 | 4 | CCS class (SR) | n/r | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 20 | 19 | NYHA class (EP/MC) | n/r | 12 mthsv | 20 | 19 | SF‐36w | n/r | 60 mths | |
| 20 | 10 | NYHA class (EP) | 6 mths | n/r | 17 | 9 | MLHFQ (EP) | 6 mths | n/r | |
| 20 | 10 | CCS class (EP/MC) | 6 mths | n/r | 13 | 10 | SF‐36 (physical/mental) (EP) | 6 mths | n/r | |
| 55 | 30 | NYHA class (MC) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 44 | 22 | CCS class (MC) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 51 | 29 | 6MWT (distance) (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 10 | 10 | NYHA class (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 10 | 10 | CCS class (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 53x | 46x | NYHA class (EP) | 6 mths | 12 mths | 53x | 46x | MLHFQ (EP) | 6 mths | 12 mths | |
| 53x | 46x | CCS class (EP) | 6 mths | 12 mths | 53x | 46x | Angina frequency (EP) | 6 mths | 12 mths | |
| 53x | 46x | 6MWT (distance) (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 19 | 9 | NYHA class (EP)y | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 19 | 9 | 6MWT (distance) (EP)y | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 15 | 15 | NYHA class (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 15 | 15 | 6MWT (distance) (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 19 | 9 | NYHA class (EP)t | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 19 | 9 | CCS class (EP)t | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 19 | 9 | Treadmill test (time; METs) (EP/MC) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 33 | 16 | NYHA class (EP) | 6 mths | 12 mths | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 24 | 25 | CCS class (EP) | 6 mths | n/r | 24 | 25 | SAQ (EP/MC) | 6 mths | n/r | |
| 24 | 25 | Bike test (workload) (EP/MC) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 16 | 16 | CCS class (MC) | 6 mths | n/r | 16 | 16 | Angina frequency (MC) | 6 mths | n/r | |
| 16 | 16 | ETT (min) (MC) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 56 | 56 | CCS class (EP/MC) | 6 mths | n/r | 56 | 56 | Angina frequency (EP/MC) | 6 mths | n/r | |
| 56 | 56 | ETT (min) (EP/MC) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| n/r | n/r | NYHA class (SR) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| n/r | n/r | 5MWT (distance) (SR) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 16 | 18 | NYHA class (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 16 | 18 | CCS class (EP) | 6 mths | n/r | ‐ | ‐ | ‐ | ‐ | ‐ | |
| CCS: Canadian Cardiovascular Society; EP: endpoint; ETT: exercise tolerance test; KCCQ‐QOL: Kansas City Cardiomyopathy Questionnaire – Quality of Life; LT: long term; MC: mean change from baseline; MET: metabolic equivalent test (mL/kg/min); MLHFQ: Minnesota Living with Heart Failure Questionnaire; n/r: not reported; NYHA: New York Heart Association; SAQ: Seattle Angina Questionnaire; SF‐36: 36‐Item Short Form Health Survey; SR: summary results; ST: short term; 5MWT: 5‐minute walk test; 6MWT: 6‐minute walk test aReported as number of participants in NYHA class III/IV. | ||||||||||
| Study ID | No. randomised participants | No. analysed participants | Baseline LVEF: Mean (SD) | Mean follow‐up of LVEF | ||||
| Cells | No cells | Cells | No cells | Cells | No cells | ST | LT | |
| Measured by MRI | ||||||||
| 42 | 21 | 18 | 7 | IM: 25.4 (8.1) IC: 28.5 (6.5) | 20.9 (8.9) | 6 mths | ‐ | |
| 43 | 39 | 15 | 12 | n/r | n/r | 4 mths | ‐ | |
| 14 | 14 | 12a | 11a | 51.0 (12.1) | 55.8 (12.4) | 3 mths | 15 mths | |
| 11 | 12 | 10 | 10 | 42.9 (10.3) | 39.5 (5.5) | 4 mths | ‐ | |
| 23 | 10 | 9 | 4 | 33.4 (SEM 12.7) | 23.3 (SEM 7.2) | 3 mths | 12 mths | |
| 31 | 29 | 31b | 28b | 23.5 (6.7) | 24.8 (5.2) | 6 mths | 12 mths | |
| 40 | 20 | 40 | 20 | 28.2 (9.3) | 25.1 (8.5) | 6 mths | ‐ | |
| 30 | 30 | 26 | 22 | 27 (6) | 26 (6) | 6 mths | ‐ | |
| 20 | 19 | 18 | 7 | 37.1 (9.5) | 38.5 (13.5) | ‐ | 60 mths | |
| 19 | 9 | 19 | 9 | 23.6 (8.4) | 26.8 (8.8) | 6 mths | ‐ | |
| 19 | 9 | 18 | 8 | 51.9 (8.5) | 45.7 (8.3) | 6 mths | ‐ | |
| 25 | 25 | 22 | 18 | 56 (12) | 54 (10) | 6 mths | ‐ | |
| 35 | 35 | 35 | 35 | 29 (7) | 28 (6) | 6 mths | ‐ | |
| Measured by echocardiography | ||||||||
| 32 | 15 | 21 | 15 | 27.5 (95% CI 25.5, 29.5) | 27.8 (95% CI 25.9, 29.8) | 6 mths | ‐ | |
| 31 | 29 | 24 | 18 | 36.0 (1.2) | 34.7 (1.4) | ‐ | 12 mths | |
| 20 | 10 | 20 | 10 | 37.0 (10.6) | 39.0 (9.1) | 6 mths | ‐ | |
| 61 | 31 | 54 | 28 | 34.7 (8.8) | 32.3 (8.6) | 6 mths | ‐ | |
| 10 | 10 | 10 | 10 | 36.1 (10.9) | 32.1 (10.6) | 6 mths | ‐ | |
| 55 | 54 | 53c | 46c | 27.8 (3.4) | 26.8 (3.8) | 6 mths | 12 mths | |
| 15 | 15 | 15 | 15 | 35.3 (3.9) | 36.5 (5.3) | 6 mths | 12 mths | |
| 25 | 25 | 24 | 25 | 50 (5) | 52 (5) | 6 mths | ‐ | |
| 45 | 45 | 45 | 45 | 39.3 (6.2) | 38.2 (8.0) | 6 mths | ‐ | |
| 18 | 18 | 16 | 18 | 35.8 (7.3) | 36.7 (9.2) | 6 mths | ‐ | |
| Measured by SPECT | ||||||||
| 24 | 24 | 22d | 23d | 26 (6) | 23 (8) | 6 mths | 12 mths | |
| 20 | 10 | 20 | 10 | 41.5 (11.2) | 43.0 (10.4) | 6 mths | ‐ | |
| 25 | 25 | 24 | 25 | 53 (12) | 54 (12) | 6 mths | 12 mths | |
| Measured by LV angiography | ||||||||
| 52 | 23 | 43 | 18 | BMMNC: 41 (11) CPC: 39 (10) | 43 (13) | 3 mths | ‐ | |
| 43 | 39 | 41 | 38 | LDSW: 37.2 (95% CI 31.7, 42.7) HDSW: 32.4 (95% CI 26.9, 37.9) | LDSW: 29.9 (95% CI 24.0, 35.7) HDSW: 32.3 (95% CI 26.5, 38.1) | 4 mths | ‐ | |
| 23 | 10 | 21 | 5 | 37.5 (SEM 12.9) | 37.6 (SEM 7.5) | 3 mths | ‐ | |
| 20 | 10 | 20 | 10 | 37.5 (8.2) | 40.0 (3.2) | 6 mths | ‐ | |
| 10 | 10 | 10 | 10 | 38.0 (17.5) | 41.9 (11.8) | 6 mths | ‐ | |
| 38 | 18 | 33 | 16 | 46 (10) | 46 (10) | 3 mths | 12 mths | |
| 95% CI: 95% confidence interval; BMMNC: bone marrow mononuclear cells; CPC: circulating progenitor cells; HDSW: high‐dose shockwave; IC: intracoronary; IM: intramyocardial; LDSW: low‐dose shockwave; LT: long term; LV: left ventricular; LVEF: left ventricular ejection fraction; SD: standard deviation; SEM: standard error of the mean; SPECT: single‐photon emission computed tomography; ST: short term a12/10 at 15 months. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 37 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short term follow‐up (< 12 months) | 33 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.87] |
| 1.2 Long term follow‐up (≥ 12 months) | 21 | 1010 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.25, 0.58] |
| 2 Non‐fatal myocardial infarction Show forest plot | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Short term follow‐up (< 12 months) | 20 | 881 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.15] |
| 2.2 Long term follow‐up (≥ 12 months) | 9 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.17, 0.93] |
| 3 Rehospitalisation due to heart failure Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Short term follow‐up (< 12 months) | 10 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.12] |
| 3.2 Long term follow‐up (≥ 12 months) | 10 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.36, 1.04] |
| 4 Arrhythmias Show forest plot | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Short term follow‐up (< 12 months) | 22 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.33, 1.45] |
| 4.2 Long term follow‐up (≥ 12 months) | 7 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.22, 0.97] |
| 5 Composite MACE Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Short term follow‐up (< 12 months) | 8 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.42] |
| 5.2 Long term follow‐up (≥ 12 months) | 5 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.41, 1.12] |
| 6 MLHFQ: short term follow‐up (< 12 months) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Mean value at endpoint | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐29.52 [‐33.76, ‐25.27] |
| 6.2 Mean change from baseline | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐9.07 [‐22.09, 3.95] |
| 6.3 Combined | 4 | 197 | Mean Difference (IV, Random, 95% CI) | ‐18.96 [‐31.97, ‐5.94] |
| 7 MLHFQ: long term follow‐up (≥ 12 months) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Mean value at endpoint | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐36.5 [‐42.21, ‐30.79] |
| 7.2 Mean change from baseline | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐7.63 [‐16.35, 1.09] |
| 7.3 Combined | 3 | 151 | Mean Difference (IV, Random, 95% CI) | ‐17.80 [‐39.87, 4.26] |
| 8 Seattle Angina Questionnaire: short term follow‐up (< 12 months) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Mean value at endpoint | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐3.21, 13.21] |
| 8.2 Mean change from baseline | 2 | 211 | Mean Difference (IV, Random, 95% CI) | 9.34 [2.62, 16.07] |
| 8.3 Combined | 2 | 211 | Mean Difference (IV, Random, 95% CI) | 9.34 [2.62, 16.07] |
| 9 Angina episodes per week: short term follow‐up (< 12 months) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Mean value at endpoint | 4 | 396 | Mean Difference (IV, Random, 95% CI) | ‐6.96 [‐11.99, ‐1.93] |
| 9.2 Mean change from baseline | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐14.61, 11.08] |
| 9.3 Combined | 5 | 428 | Mean Difference (IV, Random, 95% CI) | ‐5.11 [‐11.30, 1.09] |
| 10 NYHA classification: short‐term follow‐up (< 12 months) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Mean value at endpoint | 16 | 658 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.84, ‐0.00] |
| 10.2 Mean change from baseline | 4 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.49, 0.36] |
| 10.3 Combined | 17 | 741 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.84, ‐0.05] |
| 11 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Mean value at endpoint | 9 | 346 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.03, ‐0.10] |
| 11.2 Mean change from baseline | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐2.2 [‐2.70, ‐1.70] |
| 11.3 Combined | 9 | 346 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.23, ‐0.39] |
| 12 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Mean value at endpoint | 10 | 486 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.82, 0.18] |
| 12.2 Mean change from baseline | 6 | 318 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.40, 0.17] |
| 12.3 Combined | 13 | 608 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 13 CCS class: long term follow‐up (≥ 12 months) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Mean value at endpoint | 3 | 142 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐2.04, 0.88] |
| 14 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Mean value at endpoint | 11 | 563 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.93] |
| 14.2 Mean change from baseline | 9 | 535 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.05, 0.61] |
| 15 Exercise capacity: long term follow‐up (≥ 12 months) Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Mean value at endpoint | 5 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 1.14 [0.04, 2.25] |
| 15.2 Mean change from baseline | 3 | 227 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.07, 0.62] |
| 16 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Mean value at endpoint | 10 | 352 | Mean Difference (IV, Random, 95% CI) | 3.01 [‐0.05, 6.07] |
| 16.2 Mean change from baseline | 9 | 308 | Mean Difference (IV, Random, 95% CI) | 4.05 [2.55, 5.55] |
| 16.3 Combined | 12 | 439 | Mean Difference (IV, Random, 95% CI) | 2.92 [1.03, 4.82] |
| 17 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Mean value at endpoint | 4 | 110 | Mean Difference (IV, Random, 95% CI) | 2.37 [‐1.54, 6.29] |
| 17.2 Mean change from baseline | 3 | 97 | Mean Difference (IV, Random, 95% CI) | 3.83 [‐0.42, 8.08] |
| 17.3 Combined | 4 | 110 | Mean Difference (IV, Random, 95% CI) | 4.38 [0.82, 7.93] |
| 18 LVEF (%) measured by echocardiography: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Mean value at endpoint | 8 | 388 | Mean Difference (IV, Random, 95% CI) | 5.16 [2.87, 7.44] |
| 18.2 Mean change from baseline | 3 | 161 | Mean Difference (IV, Random, 95% CI) | 3.47 [1.59, 5.34] |
| 18.3 Combined | 9 | 470 | Mean Difference (IV, Random, 95% CI) | 5.71 [4.29, 7.13] |
| 19 LVEF (%) measured by echocardiography: long term follow‐up (≥ 12 months) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Mean value at endpoint | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 7.69 [6.47, 8.92] |
| 19.2 Mean change from baseline | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 6.1 [‐1.27, 13.47] |
| 19.3 Combined | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 7.96 [6.39, 9.54] |
| 20 LVEF (%) measured by SPECT: short term follow‐up (< 12 months) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Mean value at endpoint | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 2.41 [‐2.65, 7.46] |
| 20.2 Mean change from baseline | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.3 [‐17.33, 12.73] |
| 20.3 Combined | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 5.22 [2.60, 7.85] |
| 21 LVEF (%) measured by SPECT: long term follow‐up (≥ 12 months) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Mean value at endpoint | 2 | 88 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐2.30, 3.04] |
| 21.2 Mean change from baseline | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐6.48, 14.48] |
| 21.3 Combined | 2 | 88 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐2.48, 3.03] |
| 22 LVEF (%) measured by LV angiography: short term follow‐up (< 12 months) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Mean value at endpoint | 6 | 265 | Mean Difference (IV, Random, 95% CI) | 3.18 [0.39, 5.97] |
| 22.2 Mean change from baseline | 4 | 181 | Mean Difference (IV, Random, 95% CI) | 1.72 [0.50, 2.95] |
| 22.3 Combined | 6 | 250 | Mean Difference (IV, Random, 95% CI) | 2.00 [0.53, 3.46] |
| 23 LVEF (%) measured by LV angiography: long term follow‐up (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 Mean value at endpoint | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.0 [0.81, 11.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 107 cells | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.63] |
| 1.2 107 < 108 cells | 18 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.79] |
| 1.3 ≥ 108 cells | 8 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.35, 1.94] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 107 cells | 4 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.10, 1.09] |
| 2.2 107 < 108 cells | 7 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.53] |
| 2.3 ≥ 108 cells | 5 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.30, 1.26] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 < 107 cells | 4 | 149 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.94, 0.36] |
| 3.2 107 < 108 cells | 8 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.22, ‐0.08] |
| 3.3 ≥ 108 cells | 4 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.11] |
| 4 CCS class: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 < 107 cells | 4 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.92, 0.19] |
| 4.2 107 < 108 cells | 5 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.40, 0.32] |
| 5 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 107 < 108 cells | 7 | 357 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.03, 1.14] |
| 5.2 ≥ 108 cells | 3 | 161 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.10, 0.77] |
| 6 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 107 < 108 cells | 7 | 199 | Mean Difference (IV, Random, 95% CI) | 5.23 [3.91, 6.54] |
| 6.2 ≥ 108 cells | 3 | 101 | Mean Difference (IV, Random, 95% CI) | 2.37 [‐0.92, 5.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 30% | 11 | 508 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.09, 0.59] |
| 1.2 30 ‐ 50% | 13 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.36, 2.11] |
| 1.3 > 50% | 4 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.35] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 30% | 9 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.64] |
| 2.2 30 ‐ 50% | 7 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.21] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 < 30% | 6 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.22, 0.43] |
| 3.2 30 ‐ 50% | 9 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.54, ‐0.10] |
| 4 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 < 30% | 5 | 202 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.28, ‐0.04] |
| 4.2 30 ‐ 50% | 4 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.72, ‐0.25] |
| 5 CCS class: short term follow‐up (< 12 months) Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 < 30% | 4 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.47, 0.97] |
| 5.2 30 ‐ 50% | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.31, 0.09] |
| 6 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 < 30% | 4 | 225 | Std. Mean Difference (IV, Random, 95% CI) | 0.96 [0.37, 1.56] |
| 6.2 30 ‐ 50% | 3 | 127 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.57, 1.33] |
| 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 < 30% | 6 | 290 | Mean Difference (IV, Random, 95% CI) | 1.54 [‐1.96, 5.03] |
| 7.2 30 ‐ 50% | 3 | 60 | Mean Difference (IV, Random, 95% CI) | 3.31 [0.88, 5.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Intramyocardial | 22 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.03] |
| 1.2 Intracoronary | 12 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.23] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Intramyocardial | 13 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.50] |
| 2.2 Intracoronary | 8 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.30, 1.09] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Intramyocardial | 11 | 445 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.99, 0.03] |
| 3.2 Intracoronary | 6 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.76, 0.00] |
| 4 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Intramyocardial | 4 | 181 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.76, ‐0.41] |
| 4.2 Intracoronary | 5 | 165 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.92, ‐0.30] |
| 5 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Intramyocardial | 10 | 434 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.87, 0.22] |
| 5.2 Intracoronary | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.87, 0.86] |
| 6 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Intramyocardial | 6 | 310 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [0.19, 1.36] |
| 6.2 Intracoronary | 5 | 253 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.06, 0.72] |
| 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Intramyocardial | 8 | 309 | Mean Difference (IV, Random, 95% CI) | 2.18 [‐0.41, 4.77] |
| 7.2 Intracoronary | 5 | 137 | Mean Difference (IV, Random, 95% CI) | 3.72 [0.86, 6.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mononuclear cells | 20 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.28, 1.04] |
| 1.2 Circulating progenitor cells | 3 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.48] |
| 1.3 Haematopoietic progenitor cells | 8 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.05, 1.46] |
| 1.4 Mesenchymal stem cells | 3 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.03, 7.59] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mononuclear cells | 12 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.25, 0.70] |
| 2.2 Haematopoietic progenitor cells | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.10, 0.69] |
| 2.3 Mesenchymal stem cells | 3 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.57] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Mononuclear cells | 12 | 547 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.86, 0.02] |
| 3.2 Haematopoietic progenitor cells | 3 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.95, 1.02] |
| 4 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Mononuclear cells | 8 | 366 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.99, 0.21] |
| 4.2 Haematopoietic progenitor cells | 5 | 242 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.55, 0.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Chronic IHD | 11 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.26, 1.62] |
| 1.2 HF (secondary to IHD) | 15 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.14, 0.82] |
| 1.3 Refractory/intractable angina | 7 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.35] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Chronic IHD | 9 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 0.99] |
| 2.2 HF (secondary to IHD) | 9 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.19, 0.58] |
| 2.3 Refractory/intractable angina | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.91] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Chronic IHD | 6 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.78, ‐0.07] |
| 3.2 HF (secondary to IHD) | 10 | 417 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.02, 0.09] |
| 4 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Chronic IHD | 3 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.91, ‐0.42] |
| 4.2 HF (secondary to IHD) | 6 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.47, ‐0.37] |
| 5 CCS class: short term follow‐up (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 HF (secondary to IHD) | 8 | 363 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.90, 0.40] |
| 5.2 Refractory/intractable angina | 5 | 245 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.44, ‐0.11] |
| 6 Exercise capacity: short term follow‐up (< 12 months) Show forest plot | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Chronic IHD | 4 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.26, 1.22] |
| 6.2 HF (secondary to IHD) | 4 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.04, 1.53] |
| 6.3 Refractory/intractable angina | 3 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.03, 0.55] |
| 7 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Chronic IHD | 6 | 178 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐0.16, 5.31] |
| 7.2 HF (secondary to IHD) | 4 | 195 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐1.97, 6.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause): short term follow‐up (< 12 months) Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Co‐interventions | 8 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.32, 1.70] |
| 1.2 No co‐interventions | 25 | 1205 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.72] |
| 2 Mortality (all‐cause): long term follow‐up (≥ 12 months) Show forest plot | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Co‐interventions | 6 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.88] |
| 2.2 No co‐interventions | 15 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.19, 0.56] |
| 3 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Co‐interventions | 6 | 233 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.20, 0.05] |
| 3.2 No co‐interventions | 11 | 508 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.87, 0.13] |
| 4 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Co‐interventions | 5 | 179 | Mean Difference (IV, Random, 95% CI) | 2.01 [‐0.26, 4.29] |
| 4.2 No co‐interventions | 7 | 260 | Mean Difference (IV, Random, 95% CI) | 3.55 [0.82, 6.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short term follow‐up (< 12 months) | 14 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.32, 1.50] |
| 1.2 Long term follow‐up (≥ 12 months) | 9 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.87] |
| 2 Non‐fatal myocardial infarction Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Short term follow‐up (< 12 months) | 6 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.58] |
| 2.2 Long term follow‐up (≥ 12 months) | 5 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.97] |
| 3 Rehospitalisation due to heart failure Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Short term follow‐up (< 12 months) | 3 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.32, 1.32] |
| 3.2 Long term follow‐up (≥ 12 months) | 6 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 4 Arrhythmias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Short term follow‐up (< 12 months) | 6 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.18, 3.21] |
| 4.2 Long term follow‐up (≥ 12 months) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 0.99] |
| 5 Composite MACE Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Short term follow‐up (< 12 months) | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Long term follow‐up (≥ 12 months) | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.08] |
| 6 NYHA classification: short term follow‐up (< 12 months) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Combined | 5 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 7 NYHA classification: long term follow‐up (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Combined | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐2.2 [‐2.70, ‐1.70] |
| 8 LVEF (%) measured by MRI: short term follow‐up (< 12 months) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Combined | 7 | 249 | Mean Difference (IV, Random, 95% CI) | 2.92 [0.67, 5.17] |
| 9 LVEF (%) measured by MRI: long term follow‐up (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Combined | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.70, 5.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short term follow‐up (< 12 months) | 25 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.29, 1.16] |
| 1.2 Long term follow‐up (≥ 12 months) | 13 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.21, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (all‐cause) Show forest plot | 32 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short term follow‐up (< 12 months) | 28 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 1.2 Long term follow‐up (≥ 12 months) | 17 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |

