Antibióticos para el líquido amniótico teñido con meconio en el trabajo de parto para la prevención de las infecciones maternas y neonatales
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007772.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 06 noviembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Thitiporn Siriwachirachai and Ussanee Sangkomkamhang drafted the review, Pisake Lumbiganon and Malinee Laopaiboon revised and approved the final version of the review.
Sources of support
Internal sources
-
Khon Kaen Hospital, Ministry of Public Health, Thailand.
-
Faculty of Medicine, Khon Kaen University, Thailand.
-
Faculty of Public Health, Khon Kaen University, Thailand.
External sources
-
Thailand Research Fund, Senior Research Scholar, Thailand.
-
Thai Cochrane Network, Thailand.
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser. We thank Erika Ota and Nancy Medley for help in preparing the 'Summary of findings' table.
Erika Ota and Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Nov 06 | Antibiotics for meconium‐stained amniotic fluid in labour for preventing maternal and neonatal infections | Review | Thitiporn Siriwachirachai, Ussanee S Sangkomkamhang, Pisake Lumbiganon, Malinee Laopaiboon | |
| 2010 Dec 08 | Antibiotics for meconium‐stained amniotic fluid in labour for preventing maternal and neonatal infections | Review | Thitiporn Siriwachirachai, Ussanee S Sangkomkamhang, Pisake Lumbiganon, Malinee Laopaiboon | |
| 2009 Apr 15 | Antibiotics for meconium‐stained amniotic fluid in labour for preventing neonatal sepsis | Protocol | Thitiporn Siriwachirachai, Ussanee S Sangkomkamhang, Pisake Lumbiganon, Malinee Laopaiboon | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Amniotic Fluid;
- *Labor, Obstetric;
- *Meconium;
- Ampicillin [therapeutic use];
- Anti‐Bacterial Agents [*therapeutic use];
- Chorioamnionitis [*prevention & control];
- Endometritis [prevention & control];
- Intensive Care Units, Neonatal [statistics & numerical data];
- Randomized Controlled Trials as Topic;
- Sepsis [prevention & control];
- Sulbactam [therapeutic use];
Medical Subject Headings Check Words
Female; Humans; Infant, Newborn; Pregnancy;
PICO

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
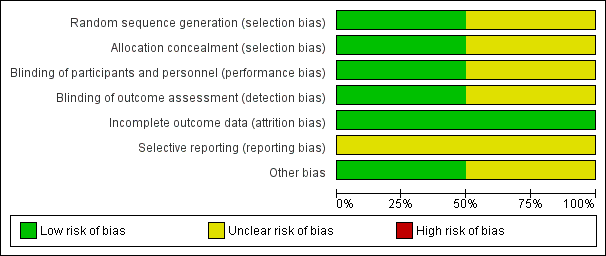
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Antibiotic versus placebo, Outcome 1 Neonatal sepsis.

Comparison 1 Antibiotic versus placebo, Outcome 2 Chorioamnionitis.
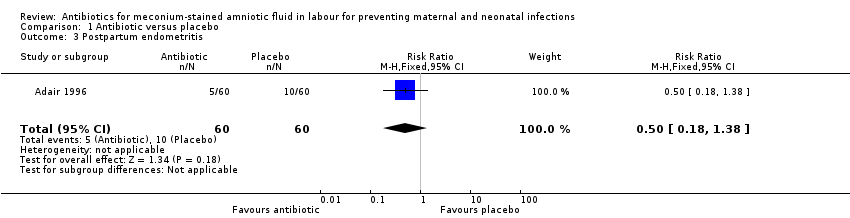
Comparison 1 Antibiotic versus placebo, Outcome 3 Postpartum endometritis.

Comparison 1 Antibiotic versus placebo, Outcome 4 Neonatal intensive care admissions.
| Antibiotic versus placebo for meconium‐stained amniotic fluid in labour for preventing maternal and neonatal infections | ||||||
| Population: Women with meconium‐stained amniotic fluid in labour, gestational age more than 24 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus placebo | |||||
| Neonatal sepsis | Study population | RR 1 | 120 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 50 per 1000 | |||||
| Moderate | ||||||
| 50 per 1000 | 50 per 1000 | |||||
| Chorioamnionitis | Study population | RR 0.36 | 362 | ⊕⊕⊕⊝ | ||
| 240 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 86 per 1000 | |||||
| Postpartum endometritis | Study population | RR 0.5 | 120 | ⊕⊕⊝⊝ | ||
| 167 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 167 per 1000 | 84 per 1000 | |||||
| Mortality and morbidity prior to discharge (Neonatal intensive care admissions) | Study population | RR 0.83 | 120 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 166 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 166 per 1000 | |||||
| Side effects of treatment | Not estimable | 0 (0 study) | See comment | This outcome was not reported in any of the included studies. | ||
| Duration of admission to neonatal intensive care unit | Not estimable | 0 (0 study) | See comment | This outcome was not reported in any of the included studies. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, small sample size and few events (‐2). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal sepsis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.76] |
| 2 Chorioamnionitis Show forest plot | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.21, 0.62] |
| 3 Postpartum endometritis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.18, 1.38] |
| 4 Neonatal intensive care admissions Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.39, 1.78] |

