Antibióticos para el líquido amniótico teñido con meconio en el trabajo de parto para la prevención de las infecciones maternas y neonatales
Resumen
Antecedentes
Es más probable que se presente corioamnionitis cuando existe líquido amniótico teñido con meconio (LATM). El meconio puede estimular el crecimiento de las bacterias en el líquido amniótico al servir como factor de crecimiento e inhibir las propiedades bacteriostáticas del líquido amniótico. Muchos resultados neonatales adversos relacionados con el LATM llevan al síndrome de aspiración de meconio (SAM). El LATM se asocia con infecciones maternas y neonatales. Los antibióticos pueden ser una opción eficaz para reducir la morbilidad.
Objetivos
El objetivo de esta revisión es evaluar la eficacia y los efectos secundarios de los antibióticos profilácticos para el LATM durante el trabajo de parto en la prevención de las infecciones maternas y neonatales.
Métodos de búsqueda
Se hicieron búsquedas en el Registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (30 de septiembre de 2014).
Criterios de selección
Ensayos controlados aleatorizados (ECA) que compararon antibióticos profilácticos con placebo o ningún tratamiento durante el trabajo de parto para mujeres con LATM.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron los ensayos para la inclusión y el riesgo de sesgo, extrajeron los datos y verificaron su exactitud.
Resultados principales
Se incluyeron dos estudios con 362 embarazadas. Ambos compararon ampicilina‐sulbactam (N = 183) versus suero fisiológico normal (N = 179) en embarazadas con LATM. Los antibióticos profilácticos no parecieron resultar en ninguna reducción estadísticamente significativa de la incidencia de sepsis neonatal (riesgo relativo [RR] 1,00; IC del 95%: 0,21 a 4,76), el ingreso a la unidad de cuidados intensivos neonatales (UCIN) (RR 0,83; IC del 95%: 0,39 a 1,78) y la endometritis posparto (RR 0,50; IC del 95%: 0,18 a 1,38). Sin embargo, se observó una disminución significativa en el riesgo de corioamnionitis (RR 0,36; IC del 95%: 0,21 a 0,62). No se informaron efectos adversos graves. No se informó acerca de la resistencia al fármaco, la duración de la ventilación mecánica y la duración del ingreso en la UCIN/hospital. La mayoría de los dominios de riesgo de sesgo tuvieron un riesgo de sesgo bajo para un estudio y un riesgo de sesgo incierto para el otro estudio. La calidad de la evidencia con el uso de GRADE fue baja para la sepsis neonatal, la endometritis posparto y la mortalidad y morbilidad neonatal (ingreso en la unidad de cuidados intensivos neonatales) y moderada para la corioamnionitis.
Conclusiones de los autores
La evidencia actual indica que, comparados con placebo, los antibióticos para el LATM en el trabajo de parto pueden disminuir la incidencia de corioamnionitis. No hubo ninguna evidencia de que los antibióticos pudieran reducir la endometritis posparto, la sepsis neonatal ni el ingreso a la UCIN. Esta revisión sistemática identifica la necesidad de ECA mejor diseñados y con una potencia estadística suficiente para evaluar el efecto de los antibióticos profilácticos en la incidencia de las complicaciones maternas y neonatales.
PICO
Resumen en términos sencillos
Antibióticos para el líquido amniótico teñido con meconio en el trabajo de parto para la prevención de las infecciones maternas y neonatales
El líquido amniótico teñido con meconio (LATM) es el resultado de los desechos materiales del colon fetal que pasan a la cavidad amniótica de la madre. Su incidencia aumenta en los embarazos postérmino. Las embarazadas con LATM tienen mayor probabilidad de presentar complicaciones maternas, incluida la inflamación de las membranas fetales causadas por una infección bacteriana (corioamnionitis), la inflamación posparto del recubrimiento del útero (endometritis), y complicaciones neonatales, como sepsis neonatal y la necesidad de ingreso a una unidad de cuidados intensivos neonatales (UCIN). La hipoxia o el estrés fetales pueden provocar una respiración jadeante en el feto, lo que resulta en la aspiración del meconio.
Esta revisión se basó en dos estudios controlados aleatorizados identificados (con 362 mujeres) y se halló que los antibióticos profilácticos podrían reducir el riesgo de infección intraamniótica en mujeres con LATM (evidencia de calidad moderada). El uso de antibióticos no redujo claramente la sepsis neonatal (evidencia de calidad baja), el ingreso en la UCIN (evidencia de calidad baja) ni la endometritis posparto (evidencia de calidad baja).. Se necesitarían estudios con un número mayor de embarazadas con LATM para examinar estos temas.
Authors' conclusions
Summary of findings
| Antibiotic versus placebo for meconium‐stained amniotic fluid in labour for preventing maternal and neonatal infections | ||||||
| Population: Women with meconium‐stained amniotic fluid in labour, gestational age more than 24 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus placebo | |||||
| Neonatal sepsis | Study population | RR 1 | 120 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 50 per 1000 | |||||
| Moderate | ||||||
| 50 per 1000 | 50 per 1000 | |||||
| Chorioamnionitis | Study population | RR 0.36 | 362 | ⊕⊕⊕⊝ | ||
| 240 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 86 per 1000 | |||||
| Postpartum endometritis | Study population | RR 0.5 | 120 | ⊕⊕⊝⊝ | ||
| 167 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 167 per 1000 | 84 per 1000 | |||||
| Mortality and morbidity prior to discharge (Neonatal intensive care admissions) | Study population | RR 0.83 | 120 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 166 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 166 per 1000 | |||||
| Side effects of treatment | Not estimable | 0 (0 study) | See comment | This outcome was not reported in any of the included studies. | ||
| Duration of admission to neonatal intensive care unit | Not estimable | 0 (0 study) | See comment | This outcome was not reported in any of the included studies. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, small sample size and few events (‐2). | ||||||
Background
Description of the condition
Meconium‐stained amniotic fluid (MSAF), as a result of the passage of fetal colonic contents into the amniotic cavity, occurs in approximately 12% of all deliveries (Cleary 1998). The incidence of intrapartum MSAF ranges from 7% to 22% for a term pregnancy but this figure increases to up to 40% in a post‐term pregnancy (Katz 1992). The composition of meconium from a term fetus is primarily water (70% to 80%). Other constituents include mucopolysaccharides, cholesterol and its precursors, proteins, lipids, bile acids and salts (giving the characteristic green colour), pancreatic enzymes, interleukin‐8, phospholipase A2, squamous cells, and vernix caseosa (white substance coating the skin of newborn babies) (Cleary 1998; Usta 2000).
MSAF may act directly and indirectly on exposed tissue. Its effects depend on the concentration of meconium, duration of exposure, and the presence of associated stress factors (hypoxia, infection). MSAF has long been associated with potentially adverse fetal outcomes including meconium aspiration syndrome (MAS), admission to neonatal intensive care unit (NICU), neonatal sepsis, cerebral palsy, seizure and pulmonary diseases (Berkus 1994; Katz 1992; Nathan 1994). Many adverse neonatal outcomes related to MSAF result from MAS. MAS occurs in 5% of the cases of MSAF and more than 4% of infants with MAS die, accounting for 2% of all perinatal deaths (Cleary 1998; Wiswell 1990). Hypoxia is the key factor that triggers gasping fetal respirations, which results in the aspiration of meconium. Most cases of MAS probably result from in utero aspiration rather than aspiration at the time of delivery. In addition to possibly contributing to respiratory distress in the neonate, MSAF has been associated with a higher risk of neonatal infection (Romero 1991). Chorioamnionitis is a risk factor for neonatal sepsis, which results in NICU admissions and potential fetal morbidity and death (Alexander 1999). Fetal microbial invasion has been proposed to cause inflammatory brain damage through the effects of elevated cytokines (e.g. tumour necrosis factor (TNF) alpha, IL‐1 beta,and IL‐6) (Hoskins 1987).
Chorioamnionitis is also more likely to occur when MSAF is present (Mazor 1995; Romero 1991; Usta 2000). The risk of clinical chorioamnionitis and histological chorioamnionitis in patients with intrapartum MSAF is significantly higher than those with clear fluid. The risk for clinically diagnosed endometritis is two‐fold (Markovitch 1993; Mazor 1995). Intrapartum chorioamnionitis is associated with dystocia and increased risk for operative delivery (Casey 1997; Mark 2000). Unrecognised or under‐treated chorioamnionitis can lead to postpartum endomyometritis, which can result in further maternal morbidity, and increased length of stay in hospital and hospital costs. MSAF is the risk factor for microbial invasion of the amniotic cavity in patients with intact membranes and preterm labour (Romero 1991). Maternal infection is also more likely in the presence of MSAF. Patients with MSAF were almost two and a half times as likely to develop postoperative endometritis (Josephson 1984). There are statistically significant associations between MSAF and puerperal infection in term deliveries (Piper 1998). Puerperal infection rates are associated with the degree of meconium staining, with rates rising as meconium thickness increases (Tran 2003). There is a three‐fold increase in positive amniotic fluid cultures in patients with MSAF compared to those with clear amniotic fluid (Mazor 1995; Romero 1991).The most common amniotic fluid isolates in MSAF are anaerobes, Ureaplasma urealyticum, Streptococci, Escherichia coli, Candida albicans and Listeria monocytogenes (Mazor 1995; Romero 1991).
Meconium may enhance the growth of bacteria in amniotic fluid by serving as a growth factor, inhibiting bacteriostatic properties of amniotic fluid, or antagonising host defence systems, thus increasing the risk of chorioamnionitis. Generally, amniotic fluid is a poor culture medium for Escherichia coli, Listeria monocytogenes and Staphylococcus aureus; however, with enough meconium, amniotic fluid becomes an excellent culture medium (Florman 1969). Meconium may alter the zinc‐to‐phosphorous ratio in amniotic fluid and facilitate bacterial growth and decrease host defences (Hoskins 1987). Light and very light MSAF significantly impair mechanisms for intracellular microbial killing. The phagocytic ability of neutrophils (a type of white blood cell) was also significantly diminished in the presence of moderate MSAF (Clark 1995). Mechanisms of meconium‐associated puerperal infections include altering the antibacterial properties of amniotic fluid and enhancing bacterial growth, impairing the host immune response through the inhibition of phagocytosis and neutrophil oxidative burst (Clark 1995; Katz 1992).
Description of the intervention
One study has shown a significant reduction in the rate of clinical chorioamnionitis when the intervention ampicillin‐sulbactam was administered prophylactically for the indication of MSAF (Edwards 1999).
How the intervention might work
Antibiotics can be bacteriostatic (they stop bacteria from multiplying) or bactericidal (they kill the bacteria). To perform either of these functions, antibiotics must be brought into contact with the bacteria. Antibiotics are thought to interfere with the surfactant of bacteria cells, causing a change in their ability to reproduce (Heizmann 2007). Gentamicin is an aminoglycoside antibiotic with bactericidal activity that acts at the 30S bacterial ribosomal subunit, inhibiting the synthesis of bacterial proteins (Ward 2008).
Why it is important to do this review
Cochrane reviews have addressed a number of issues about MAS including steroid therapy, endotracheal intubation, surfactant and antibiotics for neonates (Halliday 2001; Shivananda 2006; Ward 2003). Other interventions include amnioinfusion for MSAF in labour (Hofmeyr 2014). Prophylactic intravenous intrapartum ampicillin‐sulbactam therapy or cefazolin infusion into the amniotic cavity during amnioinfusion in mothers with MSAF did not show any benefit in reducing chorioamnionitis, endometritis and neonatal sepsis (Adair 1996; Edwards 1999). However, the role of antibiotics for MSAF during labour has not been systematically evaluated.
Objectives
The objective of this review is to assess the efficacy and side effects of prophylactic antibiotics for meconium‐stained amniotic fluid during labour in preventing maternal and neonatal infections.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of prophylactic antibiotic administration during labour for women with MSAF. We excluded quasi‐RCTs. Conference abstracts were considered eligible for inclusion.
Types of participants
Pregnant women with a gestational age of more than 22 weeks who were in labour and had MSAF.
Types of interventions
Systemic prophylactic antibiotics started during labour in women with MSAF compared with no treatment or placebo.
Types of outcome measures
The primary outcomes were the most clinically important for the neonate, whereas the secondary outcomes also included maternal and neonatal complications.
Primary outcomes
-
Neonatal sepsis.
(Definition of sepsis as defined by authors.)
Secondary outcomes
Maternal
-
Intrapartum chorioamnionitis.
-
Postpartum endometritis.
-
Side effects of treatment, e.g. drug allergy, anaphylactic shock.
-
Drug resistance.
Neonatal
-
Mortality and morbidity prior to discharge, e.g. birth asphyxia, intracranial haemorrhage, intraventricular haemorrhage, necrotising enterocolitis and admission to neonatal intensive care unit (NICU).
-
Duration of mechanical ventilation (days).
-
Duration of admission to NICU/hospital.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version of this review, please refer to Siriwachirachai 2010.
For this update (2014), the following methods were used for full 'Risk of bias' assessment and assessment of the quality of evidence using the GRADE approach.
Selection of studies
Thitiporn Siriwachirachai (TS) and Ussanee Sangkomkamhang (US) independently assessed trials for inclusion and methodological; quality. There were no disagreements.
Data extraction and management
We designed a form to extract data. For eligible studies, TS and US independently extracted the data using the agreed form. There were no discrepancies. We entered the data into Review Manager software (RevMan 2014) and checked for accuracy. We did not contact the original study authors because the reported information was sufficient in the report.
Assessment of risk of bias in included studies
TS and US independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
(1) Random sequence generation (checking for possible selection bias)
We described for the one included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for the included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that the study would be at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for the included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for the included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether the one included study was at high risk of bias, according to the criteria given in the Cochrane Handbook of Reviews of Intervention (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following the outcomes.
-
Neonatal sepsis
-
Intrapartum chorioamnionitis.
-
Postpartum endometritis.
-
Side effects of treatment, e.g. drug allergy, anaphylactic shock.
-
Mortality and morbidity prior to discharge, e.g. birth asphyxia, intracranial haemorrhage, intraventricular haemorrhage, necrotising enterocolitis and admission to NICU.
-
Duration of admission to NICU/hospital.
Although, we could not assess some outcomes listed due to lack of data, we were able to asses neonatal sepsis, chorioamnionitis, and postpartum endometritis.
GRADE profiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. In future updates, if more studies are included a summary of the intervention effect and a measure of quality for each of the above outcomes will be produced using the GRADE approach.
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios (RR) with 95% confidence intervals (CIs) .
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measure the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included only one randomised controlled trial (RCT). In future updates, if we identify cluster‐randomised trials, we will include these in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2009). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and we consider the interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For the included study, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis; i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in the included trial is the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
This review did not include meta‐analysis. In future updates, as more data become available, we will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if a Tau² is greater than zero and either an I² is greater than 30% or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In subsequent updates of this review, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). This review only included one RCT so we did not pool any data. In future updates, if more data become available, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates of this review, when sufficient data become available, we plan to carry out the following subgroup analyses:
-
intact versus rupture membrane;
-
single versus combine antibiotic regimens;
-
duration of antibiotics less than 24 hours versus more than 24 hours.
We will use the following outcomes in subgroup analysis:
-
early onset neonatal sepsis (symptomatic before 72 hours of age);
-
late onset neonatal sepsis (symptomatic after 72 hours of age).
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In subsequent updates we also plan to conduct a sensitivity analysis to compare the results using all studies and using only those of high methodological quality, i.e. comparing studies at low risk of bias versus high risk of bias.
Results
Description of studies
Results of the search
We identified four publications as potentially eligible for inclusion in this review.
Included studies
This review includes two RCT (Adair 1996; Adair 1999) in which 362 women were randomised and the results analysed; seeCharacteristics of included studies.
Excluded studies
We assessed and excluded two retrospective cohort studies (Adair 1998; Edwards 1999); seeCharacteristics of excluded studies.
Risk of bias in included studies
We have summarised the risk of bias of the included studies (Adair 1996; Adair 1999) in Figure 1 and Figure 2.
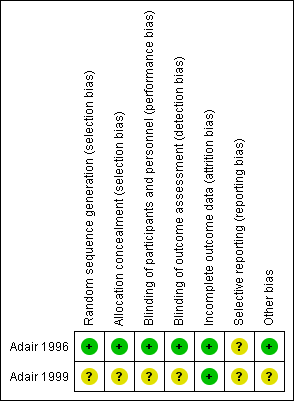
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
One trial reported clear information on allocation concealment. The randomisation schedule was generated and kept in an area away from the clinical area and was unavailable to caregivers (Adair 1996). Another trial, published as a conference abstract contained little information and so it was unclear how randomisation and allocation concealment had been performed (Adair 1999).
Blinding
Adair 1996 reported participants and all caregivers were thoroughly blinded until the study was completed. Interventions were identically prepared in 100 mL fluid bags and issued by one of two research nurses, independent to the trial investigators. The outcome assessors were also blinded to the randomisation status. The other trial report did not contain enough information to be able tell whether blinding had been undertaken (Adair 1999).
Incomplete outcome data
Two trials (Adair 1996; Adair 1999) reported no withdrawals. Adair 1996 reported analysis based on intention‐to‐treat basis.
Selective reporting
We do not have access to this study protocol; therefore we could not evaluate this risk of bias.
Other potential sources of bias
None apparent in one study (Adair 1996); and unclear in the other study published in abstract form (Adair 1999).
Effects of interventions
Results are based on two randomized controlled trials ( 362 women).
Antibiotic versus placebo
Primary outcomes
There was no significant reduction in the incidence of neonatal sepsis (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.21 to 4.76; one study; 120 women), seeAnalysis 1.1. Adair 1996 did not report their results in terms of early and late onset neonatal sepsis.
Secondary outcomes
There was a significant reduction in the incidence of chorioamnionitis in the ampicillin‐sulbactam group compared with placebo (RR 0.36, 95% CI 0.21 to 0.62; two studies; 362 women), seeAnalysis 1.2. There was no significant reduction in the incidence of endometritis (RR 0.50, 95% CI 0.18 to 1.38; one study; 120 women), seeAnalysis 1.3 or neonatal intensive care unit (NICU) admission (RR 0.83, 95% CI 0.39 to 1.78; one study; 120 women), seeAnalysis 1.4.
No serious adverse effects were reported. Drug resistance, duration of mechanical ventilation and duration of admission to NICU/hospital were not reported
Discussion
Summary of main results
There was a significant reduction in the incidence of chorioamnionitis in mothers who received ampicillin‐sulbactam compared to placebo. Neonatal sepsis was not differentiated into 'early' or 'late' onset but there was no difference in the incidence of neonatal sepsis between the two groups. Endometritis was not statistically reduced. There was no information about adverse effects.
Overall completeness and applicability of evidence
Two randomised controlled trials (RCT) from a developed country were included in this review and they did not report the primary outcome 'neonatal sepsis' in terms of early or late onset. The evidence may be insufficient to evaluate the efficacy and side effects of prophylactic antibiotics for meconium‐stained amniotic fluid in labour for preventing neonatal sepsis.
Quality of the evidence
Most of the domains for risk of bias were at low risk of bias for one study (Adair 1996) and at unclear risk of bias for the other study (Adair 1999). The quality of the evidence using GRADE was low for neonatal sepsis, postpartum endometritis, and neonatal mortality and morbidity prior to discharge (Neonatal intensive care admissions) and of moderate quality for chorioamnionitis. No serious adverse effects were reported. Drug resistance, duration of mechanical ventilation and duration of admission to NICU/hospital were not reported.
Potential biases in the review process
We followed the process of review as recommended in the Cochrane Handbook for Systematic Reviews of InterventionsHiggins 2009. We also did an exhaustive search which included many clinical trial registries.
Agreements and disagreements with other studies or reviews
There are no other reviews and studies related to the efficacy and side effects of prophylactic antibiotics for MSAF during labour in preventing maternal and neonatal infections.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
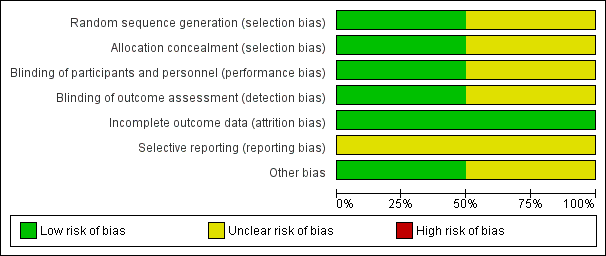
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Antibiotic versus placebo, Outcome 1 Neonatal sepsis.

Comparison 1 Antibiotic versus placebo, Outcome 2 Chorioamnionitis.
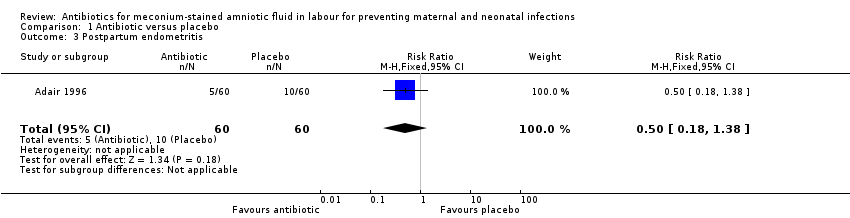
Comparison 1 Antibiotic versus placebo, Outcome 3 Postpartum endometritis.

Comparison 1 Antibiotic versus placebo, Outcome 4 Neonatal intensive care admissions.
| Antibiotic versus placebo for meconium‐stained amniotic fluid in labour for preventing maternal and neonatal infections | ||||||
| Population: Women with meconium‐stained amniotic fluid in labour, gestational age more than 24 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus placebo | |||||
| Neonatal sepsis | Study population | RR 1 | 120 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 50 per 1000 | |||||
| Moderate | ||||||
| 50 per 1000 | 50 per 1000 | |||||
| Chorioamnionitis | Study population | RR 0.36 | 362 | ⊕⊕⊕⊝ | ||
| 240 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 86 per 1000 | |||||
| Postpartum endometritis | Study population | RR 0.5 | 120 | ⊕⊕⊝⊝ | ||
| 167 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 167 per 1000 | 84 per 1000 | |||||
| Mortality and morbidity prior to discharge (Neonatal intensive care admissions) | Study population | RR 0.83 | 120 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 166 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 166 per 1000 | |||||
| Side effects of treatment | Not estimable | 0 (0 study) | See comment | This outcome was not reported in any of the included studies. | ||
| Duration of admission to neonatal intensive care unit | Not estimable | 0 (0 study) | See comment | This outcome was not reported in any of the included studies. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, small sample size and few events (‐2). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal sepsis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.76] |
| 2 Chorioamnionitis Show forest plot | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.21, 0.62] |
| 3 Postpartum endometritis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.18, 1.38] |
| 4 Neonatal intensive care admissions Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.39, 1.78] |

