Antibióticos para el líquido amniótico teñido con meconio en el trabajo de parto para la prevención de las infecciones maternas y neonatales
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized trial with allocation concealment using computer‐generated randomization list. All participants, caregivers and outcome assessors were blinded to the treatment regimen. | |
| Participants | Intervention group: 60 pregnant women (mean age 24.5, SD 6.3) with gestational age more than 24 weeks (mean 39.8, SD 1.0). Control group: 60 pregnant women (mean age 25.9, SD 6.3), (mean gestational age 39.9, SD 1.2). Inclusion criteria: gestational age more than 24 weeks with MSAF complicating the intrapartum. Exclusion criteria: patients with penicillin and/or cephalosporin allergy, evidence of active infection, presence of intrauterine death, GA < 24 weeks, or history of antibiotics use in 7 days. Location: North Carolina, United States. | |
| Interventions | Intervention: ampicillin‐sulbactam 3.0 g intravenous prepared in 100 ml fluid bags, and was repeated every 6 hours until delivery. Control: normal saline infused as an IV bolus. | |
| Outcomes | Mother Neonatal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization was performed by a computer‐generated list. |
| Allocation concealment? | Low risk | Adequate: there was randomization by computer‐generated list and both IV preparations were prepared by 1 of 2 research nurses who were not involved in this study. |
| Blinding? | Low risk | Adequate: there was blinding of participants, caregivers and outcome assessor. |
| Incomplete outcome data addressed? | Low risk | Adequate: there was no withdrawal. |
| Free of selective reporting? | Unclear risk | Unclear, because we don't have access to this trial's outcomes. |
| Free of other bias? | Low risk | Study appeared to be free of other sources. |
GA: gestational age
IV: intravenous
MSAF: meconium‐stained amniotic fluid
NICU: neonatal intensive care unit
RCT: randomized controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a RCT, this was a retrospective cohort study. | |
| This is a conference abstract. | |
| Intervention not of interest to systematic review, it is not systematic prophylactic antibiotics. |
RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal sepsis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.76] |
| Analysis 1.1  Comparison 1 Antibiotic versus placebo, Outcome 1 Neonatal sepsis. | ||||
| 2 Chorioamnionitis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.82] |
| Analysis 1.2  Comparison 1 Antibiotic versus placebo, Outcome 2 Chorioamnionitis. | ||||
| 3 Postpartum endometritis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.18, 1.38] |
| Analysis 1.3  Comparison 1 Antibiotic versus placebo, Outcome 3 Postpartum endometritis. | ||||
| 4 Neonatal intensive care admissions Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.39, 1.78] |
| Analysis 1.4  Comparison 1 Antibiotic versus placebo, Outcome 4 Neonatal intensive care admissions. | ||||

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Antibiotic versus placebo, Outcome 1 Neonatal sepsis.

Comparison 1 Antibiotic versus placebo, Outcome 2 Chorioamnionitis.
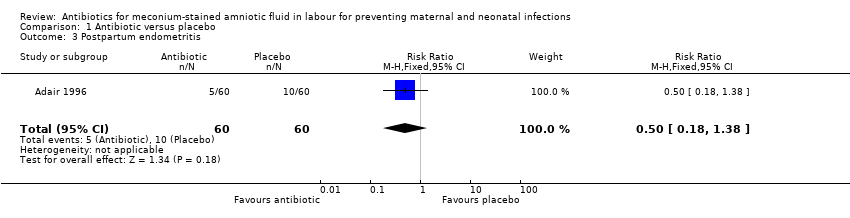
Comparison 1 Antibiotic versus placebo, Outcome 3 Postpartum endometritis.

Comparison 1 Antibiotic versus placebo, Outcome 4 Neonatal intensive care admissions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal sepsis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.76] |
| 2 Chorioamnionitis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.82] |
| 3 Postpartum endometritis Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.18, 1.38] |
| 4 Neonatal intensive care admissions Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.39, 1.78] |

