Intervenções não cirúrgicas para hordéolos internos agudos
Resumo
Introdução
Um hordéolo é uma inflamação comum e dolorosa da borda da pálpebra que geralmente é causada por uma infecção bacteriana. A infecção afeta as glândulas sebáceas da pálpebra e pode ser interna ou externa. Em muitos casos, a lesão drena espontaneamente e se resolve sem tratamento. Porém, a inflamação pode se espalhar para outras glândulas ou tecidos oculares, e as recidivas são comuns. Se não for resolvido, um hordéolo interno agudo pode tornar‐se crônico, ou pode transformar‐se em um calázio. Os hordéolos externos, também conhecido como terçol, não foram incluídos nesta revisão.
Objetivos
O objetivo desta revisão foi investigar a efetividade e, quando possível, a segurança dos tratamentos não cirúrgicos dos hordéolos internos agudos versus conduta expectante ou placebo.
Métodos de busca
Fizemos buscas nas seguintes bases de dados: CENTRAL (que contêm a base Cochrane Eyes and Vision Trials Register (2016; Issue12)), MEDLINE Ovid, MEDLINE Ovid Epub Ahead of Print, MEDLINE Ovid In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Ovid Daily (janeiro 1946 a dezembro 2016), Embase (janeiro 1947 a dezembro 2016), PubMed (1948 a dezembro 2016), Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS (janeiro 1982 a dezembro 2016)), o metaRegister of Controlled Trials (mRCT; www.controlled-trials.com (última pesquisa 26 de julho de 2012)), Clinical Trials.gov(www.clinicaltrials.gov), e World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) ((www.who.int/ictrp/search/en). Não houve restrições de data ou idiomas nas buscas eletrônicas. A data a última busca nas bases de dados foi 2 de dezembro de 2016.
Critério de seleção
Incluímos na revisão ensaios clínicos randomizados (ECR) ou quase randomizados envolvendo pessoas com hordéolo interno agudo. Excluímos os estudos envolvendo pessoas com hordéolos externos (terçol), hordéolos crônicos ou calázio. As intervenções não cirúrgicas de interesse incluíram o uso de compressas quentes ou mornas, esfoliantes palpebrais, antibióticos ou esteroides versus conduta expectante, placebo ou outras intervenções ativas.
Coleta dos dados e análises
Dois revisores, trabalhando de forma independente, avaliaram as referências identificadas pelas buscas eletrônicas para inclusão nesta revisão. Nenhum estudo relevante foi encontrado. As razões para as exclusões foram documentadas.
Principais resultados
Não encontramos nenhum ensaio clínico que pudesse ser incluído na revisão. A maioria das referências identificadas nas buscas eram sobre hordéolos externos ou hordéolos internos crônicos. As poucas referências específicas sobre hordéolos internos agudos relatavam recomendações de tratamento, eram séries de casos intervencionistas, estudos de casos ou outros tipos de desenhos de estudos observacionais, e foram publicados há mais de 20 anos.
Conclusão dos autores
Não encontramos nenhuma evidência a favor ou contra a efetividade de intervenções não cirúrgicas para o tratamento de hordéolos internos. Ensaios clínicos controlados seriam úteis para determinar quais intervenções são efetivas para o tratamento de pessoas com hordéolos internos agudos.
PICO
Resumo para leigos
Intervenções para tratar hordéolos internos agudos
Qual foi o objetivo desta revisão?
O objetivo desta revisão Cochrane foi investigar se intervenções como compressas quentes, medicamentos tópicos de venda livre e esfoliantes palpebrais, antibióticos, esteroides e massagens das pálpebras eram úteis para o tratamento de hordéolos internos (um inchaço no interior da pálpebra). Os pesquisadores da Cochrane procuraram todos os estudos relevantes para responder a esta pergunta e não encontraram nenhum estudo.
Principais achados
Muitos tratamentos comuns estão disponíveis para tratar os hordéolos internos. Atualmente, não existe evidência sobre a eficácias destes tratamentos.
O que foi estudado nesta revisão?
Um hordéolo é um caroço comum e doloroso na pálpebra que normalmente é causado por uma infecção bacteriana. A infecção afeta as glândulas sebáceas das pálpebras e resulta em um caroço. Muitas vezes, o caroço infectado drena e cicatriza por si só, sem tratamento. Porém, as vezes a infecção pode se alastrar para outras glândulas dos olhos, e pode persistir. Ela também pode se transformar em um cisto (conhecido como um calázio). O hordéolo pode ser interno (ficar na parte de dentro da pálpebra), ou externo (na parte de fora da pálpebra, perto dos cílios). O hordéolo na parte externa da pálpebra é conhecido como terçol. Os hordéolos também podem ser agudos (aparecem de repente e curar em pouco tempo), ou crônicos (longa duração). Os tratamentos comuns para hordéolos incluem compressas quentes aplicadas em casa, medicamentos tópicos e esfoliantes para as pálpebras disponíveis livremente em farmácias, antibióticos ou esteroides prescritos por médicos, e massagens na pálpebra.
Quais foram os principais resultados da revisão?
Os pesquisadores da Cochrane procuraram por estudos sobre tratamentos para pessoas com um hordéolo interno agudo. Eles não procuraram por estudos sobre tratamentos para terçol ou hordéolos de longa duração. Não encontramos nenhum estudo relevante que tenha comparado tratamentos. Assim, nenhuma evidência foi encontrada a favor ou contra o uso de qualquer um dos tratamentos comuns para hordéolos.
Quão atualizada é esta revisão?
Os pesquisadores da Cochrane buscaram por todos os estudos disponíveis até 2 de dezembro de 2016.
Authors' conclusions
Background
Description of the condition
A hordeolum is a common inflammation of the eyelid margin. It presents as a red, painful, swollen furuncle with an acute onset, and is usually caused by a staphylococcal infection (Mueller 2008; Peralejo 2008; Skorin 2002). The inflammation can be internal, affecting the meibomian glands, or external, affecting the glands of Zeis or Moll (Wald 2004). External hordeola are more commonly known as styes. In many cases, the lesion drains spontaneously and resolves untreated; however, the inflammation can spread to other ocular glands or tissues (i.e., cellulitis), and recurrences are common. If unresolved, an acute internal hordeolum can become chronic or can develop into a chalazion (De Jesus 2004; Hudson 1981; Mueller 2008; Rubin 1995).
A hordeolum is one of the most common diseases of the eye; therefore, many people can be affected, and many causative factors are known to be related to the disease. Blepharitis (Fuchs 1911; Skorin 2002), acne rosacea (De Jesus 2004), trichiasis, and cicatricial ectropion (Moriarty 1982), are conditions frequently associated with internal hordeola. Incidence rates for hordeola are not available because most cases are not reported. Hordeola tend to occur in younger people, but are not limited to any age, gender, or racial group (Fuchs 1911; Lederman 1999; Roodyn 1954). Internal hordeola tend to be more painful and longer lasting than external hordeola (Barza 1983; Fuchs 1911; Olson 1991; Wilkie 1956). Onset is spontaneous (idiopathic), but may be related to lid hygiene, an underlying condition, or a systemic infection (Mathew 1966; Wald 2004). When due to infection, the size of the swelling is a direct indicator of the severity of the infection (Lebensohn 1950). Cases of recurrent hordeola are usually the result of failure to eliminate bacteria completely, rather than resulting from new infections (Roodyn 1954).
Most cases of internal hordeola resolve on their own; therefore, people with hordeola often do not seek professional medical treatment (Olson 1991). Home therapies, including heated compresses, lid scrubs, and over‐the‐counter medications, are often used without consulting a medical professional. For times when medical care is sought, a general practitioner or a family physician may be consulted before an ophthalmologist or an optometrist is seen (Fraunfelder 1971; Lebensohn 1950).
Practice standards for the initial treatment of hordeola are conservative, typically limited to the application of warm compresses several times a day, if any treatment is recommended at all (Barza 1983; Fuchs 1911; Olson 1991; Panicharoen 2011; Sethuraman 2009; Wilkie 1956). A topical antibiotic may be prescribed in conjunction with warm compresses (Diegel 1986; Lebensohn 1950; Lederman 1999; Panicharoen 2011; Wald 2004). If the condition is severe and is resistant to topical antibiotics, systemic antibiotics, or surgical incision and drainage may be implemented (Moriarty 1982; Mueller 2008; Panicharoen 2011; Rubin 1995; Skorin 2002).
Description of the intervention
Nonsurgical treatments for hordeola include the application of warm or hot compresses, the use of lid scrubs and digital massage, the administration of antibiotics or steroids, or alternative medicine such as acupuncture and autohemotherapy. Typically, the intent of these interventions is to reduce healing time while relieving the symptoms associated with the lesion. Interventions of interest would be provided during the first week after onset. Beyond one week, it is believed that internal hordeola may resolve on their own, or may require surgical incision and curettage. In addition to resolving the presenting hordeolum, other aims of the interventions are to minimize the risk that the inflammation may worsen, may spread to other areas, or may become recurrent.
How the intervention might work
The natural history of an acute internal hordeolum generally spans one to two weeks, beginning with the appearance of an abscess and concluding with draining of the abscess. Initial treatments for hordeola have been aimed at promoting the drainage of pus from the abscess and removing the meibomian gland obstruction. The application of a warm or hot compress may facilitate drainage by softening the granuloma (Diegel 1986; Fuchs 1911; Moriarty 1982; Skorin 2002). Heated compresses are typically employed for five to 10 minutes, several times a day, until the hordeolum is resolved.
Lid scrubs consist of mild shampoos or saline solutions, and are applied while the affected area is gently massaged. The theory underlying the use of lid scrubs is that they promote lid hygiene and prepare the physical environment for drainage by clearing debris from the lid margin (Driver 1996; Skorin 2002). Creating a clear channel is believed to initiate drainage, similar to the epilation of an eyelash in cases of an external hordeolum (Hudson 1981). Also, ingredients used in shampoos break down bacterial membranes, which further decreases the presence of bacteria at the infection site (McCulley 1984). Lid scrubs are commonly recommended in the treatment of other ocular bacterial infections, such as blepharitis, and may prevent the spread of infection (Avisar 1991). In conjunction with lid scrubs, lid massage has been proposed to physically express secretions from the infected glands (Driver 1996; Scobee 1942).
Antibiotics can be administered locally, at the site of infection, or may be given systemically. Most cases of hordeola are caused by one of the staphylococcal species; therefore, antibiotics should be effective against the bacteria. Application of topical antibiotics may reduce healing time by fighting against the causative bacterial infection and reducing inflammation. Many topical medications include ingredients that relieve the symptomatic pain of an internal hordeolum. Antibiotics can also be applied locally by injection. Systemic antibiotics are sometimes used when local antibiotics are not effective, or when the infection is not localized.
Steroids can be applied topically as ointments or eyedrops. Internal hordeola have a short course; therefore, as little as one steroid treatment could be effective in reducing healing time and relieving symptoms associated with the inflammation (King 1986; Palva 1983).
Alternative medicine treatments, such as acupuncture, may be used alone or complementary to traditional interventions to treat hordeola. Acupuncture for the treatment of hordeola is evaluated in a separate Cochrane review (Cheng 2014).
Why it is important to do this review
An acute internal hordeolum is a common disease experienced by a wide population. Although the course of the disease is relatively short, instances of internal hordeola are painful and bothersome. Furthermore, improper management of the underlying cause of the infection may lead to recurrent infections, or to the development of other diseases. Despite the common recommendation to use heated compresses, their efficacy in treating hordeola has not been systematically reviewed. If heated compresses are indeed sufficient to treat hordeola, then more rigorous interventions, such as antibiotics or steroids, may not be warranted for initial treatment. Conversely, comparing the effectiveness and safety of all available interventions, to determine which may be most beneficial to the individual, is also important. A summary of the evidence should assist patients and professionals to determine preferred methods of treatment.
Objectives
The objective of this review was to investigate the effectiveness, and when possible, the safety, of non‐surgical treatments for acute internal hordeola compared with observation or placebo.
Methods
Criteria for considering studies for this review
Types of studies
This review was limited to randomized and quasi‐randomized clinical trials. Examples of quasi‐randomized allocation include using participants' birth dates, medical record numbers, or order of enrolment to determine treatment groups.
Types of participants
We were interested in studies of participants with a diagnosis of an acute internal hordeolum. Studies of participants with only an external hordeolum (stye), chronic hordeola, or chalazia were excluded.
Types of interventions
Non‐surgical interventions were the primary focus of this review. We included trials that compared the use of hot or warm compresses, lid scrubs, antibiotics, or steroids with observation, placebo, or another active intervention for the treatment of acute internal hordeola. Complementary and alternative therapies, such as acupuncture and bloodletting, were outside the scope of this review (see Cheng 2014).
Types of outcome measures
Primary outcomes
The primary outcome for this review was the proportion of participants with complete resolution of a hordeolum seven days after diagnosis. The seven‐day period for resolution was selected because most cases of hordeola resolve on their own at between one and two weeks. We had also planned to analyze the proportion of participants with complete resolution of hordeola after 14 days as a secondary outcome, when these data were available.
Secondary outcomes
-
The proportion of participants requiring surgical incision and drainage after the treatment period, or seven days after diagnosis.
-
The incidence of chalazia after the treatment period or seven days after diagnosis.
-
The incidence of recurrence of hordeola after six months, and after one year. A recurrent case was considered to be any hordeolum that occurred after one month from the resolution of the initial hordeolum, at any location on the same eyelid, or as defined by the included study.
-
The incidence of a secondary hordeolum during or after the treatment period, or seven days after diagnosis. A secondary hordeolum was defined as a hordeolum that occurred within one month of the initial hordeolum, at a different location than the initial hordeolum, or as defined by the included study.
Adverse outcomes
We had planned to report all adverse effects related to the treatment of hordeola that were reported in the primary studies. Specific adverse outcomes of interest included conjunctivitis; eye irritation; discoloration of the eyelid, conjunctiva, and lens; and corneal damage.
Economic data
We had planned to report economic data.
Quality of life data
We had planned to report quality of life data.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register (2016; Issue 12 in The Cochrane Library)), MEDLINE Ovid, MEDLINE Ovid Epub Ahead of Print, MEDLINE Ovid In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Ovid Daily (January 1946 to December 2012), Embase (January 1947 to December 2016), PubMed (1948 to December 2016), Latin American and Caribbean Literature on Health Sciences (LILACS (January 1982 to December 2016)), the metaRegister of Controlled Trials (mRCT; www.controlled‐trials.com (last searched 26 July 2012)), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 December 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), PubMed (Appendix 4), LILACS (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and the ICTRP (Appendix 8).
Searching other resources
We reviewed the reference lists from potentially eligible studies to identify further studies. In addition, we used the Science Citation Index to search for references that cited potentially eligible studies (last searched on 9 December 2016; no relevant studies were identified).
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts from the electronic literature searches and the manual search to identify possible trials of interest, according to the 'Criteria for considering studies for this review'. We designated each reference identified from the searches as (a) relevant, (b) possibly relevant, or (c) not relevant for this review. We retrieved full‐text copies of the articles if an abstract was classified as (a) or (b). Each article was then independently assessed by two people and was classified as (1) include in review, (2) awaiting classification, or (3) exclude from review. We resolved discrepancies between authors by consensus.
Data extraction and management
As no studies were identified for inclusion in this review, no data extraction or assessment of risk of bias was performed. If, in the future, relevant studies become available, we will undertake the following methods for updating this review.
Two review authors will independently extract data using the data extraction forms created by Cochrane Eyes and Vision. For each included study, we will extract data on study characteristics, interventions, outcomes, cost, quality of life, and other relevant information. One review author will enter the data into Review Manager (RevMan 2014) and a second review author will verify the data entry. Discrepancies between review authors will be resolved by the third review author.
Assessment of risk of bias in included studies
Two review authors will independently assess the risk of bias of included studies, based on the methods provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Sources of potential bias affecting the methodological quality of a study will be divided into six domains that include:
-
Selection bias: sequence generation and allocation concealment.
-
Performance bias: masking (blinding) of participants and study personnel.
-
Detection bias: masking (blinding) of outcome assessors.
-
Attrition bias: incomplete outcome data and rates of missing data among treatment groups.
-
Reporting bias: selective outcome reporting.
-
Other sources of bias: funding source and other potential sources of bias.
For every study included in the review, we will assess each domain to have (a) low risk of bias, (b) unclear or not reported risk of bias, or (c) high risk of bias. Discrepancies between review authors will be resolved by a third review author. For studies classified as unclear or not reported, we will contact the authors of the study for further information, in an attempt to reclassify the quality of the study. If no informative response is received within six weeks, we will assess the study on the basis of available information.
Measures of treatment effect
The measures of treatment effect will depend on the types of data presented in the included studies and will be identified by the definitions given in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Dichotomous data
The primary outcome of interest, the proportion of participants with complete resolution of hordeola at seven days after diagnosis, will be analyzed as a dichotomous variable: resolved versus not resolved. Data on the proportion of participants requiring surgical incision and drainage after treatment, the proportion of participants developing a chalazion after treatment, the proportion of participants with recurrent hordeola, and the number of secondary hordeola will also be analyzed as dichotomous data. We will report dichotomous data as a summarized risk ratio with 95% confidence interval.
Continuous data
We will report continuous data as a mean difference with its standard deviation. We anticipate that available economic and quality of life data will be analyzed as continuous data.
Ordinal data
We will summarize ordinal data qualitatively.
Counts and rate data
We will summarize counts and rate data in rate ratios when the event is rare, and as continuous outcome data when the event is more common. We will analyze adverse events data as counts and rates.
Unit of analysis issues
The unit of analysis for this review will be an eyelid of an individual participant.
Dealing with missing data
We will contact authors of included studies in an attempt to obtain missing data. We will set the response time at six weeks and will document any communications with study authors. If data cannot be retrieved, we will not impute data and use only the reported data in the study. We will report loss to follow‐up when available.
Assessment of heterogeneity
We will test for statistical heterogeneity using the I² statistic and will examine the overlap of confidence intervals of individual trial effects on forest plots.
Assessment of reporting biases
We will use funnel plots to assess the possibility of reporting biases, if more than ten studies are available.
Data synthesis
If limited heterogeneity is suggested (defined here as I² < 50%), we will perform meta‐analyses using the random‐effects model, unless there are three or fewer trials, in which case, we will use the fixed‐effect model. If heterogeneity is detected, and sufficient data are available, we will combine trial results by relevant, less heterogeneous subgroups. Otherwise, we will describe the results individually.
Subgroup analysis and investigation of heterogeneity
We will investigate heterogeneity by conducting subgroup analyses, provided sufficient information is available. Subgroups of interest include sex, age, use of contact lenses, including soft lenses versus hard lenses, and the frequency of hordeola occurrences, co‐infections, and other comorbidities at baseline.
Sensitivity analysis
We will investigate the impact of studies with a high likelihood of bias, or with missing data, as well as the impact of unpublished studies, using sensitivity analyses.
Summary of findings
We will present a "Summary of findings" table to summarize the comparative effects between treatments for outcomes evaluated in the review. For each outcome, we will use the GRADE approach to assess the certainty of evidence (gradeworkinggroup.org/). The GRADE approach examines five criteria that may affect the certainty of effect estimates: risk of bias in individual trials, indirectness, heterogeneity, imprecision (wide confidence intervals), and publication bias. Two review authors will independently grade the body of evidence supporting each outcome of interest as very low, low, moderate, or high, and document reasons for downgrading as indicated. We will resolve any discrepancy by discussion.
Results
Description of studies
Results of the search
The electronic searches identified a total of 517 references, as of 21 June 2010, for the original publication of the review (Lindsley 2010). After the review authors screened the titles and abstracts, they classified 19 references as being potentially relevant. After reviewing the full text, they excluded all 19 references, which reported on 18 unique studies.
For the first update of this review, as of 26 July 2012, we identified 427 additional references through electronic searches (Lindsley 2013). After we screened the titles and abstracts, we classified six references as being potentially relevant. After reviewing the full text, we excluded all six references, which reported on five unique studies.
For this update (2017), we identified 901 additional references through electronic searches (Figure 1). We excluded 899 records after screening titles and abstracts, and two records after reviewing the full‐text report (NCT01763437; NCT02338648). We excluded an additional two studies identified by searching the Science Citation Index for references that cited potentially eligible studies (Gordon 1970; Laibson 1981). No trial was included in this review.

Study flow diagram.
Excluded studies
Overall, we excluded 27 studies from this review. The reasons for exclusion are described in the 'Characteristics of excluded studies' table.
Of the 27 excluded studies, six were randomized controlled trials (RCTs) that included patients with acute internal hordeola. The first included pediatric participants with lid inflammation, and was conducted to evaluate the safety of loteprednol etabonate 0.5% and tobramycin 0.3% ophthalmic suspension (Zylet®) in the pediatric population (Comstock 2012). As safety was the primary focus of the trial, the study population comprised participants with varying ocular inflammatory conditions, and data were not collected by study investigators for specific conditions. The second study compared the effectiveness of a combined antibiotic ophthalmic solution with placebo after surgical incision and curettage, in participants with internal and external hordeola (Hirunwiwatkul 2005). All participants had been newly diagnosed and untreated before undergoing incision and curettage. A total of 14 participants were randomly assigned to each group, and results for participants with internal and external hordeola were not reported separately. The study authors concluded that there was no evidence that suggested differences in pain score, mass size, or duration of cure between groups. The remaining four RCTs evaluated complementary and alternative therapies, such as acupuncture and bloodletting (Chen 2000; Gao 2001; Takama 2006; Xu 2004), which were not within the scope of this review, but are assessed in a separate Cochrane review (Cheng 2014).
Risk of bias in included studies
No studies were included in this review, thus no 'Risk of bias' assessment was done.
Effects of interventions
No studies were included in this review, thus no effects of interventions were reported.
Discussion
Summary of main results
No trials were identified for this review.
Overall completeness and applicability of evidence
Most of the references identified from the literature search for this review were related to external hordeola (styes) or chalazia. By and large, the few references specific to acute internal hordeola either reported recommendations for treatment without cited evidence, or were reports of interventional case series, case studies, or other types of observational study designs. The only clinical trials we found that included participants with acute internal hordeola were not eligible for the review because they included multiple conditions and did not stratify by specific diagnoses, they included participants who underwent surgical treatment as a criterion for study enrolment, or they evaluated treatments that were not within the scope of this review. Furthermore, the bulk of the literature was published more than 20 years ago.
Potential biases in the review process
We had anticipated that the primary source of bias for this review would be selection bias, specifically, the identification and inclusion of relevant studies. Before beginning the review process, we expected that few trials had been published on hordeola, that various authors used different terminologies when referring to different classifications of hordeola (i.e. hordeolum, stye, chalazion, etc.), and that relevant studies may be found in older publications. Therefore, we designed a broad search strategy for the electronic databases, to facilitate the identification of potentially relevant studies. We also manually searched the reference lists of potentially relevant studies, to identify older studies that may not be included in electronic databases.
To minimize bias during the process of selecting studies for this review, two review authors screened the references from the electronic search and independently classified them for inclusion or exclusion. We included potentially relevant references that mentioned any type of hordeolum or external eye inflammation for assessment at the full‐text level. Inclusion and exclusion were determined by using the definition of the disease given in the full‐text article. Furthermore, one review author screening the studies had a clinical background (JN), and one had a methodological background (KL).
Agreements and disagreements with other studies or reviews
Although it is the most commonly recommended therapy for hordeola, the application of warm compresses has not been shown to be effective in accelerating healing time or reducing symptoms associated with a hordeolum in a controlled trial. Moreover, there is no evidence that indicates that warm compresses alone would eliminate the infection. It is also unclear whether medical treatment or lid hygiene is effective in treating acute internal hordeola.
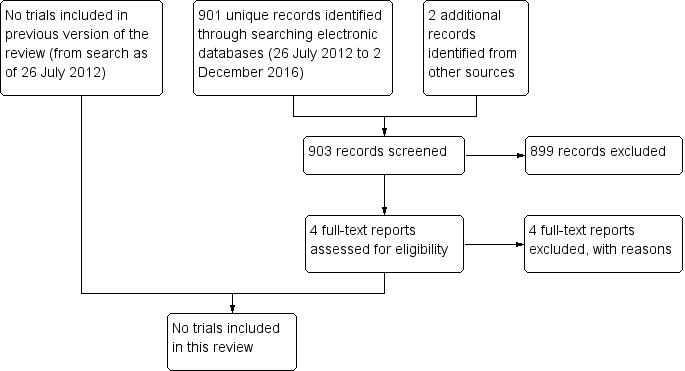
Study flow diagram.

