Absetzen im Vergleich zur Weiterbehandlung mit Antipsychotika als Langzeitbehandlung gegen herausfordernde Verhaltensweisen bei älteren Menschen mit Demenz
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | ||
| Methods | Design: double‐blind, placebo‐controlled study Duration: 3 months | |
| Participants | Country: UK Centres: 2 Setting: residents of 2 long‐term care facilities Total number of participants: 100 (46 intervention, 54 control) Analysis: completed at least 1 follow‐up randomisation Gender distribution (F%): 76 % intervention = 76%, control = 87% Mean age (years): intervention = 83.1 (SD 7.1), control = 83.6 (SD 9.3) Cognitive function (Mean MMSE): intervention = 5.5 (SD 6.8), control = 5.5 (SD 6.5) Total NPI: intervention = 13.3 (SD 9.3), control = 15.7 (SD 8.3) Inclusion criteria: older participants (aged > 65 years) care facility residents, probable or possible Alzheimer's disease by the NINCDS‐ADRDA criteria (National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association Criteria), a Clinical Dementia Rating Scale stage 1 or greater and no severe behavioural symptoms (no individual scores above 7) on the NPI at time of evaluation, taking neuroleptics (thioridazine, chlorpromazine, haloperidol, trifluoperazine or risperidone) for more than 3 months (median prescription time longer than 1 year) Exclusion criteria: no severe behavioural disturbances, taking neuroleptics for longer than 3 months, having severe behavioural symptoms (individual scores above 1) and no severe behavioural symptoms (individual scores above 7 on 1 of the 12 items of the NPI‐scale) | |
| Interventions | Intervention 1: abrupt discontinuation of antipsychotics (intervention group) Intervention 2: continuing antipsychotics (control group) No dose reduction of tapering | |
| Outcomes | Duration of follow‐up: 3 months (0, 1 and 3 months) Outcomes measured: behavioural and psychiatric symptoms (NPI), quality of life (DCM) | |
| Notes | Funding provided by Research into Aging (London, UK) and Age Concern (London, UK). Conflicts of interests were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation is not reported Quote: "Subjects were then randomised to neuroleptic (N = 54) or placebo (N = 46)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment is not described Quote: "...Dispensing was coordinated by the pharmacy departments at the 2 centres. Prescriptions were written prior to randomisation in a twice daily regimen, allocating to each participant the closest dose..." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study is described as double‐blinded, unlikely that blinding could have been broken. Encapsulation of the administered drugs ensured blinding of participants and doctors/nurses. Quote: "The study was conducted using a double‐blind design. All study neuroleptics were encapsulated by an independent company to maintain blind, and dispensing was coordinated by the pharmacy departments at the 2 centres. Prescriptions were written prior to randomisation in a twice‐daily regimen, allocating to each participant the closest dose to their pre‐existing prescription from the doses encapsulated" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: study is described as double‐blinded, unlikely that blinding could have been broken. Blinding of the outcome raters is not described. Quote: "...using a double blind design...the centre coordinator, blinded to neuroleptic status, decided whether the patient needed to be withdrawn from the study to receive "rescue" medication." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: reasons for dropouts reported and similar for both groups Quote: "All evaluations were undertaken at baseline. The NPI and DCM assessments were also completed at 1 and 3 month follow‐up. Study withdrawals and the proportion of participants developing marked (pronounced or manifest) behavioural symptoms are described and compared between groups using the chi‐square test. Fourteen participants (26% active treatment, 30% placebo) withdrew from the study in each group. There were only 6 withdrawals in the placebo‐treated group (13%) and 5 withdrawals in the active treatment group (9%) because of behavioural deterioration. Other withdrawals were because of physical health problems (active group: 3 (6%), placebo group: 3 (7%)), protocol violation (active group: 2 (4%), placebo group: 1(2%)) or withdrawal of consent (active group: 3 (6%), placebo group: 2 (4%)). Eighty‐two (82%) of the participants completed at least 1 follow‐up evaluation and were included in the primary outcome analysis. For all participants who completed at least 1 follow‐up assessment, the last evaluation was carried forward" |
| Selective reporting (reporting bias) | Low risk | Comment: behavioural and psychiatric symptoms and well‐being reported on all measured time points, cognition was only assessed at start of the study |
| Other bias | Low risk | No other bias |
| Study characteristics | ||
| Methods | Design: randomised, blinded, placebo‐controlled parallel 2‐group treatment discontinuation study Duration: 12 months | |
| Participants | Country: UK Setting: residents of 5 long‐term care facilities Total number of participants: 165 (82 discontinuation, 83 continuation) Gender distribution (women %): 75.6 % discontinuation, 77.1% continuation Mean Age, SD (years): 84.9 (SD 6.1) discontinuation, 84.4 (SD 7.0) continuation Inclusion criteria: participants lived in a nursing or residential home, patient fulfilled the NINCDS/ADRDA criteria for possible or probable Alzheimer's Disease, patient had either a MMSE score > 6 or a Severe Battery Impairment score > 30, patient was taking at least 10mg chlorpromazine equivalents of a typical neuroleptic or at least 0.5 mg daily of risperidone Exclusion criteria: participants unable to complete primary outcome measures at baseline assessment, clinician responsible for care or study clinician considered the person with any physical condition that would have made participation in the trial distressing or likely to have more physical problems, patient was currently taking thioridazine and showing a prolonged QTc on electrocardiogram, patient was likely to be unable to take capsules | |
| Interventions | Intervention 1: abrupt discontinuation of neuroleptics and switch to placebo (placebo group) Intervention 2: continuation of neuroleptics (continuation group) Three fixed dosages (very low‐low‐high) were chosen for each of the permitted neuroleptic drugs and were maintained during the 12 months | |
| Outcomes | Duration of follow‐up: 12 months Primary outcome: Cognitive function: total Severe Impairment Battery (SIB) score (0, 6, 12 months) Secondary outcomes: neuropsychiatric symptoms: NPI (0, 6, 12 months) cognitive function: Standardised Mini‐Mental State Examination (SMME) (0, 6 months) adverse effects: Modified Unified Parkinson's disease Rating Scale (M‐UPDRS) (0, 6 months) FAS test (0, 6 months) Bristol Activities of Daily Living Scale (BADLS) (0, 6 months) Sheffield Test for Acquired Language Disorders (STALD)(0, 6 months) Functional Assessment Staging (FAST)(0, 6 months) Clinician's Global Impression of Change (CGIC) (0, 6 months) | |
| Notes | Clive Ballard, first author, has received honoraria en research grants from different companies. Study was possible by a grant from the Alzheimer's Research Trust, Cambridge, UK. There are several factors limiting the generalisability of the interpretation of this trial. First, recruitment focused on participants living in residential care where moderate and severe dementia usually predominates, and the participants generally are older and frailer than their counterparts in other settings. Thus, the results are not easily extrapolated to individuals who are cared for in other community settings Second, 89% of the participants were taking haloperidol or risperidone, but pharmacological profiles of neuroleptics differ, so that the study might not adequately represent the effects of discontinuation of other neuroleptics Furthermore, polypharmacy is common in residential care, and the study did not consider other psychotropic prescriptions Finally, high participant attrition sharply reduced the statistical power and scope for analysis of outcomes at 12 months. Imputation procedures and sensitivity analyses established robustness of estimates, but they cannot account for type II errors (i.e. false‐negative interpretation) Individual participant data at three months from was kindly provided by the authors to allow pooling of the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comments: randomisation was done by computer random number generator Quote: "Randomisation was performed centrally at the Centre for Statistics in Medicine in Oxford (CSMO), using dedicated computer software (MINIM). The randomisation programme included a minimisation algorithm to ensure balanced allocation of participants across the intervention groups for important prognostic factors" |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation concealment Quote: "The statistician carrying out the randomisation had no direct contact with patients and allocation was, therefore, totally independent of patient recruitment. The clinician responsible for randomisation of a patient faxed a randomisation form to the CSMO (or sent e‐mail) in exceptional circumstances) and provided details appropriate and sufficient for establishing eligibility. If a person was eligible and informed consent/assent had been obtained and baseline assessments had been completed, the patient was randomised by the statistician either to continue taking medication or to discontinue (placebo group). The statistician directly communicated the allocation to the relevant trial pharmacy, ensuring concealment." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study is described as double‐blinded, unlikely that blinding could have been broken Quote: "The clinicians, those administering the trial medication, the carers, the relatives and the participants themselves, and those assessing the outcomes were all blinded to treatment allocation. Each antipsychotic was over‐encapsulated to maintain the double‐blind design. Placebo capsules were identical to the over‐encapsulated antipsychotics,.." |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: study is described as double‐blinded, unlikely that blinding could have been broken. Quote: "... those assessing the outcomes were blinded to treatment allocation..." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: flow of participants is included. Missing data is balanced across groups and similar reasons. Quote: "Primary analysis was done performed on patients with complete data at both baseline and week 26, including those who did not adhere tot the protocol. To give a completed data set the imputation method was used "filling in" missing data with plausible values. A sensitivity analysis was used to test the robustness of the SIB result. This analysis was limited to those participants for whom the risk of possible floor and ceiling effects was smallest, i.e. SIB baseline cut‐off values ≥ 40 but ≤ 90." |
| Selective reporting (reporting bias) | Low risk | Comment: all intended primary and secondary outcomes are reported in the first and the follow‐up study |
| Other bias | Low risk | No other bias |
| Study characteristics | ||
| Methods | Design: randomised double‐blind placebo‐controlled trial with two sub‐studies: the antidepressant discontinuation placebo‐controlled study group (Bergh 2012) and the antipsychotic discontinuation placebo controlled study group (Bergh 2011). The antipsychotic discontinuation study (Bergh 2011) met inclusion criteria and was included in the review. Duration: 25 weeks | |
| Participants | Country: Norway Setting: residents of 15 nursing homes Total number of randomised participants: 19 (9 discontinuation, 10 continuation) Mean age (years): 81.7 discontinuation, 82.6 continuation Gender distribution (% women): 37.5 discontinuation, 70 continuation Median CSDD score (range 0 to 38): 5.5 discontinuation, 6 continuation Median NPI‐10 (range 0 to 144): 22 discontinuation, 21 continuation Inclusion criteria: vascular or Alzheimer dementia, or mixed Alzheimer's disease/vascular dementia, nursing homes resident for 3 months or more, given risperidone for 3 months or more, Clinical Dementia rating 1, 2 or 3 Exclusion criteria: dementia of other origin, psychiatric disease (schizophrenia, depression or any severe), life expectancy less than 3 months, acute infection last 10 days, unstable diabetes mellitus, terminal disease | |
| Interventions | Treatment 1: discontinuation risperidone after titrated out over one week Treatment 2: continuation of risperidone (dose in each patient varied, according to the dose the participants were prescribed at inclusion, mean dose at inclusion was 0.92 mg/d) Concomitant therapy: all kinds of concomitant therapy were allowed before, during and after the study | |
| Outcomes | Primary outcomes: neuropsychiatric symptoms: changes in Neuropsychiatric Inventory‐10 (0 weeks, 25 weeks; range 0 to 120), depressive symptoms of a patient with dementia: changes in Cornell's Depression Scale (0 week, 25 weeks; range 0 to 38), safety analyses: changes on the Unified Parkinson Disease Rating Scale (UPDRS, six‐item version) (0 weeks, 25 weeks) Secondary outcomes: Quality of life – Alzheimer's disease (QoL‐AD), the Severe Impairment Battery (SIB), the Lawton & Brody's Physical Self‐Maintenance scale (PSMS), the Clinical Dementia Rating Scale (CDR) | |
| Notes | Sponsor: Innlandet Hospital Trust. There is no conflict of interest of the author reported. It is unclear if the author received grants. Provider of study drugs is not described. This was an unpublished study. We requested results by email communication with the author on 8 June 2017 (Van Leeuwen 2017 [pers comm]). We received study results on 10 June 2017. The study arm with antidepressants was published in 2012 as a separate paper. The antipsychotics discontinuation arm was never published as paper. The study results were known in 2011 and reported to the Norwegian Medicines Agency. This study was not included in the 2013 review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: participants randomised centrally Quote: "...using computer generated randomisation (1:1) in block of four..." |
| Allocation concealment (selection bias) | Low risk | Comment: pharmacist was responsible for allocation Quote: "... patients were allocated to placebo or active treatment group by centralized allocation in blocks of four (1:1) by Sykehusapotekene Gjøvik, who also kept the randomisation list, computer derived, until the statistical analyses were completed.." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study was described as double‐blinded, probably blinding will be successfully done Quote: "Study blinding was maintained as no other involved partner of the study knew the randomisation list than Sykehusapoteket Gjøvik. All statistical analyses were performed before the randomisation groups were unblended. The bottles with active medication and placebo were identical labelled...replaced by placebo in a blinded way or replaced by a study drug containing active medication (same kind, same dose) as before..." |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: probably blinding of assessment outcomes was successfully done. The study involved 57 study nurses which could have biased the results. Quote: "Data collection was done by research nurses. All statistical analyses were performed before the randomisation groups were unblended. A sealed code envelope was stored in the patient’s medical journal at the nursing homes, and could only be opened in case of medical emergencies as a serious adverse event." |
| Incomplete outcome data (attrition bias) | High risk | Comment: all randomised participants (19) are described in the flowchart. Very high dropout and withdrawal in the discontinuation group (7/9) suggests high risk of bias. Dropouts were more frequent in the ApDG (7/9, 77.8%) than in the ApCG (0/10, 0.0%) (P = 0.001). The analysis was based on modified ITT: participants in efficacy analysis: 16, participants included in safety analysis: 18. Unpublished data and high dropouts suggests high risk of attrition bias. |
| Selective reporting (reporting bias) | High risk | Comment: protocol was registered in ClinicalTrials.gov, outcome measurements were not all reported as per protocol paper. Study is unpublished, no peer reviewing to valid results suggests high risk of bias. Quote: " ... The number of primary endpoints were reduced from three to two: Cornell Scale of Depression in Dementia and the Neuropsychiatric Inventory...the changes were made prior to breaking the blind, and have limited implications for study interpretation... no observe case analysis en no interim analyses as planned in the protocol." |
| Other bias | Low risk | No other bias |
| Study characteristics | ||
| Methods | Design: double‐blind, baseline treatment neuroleptic‐controlled pilot study Duration: 4 weeks | |
| Participants | Country: USA Setting: residents of long‐term care facilities Participants: 36 (22 discontinuation, 14 continuation) Inclusion criteria: participants with diagnosis of possible or probable Alzheimer's dementia (criteria were given), participants receiving a neuroleptic (any traditional neuroleptic was acceptable) and who had been on a stable dose for 3 months prior to the study, a history of physically aggressive behaviour according to the referring nursing supervisor, participants residing in a nursing home, participants on antidepressants were permitted to participate if medication doses had been stable Exclusion criteria: participants with primary psychiatric diagnoses, mental retardation and terminal illness or other recent acute, changes in health status (e.g. recent broken hip) | |
| Interventions | Intervention 1: withdrawal neuroleptics (discontinuation) Intervention 2: no withdrawal neuroleptics (continuation) Abrupt withdrawal or tapering off a neuroleptic when baseline dose exceeded the equivalent of 50 mg of chlorpromazine. The tapering was done by dropping the baseline neuroleptic dose by half during week 1 and then discontinuing the neuroleptic completely at the beginning of week 2 Neuroleptic drugs: haloperidol (21), thioridazine (9), thiothixene (3), trifluoperazine (1), mesoridazine (1), loxapine (1) | |
| Outcomes | Primary outcome: completion of the 4 weeks of study (numbers completing the 4 week study), behavioural symptoms: change in the amount of observed physically aggressive behaviour (mean, mean difference) (0, 1, 2, 4 weeks) Secondary outcomes: use of physical restraint, verbally aggressive behaviour, walking, amount of time spent sleeping and sitting, verbal aggressiveness, physically aggressive acts observed by experienced study personnel and by using a portable barcode reader capable of storing several hours of observation (0, 1, 2, 4 weeks) | |
| Notes | Research grant from the Alzheimer's Association. There may have been selective recruitment limiting the generalisation of the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was done by random number table Quote: "Assignment was based on a predetermined sequence such that three patients were assigned to withdrawal for every two not withdrawn. At the end of week 1, subjects were randomly assigned to either withdrawal or no withdrawal." |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment is not described, and may not have been blinded. Participant groups were well matched for age, chlorpromazine‐equivalent neuroleptic dose and physically aggressive behaviour at baseline. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study is described as double‐blinded, unlikely that blinding could have been broken. Nursing staff was involved in decision to discontinue the programme. They were blinded for the treatment allocated, thus outcome assessment may have been adequately blinded. Quote: "Patients in both groups received identical‐appearing capsules prepared at the University of Minnesota Hospital Pharmacy. Patients receiving their medication in crushed form, received in the placebo group tablets of vitamin C instead of capsules. The patient receiving intramuscular mesoridazine daily was given intramuscular saline from a nurse not directly involved in the patient's care" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: study is described as double‐blinded, unlikely that blinding could have been broken. Participants were directly observed by study personnel, who were blinded to treatment assignments and recording behaviour was done by using a portable bar‐code reader capable of storing several hours of observation. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: dropouts were described Quote: "Of the 22 patients who were withdrawn, 20 (91%) completed the 4‐week double‐blinded withdrawal. Two patients were restarted on medication on the recommendation of the nursing staff; only one went back on a neuroleptic. Of the 14 patients not withdrawn, all completed the 4‐week trial. Of the 576 observation periods there were seven in which the bar‐code reader failed. Handwritten back‐up notes were used for physically aggressive behaviour frequency." |
| Selective reporting (reporting bias) | Low risk | Comment: all intended outcomes were reported |
| Other bias | Low risk | No other bias |
| Study characteristics | ||
| Methods | Design: double‐blind cross‐over study Duration: seven weeks followed by seven weeks cross‐over | |
| Participants | Country: USA Setting: residents of one nursing home Diagnosis: the diagnosis of dementia was not mentioned Number of participants: 58 Inclusion criteria: nursing home residents; aged over 70 years; had received at least four weeks haloperidol, thioridazine or lorazepam for agitation Exclusion criteria: concomitant administration of other antipsychotic or anti‐anxiety drugs other than low‐dose trazodone hydrochloride for sleep, life expectancy less than three months due to obvious causes as judged by the nursing home staff member responsible for direct care psychiatric diagnosis of a major affective disorder of schizophrenia according to DSM‐III, acute infection within 10 days before entry, expectancy of leaving the nursing home within three months, uncontrolled hyperglycaemia or hypoglycaemia | |
| Interventions | Tapering period: withdrawal of antipsychotic and lorazepam use by tapering to placebo during a three‐week period Intervention 1: seven weeks of taking placebo followed by seven weeks of taking antipsychotic medication Intervention 2: seven weeks of taking medication followed by seven weeks of placebo Antipsychotics: haloperidol, thioridazine | |
| Outcomes | Time of measurements: one week after start of dosage tapering (week 1), phase one tapering (week 3), phase one end point (week 10), phase two tapering (week 13), phase two end point (week 20) Primary outcomes: behavioural symptoms (BPRS) (mean), agitation (CMAI) (mean) Secondary outcomes (mean): adverse effects (AIMS), cognitive function (MMSE), global impression scale (GCI‐S), sleep and activity level ratings Time of assessment: one week after start of dosage tapering (week 1), phase one tapering (week 3), phase one end point (week 10), phase two tapering (week 13), phase two end point (week 20) | |
| Notes | Several different analyses were used to assess the robustness of the result. However, it was not clear if an intention‐to‐treat analysis was used. We were unable to use any data from this study. Cohen‐Mansfield 1999 did not report outcome data separately for the different medications discontinued in the trial (which included the benzodiazepine lorazepam as well as the antipsychotics haloperidol and thioridazine). This study was supported by grants AG00547 and AG10172 from the National Institute on Aging, Bethesda, MD, USA. No conflicts of interest reported. Because diagnosis of dementia was not mentioned in the paper, we emailed the first author (Declerck 2009a [pers comm] on 21 april 2009 to ask her whether the participants included had dementia and her answer was positive (referring to the MMSE scores). We have asked the author by email for more results (SDs of the means…) on 1 July 2009, but we have not received any response on our last e‐mail (Declerck 2009b [pers comm]). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation is not described Quote "...half the residents were randomly assigned to have their medication dose tapered during a 3‐week period, followed by receipt of a placebo (the other half continued their usual medication dosage). Residents were randomly assigned to the placebo versus medication group and stratified both by level of cognitive function and by psychotropic medication." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation is not described |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study is described as blinded, unlikely that blinding was broken Quote: "Study medications (usual medication and placebo) were administered as identical liquids to ensure blindness by the care team. Only the dispensing pharmacist, who was not an employee of the nursing home, knew which medication was administered. The care team, residents, family caregivers, and research team were blinded to which group a participant was assigned." |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: study is described as blinded, unlikely that blinding was broken Quote: "The care team, ... and research team were blinded to which group a participant was assigned. Primary outcome data BPRS was assessed by daytime and evening nursing staff. " |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: rates and reason for dropout were described. However we were uncertain how many participants discontinued in the discontinuation or continuation group in the first part of the study. Quote: "Twenty‐three participants discontinued participation in the study before completion for the following reasons: death or dying (3), hospitalisation (1), not eating or weight loss (3), increased agitation (9), lethargy (2), withdrawal of consent (4), facial asymmetry (1) and fall (3); some had multiple reasons. For 12 participants, discontinuation occurred during the original drug dosage, for 9 while taking placebo, and for 2 during titration from drug to placebo. Most discontinuations (20 of 23) occurred in the first part of the study, before the cross‐over." |
| Selective reporting (reporting bias) | High risk | Comment: no distinction is made for number of withdrawals in each group during the first part of the trial. it is not clear how they analyse these outcome. By not making difference in outcome reporting between discontinuation of antipsychotics, namely haloperidol and thioridazine, versus discontinuation of lorazepam, a benzodiazepine, it is impossible to retain robust conclusions from this withdrawal study. Quote: "Participants who discontinued the study were similar in demographic characteristics to those who stayed. Although their levels of agitation at baseline were higher than those who stayed in the study, these differences did not reach statistical significance. Most withdrawals from the study occurred in the first part of the study (no numbers given)." |
| Other bias | Low risk | No other bias |
| Study characteristics | ||
| Methods | Design: a six‐month, randomised, double‐blind, placebo‐controlled discontinuation trial (phase B) following response to haloperidol open treatment during 20 weeks (phase A) | |
| Participants | Country: USA Setting: participants living in the community who presented to a memory disorders clinic or an affiliated behavioural neurology practice group Total number of participants: 44 participants included in phase A, 22 responders of phase A were eligible for randomisation in phase B (discontinuation trial), 20 in phase B (10 discontinuation, 10 continuation) Gender distribution (female): 77% Mean (years): 75 (SD 8.0) Inclusion criteria: aged 50 to 95 years, clinical diagnosis of dementia by DSM‐IV criteria and probable Alzheimer's disease by NNCDS‐ADRA criteria, MMSE range between 5 and 26, current symptoms of psychosis, agitation or aggression. Exclusion criteria: acute unstable medical condition, delirium, alcohol or substance abuse or dependence during the prior year, clinical evidence of stroke, other dementias including vascular or Lewy body or frontotemporal dementia, multiple sclerosis, Parkinson's disease, Huntington's disease, tardive dyskinesia, diagnosis of a psychotic disorder predating the onset of dementia, antipsychotic medication usage during the 4 weeks before study entry, and contra‐indication to the use of haloperidol | |
| Interventions | Phase A: open treatment (20 weeks): 44 participants living in the community with Alzheimer's disease and psychosis, agitation or aggression receiving psychotropic medication had a 1‐week washout before entering phase A. During phase A flexible doses of haloperidol 0.5 to 5 mg daily were individually titrated to maximise therapeutic response and minimise side effects, especially extrapyramidal side effects. Visits occurred at 0, 2, 4, 8, 12, 16 and 20 weeks | |
| Outcomes | Phase A: the 3 most prominent targets of psychosis, agitation or aggression, scored on a 7‐point scale (0 = absent to 6 = extreme) and tracked during the study. Criteria for response (primary outcome) were minimum 50% reduction from baseline in the sum score of these 3 target symptoms, a sum score ≤ 6 on these 3 items (range 0 to 18), and minimal or greater improvement on the CGI‐C score (rated only for symptoms of psychosis, agitation or aggression). Phase B Primary outcome: relapse, assessed at any single time point during phase B. Criteria for relapse were minimum 50% worsening from the sum score of the 3 target symptoms at the end of phase A, a sum score ≥ 6 on these 3 items (range 0 to 18), and minimal or greater worsening on the CGI‐C score (rated for psychosis and agitation/aggression). Secondary outcomes: somatic side effects assessed by the TESS, extrapyramidal signs assessed by the UPDRS and tardive dyskinesia assessed by the Rockland TD scale. Cognition was assessed by change in MMSE and impairment in ADL was assessed by the modified BFAS. Time points of assessment during phase B: 0 (same as end of phase A), 2, 4, 8, 12, 16, 20 and 24 weeks (i.e. 6 months). Pretrial: a 1‐week washout prior to entering phase A. | |
| Notes | Disclosures: authors had financial links with several pharmaceutical companies. Study supported by NIH grant. The discontinuation trial included only participants who responded to haloperidol. Non‐responders after the first 20 weeks (phase A) were excluded from the discontinuation phase (B). This limits the generalisability of the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the blinding of random allocation Quote: "Responders by end‐Phase A were eligible for Phase B, a 24‐week, random assignment (1:1 assignment of haloperidol and placebo), double‐blind, trial of continuation haloperidol (same dose as end‐Phase A) versus switch to placebo." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study is described as double‐blinded, unlikely blinding could have been broken Quote: "Haloperidol and placebo were made up in identical looking opaque white capsules." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: blinding of outcome raters is not described. In these trial, they were several subjective outcomes, so a lack of blinding of outcome assessors could had an influence. The protocol (Devanand 2012a) for the subsequent study (Devanand 2012) mentions: Quote: "The blind is maintained after study exit to avoid biasing raters. A code‐break is authorized only if needed in cases of overdose or medical emergency... raters remained unaware of the group assignments of all patients during the entire study." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: non‐completers data were described and balanced between the groups Attrition at end of phase A fully accounted for; quote: "There were 15 Phase A non‐completers (34%), with all early terminations attributed either to lack of efficacy (n = 9) or side effects (n = 6)." Attrition at end of phase B accounted for and ITT included; quote: "Twenty of the 21 patients randomised in Phase B to continuation haloperidol or placebo had at least one follow‐up visit after randomisation and were included in the Phase B analysis. Among patients who did not relapse, reasons for early study termination prior to 24 weeks in Phase B were side effects (n = 2), moving out of the area (n = 1), medical illness (n = 1) and noncompliance (n = 1). All data from these patients were included in the intent‐to‐treat, last observation carried forward, analyses. " |
| Selective reporting (reporting bias) | Unclear risk | Comment: several outcomes were measured at baseline of the open haloperidol treatment and at time of the discontinuation period, but no results were reported at later times of assessment |
| Other bias | Low risk | No other bias |
| Study characteristics | ||
| Methods | Phase A: flexible dose risperidone open treatment for 16 weeks Phase B: six‐month, randomised, double‐blind, placebo‐controlled discontinuation trial, following response to phase A Duration: 48 weeks | |
| Participants | Country: USA Setting: outpatients through physician referrals and advertising; and residents of assisted‐living facilities (memory clinics (including Alzheimer’s research centres), geriatric psychiatry clinics and clinics at Veterans' Affairs medical centres) or nursing home Total number of participants: 253 participants screened Phase A: 180 received risperidone, participants who had a response in phase A entered phase B. Phase B: 110 randomised Group 1: continue risperidone: 32 participants at start, 13 received risperidone at 16 weeks, 10 completed 48 weeks without relapse Group 2: continue risperidone for 16 weeks and then placebo: 38 participants at start, 27 received placebo at 16 weeks, 14 completed 48 weeks without relapse Group 3: start placebo in phase B: 40 participants at start, 13 received placebo at week 16, 10 completed 48 weeks without relapse Gender distribution at baseline: 59% Mean (years) at baseline: 79.6 (SD 7.6) Inclusion criteria: outpatients or residents of assisted‐living facilities or nursing homes, aged 50 to 95 years, met the criteria for dementia of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) and the criteria for probable Alzheimer’s disease of the NINCDS–Alzheimer’s Disease and Related Disorders Association, a score on the Neuropsychiatric Inventory (NPI) of 4 or more at both screening and baseline on the delusions or hallucinations subscale (psychosis score) or the agitation/aggression subscale (agitation score) (with scores on all NPI subscales ranging from 0 to 12 and higher scores indicating more pronounced symptoms), a score of 5 to 26 on the Mini‐Mental State Examination (MMSE, with scores ranging from 0 to 30) in the case of outpatients, a score of 2 to 26 in the case of nursing home residents (with the lower range reflecting the greater severity of dementia in nursing homes) Exclusion criteria: history of stroke, transient Ischaemic attack, or uncontrolled atrial fibrillation | |
| Interventions | Participants with Alzheimer’s disease and psychosis, agitation or aggression received open‐label treatment with risperidone for 16 weeks. Those who had a response to risperidone therapy were then randomly assigned, in a double‐blind fashion, to one of three regimens: continued risperidone therapy for 32 weeks (group 1), risperidone therapy for 16 weeks followed by placebo for 16 weeks (group 2), or placebo for 32 weeks (group 3) Phase A: open‐label treatment with flexible dose risperidone for 16 weeks, participants who had a response entered phase B Phase B: 110 randomised in phase B Concomitant treatment: quote: "If washout was not feasible ... stable doses of selective serotonin‐reuptake inhibitors or low‐dose trazodone or of sedatives or hypnotic agents were permitted ... Lorazepam, at a dose of 1 mg or less per day, was permitted if needed... cholinesterase inhibitors and memantine at stable dose were permitted" | |
| Outcomes | Primary end point: the time to relapse of psychosis and agitation/aggression during weeks 0 to 16 of phase B, the time to relapse psychosis and agitation/aggression during weeks 17 to 32 of phase B Phase A: participants were considered to have had a response if they had a reduction of 30% or more from baseline on the NPI score (the sum of the sub‐scores for agitation–aggression, hallucinations, and delusions) and a score of one (very much improved) or two (much improved) on the Clinical Global Impression of Change (CGI‐C) scale (which ranges from one to seven, with higher scores indicating less improvement) for overall psychosis, agitation or aggression Phase B: participants were considered to have had a relapse if they had an increase in the NPI core score of 30% or more, or a 5‐point increase from the score at the end of phase A, and a score of six (much worse) or seven (very much worse) on the CGI‐C Secondary outcomes: assessments of extrapyramidal signs, with the use of the Simpson–Angus scale (which ranges from 0 to 40, with higher scores indicating more extrapyramidal signs), tardive dyskinesia, with the use of the Abnormal Involuntary Movement Scale (AIMS, which ranges from 0 to 35, with higher scores indicating more severe symptoms), general somatic symptoms developing during treatment, as assessed with the use of the Treatment Emergent Symptoms Scale (TESS, which ranges from 0 to 26, with higher scores indicating more somatic symptoms), cognitive status, as assessed with the use of the MMSE and the Alzheimer's Disease Assessment Scale (ADAS)–cognitive score (which ranges from 0 to 70, with higher scores indicating worse cognition), physical function, as assessed with the use of the Physical Self‐Maintenance Scale (PSMS, which ranges from 1 to 30, with higher scores indicating worse functioning) and adverse events | |
| Notes | Outcome symptoms slightly unevenly distributed in randomised groups in phase B: 9% agitation‐aggression in group 1 (continue risperidone) versus 19% in group 2 (switch to placebo after 16 weeks) and 18% in group 3 (placebo throughout phase B). High rates of discontinuation of risperidone (38% in phase A; 68% in group 1 and 29% in group 2). Funding sources: quote "...Johnson & Johnson, donated the risperidone tablets and matching placebo but had no role in the conduct of the study or the analysis or reporting of the data... Supported by grants from the National Institutes of Health (R01 AG021488 and R01 AG17761) and the Department of Veterans Affairs." Conflict of interest: not reported in the paper, but included in the Supplementary Appendix available online (add link). The first author received grants from several pharmaceutical companies (inside and outside the submitted work). The discontinuation trial only included only participants who responded to risperidone. Non‐responders after first 16 weeks (phase A) were excluded from the discontinuation phase (B). This limits the generalisability of the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: block randomisation is described Quote: "The study statistician prepared a randomised permuted‐blocks procedure, with blocks of three or six, to balance the group assignment in each of four (2 × 2) strata, with stratification within each site according to the presence or absence of psychosis at baseline and residence (assisted‐living facility or nursing home vs. home)." |
| Allocation concealment (selection bias) | Low risk | Comment: central allocation by pharmacist Quote: "Patients who had a response entered phase B of the study and were randomly assigned...The central pharmacy of the New York State Psychiatric Institute maintained the assignment code, and clinicians and raters remained unaware of the group assignments of all patients during the entire study." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study is described as double‐blinded and unlikely that blinding could have been broken. Quote: "...double‐blind fashion... clinicians and raters remained unaware of the group assignments of all patients during the entire study ... all tablets identical in appearance ... Immediately before the end of phase A, the pharmacy dispensed pre‐packaged blister packs of risperidone or placebo tablets that were identical in appearance for patients eligible for randomisation in phase B. The number of tablets the patient was receiving daily at the end of phase A was the number he or she received throughout phase B." |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: study is described as double‐blinded and unlikely that blinding could have been broken. The blind is maintained after study exit to avoid biasing raters. A code‐break is authorized only if needed in cases of overdose or medical emergency. Quote: "...raters remained unaware of the group assignments of all patients during the entire study..." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants accounted for in the flowchart Quote: "The dropout rates did not differ significantly among the randomised groups (Fig. 1)" |
| Selective reporting (reporting bias) | Unclear risk | Comment: the results for the CGI‐C were not reported in the study, the total NPI scores and the NPI core score were measured at baseline (phase A) and at time of randomisation (phase B), but no results were reported at later times of assessment |
| Other bias | Low risk | No other bias. |
| Study characteristics | ||
| Methods | Design: randomised double‐blind placebo‐controlled trial Duration: 4 weeks | |
| Participants | Country: UK Setting: residents from one long stay of psychogeriatric ward of hospital Total number of participants: 36 Gender distribution: 100% women Mean age (years): 65 years or older Inclusion criteria: senile dementia, Alzheimer type, according to ICD‐9, receiving a stable dose of between 10 mg and 100 mg of thioridazine per day for at least 2 months Exclusion criteria: male, multi‐infarct dementia and antipsychotic agents other than thioridazine | |
| Interventions | Intervention 1: withdrawal of thioridazine Intervention 2: continuation of thioridazine Antipsychotic drug: thioridazine Pre‐trial: tapering to half of the daily dose in the first week and to placebo over the next week Post‐trial: all participants were restored to half their original dose of thioridazine with any subsequent alterations being made by their regular medical attendant on an empirical base Concomitant treatment: chlormethiazole | |
| Outcomes | Primary outcomes: cognitive function: CAS (0, 2, 4 weeks), cognitive and behavioural dysfunction: LPRS (0, 2, 4 weeks), functioning: SCAGS (0, 2, 4 weeks) Secondary outcomes: systolic BP and heart rate (0, 2, 4 weeks) Results only given as means, ranges and numbers of observations | |
| Notes | Conflict of interest and source of funding were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: methods of sequence generation were not described Quote "...matching active and placebo (liquid) formulations of thioridazine were used, each subject being entered separately and allocated by a random code to the active or placebo group in a double‐blind manner" |
| Allocation concealment (selection bias) | Unclear risk | Comment: study described as 'randomised', the randomisation process was not completely successful Quote: "...each subject being allocated by a random code to the active or placebo group in a double‐blind manner ... The starting difference in Cognitive Assessment Scale scores between active‐continued and placebo‐substituted groups represents an artefact of the randomisation process." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study described as "double blinded", unlikely that blinding was been broken Quote: "matching active and placebo (liquid) formulations were used ... During the first week patients in the 'placebo' group received placebo substitution for half of their daily dose of thioridazine and over the next week a total substitution. Similar mock substitutions with thioridazine were given to the 'active' group, so that initial medication was continued but the trial remained double‐blind. After four weeks all patients were restored to half their original dose of thioridazine with any subsequent alterations being made by their regular medical attendant on an empirical basis ... as it was not possible to break the code in the middle of the trial " |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: study is described as double‐blinded, blinding of the outcome assessors is not described. Assessment was done by clinicians and nurses with psychiatric training. In these trial, they were several subjective outcomes, so a lack of blinding of outcome assessors could had an influence. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information of dropouts is not reported in the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: primary outcome is not described, it is unclear if a selection of measured outcomes was reported |
| Other bias | Unclear risk | Comment: the randomisation procedure unfortunately resulted in a baseline imbalance in 1 of the 3 cognitive/behavioural rating scales (starting difference in cognitive assessment scale (CAS) between active continued was 4.2 treatment and 9.8 in the placebo‐substituted groups. The author noted: "Difference represents an artefact of the randomisation process." It is unclear if this has had an impact on outcomes. |
| Study characteristics | ||
| Methods | Design: randomised, placebo‐controlled, double‐blinded study Duration: 4 weeks | |
| Participants | Country: Norway Setting: residents of 13 nursing homes Diagnosis: dementia diagnosis according to the clinical criteria of ICD‐10 Number of participants: 55 (27 intervention, 28 reference) Gender distribution (F) (number, %): 20 (74%) intervention, 23 (82%) reference Mean age (years): 83.6 (SD 8.1) intervention, 84.6 (SD 5.9) reference Inclusion criteria: older participants, aged 65 years and over, dementia diagnosis according to the clinical criteria of ICD‐10 residence in the facility for at least 3 months before inclusion, taking haloperidol, risperidone or olanzapine for nonpsychotic symptoms for at least 3 months before the study as standing medication in stable doses Exclusion criteria: participants with antipsychotic use for a primary diagnosis of major psychotic disorder, mental retardation, terminal illness with life expectancy judged to be shorter than 3 months and recent major changes in health status | |
| Interventions | Intervention group: abrupt discontinuation of antipsychotic medication Reference group: no discontinuation of antipsychotic medication Note: same dose of initial daily dose of antipsychotic drugs in intervention group: risperidone 1.0 (0.5 to 2.0) mg, haloperidol 1.0 (0.5 to 1.5) mg or olanzapine 5.0 (2.5 to 5.0) mg | |
| Outcomes | Behavioural and psychological symptoms measured by the NPI‐Q (second baseline period, week 1, week 4). The NPI‐Q covers 12 symptoms: delusions, hallucinations, agitation/aggression, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behaviour (restlessness, e.g. purposeless wandering and inappropriate activity), sleep problems and eating disorders. Information on participants' symptoms was obtained by interview with the primary nurse informant. Individual symptoms were scored as 0 (absent), 1 (mild), 2 (moderate), or 3 (severe), providing an NPI‐Q sum score rating from 0 to 36. Three separate ratings were conducted for all the participant. These ratings included symptoms occurring during the 7‐day period before assessment. Sleep/wake activity was recorded continuously during baseline and intervention (i.e. over 6 weeks) using an Actiwatch portable recorder (second baseline week, week 1, week 4). The Actiwatch is a small wrist‐worn device containing an accelerometer that is optimised for highly effective sleep‐week inference from wrist activity. Actigraphically measured wrist activity is a feasible and reliable method for sleep/wake evaluation in nursing home residents. The following actigraphic parameters were calculated: total sleep time, total wake time, sleep efficiency (proportion of sleep during night window, i.e. 11 pm to 7 am), daytime activity and night‐time activity. The ratio of day‐to‐night‐time activity was calculated and expressed as a light/dark ratio. Mean 24‐hours activity and peak times of activity were calculated. Analyses of sleep/wake activity were based on 3 x 7‐day records. | |
| Notes | Conflict of interests and funding were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation is done computer random number generator Quote: "Participants were assigned to antipsychotic drug discontinuation (intervention group) or no discontinuation (reference group) by means of computer generated, random, permuted blocks of 4... " |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was provided central, no further details were reported Quote: "...an independent researcher ... participants were consecutively assigned to antipsychotic drug discontinuation or no discontinuation..." |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: blinding of participants and personnel is described, unlikely that blinding could have been broken. Quote: "In the intervention group, patients received inert placebo capsules consisting of lactose, whereas reference group patients received identically looking capsules containing continued antipsychotic drug treatment at current dose ... all study medications were provided by an independent pharmacy to maintain blindness ... Sealed envelopes, containing details of study medication for each patient, were available for the nursing home physicians in case of serious health events." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: study is described as double‐blinded. Blinding of the assessment interviewers is not described. In these trial, they were several subjective outcomes, so a lack of blinding of outcome assessors could had an influence. Quote: "NPI rating was based on interviews with patient' prime nurse on her observations of BPSD the previous week. The interviews were conducted by specially trained medical students... Sealed envelopes... At the completion of the intervention, randomizations codes were broken." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comments: all 55 participants completed at least the week one evaluation were included in study analysis. No statistical difference in dropout between intervention and reference group. Quote: "Seven patients completed the study prematurely, due to unblinding for randomisation code, behavioural deterioration, restless legs or delirium." |
| Selective reporting (reporting bias) | Low risk | Comment: all intended outcomes reported in accordance with the methods section |
| Other bias | Unclear risk | Comment: the selection of participants may have been biased. It is not clear if the participating nursing homes participants are different from non‐participating nursing home patients. |
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, placebo‐controlled trial Duration: 26 weeks | |
| Participants | Country: Canada Setting: residents of two nursing homes and geriatric chronic care floor of an academic health science centre Total number of participants: 34 (17 placebo, 17 active) Gender distribution (male): 47.1% placebo; 56.3% active Mean (SD) age, years: 84.4 (SD 4;6) placebo, 82.9 (SD 6.9) active Inclusion criteria: any form of dementia, receiving antipsychotics for 6 months or longer, stable behaviour Exclusion criteria: history of antipsychotic discontinuation having failed within the past 6 months, a history of schizophrenia, antipsychotic use for nausea, diagnosis of delirium (DSM‐IV criteria), a global rating scale of 3 on the BEHAVE‐AD rating scale at the time of the screening, 1 week prior to the start of the study or within the 2 weeks of the pre‐trial period | |
| Interventions | Placebo group: discontinuation antipsychotics Active treatment: continuing antipsychotic treatment with the same dose Antipsychotics: risperidone, thioridazine, loxapine, perphenazine, haloperidol, olanzapine, nozinan Note: pre‐trial period: 2‐week pre‐trial period and a 2 week dose reduction period by tapering (dose reduction with original medication halved for the first week of the dose reduction period and the remaining dose halved for the second week) Concomitant medication: lorazepam 0.5 mg to 1 mg every 8 hours on a per need basis for agitation | |
| Outcomes | Behavioural outcome measures: BEHAVE‐AD, the NPI and the ROAS (each visit: 15 times) Cognitive functioning: MMSE (0, 4, 8, 12, 14, 16, 20, 24 weeks), MDRS (0, 24 weeks) Functional level: BDS, ADL and motivational behaviour subscale (0, 4, 8, 12, 14, 16, 20, 24 weeks) Extrapyramidal signs: ESRS (0, 4, 8, 12, 14, 16, 20, 24 weeks) Clinical impression of severity of behavioural disturbance: CGI (all visits). The CGI quantified the clinical impression of severity of behavioural disturbance on a 7‐point, verbally anchored scale. The degree of change from baseline was also ranked on a similar scale All outcome data were obtained by a trained research assistant upon interview of the prime nurse or the subject as appropriate for the instrument. | |
| Notes | Funding source and conflict of interests were not reported in study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: participants randomly assigned to treatment by random number table Quote: "A random number table was used to allocate subjects to receive either continued antipsychotic treatment at the current dose or to receive identical placebo" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no reference made to the method in which allocation concealment was ensured |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study is described as "double blind" and unlikely blinding could have been broken Quote: "A randomised double blind placebo controlled study design was used ... During all study periods, medications, including placebo, were placed into identical capsules to maintain blindness." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: study is described as double‐blinded, blinding of the research team is not described. In these trial, they were several subjective outcomes, so a lack of blinding of outcome assessors could had an influence. Quote: "All outcome data were obtained by a trainee research assistant upon interview of the prime nurse or the subject as appropriate for the instrument... if the clinical staff observed significant behavioural worsening that they thought warranted early withdrawal from the study, the ware asked to contact the research team immediately and the outcome measures were repeated at that time." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: rates and reasons for dropouts were reported. Analysis is done by intention‐to‐treatment principle. Quote: "The total number of subjects who were withdrawn from the study early was 10/17 in the placebo group and 6/17 in the active treatment group. The difference in the rate of early study withdrawal was not statistically significant. Subjects were withdrawn due to medical illness (3), death (3), extrapyramidal symptoms (3), and exacerbations of behavioural problems (4 in the placebo and 3 in the active group)." |
| Selective reporting (reporting bias) | High risk | Comments: some data of the continuation and the discontinuation group for several outcomes is not completely given in numerical results but only in descriptive figures. NPS assessed by NPI, aggression assessed by the ROAS, extrapyramidal signs assessed by the ESRS, cognitive functioning assessed by MMSE and functional outcome assessed by the BDS were not reported in the paper. |
| Other bias | Low risk | No other bias. |
ADL: activities of daily living; BDS: Blessed Dementia Scale; BEHAVE‐AD: Behavioural Pathology in Alzheimer’s disease; BFAS: Blessed Functional Activity Scale; BP: blood pressure; BPRS: Brief Psychiatric Rating Scale; CAS: Cognitive Assessment Scale; CGI: Clinical Global Impression; CGI‐C: Clinical Global Impression‐Change; CMAI: Cohen‐Mansfield Agitation Inventory; DCM: Dementia Care Mapping; DSM‐III: Diagnostic and Statistical Manual of Mental Disorders ‐ 3th Edition; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders ‐ 4th Edition; ESRS: Extrapyramidal Symptom Rating Scale; LPRS: London Psychogeriatric Rating Scale; MDRS: Mattis Dementia Rating Scale; MMSE: Mini‐Mental State Examination; NIH: National Institutes of Health; NPI: Neuropsychiatric Inventory; NPQ‐I: Neuropsychiatric Inventory Questionnaire; NPS: neuropsychiatric symptoms; RCT: randomised controlled trial; SCAGS: Sandoz Clinical Assessment Geriatric Scale; SIB: Severe Impairment Battery; TESS: Treatment Emergent Symptom Scale; ROAS: Retrospective Overt Aggression Scale; UPDRS: Unified Parkinson's Disease Rating Scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not an RCT but a clinical trial without a suitable control group | |
| Intervention was discontinuation of memantine | |
| Intervention was discontinuation of antidepressants | |
| Commentary | |
| Commentary | |
| Commentary | |
| Commentary | |
| Not a clinical trial, but a naturalistic study | |
| Commentary | |
| Commentary | |
| This trial reports a primary outcome (prolactin response to withdrawal of thioridazine assessed in the Findlay 1989 cohort), which has no relationship with the neuropsychiatric symptom in which we were interested | |
| Not an RCT; post hoc analysis of Devanand 2012 | |
| Commentary | |
| Commentary | |
| This reference probably refers to the registration of a discontinuation study which has not been published until now (no more information found on this reference) | |
| This trial was not a discontinuation trial | |
| This trial was a follow‐up study and not a discontinuation trial |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Behavioural assessment Show forest plot | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐5.39, 2.40] |
| Analysis 1.1  Comparison 1: Discontinuation versus continuation of long‐term antipsychotic drug use (continuous data, analysis method mean difference), Outcome 1: Behavioural assessment | ||||

Forest plot of comparison: 1 Discontinuation versus continuation of long‐term antipsychotic drug use: continuous data, analysis method: mean difference, outcome: 1.1 Behavioural assessment by using Neuropsychiatric Inventory (NPI) measuring neuropsychiatric symptoms (NPS) at 3 months (Ballard 2004 and Ballard DART‐AD) (Analysis 1.1).
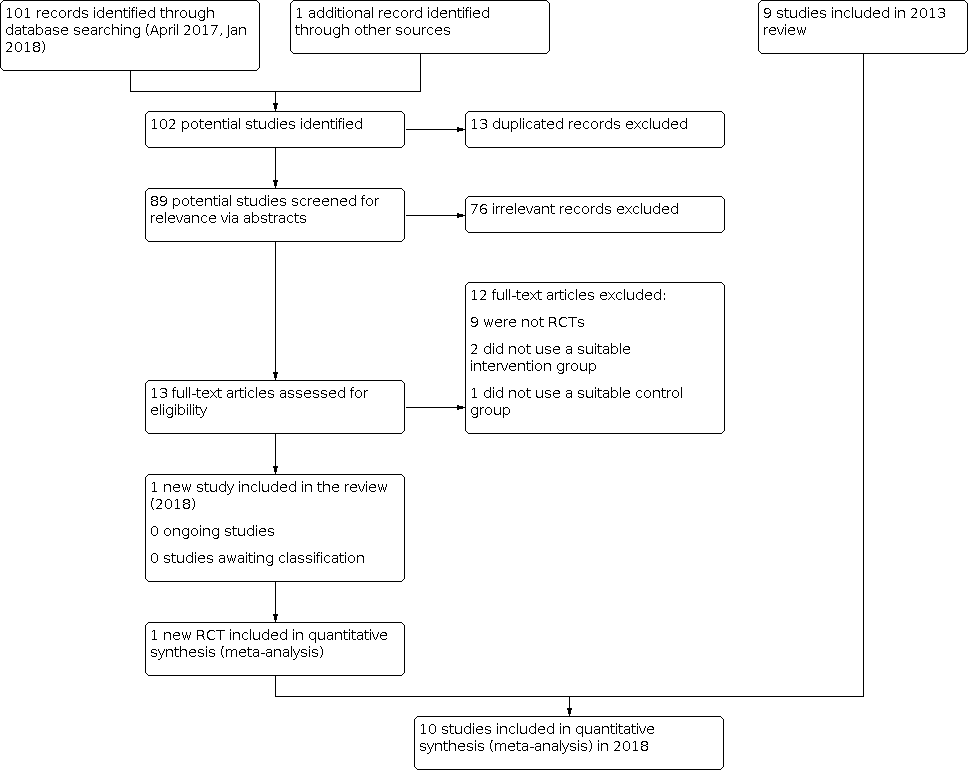
Inclusions of trials of study flow diagram 2018

Risk of bias graph for the 10 included studies in the review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study in the review.

Comparison 1: Discontinuation versus continuation of long‐term antipsychotic drug use (continuous data, analysis method mean difference), Outcome 1: Behavioural assessment
| Discontinuation compared to continuation of antipsychotic medication for behavioural and psychological symptoms in older participants with dementia | ||||||
| Patient or population: older people with dementia who had been taking an antipsychotic drug for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk Continuation antipsychotics | Corresponding risk Discontinuation antipsychotics | |||||
| Success of withdrawal from antipsychotics Measured with a variety of outcomes related to failure to complete the study Follow‐up: 1 to 8 months | In 7 studies there was no overall difference in the outcomes reported for success of withdrawal. In two studies of participants with psychosis, aggression or agitation who had responded to antipsychotic treatment, discontinuation accelerated symptomatic relapse without affecting the number of participants experiencing a relapse in one study and was associated with a higher rate of symptomatic relapse in the other study. In one small study a high proportion of the participants in the discontinuation group failed to complete the study. | 575 (9 RCTs) | ⊕⊕⊝⊝ LOWab | Our intended primary outcome, success of withdrawal defined as the ability to complete the study in the allocated study group, i.e. no failure due to worsening of NPS or relapse to antipsychotic drug use, was not reported in any study. We used the difference between groups in the number of non‐completers of the study as a proxy for our primary outcome. However, data could not be pooled due to variability in outcome measures. | ||
| Behavioural and psychological symptoms Assessed with various scales. Follow up: 1 to 8 months | In 2 pooled studies there was no difference in NPI scores between the continuation and discontinuation groups (see Data and analyses and Figure 1). In five non‐pooled studies, there was no difference in the outcomes on scales measuring overall behaviour and psychological symptoms between groups. | 519 (7 RCTs) | ⊕⊕⊝⊝ | Data could only be pooled for 2 studies due to variability in outcome measures. The two pooled studies performed subgroup analyses according to baseline NPI‐score (≤ 14 or > 14). In one study, some participants with milder symptoms at baseline were less agitated at three months in the discontinuation group. In both studies, discontinuation led to worsening of NPS in some participants with more severe baseline NPS. | ||
| Adverse events Assessed with various scales. Follow‐up: 1 to 8 months | In 5 studies, there was no evidence of a difference between groups in adverse events. | 381 (5 RCTs) | ⊕⊕⊝⊝ LOWab | Data could not be pooled due to variability in outcome measures. Adverse events of antipsychotics were not systematically reported. | ||
| Quality of life (QoL) Assessed with DCM or QoL‐AD. Follow‐up: 3 months to 25 weeks | In 2 studies, there was no evidence of an effect on quality of life. | 119 (2 RCTs) | ⊕⊕⊝⊝ | Data could not be pooled due to variability in outcome measures. There was no difference between discontinuation and continuation group in the overall cohort or in subgroups with baseline NPI score above or below the median (14). | ||
| Cognitive function Assessed with various scales. Follow‐up: 1 to 8 months | In 5 studies, there was no evidence of an impact on scales measuring overall cognitive function. In one of these trials, discontinuation improved a measure of verbal fluency. | 365 (5 RCTs) | ⊕⊕⊝⊝ | Data could not be pooled due to variability in outcome measures. | ||
| Use of physical restraint Follow‐up: 1 month | In one study there was no effect on the use of physical restraint. | 36 (1 RCT) | ⊕⊝⊝⊝ | Conclusion made by the authors but not supported by data. | ||
| Mortality Assessed with various scales. Follow‐up: 4 to 12 months | In two studies there was no evidence of an effect on mortality. | 275 (2 RCTs) | ⊕⊝⊝⊝ | Data could not be pooled due to clinical heterogeneity. In a long‐term follow‐up of 36 months after the 12 months randomised discontinuation trial (Devanand 2012), we were uncertain whether discontinuation decreased mortality. | ||
| *The basis for the assumed risk (e.g. the median control group risk across the studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded one level for indirectness. b Downgraded one level for risk of bias. c Downgraded one level for imprecision due to a small number of participants. d Downgraded two level for risk of bias. | ||||||
| Phenothiazines with aliphatic side chain Phenothiazines with piperazine structure Fhenothiazines with piperidine structure Butyrophenone derivatives Indole derivatives Thioxanthene derivatives Diphenylbutylpiperidine derivatives Diazepines, Oxazepines and Thiazepines Benzamides Other antipsychotics |
| Phenothiazines with aliphatic side‐chain N05AA01 Chlorpromazine 0.3 g per os N05AA02 Levomepromazine 0.3 g per os N05AA03 Promazine 0.3 g per os N05AA04 Acepromazine 0.1 g per os N05AA05 Triflupromazine 0.1 g per os N05AA06 Cyamemazine N05AA07 Chlorproethazine |
| Phenothiazines with piperazine structure N05AB01 Dixyrazine 50 mg per os N05AB02 Fluphenazine 10 mg per os N05AB03 Perphenazine 30 mg per os N05AB04 Prochlorperazine 0.1 g per os N05AB05 Thiopropazate 60 mg per os N05AB06 Trifluoperazine 20 mg per os N05AB07 Acetophenazine 50 mg per os N05AB08 Thioproperazine 20 mg per os N05AB09 Butaperazine 10 mg per os N05AB10 Perazine 0.1 g per os N05AB20 Homophenazine |
| Phenothiazines with piperidine structure N05AC01 Periciazine 50 mg per os N05AC02 Thioridazine 0.3 g per os N05AC03 Mesoridazine 0.2 g per os N05AC04 Pipotiazine 10 mg per os |
| Butyrophenone derivatives N05AD01 Haloperidol 8 mg per os N05AD02 Trifluperidol 2 mg per os N05AD03 Melperone* 0.3 g per os N05AD04 Moperon 20 mg per os N05AD05 Pipamperone 0.2 g per os N05AD06 Bromperidol 10 mg per os N05AD07 Benperidol 1.5 mg per os N05AD08 Droperidol N05AD09 Fluanisone |
| N05AE Indole derivatives N05AE01 Oxypertine 0.12 g per os N05AE02 Molindone 50 mg per os N05AE03 Sertindole* 16 mg per os N05AE04 Ziprasidone* 80 mg per os |
| Thioxanthene derivatives N05AF01 Flupentixol 6 mg per os N05AF02 Clopenthixol 0.1 g per os N05AF03 Chlorprothixene 0.3 g per os N05AF04 Tiotixene 30 mg per os N05AF05 Zuclopenthixol 30 mg per os |
| Diphenylbutylpiperidine derivatives N05AG01 Fluspirilene N05AG02 Pimozide 4 mg per os N05AG03 Penfluridol 6 mg per os |
| Diazepines, Oxazepines and Thiazepines N05AH01 Loxapine 0.1 g per os N05AH02 Clozapine* 0.3 g per os N05AH03 Olanzapine* 10 mg per os N05AH04 Quetiapine* 0.4 g per os |
| Benzamides N05AL01 Sulpiride 0.8 g per os N05AL02 Sultopride 1.2 g per os N05AL03 Tiapride 0.4 g per os N05AL04 Remoxipride 0.3 g per os N05AL05 Amisulpride* 0.4 g per os N05AL06 Veralipride N05AL07 Levosulpiride 0.4 g per os |
| Other antipsychotics N05AX07 Prothipendyl 0.24 g per os N05AX08 Risperidone* 5 mg per os N05AX09 Clotiapine 80 mg per os N05AX10 Mosapramine* N05AX11 Zotepine* 0.2 g per os N05AX12 Aripiprazole* 15 mg per os N05AX13 Paliperidone* *atypical antipsychotics |
| * Atypical antipsychotic agents. |
| Study IDI | Setting | Duration | Randomised number | Discontinuation group | Continuation group | Discontinuation schedule | Control | Behavioural inclusion criteria | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Residents in long‐term care facilities | 3 months | 100 | 46 | 54 | Abrupt | Typical APa or risperidone | NPIb not higher than 7 | ||
| Residents in long‐term care facilities | 6 months 12 months | 165 | 82 | 83 | Abrupt | Typical and risperidone | NRc | ||
| Residents in nursing homes | 25 weeks | 19 | 9 | 10 | Tapering over 2 week | Risperidone | NRc | Unpublished study | |
| Residents in long‐term care facilities | 1 month | 36 | 22 | 14 | Abrupt + tapering over 2 weeks | Typical APa | Physically aggressive participants identified by nurse supervisors | ||
| Residents in nursing homes | 7 weeks followed by 7 weeks cross‐over | 58 | 29 | 29 | Tapering over 3 weeks | Typical APa + lorazepam | NRc | Cross‐over study | |
| Residents in the community | 6 months (primary analysis) 12 months | 20 | 10 | 10 | Abrupt + tapering over 2 weeks | Haloperidol | Current symptoms of psychosis, agitation or aggression | Participants had a response to haloperidol open treatment for 20 weeks | |
| Residents in the community and nursing homes | 4 months 8 months | 110 | 70 | 40 | Abrupt + tapering over 2 week | Risperidone | NPIb score higher than 4 on psychosis or agitation/aggression subscale | Participants had a response to risperidone open treatment for 16 weeks | |
| Residents in nursing homes | 1 month | 36 | 18 | 18 | Tapering over 1 week | Thioridazine | NRc | ||
| Residents in nursing homes | 1 month | 55 | 27 | 28 | Abrupt | Haloperidol risperidone, olanzapine | All participants regardless individual symptoms | ||
| Residents in nursing homes | 26 weeks | 34 | 17 | 17 | Tapering over 2 weeks | Typical APa | Stable behaviour | ||
| a AP: antipsychotic drug. b NPI: Neuropsychiatric Inventory. c NR: not reported. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Behavioural assessment Show forest plot | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐5.39, 2.40] |

