Absetzen im Vergleich zur Weiterbehandlung mit Antipsychotika als Langzeitbehandlung gegen herausfordernde Verhaltensweisen bei älteren Menschen mit Demenz
Appendices
Appendix 1. Initial search: 9 February 2009
| Source | Date range searched | Hits retrieved |
|---|---|---|
| MEDLINE (PubMed) | Up to 9 Feb 2009 | 108 |
| Embase (Ovid SP) | Up to 10 Feb 2009 | 37 |
| PsycINFO (Ovid SP) | Up to 10 Feb 2009 | 20 |
| CINAHL (Ovid SP) | Up to 11 Feb 2009 | 16 |
| LILACS (Bireme) | Up to 9 Feb 2009 | 0 |
| CDCIG SR* | Searched 9 Feb 2009 | 163 |
| CENTRAL (Cochrane Library) | Issue 1 2009 | 75 |
| ISTP Conference Proceedings portal.isiknowledge.com/portal.cgi | Up to 11 Feb 2009 | 105 |
| Australian Digital Theses Program adt.caul.edu.au/ | Searched 12 Feb 2009 | 0 |
| Canadian Theses and Dissertations www.collectionscanada.ca/thesescanada/index-e.html | Searched 12 Feb 2009 | 0 |
| Searched 11 Feb 2009 | 4 | |
| Current Controlled trials: MetaRegister of Controlled trials (mRCT) www.controlled-trials.com/ | Searched 11 Feb 2009 | 1 |
| Searched 12 Feb 2009 | 0 | |
| Nederlands Trial Register www.trialregister.nl/trialreg/index.asp | Searched 12 Feb 2009 | 0 |
| ClinicalTrials.gov www.ClinicalTrials.gov | Included in WHO portal | // |
| IPFMA Clinical Trials Register www.ifpma.org/clinicaltrials.html | Searched 12 Feb 2009 | 0 |
| UMIN Japan Trial Register www.umin.ac.jp/ctr/ | Searched 12 Feb 2009 | 0 |
| OPENsigle | Searched 12 Feb 2009 | 0 |
Appendix 2. Updated search: 11 March 2011
| Source
| Search strategy | Hits retrieved |
|---|---|---|
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Antipsychotic OR neuroleptic OR APSY | 126 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 to 11 March 2011 (Ovid SP) | 1. antipsychotic*.ti,ab. 2. "anti‐psychotic*".ti,ab. 3. Antipsychotic Agents/ 4. neuroleptic*.ti,ab. 5. phenothiazines.ti,ab. 6. Phenothiazines/ 7. butyrophenones.ti,ab. 8. Butyrophenones/ 9. risperidone.ti,ab. 10. Risperidone/ 11. Risperdal*.ti,ab. 12. olanzapine.ti,ab. 13. (Zyprexa* or Zalasta* or Zolafren* or Olzapin* or Oferta* or Zypadhera*).ti,ab. 14. haloperidol.ti,ab. 15. Haloperidol/ 16. (Aloperidin* or Bioperidolo* or Brotopon* or Dozic* or Duraperidol*).ti,ab. 17. prothipendyl.ti,ab. 18. methotrimeprazine.ti,ab. 19. Methotrimeprazine/ 20. (Nosinan* or Nozinan* or Levoprome*).ti,ab. 21. clopenthixol.ti,ab. 22. Clopenthixol/ 23. (Sordinol* or clopentixol).ti,ab. 24. flupenthixol.ti,ab. 25. Flupenthixol/ 26. (flupentixol or depixol* or fluanxol*).ti,ab. 27. clothiapine.ti,ab. 28. metylperon.ti,ab. 29. melperon.ti,ab. 30. droperidol.ti,ab. 31. Droperidol/ 32. (Droleptan* or Dridol* or Inapsine* or Xomolix*).ti,ab. 33. pipamperone.ti,ab. 34. Dipiperon*.ti,ab. 35. benperidol.ti,ab. 36. Benperidol/ 37. Anquil*.ti,ab. 38. bromperidol.ti,ab. 39. Bromidol*.ti,ab. 40. fluspirilene.ti,ab. 41. Fluspirilene/ 42. (Redeptin* or Imap*).ti,ab. 43. pimozide.ti,ab. 44. Pimozide/ 45. orap*.ti,ab. 46. penfluridol.ti,ab. 47. Penfluridol/ 48. (Semap* or Micefal*).ti,ab. 49. sulpiride.ti,ab. 50. Sulpiride/ 51. veralipride.ti,ab. 52. (Agreal* or Agradil*).ti,ab. 53. levosulpiride.ti,ab. 54. sultopride.ti,ab. 55. (Barnetil* or Barnotil* or Topral*).ti,ab. 56. aripiprazole.ti,ab. 57. (Abilify* or Aripiprex*).ti,ab. 58. clozapine.ti,ab. 59. Clozapine/ 60. (Clozaril* or Azaleptin* or Leponex* or Fazaclo* or Froidir* or Denzapine* or Zaponex* or Klozapol* or Clopine*).ti,ab. 61. quetiapine.ti,ab. 62. (Seroquel* or Ketipinor*).ti,ab. 63. thioridazine.ti,ab. 64. Thioridazine/ 65. (Mellaril* or Novoridazine* or Thioril*).ti,ab. 66. or/1‐65 67. exp Dementia/ 68. Delirium/ 69. Wernicke Encephalopathy/ 70. Delirium, Dementia, Amnestic, Cognitive Disorders/ 71. dement*.mp. 72. alzheimer*.mp. 73. (lewy* adj2 bod*).mp. 74. deliri*.mp. 75. (chronic adj2 cerebrovascular).mp. 76. ("organic brain disease" or "organic brain syndrome").mp. 77. ("normal pressure hydrocephalus" and "shunt*").mp. 78. "benign senescent forgetfulness".mp. 79. (cerebr* adj2 deteriorat*).mp. 80. (cerebral* adj2 insufficient*).mp. 81. (pick* adj2 disease).mp. 82. (creutzfeldt or jcd or cjd).mp. 83. huntington*.mp. 84. binswanger*.mp. 85. korsako*.mp. 86. or/67‐85 87. 66 and 86 88. (discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*).ti,ab. 89. 87 and 88 90. randomized controlled trial.pt. 91. controlled clinical trial.pt. 92. randomized.ab. 93. placebo.ab. 94. drug therapy.fs. 95. randomly.ab. 96. trial.ab. 97. groups.ab. 98. or/90‐97 99. (animals not (humans and animals)).sh. 100. 98 not 99 101. 89 and 100 102. (2009* or 2010* or 2011*).ed. 103. 101 and 102 | 86 |
| 3. Embase 1980 to 2011 week 12 (Ovid SP) | 1. antipsychotic*.ti,ab. 2. "anti‐psychotic*".ti,ab. 3. Antipsychotic Agents/ 4. neuroleptic*.ti,ab. 5. phenothiazines.ti,ab. 6. Phenothiazines/ 7. butyrophenones.ti,ab. 8. Butyrophenones/ 9. risperidone.ti,ab. 10. Risperidone/ 11. Risperdal*.ti,ab. 12. olanzapine.ti,ab. 13. (Zyprexa* or Zalasta* or Zolafren* or Olzapin* or Oferta* or Zypadhera*).ti,ab. 14. haloperidol.ti,ab. 15. Haloperidol/ 16. (Aloperidin* or Bioperidolo* or Brotopon* or Dozic* or Duraperidol*).ti,ab. 17. prothipendyl.ti,ab. 18. methotrimeprazine.ti,ab. 19. Methotrimeprazine/ 20. (Nosinan* or Nozinan* or Levoprome*).ti,ab. 21. clopenthixol.ti,ab. 22. Clopenthixol/ 23. (Sordinol* or clopentixol).ti,ab. 24. flupenthixol.ti,ab. 25. Flupenthixol/ 26. (flupentixol or depixol* or fluanxol*).ti,ab. 27. clothiapine.ti,ab. 28. metylperon.ti,ab. 29. melperon.ti,ab. 30. droperidol.ti,ab. 31. Droperidol/ 32. (Droleptan* or Dridol* or Inapsine* or Xomolix*).ti,ab. 33. pipamperone.ti,ab. 34. Dipiperon*.ti,ab. 35. benperidol.ti,ab. 36. Benperidol/ 37. Anquil*.ti,ab. 38. bromperidol.ti,ab. 39. Bromidol*.ti,ab. 40. fluspirilene.ti,ab. 41. Fluspirilene/ 42. (Redeptin* or Imap*).ti,ab. 43. pimozide.ti,ab. 44. Pimozide/ 45. orap*.ti,ab. 46. penfluridol.ti,ab. 47. Penfluridol/ 48. (Semap* or Micefal*).ti,ab. 49. sulpiride.ti,ab. 50. Sulpiride/ 51. veralipride.ti,ab. 52. (Agreal* or Agradil*).ti,ab. 53. levosulpiride.ti,ab. 54. sultopride.ti,ab. 55. (Barnetil* or Barnotil* or Topral*).ti,ab. 56. aripiprazole.ti,ab. 57. (Abilify* or Aripiprex*).ti,ab. 58. clozapine.ti,ab. 59. Clozapine/ 60. (Clozaril* or Azaleptin* or Leponex* or Fazaclo* or Froidir* or Denzapine* or Zaponex* or Klozapol* or Clopine*).ti,ab. 61. quetiapine.ti,ab. 62. (Seroquel* or Ketipinor*).ti,ab. 63. thioridazine.ti,ab. 64. Thioridazine/ 65. (Mellaril* or Novoridazine* or Thioril*).ti,ab. 66. or/1‐65 67. exp dementia/ 68. Lewy body/ 69. delirium/ 70. Wernicke encephalopathy/ 71. cognitive defect/ 72. dement*.mp. 73. alzheimer*.mp. 74. (lewy* adj2 bod*).mp. 75. deliri*.mp. 76. (chronic adj2 cerebrovascular).mp. 77. ("organic brain disease" or "organic brain syndrome").mp. 78. "supranuclear palsy".mp. 79. ("normal pressure hydrocephalus" and "shunt*").mp. 80. "benign senescent forgetfulness".mp. 81. (cerebr* adj2 deteriorat*).mp. 82. (cerebral* adj2 insufficient*).mp. 83. (pick* adj2 disease).mp. 84. (creutzfeldt or jcd or cjd).mp. 85. huntington*.mp. 86. binswanger*.mp. 87. korsako*.mp. 88. CADASIL.mp. 89. or/67‐88 90. 66 and 89 91. (discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*).ti,ab. 92. 90 and 91 93. randomized controlled trial/ 94. randomi?ed.ab. 95. controlled clinical trial/ 96. placebo.ab. 97. randomly.ab. 98. trial.ab. 99. groups.ab. 100. or/93‐99 101. 92 and 100 102. (2009* or 2010* or 2011*).em. 103. 101 and 102 | 178 |
| 4. PsycINFO 1806 to March week 3 2011 (Ovid SP) | 1. antipsychotic*.ti,ab. 2. "anti‐psychotic*".ti,ab. 3. neuroleptic*.ti,ab. 4. phenothiazines.ti,ab. 5. butyrophenones.ti,ab. 6. risperidone.ti,ab. 7. Risperidone/ 8. Risperdal*.ti,ab. 9. olanzapine.ti,ab. 10. (Zyprexa* or Zalasta* or Zolafren* or Olzapin* or Oferta* or Zypadhera*).ti,ab. 11. haloperidol.ti,ab. 12. Haloperidol/ 13. (Aloperidin* or Bioperidolo* or Brotopon* or Dozic* or Duraperidol*).ti,ab. 14. prothipendyl.ti,ab. 15. methotrimeprazine.ti,ab. 16. (Nosinan* or Nozinan* or Levoprome*).ti,ab. 17. clopenthixol.ti,ab. 18. (Sordinol* or clopentixol).ti,ab. 19. flupenthixol.ti,ab. 20. (flupentixol or depixol* or fluanxol*).ti,ab. 21. clothiapine.ti,ab. 22. droperidol.ti,ab. 23. (Droleptan* or Dridol* or Inapsine* or Xomolix*).ti,ab. 24. pipamperone.ti,ab. 25. Dipiperon*.ti,ab. 26. benperidol.ti,ab. 27. bromperidol.ti,ab. 28. Bromidol*.ti,ab. 29. fluspirilene.ti,ab. 30. (Redeptin* or Imap*).ti,ab. 31. pimozide.ti,ab. 32. Pimozide/ 33. orap*.ti,ab. 34. penfluridol.ti,ab. 35. (Semap* or Micefal*).ti,ab. 36. sulpiride.ti,ab. 37. Sulpiride/ 38. veralipride.ti,ab. 39. levosulpiride.ti,ab. 40. sultopride.ti,ab. 41. (Barnetil* or Barnotil* or Topral*).ti,ab. 42. aripiprazole.ti,ab. 43. (Abilify* or Aripiprex*).ti,ab. 44. clozapine.ti,ab. 45. Clozapine/ 46. (Clozaril* or Azaleptin* or Leponex* or Fazaclo* or Froidir* or Denzapine* or Zaponex* or Klozapol* or Clopine*).ti,ab. 47. quetiapine.ti,ab. 48. (Seroquel* or Ketipinor*).ti,ab. 49. thioridazine.ti,ab. 50. Thioridazine/ 51. (Mellaril* or Novoridazine* or Thioril*).ti,ab. 52. (discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*).ti,ab. 53. or/1‐51 54. 52 and 53 55. exp Dementia/ 56. exp Delirium/ 57. exp Huntingtons Disease/ 58. exp Kluver Bucy Syndrome/ 59. exp Wernickes Syndrome/ 60. exp Cognitive Impairment/ 61. dement*.mp. 62. alzheimer*.mp. 63. (lewy* adj2 bod*).mp. 64. deliri*.mp. 65. (chronic adj2 cerebrovascular).mp. 66. ("organic brain disease" or "organic brain syndrome").mp. 67. "supranuclear palsy".mp. 68. ("normal pressure hydrocephalus" and "shunt*").mp. 69. "benign senescent forgetfulness".mp. 70. (cerebr* adj2 deteriorat*).mp. 71. (cerebral* adj2 insufficient*).mp. 72. (pick* adj2 disease).mp. 73. (creutzfeldt or jcd or cjd).mp. 74. huntington*.mp. 75. binswanger*.mp. 76. korsako*.mp. 77. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 78. or/55‐77 79. 54 and 78 80. (2009* or 2010* or 2011*).up. 81. 79 and 80 | 110 |
| 5. CINAHL (EBSCO host) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "cognit* impair*" S21 TX "cognit* defect*" S22 (MH "Cognition Disorders+") S23 TX MCI S24 TX ACMI S25 TX ARCD S26 TX SMC S27 TX CIND S28 TX BSF S29 TX AAMI S30 AB MD S31 AB LCD S32 AB QD OR "questionable dementia" S33 TX AACD S34 TX MNCD S35 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S36 TX "preclinical AD" S37 TX "pre‐clinical AD" S38 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S39 TX aMCI OR MCIa S40 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S41 TX "GDS 3" OR "stage 3 GDS" S42 TX "global deterioration scale" AND "stage 3" S43 TX "Benign senescent forgetfulness" S44 TX "mild neurocognit* disorder*" S45 TX prodrom* N2 dement* S46 TX "age‐related symptom*" S47 TX cognit* N2 deficit* S48 TX cognit* N2 deteriorat* S49 TX cognit* N2 declin* S50 TX cognit* N2 degenerat* S51 TX cognit* N2 complain* S52 TX cognit* N2 disturb* S53 TX cognit* N2 disorder* S54 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S55 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S56 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S57 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S58 TX "pre‐clinical dementia" or TX "preclinical dementia" S59 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 S60 S19 or S59 | 99 |
| 6. ISI Web of Knowledge – all databases (includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports) | #1 Topic=(antipsychotic* OR neuroleptic* OR phenothiazines OR butyrophenones OR risperidone OR olanzapine OR haloperidol OR prothipendyl OR methotrimeprazine OR clopenthixol OR flupenthixol) #2 Topic=(clothiapine OR melperon OR droperidol OR pipamperone OR benperidol OR bromperidol OR fluspirilene OR pimozide OR penfluridol OR sulpiride) #3 Topic=(veralipride OR levosulpiride OR sultopride OR aripiprazole OR clozapine OR quetiapine OR thioridazine) #4 Topic=(discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*) #5 #3 OR #2 OR #1 #6 #5 AND #4 #7 Topic=(dementia OR alzheimer* OR "lew* bod*" OR "parkinson disease dementia" OR VAD OR PDD) #8 #7 AND #6 #9 Topic=(randomly OR randomized OR randomised OR placebo* OR trial OR RCT) #10 #9 AND #8 #11 Topic=(#10) AND Year Published=(2009‐2011) | 90 |
| 7. LILACS (Bireme) | antipsychotic OR antipsychotics OR neuroleptic OR neuroleptics [Words] and dementia OR demenc$ OR alzheimer$ [Words] and 2009 OR 2010 OR 2011 [Country, year publication] | 17 |
| 8. CENTRAL (Cochrane Library) (Issue 1, January 2011) | #1 "anti‐psychotic*" #2 antipsychotic*:ti,ab #3 MeSH descriptor Antipsychotic Agents explode all trees #4 neuroleptic*:ti,ab #5 phenothiazines OR butyrophenones OR risperidone OR Risperdal* OR olanzapine #6 Zyprexa* OR Zalasta* OR Zolafren* OR Olzapin* OR Oferta* OR Zypadhera* #7 haloperidol #8 Aloperidin* OR Bioperidolo* OR Brotopon* OR Dozic* OR Duraperidol* #9 prothipendyl OR methotrimeprazine OR Nosinan* OR Nozinan* OR Levoprome* #10 clopenthixol OR Sordinol* OR clopentixol OR flupenthixol OR flupentixol OR depixol* OR fluanxol* #11 clothiapine OR metylperon OR melperon OR droperidol OR Droleptan* OR Dridol* OR Inapsine* OR Xomolix* #12 pipamperone OR Dipiperon* OR benperidol OR Anquil* OR bromperidol OR Bromidol* OR fluspirilene OR Redeptin* OR Imap* #13 pimozide OR orap* OR penfluridol OR Semap* OR Micefal* #14 sulpiride OR veralipride OR Agreal* OR Agradil* OR levosulpiride OR sultopride #15 Barnetil* OR Barnotil* OR Topral* #16 aripiprazole OR Abilify* OR Aripiprex* OR clozapine OR Clozaril* OR Azaleptin* OR Leponex* OR Fazaclo* OR Froidir* OR Denzapine* OR Zaponex* OR Klozapol* #17 quetiapine OR Seroquel* OR Ketipinor* #18 thioridazine OR Mellaril* OR Novoridazine* OR Thioril* #19 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 Dement* #21 Deliri* #22 alzheimer* #23 "organic brain disease" OR "organic brain syndrome" #24 creutzfeldt OR jcd OR cjd #25 huntington* #26 binswanger* #27 korsako* #28 "parkinson* disease dementia*" OR PDD #29 "lew* bod*" OR DLB OR LDB OR LBD #30 MeSH descriptor Dementia explode all trees #31 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #32 (#19 AND #31), from 2009 to 2011 | 46 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Search 1: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | antipsychotic OR neuroleptic OR risperidone OR olanzapine OR haloperidol OR prothipendyl OR clopenthixol | received from 01/01/2009 to 03/31/2011 Search 2: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | clothiapine OR droperidol OR pipamperone OR benperidol OR bromperidol OR fluspirilene OR pimozide | received from 01/01/2009 to 03/31/2011 Search 3: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | penfluridol OR sulpiride OR veralipride OR levosulpiride OR sultopride OR aripiprazole OR clozapine OR quetiapine OR thioridazine | received from 01/01/2009 to 03/31/2011 | 9 + 0 + 2 = 11 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrials.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; Netherlands National Trial Register) | Search 1: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | antipsychotic OR neuroleptic OR risperidone OR olanzapine OR haloperidol OR prothipendyl OR clopenthixol | received from 01/01/2009 to 03/31/2011 Search 2: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | clothiapine OR droperidol OR pipamperone OR benperidol OR bromperidol OR fluspirilene OR pimozide | received from 01/01/2009 to 03/31/2011 Search 3: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | penfluridol OR sulpiride OR veralipride OR levosulpiride OR sultopride OR aripiprazole OR clozapine OR quetiapine OR thioridazine | received from 01/01/2009 to 03/31/2011 | 13 |
| TOTAL before de‐duplication | 776 | |
| TOTAL after de‐duplication and first‐assess | 70 | |
Appendix 3. Top‐up searches: June 2012, November 2012, March 2017, January 2018
| Source | Search strategy | Hits retrieved |
|---|---|---|
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 10 January 2018] | Antipsychotic OR neuroleptic OR APSY | Jun 2012: 58 Nov 2012: 1 Mar 2017: 0 Jan 2018: 0 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 10 January 2018] |
1. antipsychotic*.ti,ab. 2. "anti‐psychotic*".ti,ab. 3. Antipsychotic Agents/ 4. neuroleptic*.ti,ab. 5. phenothiazines.ti,ab. 6. Phenothiazines/ 7. butyrophenones.ti,ab. 8. Butyrophenones/ 9. risperidone.ti,ab. 10. Risperidone/ 11. Risperdal*.ti,ab. 12. olanzapine.ti,ab. 13. (Zyprexa* or Zalasta* or Zolafren* or Olzapin* or Oferta* or Zypadhera*).ti,ab. 14. haloperidol.ti,ab. 15. Haloperidol/ 16. (Aloperidin* or Bioperidolo* or Brotopon* or Dozic* or Duraperidol*).ti,ab. 17. prothipendyl.ti,ab. 18. methotrimeprazine.ti,ab. 19. Methotrimeprazine/ 20. (Nosinan* or Nozinan* or Levoprome*).ti,ab. 21. clopenthixol.ti,ab. 22. Clopenthixol/ 23. (Sordinol* or clopentixol).ti,ab. 24. flupenthixol.ti,ab. 25. Flupenthixol/ 26. (flupentixol or depixol* or fluanxol*).ti,ab. 27. clothiapine.ti,ab. 28. metylperon.ti,ab. 29. melperon.ti,ab. 30. droperidol.ti,ab. 31. Droperidol/ 32. (Droleptan* or Dridol* or Inapsine* or Xomolix*).ti,ab. 33. pipamperone.ti,ab. 34. Dipiperon*.ti,ab. 35. benperidol.ti,ab. 36. Benperidol/ 37. Anquil*.ti,ab. 38. bromperidol.ti,ab. 39. Bromidol*.ti,ab. 40. fluspirilene.ti,ab. 41. Fluspirilene/ 42. (Redeptin* or Imap*).ti,ab. 43. pimozide.ti,ab. 44. Pimozide/ 45. orap*.ti,ab. 46. penfluridol.ti,ab. 47. Penfluridol/ 48. (Semap* or Micefal*).ti,ab. 49. sulpiride.ti,ab. 50. Sulpiride/ 51. veralipride.ti,ab. 52. (Agreal* or Agradil*).ti,ab. 53. levosulpiride.ti,ab. 54. sultopride.ti,ab. 55. (Barnetil* or Barnotil* or Topral*).ti,ab. 56. aripiprazole.ti,ab. 57. (Abilify* or Aripiprex*).ti,ab. 58. clozapine.ti,ab. 59. Clozapine/ 60. (Clozaril* or Azaleptin* or Leponex* or Fazaclo* or Froidir* or Denzapine* or Zaponex* or Klozapol* or Clopine*).ti,ab. 61. quetiapine.ti,ab. 62. (Seroquel* or Ketipinor*).ti,ab. 63. thioridazine.ti,ab. 64. Thioridazine/ 65. (Mellaril* or Novoridazine* or Thioril*).ti,ab. 66. or/1‐65 67. exp Dementia/ 68. Delirium/ 69. Wernicke Encephalopathy/ 70. Delirium, Dementia, Amnestic, Cognitive Disorders/ 71. dement*.mp. 72. alzheimer*.mp. 73. (lewy* adj2 bod*).mp. 74. deliri*.mp. 75. (chronic adj2 cerebrovascular).mp. 76. ("organic brain disease" or "organic brain syndrome").mp. 77. ("normal pressure hydrocephalus" and "shunt*").mp. 78. "benign senescent forgetfulness".mp. 79. (cerebr* adj2 deteriorat*).mp. 80. (cerebral* adj2 insufficient*).mp. 81. (pick* adj2 disease).mp. 82. (creutzfeldt or jcd or cjd).mp. 83. huntington*.mp. 84. binswanger*.mp. 85. korsako*.mp. 86. or/67‐85 87. 66 and 86 88. (discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*).ti,ab. 89. 87 and 88 90. randomized controlled trial.pt. 91. controlled clinical trial.pt. 92. randomized.ab. 93. placebo.ab. 94. drug therapy.fs. 95. randomly.ab. 96. trial.ab. 97. groups.ab. 98. or/90‐97 99. (animals not (humans and animals)).sh. 100. 98 not 99 101. 89 and 100 102. (2011* or 2012*).ed. 103. 101 and 102 | Jun 2012: 60 Nov 2012: 28 (plus suppl search hits) Mar 2017: 284 Jan 2018: 63 |
| 3. Embase 1980 to 2018 January 09 (Ovid SP) [Date of most recent search: 10 January 2018] | 1. antipsychotic*.ti,ab. 2. "anti‐psychotic*".ti,ab. 3. Antipsychotic Agents/ 4. neuroleptic*.ti,ab. 5. phenothiazines.ti,ab. 6. Phenothiazines/ 7. butyrophenones.ti,ab. 8. Butyrophenones/ 9. risperidone.ti,ab. 10. Risperidone/ 11. Risperdal*.ti,ab. 12. olanzapine.ti,ab. 13. (Zyprexa* or Zalasta* or Zolafren* or Olzapin* or Oferta* or Zypadhera*).ti,ab. 14. haloperidol.ti,ab. 15. Haloperidol/ 16. (Aloperidin* or Bioperidolo* or Brotopon* or Dozic* or Duraperidol*).ti,ab. 17. prothipendyl.ti,ab. 18. methotrimeprazine.ti,ab. 19. Methotrimeprazine/ 20. (Nosinan* or Nozinan* or Levoprome*).ti,ab. 21. clopenthixol.ti,ab. 22. Clopenthixol/ 23. (Sordinol* or clopentixol).ti,ab. 24. flupenthixol.ti,ab. 25. Flupenthixol/ 26. (flupentixol or depixol* or fluanxol*).ti,ab. 27. clothiapine.ti,ab. 28. metylperon.ti,ab. 29. melperon.ti,ab. 30. droperidol.ti,ab. 31. Droperidol/ 32. (Droleptan* or Dridol* or Inapsine* or Xomolix*).ti,ab. 33. pipamperone.ti,ab. 34. Dipiperon*.ti,ab. 35. benperidol.ti,ab. 36. Benperidol/ 37. Anquil*.ti,ab. 38. bromperidol.ti,ab. 39. Bromidol*.ti,ab. 40. fluspirilene.ti,ab. 41. Fluspirilene/ 42. (Redeptin* or Imap*).ti,ab. 43. pimozide.ti,ab. 44. Pimozide/ 45. orap*.ti,ab. 46. penfluridol.ti,ab. 47. Penfluridol/ 48. (Semap* or Micefal*).ti,ab. 49. sulpiride.ti,ab. 50. Sulpiride/ 51. veralipride.ti,ab. 52. (Agreal* or Agradil*).ti,ab. 53. levosulpiride.ti,ab. 54. sultopride.ti,ab. 55. (Barnetil* or Barnotil* or Topral*).ti,ab. 56. aripiprazole.ti,ab. 57. (Abilify* or Aripiprex*).ti,ab. 58. clozapine.ti,ab. 59. Clozapine/ 60. (Clozaril* or Azaleptin* or Leponex* or Fazaclo* or Froidir* or Denzapine* or Zaponex* or Klozapol* or Clopine*).ti,ab. 61. quetiapine.ti,ab. 62. (Seroquel* or Ketipinor*).ti,ab. 63. thioridazine.ti,ab. 64. Thioridazine/ 65. (Mellaril* or Novoridazine* or Thioril*).ti,ab. 66. or/1‐65 67. exp dementia/ 68. Lewy body/ 69. delirium/ 70. Wernicke encephalopathy/ 71. cognitive defect/ 72. dement*.mp. 73. alzheimer*.mp. 74. (lewy* adj2 bod*).mp. 75. deliri*.mp. 76. (chronic adj2 cerebrovascular).mp. 77. ("organic brain disease" or "organic brain syndrome").mp. 78. "supranuclear palsy".mp. 79. ("normal pressure hydrocephalus" and "shunt*").mp. 80. "benign senescent forgetfulness".mp. 81. (cerebr* adj2 deteriorat*).mp. 82. (cerebral* adj2 insufficient*).mp. 83. (pick* adj2 disease).mp. 84. (creutzfeldt or jcd or cjd).mp. 85. huntington*.mp. 86. binswanger*.mp. 87. korsako*.mp. 88. CADASIL.mp. 89. or/67‐88 90. 66 and 89 91. (discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*).ti,ab. 92. 90 and 91 93. randomized controlled trial/ 94. randomi?ed.ab. 95. controlled clinical trial/ 96. placebo.ab. 97. randomly.ab. 98. trial.ab. 99. groups.ab. 100. or/93‐99 101. 92 and 100 102. (2011* or 2012*).em. 103. 101 and 102 | Jun 2012: 109 Nov 2012: 35 (plus suppl search hits) Mar 2017: 533 Jan 2018: 133 |
| 4. PsycINFO 1806 to January week 1 2018 (Ovid SP) [Date of most recent search: 10 January 2018] | 1. antipsychotic*.ti,ab. 2. "anti‐psychotic*".ti,ab. 3. neuroleptic*.ti,ab. 4. phenothiazines.ti,ab. 5. butyrophenones.ti,ab. 6. risperidone.ti,ab. 7. Risperidone/ 8. Risperdal*.ti,ab. 9. olanzapine.ti,ab. 10. (Zyprexa* or Zalasta* or Zolafren* or Olzapin* or Oferta* or Zypadhera*).ti,ab. 11. haloperidol.ti,ab. 12. Haloperidol/ 13. (Aloperidin* or Bioperidolo* or Brotopon* or Dozic* or Duraperidol*).ti,ab. 14. prothipendyl.ti,ab. 15. methotrimeprazine.ti,ab. 16. (Nosinan* or Nozinan* or Levoprome*).ti,ab. 17. clopenthixol.ti,ab. 18. (Sordinol* or clopentixol).ti,ab. 19. flupenthixol.ti,ab. 20. (flupentixol or depixol* or fluanxol*).ti,ab. 21. clothiapine.ti,ab. 22. droperidol.ti,ab. 23. (Droleptan* or Dridol* or Inapsine* or Xomolix*).ti,ab. 24. pipamperone.ti,ab. 25. Dipiperon*.ti,ab. 26. benperidol.ti,ab. 27. bromperidol.ti,ab. 28. Bromidol*.ti,ab. 29. fluspirilene.ti,ab. 30. (Redeptin* or Imap*).ti,ab. 31. pimozide.ti,ab. 32. Pimozide/ 33. orap*.ti,ab. 34. penfluridol.ti,ab. 35. (Semap* or Micefal*).ti,ab. 36. sulpiride.ti,ab. 37. Sulpiride/ 38. veralipride.ti,ab. 39. levosulpiride.ti,ab. 40. sultopride.ti,ab. 41. (Barnetil* or Barnotil* or Topral*).ti,ab. 42. aripiprazole.ti,ab. 43. (Abilify* or Aripiprex*).ti,ab. 44. clozapine.ti,ab. 45. Clozapine/ 46. (Clozaril* or Azaleptin* or Leponex* or Fazaclo* or Froidir* or Denzapine* or Zaponex* or Klozapol* or Clopine*).ti,ab. 47. quetiapine.ti,ab. 48. (Seroquel* or Ketipinor*).ti,ab. 49. thioridazine.ti,ab. 50. Thioridazine/ 51. (Mellaril* or Novoridazine* or Thioril*).ti,ab. 52. (discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*).ti,ab. 53. or/1‐51 54. 52 and 53 55. exp Dementia/ 56. exp Delirium/ 57. exp Huntingtons Disease/ 58. exp Kluver Bucy Syndrome/ 59. exp Wernickes Syndrome/ 60. exp Cognitive Impairment/ 61. dement*.mp. 62. alzheimer*.mp. 63. (lewy* adj2 bod*).mp. 64. deliri*.mp. 65. (chronic adj2 cerebrovascular).mp. 66. ("organic brain disease" or "organic brain syndrome").mp. 67. "supranuclear palsy".mp. 68. ("normal pressure hydrocephalus" and "shunt*").mp. 69. "benign senescent forgetfulness".mp. 70. (cerebr* adj2 deteriorat*).mp. 71. (cerebral* adj2 insufficient*).mp. 72. (pick* adj2 disease).mp. 73. (creutzfeldt or jcd or cjd).mp. 74. huntington*.mp. 75. binswanger*.mp. 76. korsako*.mp. 77. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 78. or/55‐77 79. 54 and 78 80. (2011* or 2012*).up. 81. 79 and 80 | Jun 2012: 70 Nov 2012: 58 (plus suppl search hits) Mar 2017: 281 Jan 2018: 73 |
| 5. CINAHL (EBSCO host) [Date of most recent search: 10 January 2018] | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "cognit* impair*" S21 TX "cognit* defect*" S22 (MH "Cognition Disorders+") S23 TX MCI S24 TX ACMI S25 TX ARCD S26 TX SMC S27 TX CIND S28 TX BSF S29 TX AAMI S30 AB MD S31 AB LCD S32 AB QD OR "questionable dementia" S33 TX AACD S34 TX MNCD S35 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S36 TX "preclinical AD" S37 TX "pre‐clinical AD" S38 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S39 TX aMCI OR MCIa S40 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S41 TX "GDS 3" OR "stage 3 GDS" S42 TX "global deterioration scale" AND "stage 3" S43 TX "Benign senescent forgetfulness" S44 TX "mild neurocognit* disorder*" S45 TX prodrom* N2 dement* S46 TX "age‐related symptom*" S47 TX cognit* N2 deficit* S48 TX cognit* N2 deteriorat* S49 TX cognit* N2 declin* S50 TX cognit* N2 degenerat* S51 TX cognit* N2 complain* S52 TX cognit* N2 disturb* S53 TX cognit* N2 disorder* S54 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S55 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S56 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S57 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S58 TX "pre‐clinical dementia" or TX "preclinical dementia" S59 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 S60 S19 or S59 S61 EM 2011 S62 EM 2012 S63 S61 OR S62 S64 S60 AND S63 | Jun 2012: 71 Nov 2012: 51 (plus suppl search hits) Mar 2017: 26 Jan 2018: 8 |
| 6. ISI Web of Knowledge – all databases (includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports) [Date of most recent search: 10 January 2018] | #1 Topic=(antipsychotic* OR neuroleptic* OR phenothiazines OR butyrophenones OR risperidone OR olanzapine OR haloperidol OR prothipendyl OR methotrimeprazine OR clopenthixol OR flupenthixol) #2 Topic=(clothiapine OR melperon OR droperidol OR pipamperone OR benperidol OR bromperidol OR fluspirilene OR pimozide OR penfluridol OR sulpiride) #3 Topic=(veralipride OR levosulpiride OR sultopride OR aripiprazole OR clozapine OR quetiapine OR thioridazine) #4 Topic=(discontinu* or withdraw* or cessat* or reduce* or reducing or reduct* or taper* or stop*) #5 #3 OR #2 OR #1 #6 #5 AND #4 #7 Topic=(dementia OR alzheimer* OR "lew* bod*" OR "parkinson disease dementia" OR VAD OR PDD) #8 #7 AND #6 #9 Topic=(randomly OR randomized OR randomised OR placebo* OR trial OR RCT) #10 #9 AND #8 | Jun 2012: 56 Nov 2012: 260 (plus suppl search hits) Mar 2017: 290 Jan 2018: 48 |
| 7. LILACS (Bireme) [Date of most recent search: 10 January 2018] | antipsychotic OR antipsychotics OR neuroleptic OR neuroleptics [Words] and dementia OR demenc$ OR alzheimer$ [Words] | Jun 2012: 6 Nov 2012: 1 Mar 2017: 0 Jan 2018: 0 |
| 8. CENTRAL (Cochrane Library) (Issue 1, 2018) [Date of most recent search: 10 January 2018] | #1 "anti‐psychotic*" #2 antipsychotic*:ti,ab #3 MeSH descriptor Antipsychotic Agents explode all trees #4 neuroleptic*:ti,ab #5 phenothiazines OR butyrophenones OR risperidone OR Risperdal* OR olanzapine #6 Zyprexa* OR Zalasta* OR Zolafren* OR Olzapin* OR Oferta* OR Zypadhera* #7 haloperidol #8 Aloperidin* OR Bioperidolo* OR Brotopon* OR Dozic* OR Duraperidol* #9 prothipendyl OR methotrimeprazine OR Nosinan* OR Nozinan* OR Levoprome* #10 clopenthixol OR Sordinol* OR clopentixol OR flupenthixol OR flupentixol OR depixol* OR fluanxol* #11 clothiapine OR metylperon OR melperon OR droperidol OR Droleptan* OR Dridol* OR Inapsine* OR Xomolix* #12 pipamperone OR Dipiperon* OR benperidol OR Anquil* OR bromperidol OR Bromidol* OR fluspirilene OR Redeptin* OR Imap* #13 pimozide OR orap* OR penfluridol OR Semap* OR Micefal* #14 sulpiride OR veralipride OR Agreal* OR Agradil* OR levosulpiride OR sultopride #15 Barnetil* OR Barnotil* OR Topral* #16 aripiprazole OR Abilify* OR Aripiprex* OR clozapine OR Clozaril* OR Azaleptin* OR Leponex* OR Fazaclo* OR Froidir* OR Denzapine* OR Zaponex* OR Klozapol* #17 quetiapine OR Seroquel* OR Ketipinor* #18 thioridazine OR Mellaril* OR Novoridazine* OR Thioril* #19 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 Dement* #21 Deliri* #22 alzheimer* #23 "organic brain disease" OR "organic brain syndrome" #24 creutzfeldt OR jcd OR cjd #25 huntington* #26 binswanger* #27 korsako* #28 "parkinson* disease dementia*" OR PDD #29 "lew* bod*" OR DLB OR LDB OR LBD #30 MeSH descriptor Dementia explode all trees #31 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #32 (#19 AND #31) | Jun 2012: 13 Nov 2012: 2 (plus suppl search hits) Mar 2017: 105 Jan 2018: 96 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [Date of most recent search: 10 January 2018] | Search 1: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | antipsychotic OR neuroleptic OR risperidone OR olanzapine OR haloperidol OR prothipendyl OR clopenthixol Search 2: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | clothiapine OR droperidol OR pipamperone OR benperidol OR bromperidol OR fluspirilene OR pimozide Search 3: Advanced search: discontinue OR withdraw OR cessation OR reduce or reducing OR reduction OR taper OR stop | dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | penfluridol OR sulpiride OR veralipride OR levosulpiride OR sultopride OR aripiprazole OR clozapine OR quetiapine OR thioridazine | Jun 2012: 2 + 0 + 2 = 4 Nov 2012: 0 (plus suppl search hits) Mar 2017: 1 Jan 2018: 0 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; Netherlands National Trial Register) [Date of most recent search: 10 January 2018] | Search 1: Advanced search: dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | antipsychotic OR neuroleptic OR risperidone OR olanzapine OR haloperidol OR prothipendyl OR clopenthixol Search 2: Advanced search: dementia OR alzheimer OR alzhimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | clothiapine OR droperidol OR pipamperone OR benperidol OR bromperidol OR fluspirilene OR pimozide Search 3: Advanced search: dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy OR DLB OR AD OR LBD | penfluridol OR sulpiride OR veralipride OR levosulpiride OR sultopride OR aripiprazole OR clozapine OR quetiapine OR thioridazine | Jun 2012: 7 + 1 + 4 = 13 Nov 2012: 0 (plus suppl search hits) Mar 2017: 20 Jan 2018: 3 |
| TOTAL before de‐duplication and first‐assessment | Jun 2012: 454 Nov 2012: 436 (plus Nov suppl search hits) Mar 2017: 1540 Jan 2018: 424 | |
| TOTAL after de‐duplication and first‐assessment by CDCIG Information specialists based on tiles and abstarcts | Jun 2012: 11 Nov 2012: 20 Mar 2017: 75 Jan 2018: 26 | |
| Supplementary search of additional antipsychotics not covered in previous searches (all dates) | ||
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 to present (Ovid SP) | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. amisulpiride.ti,ab. 22. Chlorpromazine/ 23. chlorpromazine.ti,ab. 24. Promazine/ 25. promazine.ti,ab. 26. Trifluoperazine/ 27. trifluoperazine.ti,ab. 28. Prochlorperazine/ 29. prochlorperazine.ti,ab. 30. or/21‐29 31. 20 and 30 32. randomized controlled trial.pt. 33. controlled clinical trial.pt. 34. randomized.ab. 35. placebo.ab. 36. drug therapy.fs. 37. randomly.ab. 38. trial.ab. 39. groups.ab. 40. or/32‐39 41. 31 and 40 | 194 |
| Embase 1980 to 2012 August 03 (Ovid SP) | 1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. amisulpride/ 25. amisulpiride.ti,ab. 26. chlorpromazine/ 27. Chlorpromazine.ti,ab. 28. promazine/ 29. promazine.ti,ab. 30. trifluoperazine/ 31. trifluoperazine.ti,ab. 32. prochlorperazine/ 33. prochlorperazine.ti,ab. 34. or/24‐33 35. 23 and 34 36. randomized controlled trial/ 37. controlled clinical trial/ 38. randomi?ed.ab. 39. placebo.ab. 40. randomly.ab. 41. trial.ab. 42. groups.ab. 43. ("double‐blind*" or "single‐blind*").ti,ab. 44. or/36‐43 45. 35 and 44 | 425 |
| PsycINFO 1806 to July week 5 2012 (Ovid SP) | 1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. amisulpiride.ti,ab. 26. Chlorpromazine/ 27. chlorpromazine.ti,ab. 28. Promazine/ 29. promazine.ti,ab. 30. Trifluoperazine/ 31. trifluoperazine.ti,ab. 32. Prochlorperazine/ 33. prochlorperazine.ti,ab. 34. or/25‐33 35. 24 and 34 36. randomized.ab. 37. placebo.ab. 38. randomly.ab. 39. trial.ab. 40. groups.ab. 41. "control group".ab. 42. ("double‐blind*" or "single‐blind*").ti,ab. 43. exp Clinical Trials/ 44. or/36‐43 45. 35 and 44 | 27 |
| Total for supplementary searches | 646 | |
| Total for pre‐publication and supplementary search | 1100 | |
| Total post first assess and de‐duplication | 33 | |
Appendix 4. Abbreviations
| ADAS | Alzheimer's Disease Assessment Scale (ADAS) |
| AIMS | Abnormal Involuntary Movement Scale |
| BADLS | Bristol Activities of Daily Living Scale |
| BCRS | Brief Cognitive Rating Scale |
| BDS | Blessed Dementia Scale |
| BEHAVE‐AD | Behavioural Pathology in Alzheimer's disease Rating Scale |
| BFAS | Blessed Functional Activity Scale |
| BPRS | Brief Psychiatric Rating Scale |
| CAS | Cognitive Assessment Scale |
| CDR | Clinical Dementia Rating Scale |
| CERAD | Consortium to Establish a Registry for Alzheimer's Disease |
| CGI‐C | Clinical Global Impression‐Change |
| CMAI | Cohen‐Mansfield Agitation inventory |
| CUSPAD | Columbia University Scale for Psychopathology in Alzheimer's Disease |
| DCM | Dementia Care Mapping |
| Diagnostic and Statistical Manual of Mental Disorders, 3th Edition | |
| Diagnostic and Statistical Manual of Mental Disorders, 4th Edition | |
| ESRS | Extrapyramidal Symptom Rating Scale |
| FAS | F‐A‐S scale, assessing phonemic verbal fluency |
| FAST | Functional Assessment Staging |
| FDA | Food and Drug Administration |
| International Statistical Classification of Diseases and Related Health Problems, Ninth Revision | |
| International Statistical Classification of Diseases and Related Health Problems, Tenth Revision | |
| LPRS | London Psychogeriatric Rating Scale Score |
| MDRS | Mattis Dementia Rating Scale |
| mITT | modified intention‐to‐treat |
| MMSE | Mini‐Mental State Examination |
| M‐UPDRS | Modified Unified Parkinson's Disease Rating Scale |
| NINCDS‐ADRDA | National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association |
| NPI | Neuropsychiatric Inventory Score |
| NPS | Neuropsychiatric symptoms |
| PAB | Physical Aggressive Behaviour |
| PSMS | Physical Self‐Maintenance Scale |
| QoL | Quality of life |
| ROAS | Retrospective Overt Aggression scale |
| RTD | Rockland Tardive Dyskinesia |
| SCAGS | Sandoz Clinical Assessment Geriatric Scale |
| SIB | Severe Impairment Battery |
| SMMSE | Standardised Mini‐Mental State Examination |
| STALD | Sheffield Test for Acquired Language Disorder = STALD receptive and STALD expressive skill |
| TESS | Treatment Emergent Symptom Scale |
| UPDRS | Unified Parkinson's Disease Rating Scale |

Forest plot of comparison: 1 Discontinuation versus continuation of long‐term antipsychotic drug use: continuous data, analysis method: mean difference, outcome: 1.1 Behavioural assessment by using Neuropsychiatric Inventory (NPI) measuring neuropsychiatric symptoms (NPS) at 3 months (Ballard 2004 and Ballard DART‐AD) (Analysis 1.1).
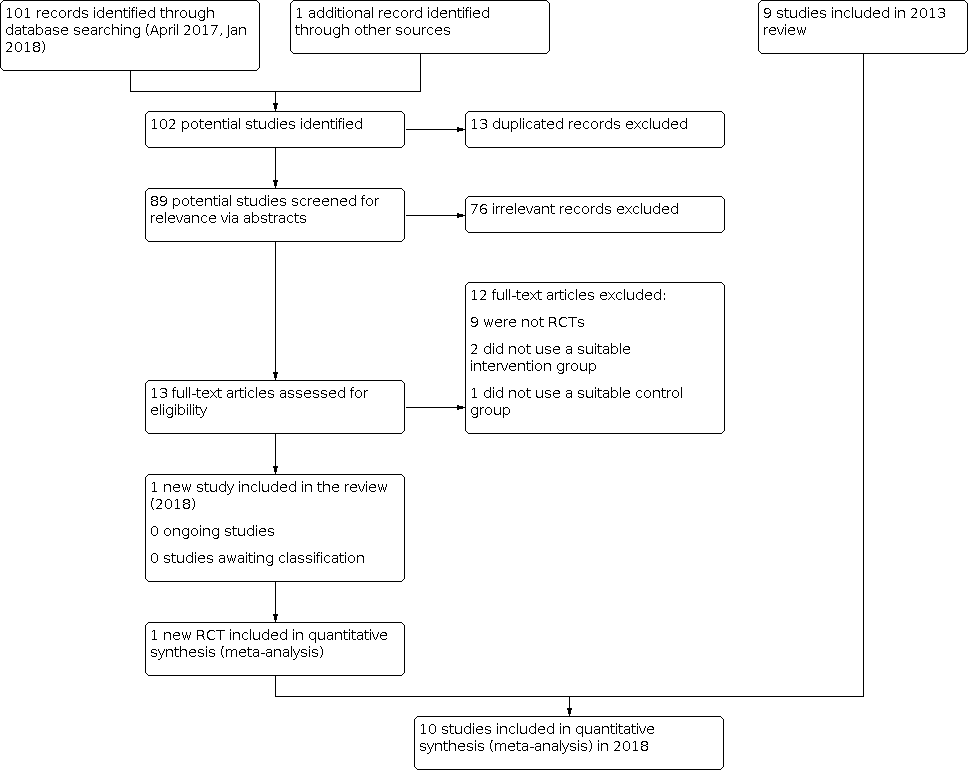
Inclusions of trials of study flow diagram 2018

Risk of bias graph for the 10 included studies in the review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study in the review.

Comparison 1: Discontinuation versus continuation of long‐term antipsychotic drug use (continuous data, analysis method mean difference), Outcome 1: Behavioural assessment
| Discontinuation compared to continuation of antipsychotic medication for behavioural and psychological symptoms in older participants with dementia | ||||||
| Patient or population: older people with dementia who had been taking an antipsychotic drug for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk Continuation antipsychotics | Corresponding risk Discontinuation antipsychotics | |||||
| Success of withdrawal from antipsychotics Measured with a variety of outcomes related to failure to complete the study Follow‐up: 1 to 8 months | In 7 studies there was no overall difference in the outcomes reported for success of withdrawal. In two studies of participants with psychosis, aggression or agitation who had responded to antipsychotic treatment, discontinuation accelerated symptomatic relapse without affecting the number of participants experiencing a relapse in one study and was associated with a higher rate of symptomatic relapse in the other study. In one small study a high proportion of the participants in the discontinuation group failed to complete the study. | 575 (9 RCTs) | ⊕⊕⊝⊝ LOWab | Our intended primary outcome, success of withdrawal defined as the ability to complete the study in the allocated study group, i.e. no failure due to worsening of NPS or relapse to antipsychotic drug use, was not reported in any study. We used the difference between groups in the number of non‐completers of the study as a proxy for our primary outcome. However, data could not be pooled due to variability in outcome measures. | ||
| Behavioural and psychological symptoms Assessed with various scales. Follow up: 1 to 8 months | In 2 pooled studies there was no difference in NPI scores between the continuation and discontinuation groups (see Data and analyses and Figure 1). In five non‐pooled studies, there was no difference in the outcomes on scales measuring overall behaviour and psychological symptoms between groups. | 519 (7 RCTs) | ⊕⊕⊝⊝ | Data could only be pooled for 2 studies due to variability in outcome measures. The two pooled studies performed subgroup analyses according to baseline NPI‐score (≤ 14 or > 14). In one study, some participants with milder symptoms at baseline were less agitated at three months in the discontinuation group. In both studies, discontinuation led to worsening of NPS in some participants with more severe baseline NPS. | ||
| Adverse events Assessed with various scales. Follow‐up: 1 to 8 months | In 5 studies, there was no evidence of a difference between groups in adverse events. | 381 (5 RCTs) | ⊕⊕⊝⊝ LOWab | Data could not be pooled due to variability in outcome measures. Adverse events of antipsychotics were not systematically reported. | ||
| Quality of life (QoL) Assessed with DCM or QoL‐AD. Follow‐up: 3 months to 25 weeks | In 2 studies, there was no evidence of an effect on quality of life. | 119 (2 RCTs) | ⊕⊕⊝⊝ | Data could not be pooled due to variability in outcome measures. There was no difference between discontinuation and continuation group in the overall cohort or in subgroups with baseline NPI score above or below the median (14). | ||
| Cognitive function Assessed with various scales. Follow‐up: 1 to 8 months | In 5 studies, there was no evidence of an impact on scales measuring overall cognitive function. In one of these trials, discontinuation improved a measure of verbal fluency. | 365 (5 RCTs) | ⊕⊕⊝⊝ | Data could not be pooled due to variability in outcome measures. | ||
| Use of physical restraint Follow‐up: 1 month | In one study there was no effect on the use of physical restraint. | 36 (1 RCT) | ⊕⊝⊝⊝ | Conclusion made by the authors but not supported by data. | ||
| Mortality Assessed with various scales. Follow‐up: 4 to 12 months | In two studies there was no evidence of an effect on mortality. | 275 (2 RCTs) | ⊕⊝⊝⊝ | Data could not be pooled due to clinical heterogeneity. In a long‐term follow‐up of 36 months after the 12 months randomised discontinuation trial (Devanand 2012), we were uncertain whether discontinuation decreased mortality. | ||
| *The basis for the assumed risk (e.g. the median control group risk across the studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded one level for indirectness. b Downgraded one level for risk of bias. c Downgraded one level for imprecision due to a small number of participants. d Downgraded two level for risk of bias. | ||||||
| Phenothiazines with aliphatic side chain Phenothiazines with piperazine structure Fhenothiazines with piperidine structure Butyrophenone derivatives Indole derivatives Thioxanthene derivatives Diphenylbutylpiperidine derivatives Diazepines, Oxazepines and Thiazepines Benzamides Other antipsychotics |
| Phenothiazines with aliphatic side‐chain N05AA01 Chlorpromazine 0.3 g per os N05AA02 Levomepromazine 0.3 g per os N05AA03 Promazine 0.3 g per os N05AA04 Acepromazine 0.1 g per os N05AA05 Triflupromazine 0.1 g per os N05AA06 Cyamemazine N05AA07 Chlorproethazine |
| Phenothiazines with piperazine structure N05AB01 Dixyrazine 50 mg per os N05AB02 Fluphenazine 10 mg per os N05AB03 Perphenazine 30 mg per os N05AB04 Prochlorperazine 0.1 g per os N05AB05 Thiopropazate 60 mg per os N05AB06 Trifluoperazine 20 mg per os N05AB07 Acetophenazine 50 mg per os N05AB08 Thioproperazine 20 mg per os N05AB09 Butaperazine 10 mg per os N05AB10 Perazine 0.1 g per os N05AB20 Homophenazine |
| Phenothiazines with piperidine structure N05AC01 Periciazine 50 mg per os N05AC02 Thioridazine 0.3 g per os N05AC03 Mesoridazine 0.2 g per os N05AC04 Pipotiazine 10 mg per os |
| Butyrophenone derivatives N05AD01 Haloperidol 8 mg per os N05AD02 Trifluperidol 2 mg per os N05AD03 Melperone* 0.3 g per os N05AD04 Moperon 20 mg per os N05AD05 Pipamperone 0.2 g per os N05AD06 Bromperidol 10 mg per os N05AD07 Benperidol 1.5 mg per os N05AD08 Droperidol N05AD09 Fluanisone |
| N05AE Indole derivatives N05AE01 Oxypertine 0.12 g per os N05AE02 Molindone 50 mg per os N05AE03 Sertindole* 16 mg per os N05AE04 Ziprasidone* 80 mg per os |
| Thioxanthene derivatives N05AF01 Flupentixol 6 mg per os N05AF02 Clopenthixol 0.1 g per os N05AF03 Chlorprothixene 0.3 g per os N05AF04 Tiotixene 30 mg per os N05AF05 Zuclopenthixol 30 mg per os |
| Diphenylbutylpiperidine derivatives N05AG01 Fluspirilene N05AG02 Pimozide 4 mg per os N05AG03 Penfluridol 6 mg per os |
| Diazepines, Oxazepines and Thiazepines N05AH01 Loxapine 0.1 g per os N05AH02 Clozapine* 0.3 g per os N05AH03 Olanzapine* 10 mg per os N05AH04 Quetiapine* 0.4 g per os |
| Benzamides N05AL01 Sulpiride 0.8 g per os N05AL02 Sultopride 1.2 g per os N05AL03 Tiapride 0.4 g per os N05AL04 Remoxipride 0.3 g per os N05AL05 Amisulpride* 0.4 g per os N05AL06 Veralipride N05AL07 Levosulpiride 0.4 g per os |
| Other antipsychotics N05AX07 Prothipendyl 0.24 g per os N05AX08 Risperidone* 5 mg per os N05AX09 Clotiapine 80 mg per os N05AX10 Mosapramine* N05AX11 Zotepine* 0.2 g per os N05AX12 Aripiprazole* 15 mg per os N05AX13 Paliperidone* *atypical antipsychotics |
| * Atypical antipsychotic agents. |
| Study IDI | Setting | Duration | Randomised number | Discontinuation group | Continuation group | Discontinuation schedule | Control | Behavioural inclusion criteria | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Residents in long‐term care facilities | 3 months | 100 | 46 | 54 | Abrupt | Typical APa or risperidone | NPIb not higher than 7 | ||
| Residents in long‐term care facilities | 6 months 12 months | 165 | 82 | 83 | Abrupt | Typical and risperidone | NRc | ||
| Residents in nursing homes | 25 weeks | 19 | 9 | 10 | Tapering over 2 week | Risperidone | NRc | Unpublished study | |
| Residents in long‐term care facilities | 1 month | 36 | 22 | 14 | Abrupt + tapering over 2 weeks | Typical APa | Physically aggressive participants identified by nurse supervisors | ||
| Residents in nursing homes | 7 weeks followed by 7 weeks cross‐over | 58 | 29 | 29 | Tapering over 3 weeks | Typical APa + lorazepam | NRc | Cross‐over study | |
| Residents in the community | 6 months (primary analysis) 12 months | 20 | 10 | 10 | Abrupt + tapering over 2 weeks | Haloperidol | Current symptoms of psychosis, agitation or aggression | Participants had a response to haloperidol open treatment for 20 weeks | |
| Residents in the community and nursing homes | 4 months 8 months | 110 | 70 | 40 | Abrupt + tapering over 2 week | Risperidone | NPIb score higher than 4 on psychosis or agitation/aggression subscale | Participants had a response to risperidone open treatment for 16 weeks | |
| Residents in nursing homes | 1 month | 36 | 18 | 18 | Tapering over 1 week | Thioridazine | NRc | ||
| Residents in nursing homes | 1 month | 55 | 27 | 28 | Abrupt | Haloperidol risperidone, olanzapine | All participants regardless individual symptoms | ||
| Residents in nursing homes | 26 weeks | 34 | 17 | 17 | Tapering over 2 weeks | Typical APa | Stable behaviour | ||
| a AP: antipsychotic drug. b NPI: Neuropsychiatric Inventory. c NR: not reported. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Behavioural assessment Show forest plot | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐5.39, 2.40] |

