Tratamientos médicos para la púrpura trombocitopénica idiopática durante el embarazo
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial. Open label. Analysis results: per‐protocol analysis. Lost post‐randomisation: 13 (32%) (not included the final analysis).
| |
| Participants | Recruited participants: 59 women (64 pregnancies). Number randomised: 38 women (41 pregnancies). Number analysed: 26 women (28 pregnancies). Mean (SD) age of experimental group (n = 14): 29 (6) years. Inclusion criteria: pregnant women with idiopathic thrombocytopenic purpura (thrombocytopenia (maternal platelet count < 150 x 109/l) on more than 1 occasion, the presence of a normal or increased number of megakaryocytes in bone marrow, and the absence of other diseases, of splenomegaly, of antinuclear antibodies and of the use of drugs known to induce thrombocytopenia). Exclusion criteria: pregnant women already using corticosteroids. | |
| Interventions | Bethametasone: 0.5 mg thrice/day (1.5 mg/day) by the first 2 weeks and 0.5 mg twice/day/ during the third week. | |
| Outcomes | Primary:
| |
| Notes | Funding/support: no special funding required. 8 pregnant women were included with normal platelet counts with diagnosis of ITP made before the current pregnancy and who had had a splenectomy. Trial period: March 1984 to March 1987. Reasons for non‐admitted participants during recruitment period: use of corticosteroids (10 participants), concomitant disease (7 participants) and refused to participate (6 participants). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate: quote: "...according to a computer‐generated table of random numbers". |
| Allocation concealment? | Unclear risk | Insufficient information to permit judgement of ‘yes’ or ‘no’. |
| Blinding? | Unclear risk | Insufficient information to permit judgement of ‘yes’ or ‘no’. |
| Blinding? | High risk | Inadequate. Comment: treatment received by mother were known by paediatricians who assessed newborns. |
| Blinding? | Unclear risk | Insufficient information to permit judgement of ‘yes’ or ‘no’. |
| Incomplete outcome data addressed? | High risk | Inadequate. Comment: study excluded 13 pregnancies (32%). |
| Free of selective reporting? | Unclear risk | Unclear. |
| Free of other bias? | High risk | Inadequate. There is a chance of bias due to inadequate sample size for estimating a real effect. This trial may be affected by early stopping bias: |
| Free of baseline imbalance? | Unclear risk | Inadequate. Comments: it only shows the baseline characteristics of the analysed participants. |
| Free of academic bias? | Low risk | Adequate. No previous published RCTs of the same medical condition. This RCT reports a retrospective study which used prednisone and suggested a beneficial effect on the neonatal platelet count. |
| Free of attrition bias? | High risk | Inadequate. Comment: there were 32% (13/41) post‐randomisation drop‐outs. |
ITP: idiopathic thrombocytopenia purpura
RCT: randomised controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal thrombocytopenia Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Betamethasone versus no treatment, Outcome 1 Neonatal thrombocytopenia. | ||||
| 1.1 Overall | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [0.62, 2.05] |
| 1.2 Severe | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.16, 6.14] |
| 1.3 Mild | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.52, 2.60] |
| 2 Neonatal bleeding Show forest plot | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.24, 4.13] |
| Analysis 1.2  Comparison 1 Betamethasone versus no treatment, Outcome 2 Neonatal bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall neonatal thrombocytopenia Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.97 [1.08, 3.59] |
| Analysis 2.1  Comparison 2 Betamethasone versus no treatment (worse‐case scenario)., Outcome 1 Overall neonatal thrombocytopenia. | ||||
| 2 Neonatal Bleeding Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 3.15 [0.99, 9.99] |
| Analysis 2.2  Comparison 2 Betamethasone versus no treatment (worse‐case scenario)., Outcome 2 Neonatal Bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal bleeding Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 0.32 [0.10, 0.98] |
| Analysis 3.1  Comparison 3 Betamethasone versus no medication (Best‐case scenario), Outcome 1 Neonatal bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal thrombocytopenia Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Betamethasone versus no treatment (Intention‐to treat analysis)., Outcome 1 Neonatal thrombocytopenia. | ||||
| 1.1 Overall | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [0.57, 2.45] |
| 1.2 Severe | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.16, 6.76] |
| 1.3 Mild | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.50, 3.02] |
| 2 Neonatal bleeding Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.24, 4.61] |
| Analysis 4.2  Comparison 4 Betamethasone versus no treatment (Intention‐to treat analysis)., Outcome 2 Neonatal bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall neonatal thrombocytopenia Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 0.63 [0.36, 1.10] |
| Analysis 5.1  Comparison 5 Bethamethasone versus no treatment (best‐case scenario), Outcome 1 Overall neonatal thrombocytopenia. | ||||
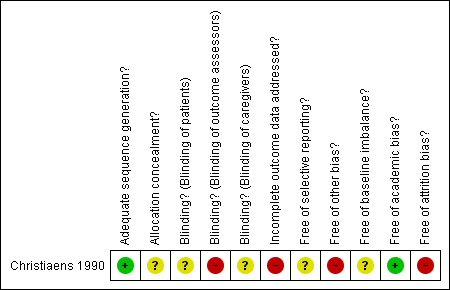
Methodological quality summary: review authors' judgements about each methodological quality item for Christiaens 1990.

Comparison 1 Betamethasone versus no treatment, Outcome 1 Neonatal thrombocytopenia.

Comparison 1 Betamethasone versus no treatment, Outcome 2 Neonatal bleeding.

Comparison 2 Betamethasone versus no treatment (worse‐case scenario)., Outcome 1 Overall neonatal thrombocytopenia.

Comparison 2 Betamethasone versus no treatment (worse‐case scenario)., Outcome 2 Neonatal Bleeding.

Comparison 3 Betamethasone versus no medication (Best‐case scenario), Outcome 1 Neonatal bleeding.

Comparison 4 Betamethasone versus no treatment (Intention‐to treat analysis)., Outcome 1 Neonatal thrombocytopenia.
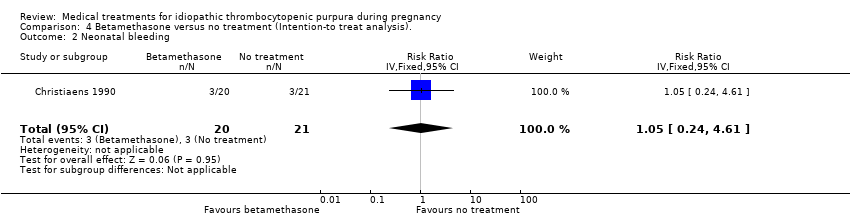
Comparison 4 Betamethasone versus no treatment (Intention‐to treat analysis)., Outcome 2 Neonatal bleeding.

Comparison 5 Bethamethasone versus no treatment (best‐case scenario), Outcome 1 Overall neonatal thrombocytopenia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal thrombocytopenia Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [0.62, 2.05] |
| 1.2 Severe | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.16, 6.14] |
| 1.3 Mild | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.52, 2.60] |
| 2 Neonatal bleeding Show forest plot | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.24, 4.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall neonatal thrombocytopenia Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.97 [1.08, 3.59] |
| 2 Neonatal Bleeding Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 3.15 [0.99, 9.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal bleeding Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 0.32 [0.10, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal thrombocytopenia Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [0.57, 2.45] |
| 1.2 Severe | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.16, 6.76] |
| 1.3 Mild | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.50, 3.02] |
| 2 Neonatal bleeding Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.24, 4.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall neonatal thrombocytopenia Show forest plot | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 0.63 [0.36, 1.10] |


