Dugoročni učinci lijekova za smanjenje tjelesne težine u osoba s hipertenzijom
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | DESIGN: parallel, randomised, double‐blind DURATION: 12 months NUMBER OF STUDY CENTRES: 41 COUNTRY OF PUBLICATION: USA | |
| Participants | WHO PARTICIPATED: obese people with insufficiently controlled hypertension SETTING: outpatient clinic MAIN INCLUSION CRITERIA: age ≥ 40 years, BMI 28 to 43 kg/m2, at least 1 antihypertensive medication, sitting DBP 96 to 109 mm Hg MAIN EXCLUSION CRITERIA: recent initiation or change in diuretic therapy, previous gastrointestinal surgery for weight reduction, active gastrointestinal disorders, use of nicotine replacement therapy, appetite suppressants, fish oil supplements, chronic systemic steroids, acute antidepressant or anxiolytic therapy GENERAL BASELINE CHARACTERISTICS (orlistat vs placebo) NUMBER: 278 vs 276 were randomised, 267 vs 265 were analysed MEAN AGE [YEARS]: 53 vs 53 GENDER [% MALE]: 37% vs 41% NATIONALITY: US ETHNICITY: 85% to 86% white; 11% to 12% black, 1% to 4% Hispanic, 0% to 1% other WEIGHT [kg]: 101 vs 102 BODY MASS INDEX [kg/m2]: 36 vs 35 SITTING SYSTOLIC BLOOD PRESSURE [mm Hg]: 154 vs 151 SITTING DIASTOLIC BLOOD PRESSURE [mm Hg]: 98 vs 98 COMORBID CONDITIONS: 8% diabetes in both groups ANTIHYPERTENSIVE TREATMENT. 95% vs 94% DURATION OF HYPERTENSION: ‐
SUBGROUP: diabetic participants (no information on reasons and method are noted) | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Orlistat 120 mg 3 times a day with meals CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo 3 times a day with meals ADDITIONAL TREATMENT: Hypocaloric diet, lifestyle intervention, moderate physical activity | |
| Outcomes | LENGTH OF FOLLOW‐UP: 12 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: reported were serious adverse events as necessitating or prolonging hospitalisation; withdrawals due to adverse events; gastrointestinal and musculoskeletal symptoms SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: sitting SBP change from baseline to endpoint visit 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: sitting DBP change from baseline to endpoint visit 3. BODY WEIGHT [kg] Definition: body weight change from baseline to endpoint visit ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: Roche Laboratories, New Jersey, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on sequence generation are provided |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "... randomized, double‐blind, placebo‐controlled study ..." No details provided to ensure that blinding of participants and key study personnel were not broken |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "... statistical analyses were performed on an ITT basis ..." Last observation was carried forward and reasons and description for withdrawals are provided ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ WITHDRAWALS: 116 vs 168 (orlistat vs placebo) REASONS/DESCRIPTIONS (orlistat vs placebo):
|
| Selective reporting (reporting bias) | High risk | No study protocol, outcomes reported that were not prespecified (e.g. systolic blood pressure) |
| Other bias | High risk | 1. The combination of a high withdrawal rate and the unknown duration of participation in the trial increases risk of bias even using the LOCF analysis. 2. There are inconsistencies between text and flowchart for numbers of participants who finished the study |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 6 months NUMBER OF STUDY CENTRES: 1 COUNTRY OF PUBLICATION: Switzerland | |
| Participants | WHO PARTICIPATED: obese people with metabolic syndrome, diabetes type 2, hypertension, mostly with coronary heart disease and concomitant cardiac dysfunction SETTING: outpatient clinic MAIN INCLUSION CRITERIA: age ≥ 35 years, BMI 31 to 40 kg/m2, left ventricular function 42% to 50% MAIN EXCLUSION CRITERIA: inability of participants to monitor their own glucose and blood pressure, active participation in a dietary program, use of weight‐losing medication GENERAL BASELINE CHARACTERISTICS (orlistat vs placebo) NUMBER: 45 vs 45 MEAN AGE [YEARS]: 55 vs 55 GENDER [% MALE]: 49% vs 49% NATIONALITY: probably Swiss ETHNICITY: 100% white WEIGHT [kg]: 107 vs 106 HbA1c: 7.3% vs 6.9% BODY MASS INDEX [kg/m2]: 37 vs 36 SYSTOLIC BLOOD PRESSURE [mm Hg]: 146 vs 142 DIASTOLIC BLOOD PRESSURE [mm Hg]: 88 vs 85 COMORBID CONDITIONS: 100% metabolic syndrome and diabetes, 77% coronary heart disease, 47% myocardial infarction ANTIHYPERTENSIVE TREATMENT: 100% DURATION OF HYPERTENSION: ‐ SUBGROUP: ‐ | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Orlistat 120 mg 3 times a day CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo 3 times a day ADDITIONAL TREATMENT: Hypocaloric diet, teaching sessions for lifestyle intervention, moderate physical activity | |
| Outcomes | LENGTH OF FOLLOW‐UP: 6 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: gastrointestinal side effects were reported (no severe effects) SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: SBP change from baseline to endpoint visit 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: DBP change from baseline to endpoint visit 3. BODY WEIGHT [kg] Definition: body weight change from baseline to endpoint visit ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure that each group would contain approximately equal numbers of risk factors, stratified randomisation was used with an algorithm generated from a random set of numbers." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding (performance bias and detection bias) | Low risk | Authors' reply gave further detailed information that participants, study personnel, and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | No study protocol or reporting on prespecified endpoint, no primary endpoint reported |
| Other bias | Low risk | Similar baselines Small inconsistencies between changes in body weight reported in the table and text in comparison with changes in body weight calculated from "baseline" and "after therapy" measures reported in the table |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 6 months NUMBER OF STUDY CENTRES: 253 COUNTRY OF PUBLICATION: France | |
| Participants | WHO PARTICIPATED: obese people with diabetes type 2, hypertension, or hypercholesterolaemia; only the predefined subgroup of hypertensive participants is reported here SETTING: private endocrinologists MAIN INCLUSION CRITERIA: age 18 to 65 years, BMI 28 to 40 kg/m2, DBP 90 to 110 mm Hg or untreated hypertensives or treatment stopped for more than 3 months or insufficiently controlled by the last 6 months of treatment MAIN EXCLUSION CRITERIA: secondary hypertension; history or presence of drug and alcohol abuse; significant cardiac, renal, hepatic gastrointestinal, endocrine. and psychiatric disorders GENERAL BASELINE CHARACTERISTICS (orlistat vs placebo) NUMBER: 304 vs 310 MEAN AGE [YEARS]: 49 vs 50 GENDER [% MALE]: 31% vs 35% NATIONALITY: probably French ETHNICITY: ‐ WEIGHT [kg]: 94 vs 94 BODY MASS INDEX [kg/m2]: 34 vs 34 SITTING SYSTOLIC BLOOD PRESSURE [mm Hg]: 150 vs 152 DIASTOLIC BLOOD PRESSURE [mm Hg]: 97 vs 97 COMORBID CONDITIONS: ‐ ANTIHYPERTENSIVE TREATMENT: 70% DURATION OF HYPERTENSION: ‐ SUBGROUP: ‐ | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Orlistat 120 mg 3 times a day with meals CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo 3 times a day with meals ADDITIONAL TREATMENT: Hypocaloric diet | |
| Outcomes | LENGTH OF FOLLOW‐UP: 6 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: Gastrointestinal events are only reported for the whole study population, which had diabetes type 2, hypertension, or hypercholesterolaemia. SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: SBP change from baseline to endpoint visit 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: DBP change from baseline to endpoint visit 3. BODY WEIGHT [kg] Definition: body weight change from baseline to endpoint visit ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: Roche Pharma, Paris, France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a centralized procedure in which patients were stratified by both centre and comorbidity. According to minimization procedure, patients were randomized to ensure that treatment groups were well balanced by both centre and by concomitant disease." |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "... randomized, double‐blind, placebo controlled, parallel‐group study ..." No details provided to ensure that blinding of participants and key study personnel was not broken |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Efficacy was assessed on an intent‐to‐treat (ITT) basis. The ITT population included all randomized patients and the safety population consisted of all randomized patients who had received at least one dose of study treatment. Principal criteria missing at six months were replaced by the last‐measured value (last observation carried forward, LOCF)." ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ WITHDRAWALS and LOST TO FOLLOW‐UP: REASONS/DESCRIPTIONS: only reported for the whole study population, which had diabetes type 2, hypertension, or hypercholesterolaemia |
| Selective reporting (reporting bias) | High risk | No study protocol |
| Other bias | Low risk | Similar baselines Power calculation is provided |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 48 months NUMBER OF STUDY CENTRES: 22 COUNTRY OF PUBLICATION: Sweden | |
| Participants | WHO PARTICIPATED: obese people with normal or impaired glucose tolerance. Only the predefined subgroup of hypertensive participants is reported here. Data were obtained from the publicly available scientific report of the Institute for Quality and Efficiency in Health Care (IQWiG 2006) SETTING: medical centres MAIN INCLUSION CRITERIA: age 30 to 60 years, BMI ≥ 30 kg/m2, normal or impaired glucose tolerance, either DBP ≥ 90 mm Hg (first predefined subgroup) or SBP ≥ 140 mm Hg (second predefined subgroup) MAIN EXCLUSION CRITERIA: SBP > 165 mm Hg or DBP > 105 mm Hg, diabetes mellitus, ongoing and active cardiovascular and gastrointestinal disease BASELINE CHARACTERISTICS OF THE FIRST SUBGROUP: (orlistat vs placebo) NUMBER: 408 vs 441 were randomised and 407 vs 437 were analysed MEAN AGE [YEARS]: 46 vs 46 GENDER [% MALE]: 62% vs 56% NATIONALITY: Swedish ETHNICITY: ‐ WEIGHT [kg]: 116 vs 114 BODY MASS INDEX [kg/m2]: ‐ SYSTOLIC BLOOD PRESSURE [mm Hg]: 146 vs 146 DIASTOLIC BLOOD PRESSURE [mm Hg]: 95 vs 95 COMORBID CONDITIONS: ‐ ANTIHYPERTENSIVE TREATMENT: ‐ DURATION OF HYPERTENSION: ‐ BASELINE CHARACTERISTICS OF THE SECOND SUBGROUP: (orlistat vs placebo) NUMBER: 516 vs 509 were randomised and 513 vs 508 were analysed MEAN AGE [YEARS]: 47 vs 47 GENDER [% MALE]: 58% vs 58% NATIONALITY: Swedish ETHNICITY: ‐ WEIGHT [kg]: 116 vs 115 BODY MASS INDEX [kg/m2]: ‐ SYSTOLIC BLOOD PRESSURE [mm Hg]: 149 vs 149 DIASTOLIC BLOOD PRESSURE [mm Hg]: 91 vs 91 COMORBID CONDITIONS: ‐ ANTIHYPERTENSIVE TREATMENT: ‐ DURATION OF HYPERTENSION: ‐ | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Orlistat 120 mg 3 times a day with meals CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo 3 times a day with meals ADDITIONAL TREATMENT: Hypocaloric diet, teaching sessions for lifestyle intervention, moderate physical activity | |
| Outcomes | LENGTH OF FOLLOW‐UP: 48 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: severe, overall, and withdrawals were reported, further reported were gastrointestinal, musculoskeletal, dermatological, vascular, and nervous side effects. SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: SBP change from baseline to endpoint visit 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: DBP change from baseline to endpoint visit 3. BODY WEIGHT [kg] Definition: body weight change from baseline to endpoint visit ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: Hoffmann‐La Roche, Nutley, (NJ) USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information obtained from the scientific report (IQWiG 2006) |
| Allocation concealment (selection bias) | Low risk | Information obtained from the scientific report (IQWiG 2006) |
| Blinding (performance bias and detection bias) | Unclear risk | Information obtained from the scientific report (IQWiG 2006) |
| Incomplete outcome data (attrition bias) | Unclear risk | Information obtained from the scientific report (IQWiG 2006) Quote: "Efficacy was assessed on an intention‐to‐treat (ITT) basis with a last observation carried forward principle (LOCF). The ITT population included all randomized patients who had received at least one dose of study treatment and one follow up examination." ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ First subgroup WITHDRAWALS: 183 (45%) vs 268 (61%) (orlistat vs placebo) REASONS/DESCRIPTIONS (orlistat vs placebo)
Second subgroup WITHDRAWALS: 215 (42%) vs 307 (60%) (orlistat vs placebo) REASONS/DESCRIPTIONS (orlistat vs placebo)
|
| Selective reporting (reporting bias) | Unclear risk | Information was obtained only from the scientific report (IQWiG 2006); no full publication was available to allow judgement of either yes or no |
| Other bias | High risk | Taken together, a high withdrawal rate and the unknown length of stay of participants in the trial increases risk of bias, even using the LOCF analysis Similar baselines Power calculation is only provided for the whole group |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 6 months NUMBER OF STUDY CENTRES: 1 COUNTRY OF PUBLICATION: Mexico | |
| Participants | WHO PARTICIPATED: obese people with hypertension SETTING: Endocrinology Service of the General Hospital of Mexico MAIN INCLUSION CRITERIA: BMI > 27 kg/m2, after 2 weeks of antihypertensive wash‐out DBP 90 to 109 mm Hg or SBP ≥ 140 mm Hg, then start with antihypertensive treatment unless blood pressure was < 140/90 mm Hg MAIN EXCLUSION CRITERIA: uncontrolled hypertension, thyroid‐replacement drugs, endocrine (other than type 2 diabetes mellitus) disease, autoimmune or ischaemic heart diseases, arrhythmia, or diuretic treatment GENERAL BASELINE CHARACTERISTICS (sibutramine vs placebo) NUMBER: 66 were randomised (no details to which treatment group), 29 vs 28 were analysed MEAN AGE [YEARS]: 49 vs 46 GENDER [% MALE]: 17% vs 25% NATIONALITY: Mexican ETHNICITY: Hispanic (Latin Americans) WEIGHT [kg]: 75 vs 78 BODY MASS INDEX [kg/m2]: 31 vs 31 SYSTOLIC BLOOD PRESSURE [mm Hg]: 139 vs 139 DIASTOLIC BLOOD PRESSURE [mm Hg]: 93 vs 92 COMORBID CONDITIONS: hypertension ANTIHYPERTENSIVE TREATMENT: 100% DURATION OF HYPERTENSION: ‐ SUBGROUP: ‐ | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Sibutramine 10 mg once a day CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo once a day ADDITIONAL TREATMENT: Hypocaloric diet | |
| Outcomes | LENGTH OF FOLLOW‐UP: 6 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: reported adverse events such as headache, gastrointestinal events, insomnia SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg]: Definition: baseline and follow‐up measurements are provided and change can be calculated 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg]: Definition: baseline and follow‐up measurements are provided and change can be calculated 3. BODY WEIGHT [kg] Definition: body weight change from baseline to endpoint visit ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: Química Knoll de México, Mexico | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... prepared a computer list of 70 random numbers in seven blocks of 10 ..." |
| Allocation concealment (selection bias) | Low risk | Quote: "... opaque sealed envelope with drug code ..." |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants and key study personnel can be assured based on publication text. It was unclear if outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | High risk | 66 participants were randomised and 9 participants withdrew during run‐in period without the participants knowing to which group they had been randomised ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ WITHDRAWALS and LOST TO FOLLOW‐UP (sibutramine vs placebo): only reported for participants included in the completers analysis REASONS/DESCRIPTIONS (sibutramine vs placebo):
|
| Selective reporting (reporting bias) | High risk | No study protocol and participant flow is inconsistent |
| Other bias | High risk | As 14% of randomised participants (unclear from which treatment group) were not included in the analysis, the handling of dropouts can be judged inadequate |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 6 months NUMBER OF STUDY CENTRES: 1 COUNTRY OF PUBLICATION: Brazil | |
| Participants | WHO PARTICIPATED: obese hypertensive people SETTING: hypertension outpatient clinic of Hospital do Rim e da Hipertensão MAIN INCLUSION CRITERIA: BMI ≥ 30 to < 50 kg/m2, office SBP ≥ 140 mm Hg and DBP > 90 and < 110 mm Hg or < 95 mm Hg if on antihypertensive therapy, WHR ≥ 0.85 for women and ≥ 0.95 for men MAIN EXCLUSION CRITERIA: diabetes (fasting glucose of over 7.05 mmol/l), severe dyslipidaemia (cholesterol levels of ≥ 7.74 mmol/l and triglyceride levels of ≥ 4.51 mmol/l), congestive heart failure, coronary heart disease, renal or hepatic insufficiency GENERAL BASELINE CHARACTERISTICS for the analysed participants (sibutramine vs placebo) NUMBER: 56 vs 53 were randomised, 43 vs 43 were analysed MEAN AGE [YEARS]: 46 vs 51 GENDER [% MALE]: 17% vs 12% NATIONALITY: Brazilian ETHNICITY: ‐ WEIGHT [kg]: 100 vs 97 BODY MASS INDEX [kg/m2]: 40 vs 39 24‐h SYSTOLIC BLOOD PRESSURE [mm Hg]: 150 vs 150 24‐h DIASTOLIC BLOOD PRESSURE [mm Hg]: 91 vs 94 COMORBID CONDITIONS: ‐ ANTIHYPERTENSIVE TREATMENT: 81% vs 81% DURATION OF HYPERTENSION: ‐ SUBGROUP: ‐ | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Sibutramine 10 mg once a day in the morning CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo once a day in the morning ADDITIONAL TREATMENT: Hypocaloric diet, increased physical activity | |
| Outcomes | LENGTH OF FOLLOW‐UP: 6 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: ‐ Definition: reported as symptoms such as dry mouth and joint pain SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: SBP at baseline and at endpoint visit 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: DBP at baseline and at endpoint visit 3. BODY WEIGHT [kg] Definition: body weight at baseline and at endpoint visit ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: Abbott Laboratories of Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on sequence generation are provided |
| Allocation concealment (selection bias) | Low risk | Quote: "... administration of sequential numbers ..." |
| Blinding (performance bias and detection bias) | Low risk | According to personal communication received by Ulrich Siering (one of the authors of original version of this Cochrane review) "blinding of participants, study personnel, and outcome assessors can be guaranteed" |
| Incomplete outcome data (attrition bias) | High risk | No missing data, but no ITT analysis, only a completers analysis was performed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ WITHDRAWALS: 13 vs 10 (sibutramine vs placebo) REASONS/DESCRIPTIONS (Sibutramine vs Placebo)
|
| Selective reporting (reporting bias) | High risk | No study protocol, small differences in reporting between the 2002 and 2005 publications |
| Other bias | High risk | Handling of dropouts in the analyses was inadequate, it is unclear whether participants were comparable for prognosis‐relevant factors at baseline |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 12 months NUMBER OF STUDY CENTRES: multicentre (numbers unclear) COUNTRY OF PUBLICATION: USA | |
| Participants | WHO PARTICIPATED: obese hypertensive people SETTING: clinic visits MAIN INCLUSION CRITERIA: age > 18 years, BMI between 27 and 40 kg/m2, diagnosis of hypertension for at least 12 months before screening with adequate medical control (a constant dose of a calcium channel blocker for at least 60 days and stable dose of a single thiazide diuretic was allowed): mean DBP ≤ 95 mm Hg during run‐in period and variations in mean DBP measured at 3 consecutive run‐in visits had to be within 10 mm Hg MAIN EXCLUSION CRITERIA: secondary elevated blood pressure, pulse rate > 95/min at baseline, DBP > 95 mm Hg during run‐in period, significant cardiac disease, endocrine abnormalities, severe cerebral trauma, stroke, substance abuse within 2 years before screening GENERAL BASELINE CHARACTERISTICS (sibutramine vs placebo) NUMBER: 150 vs 74 were randomised, 142 vs 69 were analysed MEAN AGE [YEARS]: 52 vs 53 GENDER [% MALE]: 39% vs 40% NATIONALITY: Americans ETHNICITY: 55% vs 64% white, 39% vs 30% African American, 7% vs 6% others WEIGHT [kg]: 97 vs 96 BODY MASS INDEX [kg/m2]: 35 vs 34 MEAN SYSTOLIC BLOOD PRESSURE [mm Hg]: 134 vs 134 MEAN DIASTOLIC BLOOD PRESSURE [mm Hg]: 84 vs 84 COMORBID CONDITIONS: ‐ ANTIHYPERTENSIVE TREATMENT: 100% (calcium channel blocker), 37% vs 38% (diuretics), 3% vs 4% (β‐adrenergic receptor antagonists) DURATION OF HYPERTENSION: at least 12 months SUBGROUP: African Americans | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Sibutramine initial dosage of 5 mg once daily titrated up to 20 mg per day in 5 mg increments every 2 weeks, maintained at 20 mg per day between weeks 8 and 52 CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo once a day ADDITIONAL TREATMENT: General dietary advice | |
| Outcomes | LENGTH OF FOLLOW‐UP: 12 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: study‐related withdrawals and adverse events such as dry mouth and headache are reported SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: mean change in SBP 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: mean change in DBP 3. BODY WEIGHT [kg] Definition: mean change in body weight ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: Knoll Pharmaceutical Co, Mount Olive (NJ) USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only information was a 2:1 randomisation ratio |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | Quote: "... statistical analyses were performed on an ITT basis ... last observation was carried forward (LOCF) ..." The ITT population included all randomised participants who had at least 1 measurement after baseline ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ WITHDRAWALS: 71 vs 33 (sibutramine vs placebo) REASONS/DESCRIPTIONS (sibutramine vs placebo)
|
| Selective reporting (reporting bias) | Unclear risk | No study protocol provided |
| Other bias | High risk | 1. Taken together, a high withdrawal rate and the unknown length of stay of participants in the trial increases risk of bias, even using the LOCF analysis 2. It is unclear whether participants were comparable for prognostic factors at baseline |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 12 months NUMBER OF STUDY CENTRES: multicentre (numbers unclear) COUNTRY OF PUBLICATION: USA | |
| Participants | WHO PARTICIPATED: obese hypertensive people treated with ACE inhibitors SETTING: outpatient clinic MAIN INCLUSION CRITERIA: age ≥ 18 years, BMI ≥ 27 and ≤ 40 kg/m2, history of well‐controlled hypertension for ≥ 60 days preceding the screening visit with a constant dose of an ACE inhibitor, with or without a thiazide diuretic (dose and nature unchanged for ≥ 60 days preceding the screening visit) Well‐controlled hypertension was protocol‐defined as a mean supine DBP ≤ 95 mm Hg at each "qualifying" visit, with an overall difference of 10 mm Hg or less between visits, without changes to the dose of the ACE inhibitor or thiazide diuretic MAIN EXCLUSION CRITERIA: secondary elevated blood pressure, mean supine pulse rate > 95/min at baseline, mean supine DBP > 95 mm Hg at any run‐in visit, significant cardiac disease, gastric surgery to reduce obesity, previous treatment with sibutramine GENERAL BASELINE CHARACTERISTICS (sibutramine vs placebo) NUMBER: 146 vs 74 were randomised, 84 vs 36 were analysed MEAN AGE [YEARS]: 52 vs 51 GENDER [% MALE]: 42% vs 43% NATIONALITY: Americans ETHNICITY: 80% vs 87% white, 18% vs 11% black, 2% vs 3% Mexican American, 1% vs 0% others WEIGHT [kg]: 97 vs 99 BODY MASS INDEX [kg/m2]: 34 vs 34 SUPINE SYSTOLIC BLOOD PRESSURE [mm Hg]: 129 vs 129 SUPINE DIASTOLIC BLOOD PRESSURE [mm Hg]: 82 vs 83 COMORBID CONDITIONS: ‐ ANTIHYPERTENSIVE TREATMENT: 100% (ACE inhibitors), 49% vs 55% (diuretics) DURATION OF HYPERTENSION: controlled for ≥ 60 days SUBGROUP: ‐ | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Sibutramine initial dosage of 5 mg once daily in the morning titrated up to 20 mg per day in 5 mg increments every 2 weeks, maintained at 20 mg per day between weeks 8 and 52 CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo once daily in the morning ADDITIONAL TREATMENT: General dietary advice | |
| Outcomes | LENGTH OF FOLLOW‐UP: 12 months PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: study‐related withdrawals, severe adverse events, and symptoms such as dry mouth and headache are reported SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: change in SBP from baseline 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: change in DBP from baseline 3. BODY WEIGHT [kg] Definition: mean change in body weight ADDITIONAL OTUCOMES MEASURED IN THE STUDY:
| |
| Notes | SPONSOR: Abbott Laboratories, Abbott Park (IL) USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only information was a 2:1 randomisation ratio |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | Quote: "... statistical analyses were performed on an ITT basis ... last observation was carried forward (LOCF) ..." The ITT population included all randomised participants who had efficacy measurements after baseline ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ WITHDRAWALS: 62 vs 38 (sibutramine vs placebo) REASONS/DESCRIPTIONS (sibutramine vs placebo)
No further reasons mentioned: 31 vs 21 |
| Selective reporting (reporting bias) | Unclear risk | No study protocol provided |
| Other bias | High risk | 1. Taken together, a high withdrawal rate and the unknown length of stay of participants in the trial increases risk of bias, even using the LOCF analysis 2. It is unclear whether participants were comparable for prognostic factors at baseline |
| Methods | DESIGN: parallel, randomised, double‐blind DURATION OF INTERVENTION: 56 weeks NUMBER OF STUDY CENTRES: 93 COUNTRY OF PUBLICATION: USA | |
| Participants | WHO PARTICIPATED: obese or overweight people with 2 or more comorbidities; only the predefined subgroup of hypertensive participants is reported here SETTING: outpatient clinic MAIN INCLUSION CRITERIA: age 18 to 70 years, BMI 27 to 45 kg/m2, 2 or more of the following comorbidities at baseline: SBP 140 to 160 mm Hg (130 to 160 mm Hg in people with diabetes), DBP 90 to 100 mm Hg (85 to 100 mm Hg in people with diabetes), or taking at least 2 antihypertensive drugs; triglycerides 2.26 to 4.52 mmol/l or using at least 2 lipid‐lowering drugs; fasting blood glucose > 5.55 mmol/l, blood glucose > 7.77 mmol/l at 2 h after oral glucose load during oral glucose tolerance test, or diagnosed type 2 diabetes managed with lifestyle changes or metformin monotherapy; and waist circumference of at least 102 cm for men or at least 88 cm for women MAIN EXCLUSION CRITERIA: blood pressure > 160/100 mm Hg, fasting glucose > 13.32 mmol/l or triglycerides > 4.52 mmol/l at randomisation, type 1 diabetes, use of antidiabetic drugs other than metformin, history of nephrolithiasis, recurrent major depression, presence or history of suicidal behaviour or ideation with intent to act, and current substantial depressive symptoms (Patient Health Questionnaire (PHQ‐9) total score ≥ 10) GENERAL BASELINE CHARACTERISTICS (predefined subgroup of hypertensive participants) (phentermine 7.5 mg/topiramate 46.0 mg vs phentermine 15.0 mg/topiramate 92.0 mg vs placebo) NUMBER: 261 vs 520 vs 524 were randomised, 256 vs 514 vs 516 were analysed (ITT LOCF) NATIONALITY: Americans ETHNICITY: 83% white, 15% black, 10% Hispanic or Latino WEIGHT [kg]: 104 BODY MASS INDEX [kg/m2]: 37 SYSTOLIC BLOOD PRESSURE [mm Hg]: 134 vs 133 vs 135 DIASTOLIC BLOOD PRESSURE [mm Hg]: 83 vs 83 vs 85 COMORBID CONDITIONS: ‐ ANTIHYPERTENSIVE TREATMENT: 27% (ACE inhibitors alone), 24% (β‐blockers alone), 16% (AT‐II‐receptor antagonists alone), 6% (ACE inhibitors + diuretics), 4% (ACE inhibitors + calcium channel blockers), 12% (AT‐II‐receptor antagonists + diuretics), 1% (AT‐II‐receptor antagonists + calcium channel blockers) DURATION OF HYPERTENSION: ‐ SUBGROUP: ‐ | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Phentermine/topiramate: dose titration during the first 4 weeks; initial dosage of 3.75 mg phentermine and 23 mg topiramate, increasing weekly (3.75 mg phentermine and 23 mg topiramate) until the assigned dosage of phentermine 7.5 mg/topiramate 46.0 mg (group 1) or phentermine 15 mg/topiramate 92.0 mg (group 2) was achieved, maintained at assigned dosages once daily for 52 weeks CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo once daily ADDITIONAL TREATMENT: Standardised counselling for diet (to reduce caloric intake by 500 kcal/day) and lifestyle modification | |
| Outcomes | LENGTH OF FOLLOW‐UP: 56 weeks PRIMARY OUTCOMES: 1. MORTALITY: ‐ 2. CARDIOVASCULAR MORBIDITY: ‐ 3. ADVERSE EVENTS: Definition: Serious adverse events and the most common treatment‐emergent adverse events are reported SECONDARY OUTCOMES: 1. CHANGES IN SYSTOLIC BLOOD PRESSURE [mm Hg] Definition: change in SBP from baseline 2. CHANGES IN DIASTOLIC BLOOD PRESSURE [mm Hg] Definition: change in DBP from baseline 3. BODY WEIGHT [kg] Definition: mean percentage change in body weight ADDITIONAL OUTCOMES MEASURED IN THE STUDY:
Response to Columbia Suicide Severity Rating Scale (C‐SSRS) | |
| Notes | SPONSOR: VIVUS Inc., Mountain View (CA) USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... used a computer‐generated algorithm that was implemented through an interactive voice response system to assign patients according to the random allocation sequence, with a block size of eight." |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment. " |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "The primary analyses were done on the intention‐to‐treat sample, consisting of all patients who were randomly assigned, took at least one dose of the study drug or placebo, and had one post baseline bodyweight measurement." WITHDRAWALS and REASONS/DESCRIPTIONS:
Total number of withdrawals and other reasons only reported for the whole study population having hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity |
| Selective reporting (reporting bias) | Unclear risk | No study protocol provided |
| Other bias | Low risk | None identified |
ACE inhibitors: angiotensin‐converting enzyme inhibitors
AT‐II‐receptor antagonists: angiotensin II‐receptor antagonists
BMI: body mass index
DBP: diastolic blood pressure
ITT: intention to treat
LOCF: last observation carried forward analysis
SBP: systolic blood pressure
WHR: waist‐to‐hip ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study does not include participants with essential hypertension | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study does not include participants with essential hypertension | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study does not include participants with essential hypertension | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study is not a randomised controlled trial | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study is not a randomised controlled trial | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study is not a randomised controlled trial | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The duration of the intervention is less than 24 weeks | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The duration of the intervention is less than 24 weeks | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The reported outcomes in this study are not relevant for this review | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study is not a randomised controlled trial | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study is not a randomised controlled trial | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study does not include participants with essential hypertension | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes different accompanying antihypertensive therapies in the study groups | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup | |
| The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results ‐ A Long Term Evaluation (LEADER) |
| Methods | randomised controlled trial, double‐blinded, placebo‐controlled |
| Participants | 9340 men and women aged 50 years and older with diabetes mellitus type 2 and with or without prior cardiovascular disease |
| Interventions | Liraglutide 1.8 mg once daily versus placebo |
| Outcomes | Primary outcome: Time from randomisation to first occurrence of cardiovascular death, non‐fatal myocardial infarction, or non‐fatal stroke (a composite cardiovascular outcome) Secondary outcomes:
|
| Starting date | August 2010 Estimated primary completion date: November 2015 |
| Contact information | |
| Notes | Sponsor: Novo Nordisk A/S Registration number: NCT01179048 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in systolic blood pressure from baseline to endpoint Show forest plot | 4 | 2058 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐4.01, ‐0.90] |
| Analysis 1.1  Comparison 1 Orlistat versus placebo, Outcome 1 Change in systolic blood pressure from baseline to endpoint. | ||||
| 2 Change in diastolic blood pressure from baseline to endpoint Show forest plot | 4 | 2058 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐2.99, ‐0.85] |
| Analysis 1.2  Comparison 1 Orlistat versus placebo, Outcome 2 Change in diastolic blood pressure from baseline to endpoint. | ||||
| 3 Change in body weight from baseline to endpoint Show forest plot | 4 | 2080 | Mean Difference (IV, Random, 95% CI) | ‐3.73 [‐4.65, ‐2.80] |
| Analysis 1.3  Comparison 1 Orlistat versus placebo, Outcome 3 Change in body weight from baseline to endpoint. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in diastolic blood pressure from baseline to endpoint Show forest plot | 2 | 428 | Mean Difference (IV, Fixed, 95% CI) | 3.16 [1.40, 4.92] |
| Analysis 2.1  Comparison 2 Sibutramine versus placebo, Outcome 1 Change in diastolic blood pressure from baseline to endpoint. | ||||
| 2 Change in body weight from baseline to endpoint Show forest plot | 4 | 574 | Mean Difference (IV, Fixed, 95% CI) | ‐3.74 [‐4.84, ‐2.64] |
| Analysis 2.2  Comparison 2 Sibutramine versus placebo, Outcome 2 Change in body weight from baseline to endpoint. | ||||
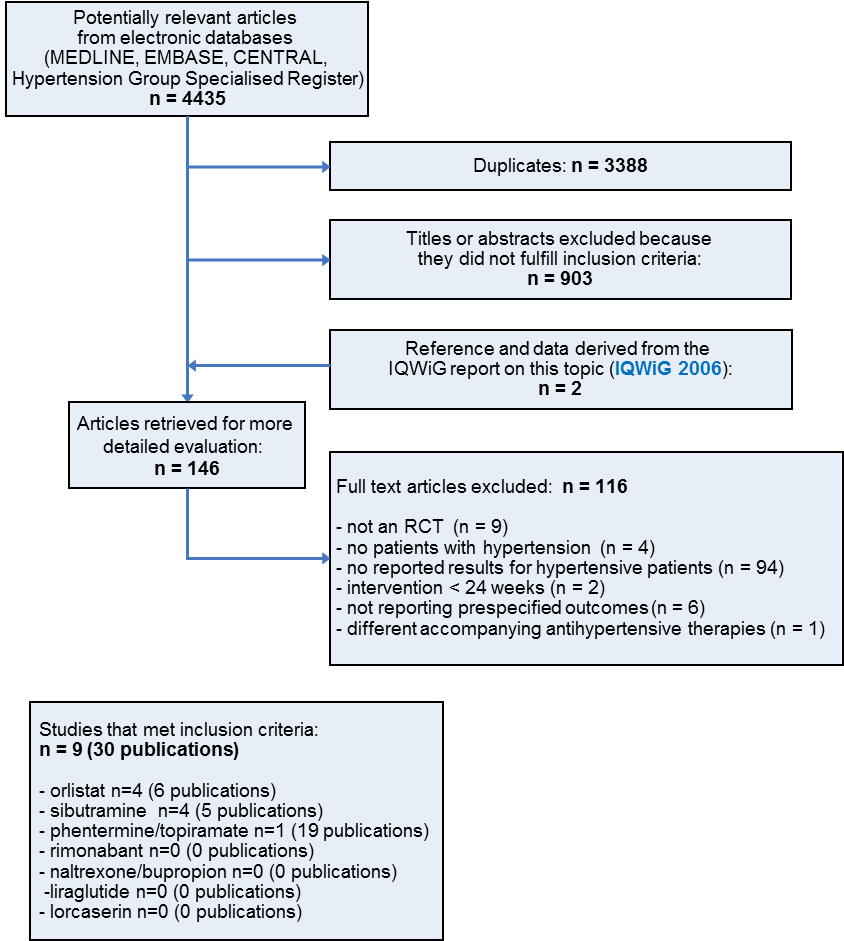
Study flow diagram
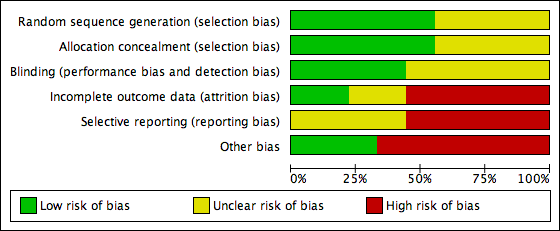
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.1 Change in systolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG04.png)
Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.1 Change in systolic blood pressure from baseline to endpoint [mm Hg].
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.2 Change in diastolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG05.png)
Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.2 Change in diastolic blood pressure from baseline to endpoint [mm Hg].
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.3 Change in body weight from baseline to endpoint [kg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG06.png)
Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.3 Change in body weight from baseline to endpoint [kg].
![Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.1 Change in diastolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG07.png)
Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.1 Change in diastolic blood pressure from baseline to endpoint [mm Hg].
![Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.2 Change in body weight from baseline to endpoint [kg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG08.png)
Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.2 Change in body weight from baseline to endpoint [kg].

Comparison 1 Orlistat versus placebo, Outcome 1 Change in systolic blood pressure from baseline to endpoint.

Comparison 1 Orlistat versus placebo, Outcome 2 Change in diastolic blood pressure from baseline to endpoint.

Comparison 1 Orlistat versus placebo, Outcome 3 Change in body weight from baseline to endpoint.

Comparison 2 Sibutramine versus placebo, Outcome 1 Change in diastolic blood pressure from baseline to endpoint.

Comparison 2 Sibutramine versus placebo, Outcome 2 Change in body weight from baseline to endpoint.
| Orlistat compared with placebo for weight reduction | |||||
| Patient or population: Men and non‐pregnant women ≥ 18 years old with essential hypertension Intervention: Orlistat Comparison: Placebo | |||||
| Outcomes | Illustrative comparative risks (per 1000 patients) | Effect estimate | No of Participants | Quality of the evidence | Comments |
| Change in systolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | MD ‐2.46 [‐4.01, ‐0.90] | 2058 | ⊕⊕⊝⊝ | ‐ |
| Change in diastolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | MD ‐1.92 [‐2.99, ‐0.85] | 2058 | ⊕⊕⊝⊝ | ‐ |
| Change in body weight as compared to placebo [kg] from baseline to end of study | Not applicable | MD ‐3.73 [‐4.65, ‐2.80] | 2080 | ⊕⊕⊕⊝ | ‐ |
| CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence | |||||
| 1High risk of bias in included studies. 2Wide confidence intervals include non‐clinically important effect. | |||||
| Sibutramine compared with placebo for weight reduction | |||||
| Patient or population: Men and non‐pregnant women ≥ 18 years old with essential hypertension Intervention: Sibutramine Comparison: Placebo | |||||
| Outcomes | Illustrative comparative risks (per 1000 patients) | Effect estimates | No of Participants | Quality of the evidence | Comments |
| Change in systolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | Not estimable | See comment | See comment | Variability measurements not available; |
| Change in diastolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | MD 3.16 [1.40, 4.92] | 428 | ⊕⊕⊝⊝ | ‐ |
| Change in body weight as compared to placebo [kg] from baseline to end of study | Not applicable | MD ‐3.74 [‐4.84, ‐2.64] | 574 | ⊕⊕⊝⊝ | ‐ |
| CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence | |||||
| 1High risk of bias in included studies. 2Small number of participants and studies. | |||||
| Study | Adverse events | Results |
| Orlistat vs placebo | ||
| Bakris 2002 | total thereof leading to withdrawal serious gastrointestinal thereof leading to withdrawal musculoskeletal | 89% of P [O] vs 71% of P [P], P < 0.001 7% [O] vs 7% [P] 14 P (12%) [O] vs 15 P (9%) [P] 200 P (73%) [O] vs 120 P (44%) [P], P < 0.001 15 P (8%) [O] vs 6 P (5%) [P] 23% of P [O] vs 16% [P], P < 0.05 |
| Cocco 2005 | total serious gastrointestinal | nr 0 P [O] vs 0 P [P] 16 P (36%) [O]a vs 11 P (24%) [P]a |
| Guy‐Grand 2004 | total serious | nrb nrb |
| XENDOS 2001‐2006 | total leading to withdrawal serious gastrointestinal musculoskeletal nervous system dermatological vascular | 99% of P [OD] vs 96% of P [PD] 99% of P [OS] vs 97% of P [PS] 9% of P [OD] vs 4% of P [PD] 9% of P [OS] vs 4% of P [PS] 18% of P [OD] vs 12% of P [PD] 18% of P [OS] vs 12% of P [PS] 93% of P [OD] vs 70% of P [PD] 93% of P [OS] vs 71% of P [PS] 65% of P [OD] vs 62% of P [PD] 65% of P [OS] vs 63% of P [PS] 39% of P [OD] vs 39% of P [PD] 40% of P [OS] vs 37% of P [PS] 20% of P [OD] vs 17% of P [PD] 22% of P [OS] vs 17% of P [PS] 17% of P [OD] vs 19% of P [PD] 17% of P [OS] vs 19% of P [PS] |
| Sibutramine vs placebo | ||
| Fanghaenel 2003 | total constipation dizziness dry mouth headache insomnia restlessness | 14 P (21 E) [S] vs 13 P (20 E) [P] 4 P [S] vs 2 P [P] 1 P [S] vs 1 P [P] 4 P [S] vs 2 P [P] 5 P [S] vs 2 P [P] 1 P [S] vs 1 P [P] 1 P [S] vs 0 P [P] |
| Faria 2002‐2005 | total dry mouth arthralgia | nr 37% of P [S] vs 9% of P [P], P < 0.005 16% of P [S] vs 2% of P [P], P = 0.03 |
| McMahon 2002 | total serious treatment related leading to withdrawal (mostly hypertension) dry mouth headache | 141 P (97%) [S] vs 65 P (88%) [P] 9 P (6%) [S] vs 5 P (7%) [P] 2 E [S] vs 0 E [P] 23 P (16%) [S] vs 4 P (5%) [P] 30 P (21%) [S] vs 0 P [P] 41 P (28%) [S] vs 17 P (23%) [P] |
| McMahon 2000 | total leading to withdrawal (mostly hypertension) dry mouth headache constipation rash | nr 30 P (20%) [S] vs 8 P (11%) [P] 29 P (19%) [S] vs 2 P (3%) [P], P < 0.05 37 P (25%) [S] vs 21 P (28%) [P] 25 P (17%) [S] vs 2 P (3%) [P], P < 0.05 16 P (11%) [S] vs 2 P (3%) [P] |
| Phentermine/topiramate vs placebo | ||
| CONQUER 2013 | total leading to withdrawal serious cardiac adverse events dry mouth paresthaesia constipation upper respiratory tract infection nasopharyngitis dysgeusia insomnia headache dizziness sinusitis | 85.4% vs 88.8% vs 77.3% 11.9% vs 19.8% vs 9.7% 3.4% (Phen/Top [LD]) vs 3.7% (Phen/Top [HD]) vs 4.2% [P] 0.8% vs 1.2% vs 0.6% 14.2% (Phen/Top [LD]) vs 22.7% (Phen/Top [HD]) vs 2.3% [P] 14.2% (Phen/Top [LD]) vs 22.3% (Phen/Top [HD]) vs 2.3% [P] 15.7% (Phen/Top [LD]) vs 18.1% (Phen/Top [HD]) vs 5.5% [P] 12.6% (Phen/Top [LD]) vs 12.1% (Phen/Top [HD]) vs 11.8% [P] 10.3% (Phen/Top [LD]) vs 10.2% (Phen/Top [HD]) vs 8.8% [P] 7.7% (Phen/Top [LD]) vs 11.0% (Phen/Top [HD]) vs 0.8% [P] 5.7% (Phen/Top [LD]) vs 11.0% (Phen/Top [HD]) vs 4.8% [P] 5.0% (Phen/Top [LD]) vs 10.8% (Phen/Top [HD]) vs 8.4% [P] 6.5% (Phen/Top [LD]) vs 12.1% (Phen/Top [HD]) vs 3.1% [P] 5.4% (Phen/Top [LD]) vs 8.3% (Phen/Top [HD]) vs 6.5% [P] |
| E: events. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. aNo data on adverse events were reported for the whole study duration. The data above refer to 4 and 3 weeks of treatment in the orlistat and placebo group, respectively. After 3 months, the number of participants with events decreased to 5(11%)[O] with flatulence and mild abdominal cramps versus 6(13%)[P] with nausea and hunger feeling. bData were not available for the hypertensive subgroup, only for the whole study population (withdrawal due to defecation troubles in 10 [O] versus 2 [P] participants). | ||
| Study | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | |||||
| Bakris 2002b Orlistat Placebo | 101 (1)c 102 (1)c | nr nr | nr nr | ‐ ‐ | P < 0.001 ‐5.4 (6.4) ‐2.7 (6.4) |
| Cocco 2005 Orlistat Placebo | 107 (6) 106 (6) | 102 (4) 104 (5) | ‐ ‐ | ‐ ‐ | P < 0.001 ‐5.4d ‐2.5d |
| Guy‐Grand 2004 Orlistat Placebo | 94 (1)c 94 (1)c | nr nr | ‐ ‐ | ‐ ‐ | P < 0.0001 ‐5.8 (0.3) ‐1.8 (0.2) |
| XENDOS 2001‐2006 Orlistat [OD] Placebo [PD] Orlistat [OS] Placebo [PS] | 117 (18) 115 (18) 117 (17) 116 (18) | 106 (17) 108 (18) 106 (17) 109 (18) | 105 (18) 108 (19) 105 (17) 110 (19) | 110 (19) 111 (20) 110 (18) 113 (19) | P < 0.001 ‐6.6 (8.6) ‐3.8 (7.8) P < 0.001 ‐6.8 (8.7) ‐3.2 (7.4) |
| Sibutramine vs placebo | |||||
| Fanghaenel 2003 Sibutramine Placebo | 75 (10) 78 (9) | 70 (10) 75 (9) | ‐ ‐ | ‐ ‐ | significant ‐5.5 (‐3.8; ‐7.1)e ‐3.4 (‐1.9; ‐5.0)e |
| Faria 2002‐2005 Sibutramine Placebo | 100 (19) 97 (14) | 93 (18) 94 (15) | ‐ ‐ | ‐ ‐ | P < 0.001 ‐6.8 (2.3) ‐2.4 (4.2) |
| McMahon 2002 Sibutramine Placebo | 97 (16) 99 (14) | nr nr | nr nr | ‐ ‐ | P < 0.05 ‐4.5 ‐0.4 |
| McMahon 2000 Sibutramine Placebo | 97 (13) 96 (17) | nr nr | nr nr | ‐ ‐ | P < 0.05 ‐4.4 ‐0.5 |
| Phentermine/topiramate vs placebo | |||||
| CONQUER 2013 Phen/Top [LD] Phen/Top [HD] Placebo | 104 (18)f | nr nr nr | nr nr nr | ‐ ‐ ‐ | P < 0.0001g ‐8.1% ‐10.1% ‐1.9% |
| Mo: months. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. SD: standard deviation. aMean kg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cReported as being the standard deviation but probably the standard error due to its small number. dPublished values are different, but data were corrected after personal communication with the author. e95% confidence interval. fReported only combined for all three study groups. gFor each intervention group versus placebo. | |||||
| Study | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | |||||
| Bakris 2002b Orlistat Placebo | 154 (13) 151 (13) | nr nr | nr nr | ‐ ‐ | ns ‐13.3 (15.2) ‐11.0 (15.0) |
| Cocco 2005 Orlistat Placebo | 146 (10) 142 (6) | 142 (13) 141 (9) | ‐ ‐ | ‐ ‐ | P = 0.025 ‐4.3 ‐0.9 |
| Guy‐Grand 2004 Orlistat Placebo | 150 (1)c 152 (1)c | nr nr | ‐ ‐ | ‐ ‐ | ns ‐9.8 (1) ‐9.8 (1) |
| XENDOS 2001‐2006 Orlistat [OD]d Placebo [PD]d Orlistat [OS]d Placebo [PS]d | 146 (13) 146 (12) 149 (10) 149 (8) | 135 (14) 136 (15) 125 (14) 138 (14) | 135 (14) 138 (16) 135 (14) 140 (14) | 137 (15) 139 (16) 138 (15) 140 (15) | P = 0.024 ‐8.8 (14.8) ‐6.4 (15.1) P < 0.002 ‐11.5 (14.9) ‐8.6 (14.3) |
| Sibutramine vs placebo | |||||
| Fanghaenel 2003e Sibutramine Placebo | 139 (9) 139 (13) | 125 (9) 123 (10) | ‐ ‐ | ‐ ‐ | ns ‐13.9f ‐16.5f |
| Faria 2002‐2005 Sibutramine Placebo | 150 (18) 150 (15) | 146 (15) 149 (22) | ‐ ‐ | ‐ ‐ | ns ‐4.6f ‐0.6f |
| McMahon 2002 Sibutramine Placebo | 129 (11) 129 (11) | nr nr | 133 130 | ‐ ‐ | P = 0.0497 3.8 1.1 |
| McMahon 2000 Sibutramine Placebo | 134 (10) 134 (11) | nr nr | nr nr | ‐ ‐ | ns 2.7 1.5 |
| Phentermine/topiramate vs placebo | |||||
| CONQUER 2013 Phen/Top [LD] Phen/Top [HD] Placebo | 134 (nr) 133 (nr) 135 (nr) | nr nr nr | nr nr nr | ‐ ‐ ‐ | P = 0.0475 [LD] P < 0.0001 [HD] ‐6.9 ‐9.1 ‐4.9 |
| Mo: months. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. SD: standard deviation. aMean mm Hg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cReported as being the standard deviation but probably the standard error due to its small number. dBased on last observation carried forward data on 399 [OD], 423 [PD], 493 [OS], and 504 [PS] participants. eData at baseline were recorded after a two‐week wash‐out period of antihypertensive drugs for diagnostic confirmation of hypertension. fCalculated. | |||||
| Study | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | |||||
| Bakris 2002b Orlistat Placebo | 98 (4) 98 (4)c | nr nr | nr nr | ‐ ‐ | P = 0.002 ‐11.4 (8.3) ‐9.2 (8.4) |
| Cocco 2005 Orlistat Placebo | 88 (7) 85 (6) | 84 (9) 85 (7) | ‐ ‐ | ‐ ‐ | P = 0.012 ‐3.6 ‐0.8 |
| Guy‐Grand 2004 Orlistat Placebo | 97 (0)d 97 (0)d | nr nr | ‐ ‐ | ‐ ‐ | ns ‐7.5 (0.6) ‐7.3 (0.6) |
| XENDOS 2001‐2006 Orlistat [OD]e Placebo [PD]e Orlistat [OS]e Placebo [PS]e | 95 (6) 95 (5) 91 (9) 91 (8) | 86 (8) 88 (9) 84 (9) 87 (9) | 86 (8) 88 (10) 85 (9) 88 (10) | 87 (9) 89 (10) 86 (9) 88 (10) | P < 0.006 ‐8.1 (9.3) ‐6.2 (9.9) P < 0.001 ‐5.0 (9.9) ‐3.0 (10.4) |
| Sibutramine vs placebo | |||||
| Fanghaenel 2003f Sibutramine Placebo | 93 (7) 92 (8) | 82 (5) 80 (5) | ‐ ‐ | ‐ ‐ | ns ‐11.4g ‐11.7g |
| Faria 2002‐2005 Sibutramine Placebo | 91 (12) 94 (12) | 92 (13) 92 (14) | ‐ ‐ | ‐ ‐ | ns 1.0g ‐2.06g |
| McMahon 2002 Sibutramine Placebo | 82 (6) 83 (6) | nr nr | 86 83 | ‐ ‐ | P = 0.004 3.0 ‐0.1 |
| McMahon 2000 Sibutramine Placebo | 84 (5) 84 (6) | nr nr | nr nr | ‐ ‐ | P < 0.05 2.0 ‐1.3 |
| Phentermine/topiramate vs placebo | |||||
| CONQUER 2013 Phen/Top [LD] Phen/Top [HD] Placebo | 83 (nr) 83 (nr) 85 (nr) | nr nr nr | nr nr nr | ‐ ‐ ‐ | P = 0.0400 [LD] P = 0.0003 [HD] ‐5.2 ‐5.8 ‐3.9 |
| Mo: months. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. SD: standard deviation. aMean mm Hg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cThe standard deviation was published as being 35 but should probably be 3.5. dReported as being the standard deviation but probably the standard error due to its small number. eBased on last observation carried forward data on 399 [OD], 423 [PD], 493 [OS], and 504 [PS] participants. fData at baseline were recorded after a two‐week wash‐out period of antihypertensive drugs for diagnostic confirmation of hypertension. gCalculated. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in systolic blood pressure from baseline to endpoint Show forest plot | 4 | 2058 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐4.01, ‐0.90] |
| 2 Change in diastolic blood pressure from baseline to endpoint Show forest plot | 4 | 2058 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐2.99, ‐0.85] |
| 3 Change in body weight from baseline to endpoint Show forest plot | 4 | 2080 | Mean Difference (IV, Random, 95% CI) | ‐3.73 [‐4.65, ‐2.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in diastolic blood pressure from baseline to endpoint Show forest plot | 2 | 428 | Mean Difference (IV, Fixed, 95% CI) | 3.16 [1.40, 4.92] |
| 2 Change in body weight from baseline to endpoint Show forest plot | 4 | 574 | Mean Difference (IV, Fixed, 95% CI) | ‐3.74 [‐4.84, ‐2.64] |

