Les effets à long terme des médicaments amaigrissants chez les personnes hypertendues
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update
Search Date: 13 April 2015
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (orlistat$ or tetrahydrolipstatin or thlp or "96829‐58‐2").mp. (1430)
2 (ro180647 or "ro 180647" or "ro 18‐0647").mp. (4)
3 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical).mp. (10125)
4 or/1‐3 (11468)
5 (sibutramin$ or "106650‐56‐0").mp. (1147)
6 (bts54524 or "bts 54524" or "bts‐54524" or "bts‐54‐524" or "bts54‐524").mp. (6)
7 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex).mp. (84)
8 or/5‐7 (1194)
9 (rimonabant$ or "168273‐06‐1" or "158681‐13‐1").mp. (2270)
10 (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).mp. (1528)
11 (acomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti).mp. (30)
12 or/9‐11 (2764)
13 (lorcaserin or "616202‐92‐7").mp. (129)
14 ("apd 356" or apd356).mp. (12)
15 (beliviq or lorqess).mp. (0)
16 or/13‐15 (134)
17 (liraglutide or "204656‐20‐2").mp. (724)
18 ("nn 2211" or nn2211 or "nnc 90‐1170" or "nnc90 1170").mp. (27)
19 (saxenda or victoza).mp. (36)
20 or/17‐19 (732)
21 (phentermine or "1197‐21‐3" or "122‐09‐8").mp. (879)
22 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl).mp. (920)
23 or/21‐22 (920)
24 (topiramate or "97240‐79‐4").mp. (3336)
25 (mcn4853 or "mcn 4853" or rwj17021 or "rwj17021‐000" or "rwj 17021" or "rwj 17021‐000").mp. (2)
26 (epitomax or qudexy or topamax or topimax or trokendi).mp. (87)
27 or/24‐26 (3340)
28 (qnexa or qsiva or qsymia).mp. (30)
29 (23 and 27) or 28 (105)
30 (bupropion or "31677‐93‐7" or "34911‐55‐2").mp. (3538)
31 (bw323 or "bw 323" or "bw323u66 bw 323u66").mp. (1)
32 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban).mp. (3549)
33 or/30‐32 (3550)
34 (naltrexone or "16590‐41‐3" or "16676‐29‐2").mp. (8103)
35 (en1639a or "en 1639a").mp. (2)
36 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol).mp. (71)
37 or/34‐36 (8107)
38 (contrave or mysimba).mp. (13)
39 (33 and 37) or 38 (97)
40 4 or 8 or 12 or 16 or 20 or 29 or 39 (15783)
41 hypertension/ (195598)
42 (antihypertens$ or hypertens$).ti,ab,ot. (321602)
43 exp blood pressure/ (250853)
44 (blood pressure$ or bloodpressure$).ti,ab,ot. (219802)
45 or/41‐44 (600660)
46 randomized controlled trial.pt. (390929)
47 controlled clinical trial.pt. (89138)
48 randomi?ed.ab. (344544)
49 placebo.ab. (151113)
50 clinical trials as topic/ (172188)
51 randomly.ab. (203716)
52 trial.ti. (124609)
53 or/46‐52 (912248)
54 animals/ not (humans/ and animals/) (3929323)
55 53 not 54 (837627)
56 40 and 45 and 55 (351)
57 remove duplicates from 56 (340)
***************************
Database: Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 4 via the Cochrane Register of Studies Online
Search Date: 13 April 2015
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 (orlistat* or tetrahydrolipstatin or thlp) 303
#2 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical) 133
#3 #1 OR #2 420
#4 sibutramin* 243
#5 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex) 20
#6 #4 OR #5 256
#7 rimonabant* 74
#8 (acomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti) 1
#9 #7 OR #8 74
#10 lorcaserin 19
#11 (belviq or lorqess) 1
#12 #10 OR #11 19
#13 liraglutide 171
#14 (saxenda or victoza) 7
#15 #13 OR #14 171
#16 phentermine 89
#17 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl) 93
#18 #16 OR #17 93
#19 topiramate 664
#20 (epitomax or qudexy or topamax or topimax or trokendi) 9
#21 #19 OR #20 665
#22 (qnexa or qsiva or qsymia) 0
#23 #18 AND #21 OR #22 26
#24 bupropion 883
#25 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban) 976
#26 #24 OR #25 976
#27 naltrexone 1220
#28 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol) 32
#29 #27 OR #28 1220
#30 (contrave or mysimba) 2
#31 #26 AND #29 OR #30 19
#32 #3 OR #6 OR #9 OR #12 OR #15 OR #23 OR #31 937
#33 antihypertens* or hypertens*36 146
#34 blood pressure* or bloodpressure* 47990
#35 #33 OR #34 65003
#36 #32 AND #35 287
***************************
Database: Embase <1980 to 2015 April 10>
Search Date: 13 April 2015
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (orlistat$ or tetrahydrolipstatin or thlp or "96829‐58‐2").mp. (5167)
2 (ro180647 or "ro 180647" or "ro 18‐0647").mp. (8)
3 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical).mp. (41033)
4 or/1‐3 (45094)
5 (sibutramin$ or "106650‐56‐0").mp. (4197)
6 (bts54524 or "bts 54524" or "bts‐54524" or "bts‐54‐524" or "bts54‐524").mp. (20)
7 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex).mp. (757)
8 or/5‐7 (4256)
9 (rimonabant$ or "168273‐06‐1" or "158681‐13‐1").mp. (5934)
10 (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).mp. (2756)
11 (acomplia or accomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti).mp. (485)
12 or/9‐11 (6069)
13 (lorcaserin or "616202‐92‐7").mp. (515)
14 ("apd 356" or apd356).mp. (67)
15 (beliviq or lorqess).mp. (11)
16 or/13‐15 (544)
17 (liraglutide or "204656‐20‐2").mp. (3601)
18 ("nn 2211" or nn2211 or "nnc 90‐1170" or "nnc90 1170").mp. (163)
19 (saxenda or victoza).mp. (372)
20 or/17‐19 (3614)
21 (phentermine or "1197‐21‐3" or "122‐09‐8").mp. (2463)
22 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl).mp. (2535)
23 or/21‐22 (2535)
24 (topiramate or "97240‐79‐4").mp. (16436)
25 (mcn4853 or "mcn 4853" or rwj17021 or "rwj17021‐000" or "rwj 17021" or "rwj 17021‐000").mp. (7)
26 (epitomax or qudexy or topamax or topimax or trokendi).mp. (1257)
27 or/24‐26 (16441)
28 (qnexa or qsiva or qsymia).mp. (185)
29 (23 and 27) or 28 (658)
30 (bupropion or "31677‐93‐7" or "34911‐55‐2").mp. (4519)
31 (bw323 or "bw 323" or "bw323u66 bw 323u66").mp. (1)
32 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban).mp. (14889)
33 or/30‐32 (14890)
34 (naltrexone or "16590‐41‐3" or "16676‐29‐2").mp. (12198)
35 (en1639a or "en 1639a").mp. (3)
36 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol).mp. (607)
37 or/34‐36 (12205)
38 (contrave or mysimba).mp. (123)
39 (33 and 37) or 38 (792)
40 4 or 8 or 12 or 16 or 20 or 29 or 39 (56071)
41 exp hypertension/ (504630)
42 (antihypertens$ or hypertens$).ti,ab,ot. (455177)
43 exp blood pressure/ (401991)
44 (blood pressure$ or bloodpressure$).ti,ab,ot. (295039)
45 or/41‐44 (947677)
46 randomized controlled trial/ (366629)
47 crossover procedure/ (42215)
48 double‐blind procedure/ (119304)
49 (randomi?ed or randomly).tw. (768833)
50 (crossover$ or cross‐over$).tw. (72794)
51 placebo.ab. (203233)
52 (doubl$ adj blind$).tw. (148884)
53 assign$.ab. (250126)
54 allocat$.ab. (88408)
55 or/46‐54 (1161272)
56 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5243201)
57 55 not 56 (1009122)
58 40 and 45 and 57 (809)
59 remove duplicates from 58 (802)
***************************
Database: Hypertension Group Specialised Register
Search Date: 13 April 2015
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 ((orlistat* or tetrahydrolipstatin or thlp)) 100
#2 ((alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical)) 34
#3 #1 OR #2 130
#4 sibutramin* 110
#5 ((adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex)) 3
#6 #4 OR #5 110
#7 rimonabant* 25
#8 ((acomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti)) 0
#9 #7 OR #8 25
#10 lorcaserin 18
#11 (belviq or lorqess) 0
#12 #10 OR #11 18
#13 liraglutide 108
#14 (saxenda or victoza) 4
#15 #13 OR #14 108
#16 phentermine 41
#17 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl) 43
#18 #16 OR #17 43
#19 topiramate 77
#20 (epitomax or qudexy or topamax or topimax or trokendi) 3
#21 #19 OR #20 77
#22 (qnexa or qsiva or qsymia) 1
#23 (#18 AND #21) OR #22 32
#24 bupropion 38
#25 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban) 42
#26 #24 OR #25 42
#27 naltrexone 46
#28 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol) 3
#29 #27 OR #28 46
#30 (contrave or mysimba) 3
#31 (#26 AND #29) OR #30 14
#32 #3 OR #6 OR #9 OR #12 OR #15 OR #23 OR #31 398
#33 #32 AND (RCT OR Review OR Meta‐Analysis):DE 194
***************************
Database: ClinicalTrials.gov (via Cochrane Register of Studies)
Search Date: 13 April 2015
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Search terms: randomized
Study type: Interventional Studies
Conditions: hypertension
Intervention: liraglutide OR lorcaserin OR orlistat OR rimonabant OR sibutramine OR phentermine‐topiramate OR (phentermine AND topiramate) OR qnexa OR qsiva or qsymia (9)
***************************
Appendix 2. Search strategies used in the last update of the review
Ovid MEDLINE(R) <1946 to August Week 2 2012>
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations <August 16, 2012>
Embase <1988 to 2012 Week 32>
EBM Reviews ‐ Cochrane Central Register of Controlled Trials <August 2012>
Search Date: 17 August 2012
1. exp hypertension/ or exp blood pressure/
2. (hypertens$ or antihypertens$ or anti hypertens$).ti,ab,ot.
3. ((systolic or diastolic or arterial) adj pressur$).ti,ab,ot.
4. (blood pressur$ or bloodpressur$).ti,ab,ot.
5. or/1‐4
6. (orlistat$ or tetrahydrolipstatin or thlp).ti,ab,ot,tn,sh.
7. (orlistat or 96829‐58‐2).rn.
8. (ro180647 or "ro 180647" or "ro 18‐0647").ti,ab,ot,tn.
9. (xenical or alli).ti,ab,ot,tn.
10. or/6‐9
11. sibutramin$.ti,ab,ot,tn,sh.
12. (sibutramine or 106650‐56‐0).rn.
13. (BTS‐54524 or BTS‐54‐524 or BTS54‐524).ti,ab,ot,tn.
14. (reductil or medaria or meridia or arcalion).ti,ab,ot,tn.
15. or/11‐14
16. rimonabant$.ti,ab,ot,tn,sh.
17. (rimonabant or "168273‐06‐1" or "158681‐13‐1").rn.
18. (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).ti,ab,ot,tn.
19. (acomplia or accomplia or zimulti).ti,ab,ot,tn.
20. or/16‐19
21. or/10,15,20
22. randomized controlled trial.pt.
23. controlled clinical trial.pt.
24. randomized.ab.
25. placebo.ab.
26. clinical trials as topic.sh.
27. randomly.ab.
28. trial.ti.
29. or/22‐28
30. exp animals/ not humans.sh.
31. 29 not 30
32. crossover procedure/
33. Double Blind Procedure/
34. Randomized Controlled Trial/
35. Single Blind Procedure/
36. random$.ti,ab.
37. factorial$.ti,ab.
38. (crossover$ or cross‐over$).ti,ab.
39. placebo$.ti,ab.
40. (doubl$ adj blind$).ti,ab.
41. (singl$ adj blind$).ti,ab.
42. assign$.ti,ab.
43. allocat$.ti,ab.
44. volunteer$.ti,ab.
45. or/32‐44
46. 5 and 21 and 31 use prem
47. 5 and 21 and 31 use mesz
48. 5 and 21 and 45 use emed
49. 5 and 21 use cctr
50. or/46‐49
Appendix 3. Search strategy used in original review
1. exp hypertension/ or exp blood pressure/
2. (hypertens$ or antihypertens$ or anti hypertens$).ti,ab,ot.
3. ((systolic or diastolic or arterial) adj pressur$).ti,ab,ot.
4. (blood pressur$ or bloodpressur$).ti,ab,ot.
5. or/1‐4
6. (orlistat$ or tetrahydrolipstatin or thlp).ti,ab,ot,tn,sh.
7. (xenical or alli).ti,ab,ot,tn.
8. or/6,7
9. sibutramin$.ti,ab,ot,tn,sh.
10. (reductil or medaria or meridia or arcalion).ti,ab,ot,tn.
11. or/9,10
12. rimonabant$.ti,ab,ot,tn,sh.
13. (acomplia or zimulti).ti,ab,ot,tn.
14. or/12,13
15. or/8,11,14
16. controlled clinical trial.pt.
17. controlled clinical trials/
18. randomized controlled trial.pt.
19. randomized controlled trials/
20. random allocation/
21. cross‐over studies/
22. double‐blind method/
23. single‐blind method/
24. or/16‐23
25. ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ti,ab,ot.
26. ((random$ or cross‐over or crossover) adj25 (trial$ or study or studies or intervention$ or investigat$ or experiment$ or design$ or method$ or group$ or evaluation$ or evidenc$ or data or test$ or condition$)).ti,ab,ot.
27. (random$ adj25 (cross over or crossover)).ti,ab,ot.
28. (RCT or placebo$).ti,ab,ot.
29. or/25‐28
30. 24 or 29
31. 5 and 15
32. 31 use prem
33. 31 use mesz
34. 31 use emed
35. or/32‐34
36. 35 and 30
37. 31 use cctr
38. 36 or 37
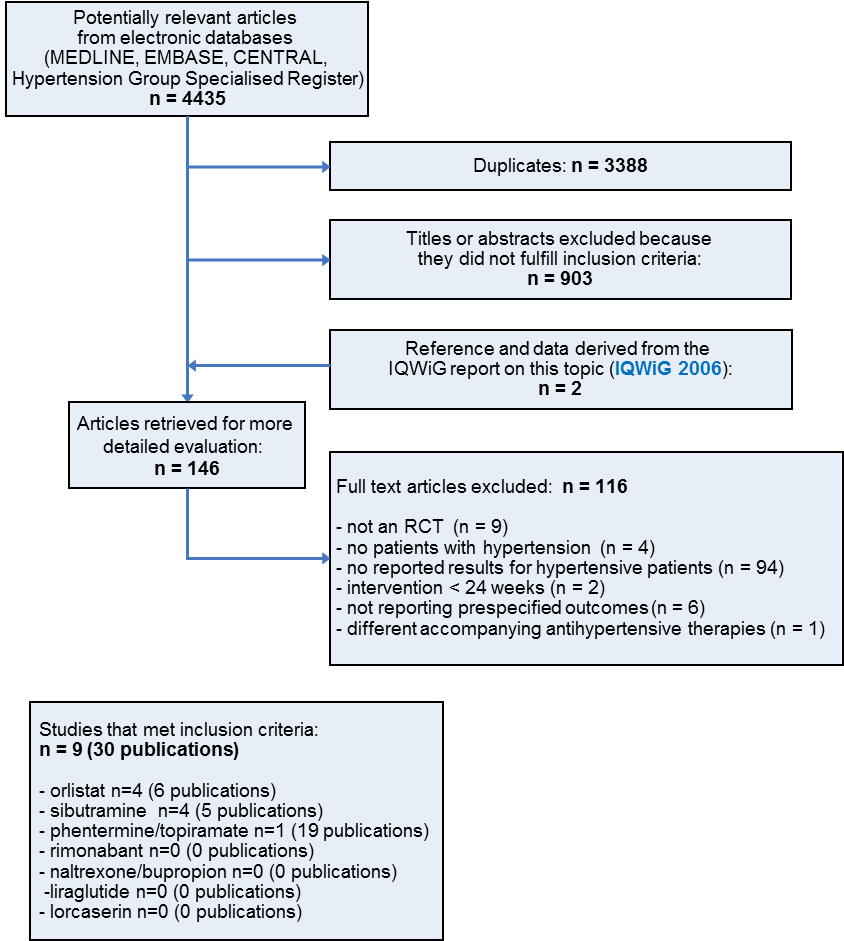
Study flow diagram
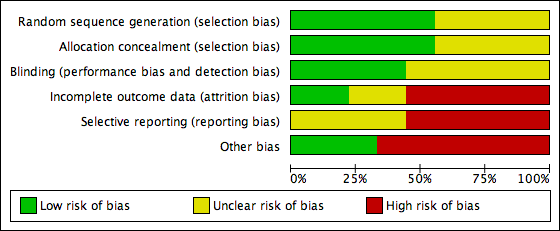
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.1 Change in systolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG04.png)
Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.1 Change in systolic blood pressure from baseline to endpoint [mm Hg].
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.2 Change in diastolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG05.png)
Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.2 Change in diastolic blood pressure from baseline to endpoint [mm Hg].
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.3 Change in body weight from baseline to endpoint [kg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG06.png)
Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.3 Change in body weight from baseline to endpoint [kg].
![Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.1 Change in diastolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG07.png)
Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.1 Change in diastolic blood pressure from baseline to endpoint [mm Hg].
![Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.2 Change in body weight from baseline to endpoint [kg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG08.png)
Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.2 Change in body weight from baseline to endpoint [kg].

Comparison 1 Orlistat versus placebo, Outcome 1 Change in systolic blood pressure from baseline to endpoint.

Comparison 1 Orlistat versus placebo, Outcome 2 Change in diastolic blood pressure from baseline to endpoint.

Comparison 1 Orlistat versus placebo, Outcome 3 Change in body weight from baseline to endpoint.

Comparison 2 Sibutramine versus placebo, Outcome 1 Change in diastolic blood pressure from baseline to endpoint.

Comparison 2 Sibutramine versus placebo, Outcome 2 Change in body weight from baseline to endpoint.
| Orlistat compared with placebo for weight reduction | |||||
| Patient or population: Men and non‐pregnant women ≥ 18 years old with essential hypertension Intervention: Orlistat Comparison: Placebo | |||||
| Outcomes | Illustrative comparative risks (per 1000 patients) | Effect estimate | No of Participants | Quality of the evidence | Comments |
| Change in systolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | MD ‐2.46 [‐4.01, ‐0.90] | 2058 | ⊕⊕⊝⊝ | ‐ |
| Change in diastolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | MD ‐1.92 [‐2.99, ‐0.85] | 2058 | ⊕⊕⊝⊝ | ‐ |
| Change in body weight as compared to placebo [kg] from baseline to end of study | Not applicable | MD ‐3.73 [‐4.65, ‐2.80] | 2080 | ⊕⊕⊕⊝ | ‐ |
| CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence | |||||
| 1High risk of bias in included studies. 2Wide confidence intervals include non‐clinically important effect. | |||||
| Sibutramine compared with placebo for weight reduction | |||||
| Patient or population: Men and non‐pregnant women ≥ 18 years old with essential hypertension Intervention: Sibutramine Comparison: Placebo | |||||
| Outcomes | Illustrative comparative risks (per 1000 patients) | Effect estimates | No of Participants | Quality of the evidence | Comments |
| Change in systolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | Not estimable | See comment | See comment | Variability measurements not available; |
| Change in diastolic blood pressure as compared to placebo [mm Hg] from baseline to end of study | Not applicable | MD 3.16 [1.40, 4.92] | 428 | ⊕⊕⊝⊝ | ‐ |
| Change in body weight as compared to placebo [kg] from baseline to end of study | Not applicable | MD ‐3.74 [‐4.84, ‐2.64] | 574 | ⊕⊕⊝⊝ | ‐ |
| CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence | |||||
| 1High risk of bias in included studies. 2Small number of participants and studies. | |||||
| Study | Adverse events | Results |
| Orlistat vs placebo | ||
| Bakris 2002 | total thereof leading to withdrawal serious gastrointestinal thereof leading to withdrawal musculoskeletal | 89% of P [O] vs 71% of P [P], P < 0.001 7% [O] vs 7% [P] 14 P (12%) [O] vs 15 P (9%) [P] 200 P (73%) [O] vs 120 P (44%) [P], P < 0.001 15 P (8%) [O] vs 6 P (5%) [P] 23% of P [O] vs 16% [P], P < 0.05 |
| Cocco 2005 | total serious gastrointestinal | nr 0 P [O] vs 0 P [P] 16 P (36%) [O]a vs 11 P (24%) [P]a |
| Guy‐Grand 2004 | total serious | nrb nrb |
| XENDOS 2001‐2006 | total leading to withdrawal serious gastrointestinal musculoskeletal nervous system dermatological vascular | 99% of P [OD] vs 96% of P [PD] 99% of P [OS] vs 97% of P [PS] 9% of P [OD] vs 4% of P [PD] 9% of P [OS] vs 4% of P [PS] 18% of P [OD] vs 12% of P [PD] 18% of P [OS] vs 12% of P [PS] 93% of P [OD] vs 70% of P [PD] 93% of P [OS] vs 71% of P [PS] 65% of P [OD] vs 62% of P [PD] 65% of P [OS] vs 63% of P [PS] 39% of P [OD] vs 39% of P [PD] 40% of P [OS] vs 37% of P [PS] 20% of P [OD] vs 17% of P [PD] 22% of P [OS] vs 17% of P [PS] 17% of P [OD] vs 19% of P [PD] 17% of P [OS] vs 19% of P [PS] |
| Sibutramine vs placebo | ||
| Fanghaenel 2003 | total constipation dizziness dry mouth headache insomnia restlessness | 14 P (21 E) [S] vs 13 P (20 E) [P] 4 P [S] vs 2 P [P] 1 P [S] vs 1 P [P] 4 P [S] vs 2 P [P] 5 P [S] vs 2 P [P] 1 P [S] vs 1 P [P] 1 P [S] vs 0 P [P] |
| Faria 2002‐2005 | total dry mouth arthralgia | nr 37% of P [S] vs 9% of P [P], P < 0.005 16% of P [S] vs 2% of P [P], P = 0.03 |
| McMahon 2002 | total serious treatment related leading to withdrawal (mostly hypertension) dry mouth headache | 141 P (97%) [S] vs 65 P (88%) [P] 9 P (6%) [S] vs 5 P (7%) [P] 2 E [S] vs 0 E [P] 23 P (16%) [S] vs 4 P (5%) [P] 30 P (21%) [S] vs 0 P [P] 41 P (28%) [S] vs 17 P (23%) [P] |
| McMahon 2000 | total leading to withdrawal (mostly hypertension) dry mouth headache constipation rash | nr 30 P (20%) [S] vs 8 P (11%) [P] 29 P (19%) [S] vs 2 P (3%) [P], P < 0.05 37 P (25%) [S] vs 21 P (28%) [P] 25 P (17%) [S] vs 2 P (3%) [P], P < 0.05 16 P (11%) [S] vs 2 P (3%) [P] |
| Phentermine/topiramate vs placebo | ||
| CONQUER 2013 | total leading to withdrawal serious cardiac adverse events dry mouth paresthaesia constipation upper respiratory tract infection nasopharyngitis dysgeusia insomnia headache dizziness sinusitis | 85.4% vs 88.8% vs 77.3% 11.9% vs 19.8% vs 9.7% 3.4% (Phen/Top [LD]) vs 3.7% (Phen/Top [HD]) vs 4.2% [P] 0.8% vs 1.2% vs 0.6% 14.2% (Phen/Top [LD]) vs 22.7% (Phen/Top [HD]) vs 2.3% [P] 14.2% (Phen/Top [LD]) vs 22.3% (Phen/Top [HD]) vs 2.3% [P] 15.7% (Phen/Top [LD]) vs 18.1% (Phen/Top [HD]) vs 5.5% [P] 12.6% (Phen/Top [LD]) vs 12.1% (Phen/Top [HD]) vs 11.8% [P] 10.3% (Phen/Top [LD]) vs 10.2% (Phen/Top [HD]) vs 8.8% [P] 7.7% (Phen/Top [LD]) vs 11.0% (Phen/Top [HD]) vs 0.8% [P] 5.7% (Phen/Top [LD]) vs 11.0% (Phen/Top [HD]) vs 4.8% [P] 5.0% (Phen/Top [LD]) vs 10.8% (Phen/Top [HD]) vs 8.4% [P] 6.5% (Phen/Top [LD]) vs 12.1% (Phen/Top [HD]) vs 3.1% [P] 5.4% (Phen/Top [LD]) vs 8.3% (Phen/Top [HD]) vs 6.5% [P] |
| E: events. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. aNo data on adverse events were reported for the whole study duration. The data above refer to 4 and 3 weeks of treatment in the orlistat and placebo group, respectively. After 3 months, the number of participants with events decreased to 5(11%)[O] with flatulence and mild abdominal cramps versus 6(13%)[P] with nausea and hunger feeling. bData were not available for the hypertensive subgroup, only for the whole study population (withdrawal due to defecation troubles in 10 [O] versus 2 [P] participants). | ||
| Study | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | |||||
| Bakris 2002b Orlistat Placebo | 101 (1)c 102 (1)c | nr nr | nr nr | ‐ ‐ | P < 0.001 ‐5.4 (6.4) ‐2.7 (6.4) |
| Cocco 2005 Orlistat Placebo | 107 (6) 106 (6) | 102 (4) 104 (5) | ‐ ‐ | ‐ ‐ | P < 0.001 ‐5.4d ‐2.5d |
| Guy‐Grand 2004 Orlistat Placebo | 94 (1)c 94 (1)c | nr nr | ‐ ‐ | ‐ ‐ | P < 0.0001 ‐5.8 (0.3) ‐1.8 (0.2) |
| XENDOS 2001‐2006 Orlistat [OD] Placebo [PD] Orlistat [OS] Placebo [PS] | 117 (18) 115 (18) 117 (17) 116 (18) | 106 (17) 108 (18) 106 (17) 109 (18) | 105 (18) 108 (19) 105 (17) 110 (19) | 110 (19) 111 (20) 110 (18) 113 (19) | P < 0.001 ‐6.6 (8.6) ‐3.8 (7.8) P < 0.001 ‐6.8 (8.7) ‐3.2 (7.4) |
| Sibutramine vs placebo | |||||
| Fanghaenel 2003 Sibutramine Placebo | 75 (10) 78 (9) | 70 (10) 75 (9) | ‐ ‐ | ‐ ‐ | significant ‐5.5 (‐3.8; ‐7.1)e ‐3.4 (‐1.9; ‐5.0)e |
| Faria 2002‐2005 Sibutramine Placebo | 100 (19) 97 (14) | 93 (18) 94 (15) | ‐ ‐ | ‐ ‐ | P < 0.001 ‐6.8 (2.3) ‐2.4 (4.2) |
| McMahon 2002 Sibutramine Placebo | 97 (16) 99 (14) | nr nr | nr nr | ‐ ‐ | P < 0.05 ‐4.5 ‐0.4 |
| McMahon 2000 Sibutramine Placebo | 97 (13) 96 (17) | nr nr | nr nr | ‐ ‐ | P < 0.05 ‐4.4 ‐0.5 |
| Phentermine/topiramate vs placebo | |||||
| CONQUER 2013 Phen/Top [LD] Phen/Top [HD] Placebo | 104 (18)f | nr nr nr | nr nr nr | ‐ ‐ ‐ | P < 0.0001g ‐8.1% ‐10.1% ‐1.9% |
| Mo: months. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. SD: standard deviation. aMean kg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cReported as being the standard deviation but probably the standard error due to its small number. dPublished values are different, but data were corrected after personal communication with the author. e95% confidence interval. fReported only combined for all three study groups. gFor each intervention group versus placebo. | |||||
| Study | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | |||||
| Bakris 2002b Orlistat Placebo | 154 (13) 151 (13) | nr nr | nr nr | ‐ ‐ | ns ‐13.3 (15.2) ‐11.0 (15.0) |
| Cocco 2005 Orlistat Placebo | 146 (10) 142 (6) | 142 (13) 141 (9) | ‐ ‐ | ‐ ‐ | P = 0.025 ‐4.3 ‐0.9 |
| Guy‐Grand 2004 Orlistat Placebo | 150 (1)c 152 (1)c | nr nr | ‐ ‐ | ‐ ‐ | ns ‐9.8 (1) ‐9.8 (1) |
| XENDOS 2001‐2006 Orlistat [OD]d Placebo [PD]d Orlistat [OS]d Placebo [PS]d | 146 (13) 146 (12) 149 (10) 149 (8) | 135 (14) 136 (15) 125 (14) 138 (14) | 135 (14) 138 (16) 135 (14) 140 (14) | 137 (15) 139 (16) 138 (15) 140 (15) | P = 0.024 ‐8.8 (14.8) ‐6.4 (15.1) P < 0.002 ‐11.5 (14.9) ‐8.6 (14.3) |
| Sibutramine vs placebo | |||||
| Fanghaenel 2003e Sibutramine Placebo | 139 (9) 139 (13) | 125 (9) 123 (10) | ‐ ‐ | ‐ ‐ | ns ‐13.9f ‐16.5f |
| Faria 2002‐2005 Sibutramine Placebo | 150 (18) 150 (15) | 146 (15) 149 (22) | ‐ ‐ | ‐ ‐ | ns ‐4.6f ‐0.6f |
| McMahon 2002 Sibutramine Placebo | 129 (11) 129 (11) | nr nr | 133 130 | ‐ ‐ | P = 0.0497 3.8 1.1 |
| McMahon 2000 Sibutramine Placebo | 134 (10) 134 (11) | nr nr | nr nr | ‐ ‐ | ns 2.7 1.5 |
| Phentermine/topiramate vs placebo | |||||
| CONQUER 2013 Phen/Top [LD] Phen/Top [HD] Placebo | 134 (nr) 133 (nr) 135 (nr) | nr nr nr | nr nr nr | ‐ ‐ ‐ | P = 0.0475 [LD] P < 0.0001 [HD] ‐6.9 ‐9.1 ‐4.9 |
| Mo: months. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. SD: standard deviation. aMean mm Hg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cReported as being the standard deviation but probably the standard error due to its small number. dBased on last observation carried forward data on 399 [OD], 423 [PD], 493 [OS], and 504 [PS] participants. eData at baseline were recorded after a two‐week wash‐out period of antihypertensive drugs for diagnostic confirmation of hypertension. fCalculated. | |||||
| Study | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | |||||
| Bakris 2002b Orlistat Placebo | 98 (4) 98 (4)c | nr nr | nr nr | ‐ ‐ | P = 0.002 ‐11.4 (8.3) ‐9.2 (8.4) |
| Cocco 2005 Orlistat Placebo | 88 (7) 85 (6) | 84 (9) 85 (7) | ‐ ‐ | ‐ ‐ | P = 0.012 ‐3.6 ‐0.8 |
| Guy‐Grand 2004 Orlistat Placebo | 97 (0)d 97 (0)d | nr nr | ‐ ‐ | ‐ ‐ | ns ‐7.5 (0.6) ‐7.3 (0.6) |
| XENDOS 2001‐2006 Orlistat [OD]e Placebo [PD]e Orlistat [OS]e Placebo [PS]e | 95 (6) 95 (5) 91 (9) 91 (8) | 86 (8) 88 (9) 84 (9) 87 (9) | 86 (8) 88 (10) 85 (9) 88 (10) | 87 (9) 89 (10) 86 (9) 88 (10) | P < 0.006 ‐8.1 (9.3) ‐6.2 (9.9) P < 0.001 ‐5.0 (9.9) ‐3.0 (10.4) |
| Sibutramine vs placebo | |||||
| Fanghaenel 2003f Sibutramine Placebo | 93 (7) 92 (8) | 82 (5) 80 (5) | ‐ ‐ | ‐ ‐ | ns ‐11.4g ‐11.7g |
| Faria 2002‐2005 Sibutramine Placebo | 91 (12) 94 (12) | 92 (13) 92 (14) | ‐ ‐ | ‐ ‐ | ns 1.0g ‐2.06g |
| McMahon 2002 Sibutramine Placebo | 82 (6) 83 (6) | nr nr | 86 83 | ‐ ‐ | P = 0.004 3.0 ‐0.1 |
| McMahon 2000 Sibutramine Placebo | 84 (5) 84 (6) | nr nr | nr nr | ‐ ‐ | P < 0.05 2.0 ‐1.3 |
| Phentermine/topiramate vs placebo | |||||
| CONQUER 2013 Phen/Top [LD] Phen/Top [HD] Placebo | 83 (nr) 83 (nr) 85 (nr) | nr nr nr | nr nr nr | ‐ ‐ ‐ | P = 0.0400 [LD] P = 0.0003 [HD] ‐5.2 ‐5.8 ‐3.9 |
| Mo: months. nr: not reported. [O]: orlistat. [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg. [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg. P: participants. [P]: placebo. Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg). Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg). [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg. [PS]: placebo and systolic blood pressure ≥ 140 mm Hg. [S]: sibutramine. SD: standard deviation. aMean mm Hg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cThe standard deviation was published as being 35 but should probably be 3.5. dReported as being the standard deviation but probably the standard error due to its small number. eBased on last observation carried forward data on 399 [OD], 423 [PD], 493 [OS], and 504 [PS] participants. fData at baseline were recorded after a two‐week wash‐out period of antihypertensive drugs for diagnostic confirmation of hypertension. gCalculated. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in systolic blood pressure from baseline to endpoint Show forest plot | 4 | 2058 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐4.01, ‐0.90] |
| 2 Change in diastolic blood pressure from baseline to endpoint Show forest plot | 4 | 2058 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐2.99, ‐0.85] |
| 3 Change in body weight from baseline to endpoint Show forest plot | 4 | 2080 | Mean Difference (IV, Random, 95% CI) | ‐3.73 [‐4.65, ‐2.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in diastolic blood pressure from baseline to endpoint Show forest plot | 2 | 428 | Mean Difference (IV, Fixed, 95% CI) | 3.16 [1.40, 4.92] |
| 2 Change in body weight from baseline to endpoint Show forest plot | 4 | 574 | Mean Difference (IV, Fixed, 95% CI) | ‐3.74 [‐4.84, ‐2.64] |

