Aromaterapia para el tratamiento de las náuseas y los vómitos posoperatorios
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007598.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: Sonia Hines (SH)
Designing the review: SH
Co‐ordinating the review: SH
Undertaking manual searches: SH
Screening search results: SH, Elizabeth Steels (ES)
Organizing retrieval of papers: SH
Screening retrieved papers against inclusion criteria: SH, ES, Anne Chang (AC)
Appraising quality of papers: SH, ES, AC
Abstracting data from papers: SH, ES, Kirsten Gibbons (KG)
Writing to authors of papers for additional information: SH
Providing additional data about papers: SH, AC
Obtaining and screening data from unpublished studies: SH, ES
Data management for the review: SH
Entering data into Review Manager 5 (RevMan 2014): SH, KG
Analysis of data: SH, ES, KG
Interpretation of data: SH, ES, AC, KG
Writing the review: SH, AC, KG
Securing funding for the review: SH
Performing previous work that was the foundation of the present study: SH
Guarantor for the review (one author): SH
Statistical analysis: KG, AC, SH
Sources of support
Internal sources
-
Nursing Research Centre, Mater Health Services, Australia.
Time and facilities.
External sources
-
Queensland Health, Australia.
Nursing and Midwifery Research Grant ($5906) awarded to Sonia Hines
Declarations of interest
Sonia Hines: Queensland Health Nursing and Midwifery Research Grant received by Sonia Hines in 2008 to assist with the conduct of the original review (AUD 5906) (Hines 2012). The granting body had no influence on the findings of this review.
Elizabeth Steels: no conflict of interest is known
Anne Chang: no conflict of interest is known
Kristen Gibbons: no conflict of interest is known
Acknowledgements
We thank Mathew Zacharias, Jung T Kim, NL Pace, Peter Kranke and Anne Lyddiatt for their help and advice during the preparation of the 2012 systematic review (Hines 2012), and Janet Wale, Winnie Schats, Jacqueline Dienemann, Fakher Rahim, Janet Roseman, Jing Xie and Anna Lee for their advice on the 2017 update.
We also thank Mathew Zacharias, Katrina Farber, Milli Reddy, Jung T Kim and Janet Wale for their help and editorial advice during the preparation of the protocol (Hines 2009), for the systematic review.
The authors wish to acknowledge Kathy Hibberd (Librarian, University of Queensland Medical Library) for her invaluable assistance in preparing and conducting the searches for the 2012 review, and Leandra Blake for her comments on the protocol and 2012 review. We also thank Kate Kynoch and Lisa Brown for assisting with the testing of the data extraction tool.
Thanks to Marie Kristensson for the Swedish translations, Abbas Breesem for the Farsi translation, and Laurie Bay at the Institute of Modern Languages at the University of Queensland for the French translation.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 10 | Aromatherapy for treatment of postoperative nausea and vomiting | Review | Sonia Hines, Elizabeth Steels, Anne Chang, Kristen Gibbons | |
| 2012 Apr 18 | Aromatherapy for treatment of postoperative nausea and vomiting | Review | Sonia Hines, Elizabeth Steels, Anne Chang, Kristen Gibbons | |
| 2009 Jan 21 | Aromatherapy for treatment of postoperative nausea and vomiting | Protocol | Sonia Hines, Elizabeth Steels, Anne Chang, Kristen Gibbons | |
Differences between protocol and review
The original protocol (Hines 2009) stated "We will judge the study quality using a validated critical appraisal checklist developed by the Joanna Briggs Institute and based on the work of The Cochrane Collaboration and the Centre for Reviews and Dissemination (Figure 2). This checklist assesses selection, allocation, treatment, and attrition biases". Due to changes in Cochrane requirements, we have used the Cochrane 'Risk of bias' assessment instead.
We had originally planned to search the website www.nhmrc.gov.au/nics/asp/index.asp, however this no longer exists and we searched www.anzctr.org.au/Default.aspx instead.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- 2-Propanol [*administration & dosage];
- Administration, Inhalation;
- Antiemetics [*administration & dosage];
- Aromatherapy [*methods];
- Controlled Clinical Trials as Topic;
- Plant Oils [*administration & dosage];
- Postoperative Nausea and Vomiting [*therapy];
- Randomized Controlled Trials as Topic;
- Salvage Therapy [methods];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram
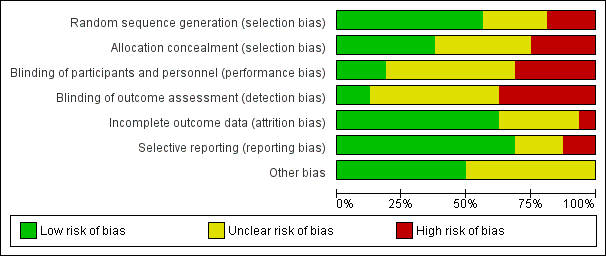
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
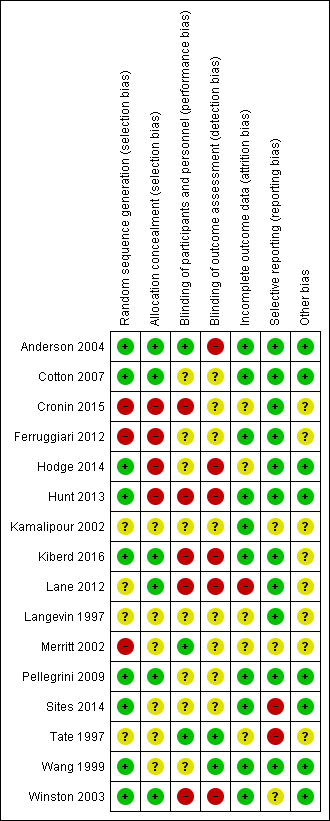
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
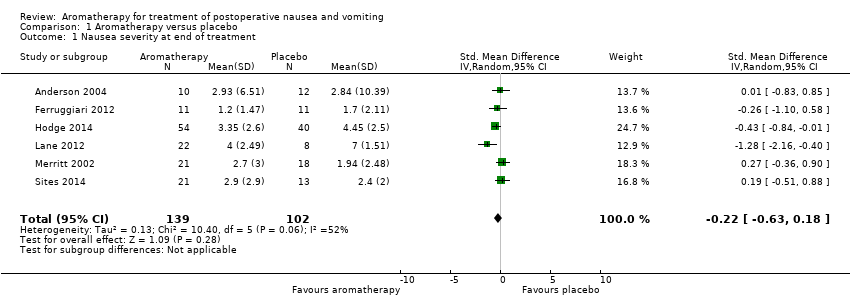
Comparison 1 Aromatherapy versus placebo, Outcome 1 Nausea severity at end of treatment.
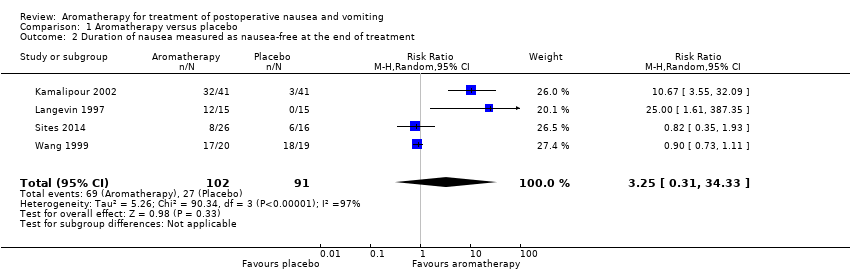
Comparison 1 Aromatherapy versus placebo, Outcome 2 Duration of nausea measured as nausea‐free at the end of treatment.

Comparison 1 Aromatherapy versus placebo, Outcome 3 Proportion requiring rescue antiemetics.

Comparison 2 Peppermint versus placebo, Outcome 1 Nausea severity at 5 minutes post‐initial treatment.
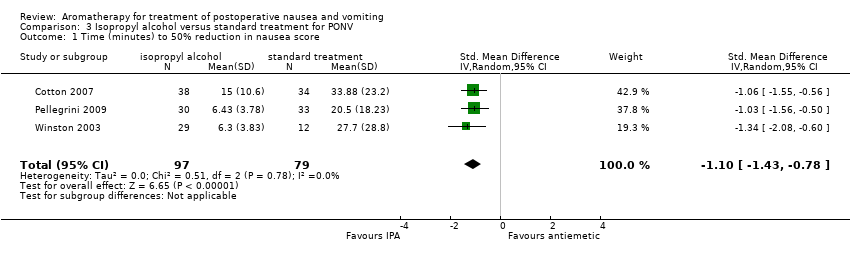
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 1 Time (minutes) to 50% reduction in nausea score.
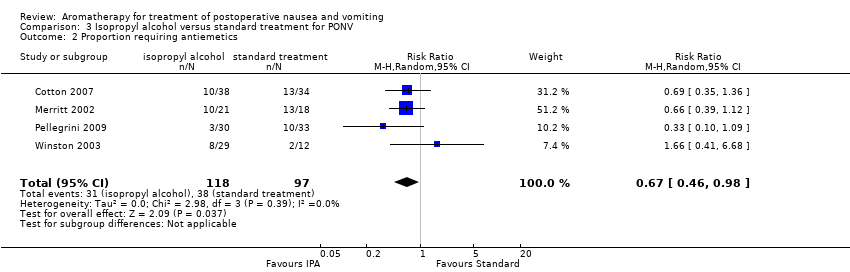
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 2 Proportion requiring antiemetics.
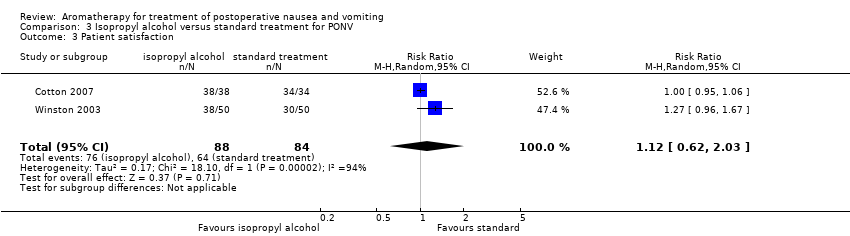
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 3 Patient satisfaction.

Comparison 4 Isopropyl alcohol versus saline, Outcome 1 Proportion requiring rescue antiemetics.
| Aromatherapy compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, either as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with aromatherapy | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.22 SD lower | ‐ | 241 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | Study population | RR 3.25 | 193 | ⊕⊝⊝⊝ | ||
| 30 per 100 | 96 per 100 | |||||
| Proportion requiring rescue antiemetics | Study population | RR 0.60 | 609 | ⊕⊕⊝⊝ | ||
| 68 per 100 | 41 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias across all studies due to study designs, downgraded one level. | ||||||
| Peppermint compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with peppermint | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.18 SD lower | ‐ | 115 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias in included studies due to study designs, downgraded one level. | ||||||
| Isopropyl alcohol compared to standard treatment for postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard treatment for PONV | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (measured as nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | The mean time to 50% reduction in nausea score was 20.5 minutes | SMD 1.10 SD lower | ‐ | 176 | ⊕⊕⊕⊝ | Risk in placebo group based |
| Use of rescue antiemetics | Study population | RR 0.67 | 215 | ⊕⊕⊕⊝ | ||
| 39 per 100 | 26 per 100 | |||||
| Patient satisfaction with treatment Measured by a validated scale | Study population | RR 1.12 | 172 | ⊕⊝⊝⊝ | ||
| 76 per 100 | 85 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No or unclear blinding in all included studies, downgraded one level. | ||||||
| Isopropyl alcohol compared to saline for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with saline | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | Study population | RR 0.39 | 291 | ⊕⊝⊝⊝ | ||
| 90 per 100 | 35 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Poor reporting in Kamalipour 2002 and Langevin 1997 affect confidence in results, downgraded one level. | ||||||
| Study | Design | Intervention/comparison | Measure | Satisfied |
| RCT | IPA/Saline/Peppermint | 100 mm VAS (0 mm extremely dissatisfied; 100 mm fully satisfied) | IPA: 90.3 (SD: 14.9) peppermint: 86.3 (SD: 32.3) saline: 83.7 (SD: 25.6) | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/38 Comparison: 34/34 | |
| RCT | IPA/Promethazine | 5‐point DOS (1 = totally unsatisfied, 5 = totally satisfied) | Both groups reported median score 4 | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/50 Comparison: 30/50 | |
| DOS: descriptive ordinal scale; IPA: isopropyl alcohol; RCT: randomized controlled trial; SD: standard deviation; VAS: visual analogue scale | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea severity at end of treatment Show forest plot | 6 | 241 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.63, 0.18] |
| 2 Duration of nausea measured as nausea‐free at the end of treatment Show forest plot | 4 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.31, 34.33] |
| 3 Proportion requiring rescue antiemetics Show forest plot | 7 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.37, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea severity at 5 minutes post‐initial treatment Show forest plot | 4 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.86, 0.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time (minutes) to 50% reduction in nausea score Show forest plot | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.43, ‐0.78] |
| 2 Proportion requiring antiemetics Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.98] |
| 3 Patient satisfaction Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.62, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue antiemetics Show forest plot | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.24] |

