Aromatherapie zur Behandlung von postoperativer Übelkeit und Erbrechen
Abstract
Background
Postoperative nausea and vomiting (PONV) is a common, unpleasant phenomenon and current therapies are not always effective for all patients. Aromatherapy has been suggested as an addition to the available treatment strategies. This review was originally published in 2012 and updated in 2017.
Objectives
The main objective was to establish the efficacy and safety of aromatherapy comparable to standard pharmacological treatments for PONV in adults and children.
Search methods
We searched CENTRAL; MEDLINE; Embase; CINAHL; CAM on PubMed; Informit; LILACS; and ISI Web of Science as well as grey literature sources and the reference lists of retrieved articles up to March 2017. The original search was performed in August 2011.
Selection criteria
We included all randomized controlled trials (RCTs) and controlled clinical trials (CCTs) where aromatherapy was used to treat PONV. Interventions were all types of aromatherapy compared to placebo or with standard antiemetics. Primary outcomes were severity and duration of PONV. Secondary outcomes were adverse reactions, use of rescue antiemetics and patient satisfaction.
Data collection and analysis
Two review authors independently assessed risk of bias in the included studies and extracted data. For dichotomous outcome variables, we used a random‐effects model and calculated risk ratio (RR) with associated 95% confidence interval (95% CI). For continuous outcome variables, we used a random‐effects model and calculated standardized mean difference (SMD) with associated 95% CI. We used the GRADE software to compile 'Summary of findings' tables.
Main results
We included seven new studies with 663 participants in the 2017 update; five RCTs and two CCTs. These were added to the nine previously included studies (six RCTs and three CCTs with a total of 373 participants) for a total of 16 included studies and 1036 participants in this updated review. The mean age and range data for all participants were not reported for all studies. We identified two registered trials that met the inclusion criteria for this review; however there are no results for these studies yet.
Overall, the GRADE assessment of evidence quality ranged from moderate to very low. The method of randomization in 11 of the 12 included RCTs was explicitly stated and adequate. Incomplete or methodologically diverse reporting of data affected the completeness of the analysis. Data on additional aromatherapies were added in the 2017 update (blended aromatherapy products, and peppermint products). Heterogeneity of outcome measures and time points between studies affected the completeness of the analysis.
In the summary of the findings of six studies, we did not find aromatherapy to be effective in reducing nausea severity in comparison to placebo (SMD ‐0.22, 95% CI ‐0.63 to 0.18, P value = 0.28, 241 participants, level of evidence: low). Those participants receiving aromatherapy were no more likely to be free of nausea at the end of the treatment period than those receiving placebo (RR 3.25, 95% CI 0.31 to 34.33, P value = 0.33, 4 trials, 193 participants, evidence level: very low), however they were less likely to require rescue antiemetics (RR 0.60, 95% CI 0.37 to 0.97, P value = 0.04, 7 trials, 609 participants, evidence level: low). There were no data reported on adverse events or patient satisfaction for this comparison.
A specific comparison of peppermint aromatherapy to placebo did not show evidence of an effect on nausea severity at five minutes post‐treatment in the pooled results (SMD ‐0.18, 95% CI ‐0.86 to 0.49, P value = 0.59, 4 trials, 115 participants, evidence level: low). There were no data reported on nausea duration, use of rescue antiemetics, adverse events or patient satisfaction for this comparison.
When we pooled studies comparing isopropyl alcohol to standard antiemetic treatment in a GRADE summary of findings, in terms of nausea duration, there was a significant effect on the time in minutes to a 50% reduction in nausea scores (SMD ‐1.10, 95% CI ‐1.43 to ‐0.78, P value < 0.00001, 3 trials, 176 participants, evidence level: moderate). Fewer participants who received isopropyl alcohol required rescue antiemetics (RR 0.67, 95% CI 0.46 to 0.98, P value = 0.04, 215 participants, 4 trials, evidence level: moderate). Two studies with 172 participants measured patient satisfaction; there were high levels of satisfaction across both aromatherapy and standard treatment groups and no differences found (evidence level: low). There were no data reported on nausea severity or adverse events for this comparison.
There was no difference in effectiveness between isopropyl alcohol vapour inhalation and placebo for reducing the proportion of participants requiring rescue antiemetics (RR 0.39, 95% CI 0.12 to 1.24, P value = 0.11, 291 participants, 4 trials, evidence level: very low). There were no data reported on nausea severity, nausea duration, adverse events or patient satisfaction for this comparison.
Authors' conclusions
Overall, for nausea severity at the end of treatment, aromatherapy may have similar effectiveness to placebo and similar numbers of participants were nausea‐free. However, this finding is based on low‐quality evidence and therefore very uncertain. Low‐quality evidence also suggests that participants who received aromatherapy may need fewer antiemetic medications, but again, this is uncertain. Participants receiving either aromatherapy or antiemetic medications may report similar levels of satisfaction with their treatment, according to low‐quality evidence.
PICO
Laienverständliche Zusammenfassung
Aromatherapie gegen postoperative Übelkeit und Erbrechen
Fragestellung
In diesem Review wurde versucht, die Wirkung von Aromatherapie auf die Schwere und Dauer von Übelkeit und Erbrechen, die bei einigen Personen direkt nach einer Operation auftreten, zu beurteilen.
Hintergrund
Postoperative Übelkeit und Erbrechen (postoperative nausea and vomiting, PONV) ist eine häufige Nebenwirkung, die nach einer Operation auftritt. Bis zu einem Drittel aller Patienten leiden unter moderater bis schwerer Übelkeit und Erbrechen infolge einer Allgemeinnarkose mit Inhalationsanästhetika. Übelkeit ist ein Unbehagen in der Magengegend, das mit Erbrechen einhergehen kann. Derzeitige medikamentöse Behandlungen sind nicht immer wirksam oder können unangenehme unerwünschte Wirkungen auslösen. Bei der Aromatherapie wird der Dampf ätherischer Öle oder anderer Substanzen inhaliert, um körperliche und emotionale Symptome zu behandeln oder zu lindern. Aromatherapie wird manchmal zur Behandlung von Übelkeit und Erbrechen empfohlen, obwohl derzeit keine ausreichende Evidenz vorliegt, die ihre Wirksamkeit bestätigt. Dieser Review ist die Aktualisierung eines 2012 veröffentlichten Reviews.
Studienmerkmale
Wir untersuchten insgesamt 16 kontrollierte, klinische Studien über Aromatherapie zur Behandlung von PONV mit insgesamt 1036 Teilnehmern (zu den neun Studien des ursprünglichen Reviews wurden durch die Suche im März 2017 sieben neue Studien hinzugefügt). Die Teilnehmer waren Erwachsene, mit Ausnahme von zwei Studien mit Kindern. In den Studien wurde bei der ersten Beschwerde über Übelkeit in der Zeit unmittelbar nach der Operation Aromatherapie angewendet und die Übelkeit bis zu zwei Tage lang gemessen. Für die Aromatherapie wurden verwendet: Isopropylalkohol (Isopropanol), Pfefferminzöl, Ingwer oder Mischungen, die Ingwer, Grüne Minze, Pfefferminz und Kardamom enthielten; oder Lavendel, Pfefferminz, Ingwer und Öle aus Grüner Minze.
Die Studien verglichen Aromatherapie mit Kochsalzlösung‐ oder Wasser‐Placebos, mit kontrollierter Atmung, mit anderen Aromatherapie‐Substanzen, mit Medikamenten gegen Übelkeit oder mit einer Kombination aus den eben genannten. Manche Studien enthielten bis zu vier Gruppen.
Hauptergebnisse
Insgesamt zeigte Aromatherapie nach über drei Minuten nach der Behandlung keine Wirkung bei der Verringerung der Schwere der Übelkeit im Vergleich zu Kochsalzlösung, Wasser oder kontrollierter Atmung (6 Studien mit 241 Teilnehmern), aber mehr Teilnehmer, die Aromatherapie erhielten, verspürten am Ende der Behandlung keine Übelkeit (4 Studien, 193 Teilnehmer) und weniger Teilnehmer, die Aromatherapie erhielten, benötigten Medikamente gegen Übelkeit (7 Studien mit 609 Teilnehmern).
Pfefferminzöl zeigte fünf Minuten nach der Behandlung keine Wirkung auf die Schwere der Übelkeit (4 Studien, 115 Teilnehmer).
Wir konnten keine Daten für einen Vergleich von Isopropylalkohol mit Standardmedikation gegen Übelkeit in Hinblick auf die Schwere der Übelkeit zusammenführen. In Bezug auf die Dauer der Übelkeit wurde eine Linderung der Symptome um 50% mit Isopropylalkohol‐Dampf schneller erreicht als mit Standard‐Antiemetika (Ondansetron und Promethazin) (3 Studien, 176 Teilnehmer). Aromatherapie mit Isopropylalkohol‐Dämpfen schuf eine schnelle, kurzfristige Befreiung von Übelkeit und senkte die Notwendigkeit der Gabe von Notfallmedikation gegen Übelkeit (4 Studien, 215 Teilnehmer). In den vier Studien, die den Endpunkt gemessen haben, war die Zufriedenheit der Patienten mit der Aromatherapie hoch.
Im Vergleich zu Teilnehmern, die Kochsalzlösung erhielten, benötigten weniger Teilnehmer, die Isopropylalkohol‐Aromatherapie anwendeten, Notfallmedikation gegen Übelkeit (4 Studien, 291 Teilnehmer). Die Teilnehmer, die Aromatherapie erhielten, waren am Ende der Behandlungszeit nicht weniger häufig von Übelkeit betroffen, doch sie verlangten seltener Notfallmedikation gegen Übelkeit.
Alle Teilnehmer dieser Studien (Behandlungs‐ und Vergleichsgruppe) gaben ein hohes Maß an Zufriedenheit an. Möglicherweise deutet das darauf hin, dass erhöhte Aufmerksamkeit gegenüber der Behandlung postoperativer Übelkeit und Erbrechen die Zufriedenheit mit der Versorgung steigerte. Aromatherapie könnte eine sinnvolle therapeutische Option sein, insbesondere wenn die Alternative aus gar keiner Behandlung besteht.
Keine der eingeschlossenen Studien berichtete von unerwünschte Wirkungen der angewendeten Aromatherapien.
Qualität der Evidenz
Laut der Bewertung durch den GRADE‐Ansatz war die Qualität der Evidenz insgesamt moderat bis sehr niedrig. Aufgrund der Gestaltung einiger Studien war ein hohes Risiko für Bias gegeben. Die eingeschlossenen Studien bestanden aus zwölf randomisierten, kontrollierten Studien und vier kontrollierten, klinischen Studien, in denen die Teilnehmer nicht zufällig den Gruppen zugeteilt wurden. In den meisten Studien wussten Teilnehmer und Forscher über die Gruppenzuordnung Bescheid, was möglicherweise einen Einfluss auf die Ergebnisse hatte. Weil intensive Gerüche bei der Aromatherapie entstehen, handelt es sich um eine Intervention, die schwer vor Teilnehmern, Forschungspersonal und Personen, die die Ergebnisse messen, verborgen werden kann. Die unterschiedlichen Vergleiche, Zeitpunkte und Messskalen begrenzten die Daten, die gepoolt werden konnten. Einige Daten wurden als standardisierte Skalen und Maße dargestellt, was das Poolen von Ergebnissen in Meta‐Analysen ermöglichte. Die Daten zur Wirkung nach mehr als 60 Minuten waren unvollständig.
Authors' conclusions
Summary of findings
| Aromatherapy compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, either as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with aromatherapy | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.22 SD lower | ‐ | 241 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | Study population | RR 3.25 | 193 | ⊕⊝⊝⊝ | ||
| 30 per 100 | 96 per 100 | |||||
| Proportion requiring rescue antiemetics | Study population | RR 0.60 | 609 | ⊕⊕⊝⊝ | ||
| 68 per 100 | 41 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias across all studies due to study designs, downgraded one level. | ||||||
| Peppermint compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with peppermint | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.18 SD lower | ‐ | 115 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias in included studies due to study designs, downgraded one level. | ||||||
| Isopropyl alcohol compared to standard treatment for postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard treatment for PONV | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (measured as nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | The mean time to 50% reduction in nausea score was 20.5 minutes | SMD 1.10 SD lower | ‐ | 176 | ⊕⊕⊕⊝ | Risk in placebo group based |
| Use of rescue antiemetics | Study population | RR 0.67 | 215 | ⊕⊕⊕⊝ | ||
| 39 per 100 | 26 per 100 | |||||
| Patient satisfaction with treatment Measured by a validated scale | Study population | RR 1.12 | 172 | ⊕⊝⊝⊝ | ||
| 76 per 100 | 85 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No or unclear blinding in all included studies, downgraded one level. | ||||||
| Isopropyl alcohol compared to saline for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with saline | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | Study population | RR 0.39 | 291 | ⊕⊝⊝⊝ | ||
| 90 per 100 | 35 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Poor reporting in Kamalipour 2002 and Langevin 1997 affect confidence in results, downgraded one level. | ||||||
Background
Aromatherapy has been recommended for the treatment of postoperative nausea and vomiting (PONV) (Huntley 2014; Lindquist 2013). It is known that this therapy is inexpensive, non‐invasive and generally has low levels of adverse effects (Lua 2012), particularly in comparison to standard pharmacological treatments. What is not known is whether the clinical effectiveness justifies its use.
Description of the condition
Nausea is an abdominal discomfort or queasiness that may be accompanied by vomiting. Postoperative nausea and vomiting (PONV) is one of the most common adverse reactions to surgery and all types of anaesthesia, with 30% to 50% of all patients suffering moderate to severe nausea and vomiting following general anaesthesia using volatile agents (Gan 2014).
Aside from the distressing nature of PONV itself, patients may experience such adverse effects as wound dehiscence, dehydration, electrolyte imbalances or aspiration pneumonia as a result of PONV (Apfel 2010). Other adverse effects may include increased patient bed days, and unplanned readmissions (particularly in the case of day surgery) (Gan 2014). Certain patients are more pre‐disposed than others to suffering from PONV and risk factors include being female, a non‐smoker, and having a history of PONV or perioperative opioid exposure (Gan 2014). Along with postoperative pain, PONV is one of the main concerns of people facing surgery and one of the main causes of patient dissatisfaction (Myles 2000).
Current treatment involves either the prophylactic or symptomatic administration of antiemetic drugs such as droperidol, metoclopramide or 5‐HT3 receptor antagonists such as ondansetron (Gan 2014). Multi‐modal treatment using a range of drugs is now recognized as more effective (Gan 2014; Jokinen 2012). Despite a wide range of available treatments, some patients will still experience PONV in varying levels of severity (Jokinen 2012). Clinically, the severity of PONV is generally measured by means of a visual analogue scale (VAS), which provides a visual representation of the patient's condition over a numerical range (for example 0 to 5), or verbal descriptive scales (for example no nausea, some nausea, very nauseated, retching, vomiting) (Boogaerts 2000).
Description of the intervention
The use of aromatherapy oils has been recommended as a treatment for nausea (Lindquist 2013; Mamaril 2006; Safajou 2014). Aromatherapy uses the application of essential oils or other substances to any part of the body for the purpose of inhalation of the vapours or absorption of the oil into the skin to treat or alleviate physical and emotional symptoms (Lindquist 2013). Essential oils can be absorbed through the skin and may exert a physiological effect on cellular and organ function, although this is not clinically understood (Ernst 2001). Aromatherapy is well accepted by many health consumers; a meta‐analysis of survey data from the UK shows it to be one of the most commonly used complementary therapies (Posadzki 2013). A significant number of health consumers already self‐prescribe and administer aromatherapy products for various common conditions, or consult qualified or unqualified aromatherapy practitioners for health advice (Eisenberg 1998).
In particular, ginger, fennel and peppermint, as either a topical application (massage or a compress) or via inhalation, are well‐known treatments (Lindquist 2013). The effectiveness of the oils may be due to analgesic and antiemetic properties (with peppermint oil and ginger oil) or anti‐spasmodic properties (peppermint oil and fennel oil). Peppermint oil is well recognized for its role in digestion disorders, due principally to the presence of menthols (see Appendix 1 for details). There have been a number of studies conducted using ginger oil, with conflicting results (Arfeen 1995; Bone 1990; Meyer 1995; Phillips 1993). Isopropyl alcohol is said to be a traditional nausea remedy from South America (Anderson 2004; Mamaril 2006; Spencer 2004), however none of the papers citing this provided a primary source for this information. Isopropyl alcohol, also known as rubbing alcohol and commonly found in the type of 'prep‐pad' used to clean skin prior to injection, does appear to be widely used in some postanaesthesia care units to treat PONV (Cotton 2007; Hunt 2013; Merritt 2002; Pellegrini 2009; Spencer 2004; Wang 1999; Winston 2003).
How the intervention might work
The mechanism of action for aromatherapy is not well understood. Essential oils are reported to have effects at the psychological, physiological and cellular level (Dobetsberger 2011) but there are currently no human studies to show that any ingredient from the inhaled vapours of essential oils are present in the blood or plasma (Herz 2009). Herz’s critique of the current state of aromatherapy science highlights many of the poorly supported claims that are made about these substances and suggests that rather than there being a pharmacological action for aromatherapy, it is more likely that aromatherapy’s effects are psychologically or culturally based (Ferdenzi 2011; Herz 2009). The theory that the action of aromatherapy is pharmacological, Herz suggests, may be disproved by the immediacy of its effect, as pharmacological substances require time for absorption within the body (usually a minimum of 20 minutes) (Herz 2009). This position does not take into account the more rapid absorption of inhaled drugs; for example, drugs commonly used to treat asthma begin to take effect as early as five minutes postadministration (Balint 2010) and it may be possible that the vapours of essential oils act with similar rapidity. Essential oils can be absorbed through the skin and some may exert a physiological effect on cellular and organ function (Ernst 2001), but this type of absorption is different to the olfactory mechanism of action disputed by Herz 2009.
One proposed mechanism of action that seems more likely is that the scent activates the olfactory system, which in turn triggers the limbic system (Lis‐Balchin 2006). This in turn may produce emotional responses and may enhance the retrieval of learnt memories (Lis‐Balchin 1997). Brain activation associated with emotional response in connection to odour exposure has been recorded on functional MRI imaging, although this was a brief report of a small study with incomplete detailing of its methods and the findings should be taken with due scepticism (Lowe 2010). It is known that olfactory pathways reach into the hypothalamus, which may be the route for emotional responses to aromas (Linck 2010).
Why it is important to do this review
The effectiveness of the various drugs for PONV has already been the subject of a Cochrane Review (Carlisle 2006), however, prior to the original review in 2012, no existing review had examined the effectiveness of aromatherapy to treat this condition for a broad range of surgical patients. It was important to update this review as several new studies have been published since our original review (Hines 2012).
Objectives
The main objective was to establish the efficacy and safety of aromatherapy comparable to standard pharmacological treatments for PONV in adults and children.
In particular, we wanted to establish:
-
what effect the use of aromatherapy has on the severity of established PONV;
-
what effect the use of aromatherapy has on the duration of established PONV;
-
whether aromatherapy can be used with safety and clinical effectiveness comparable to standard pharmacological treatments to treat established PONV.
Methods
Criteria for considering studies for this review
Types of studies
We considered any randomized controlled trials (RCTs) or controlled clinical trials (CCTs) that evaluated the effect of aromatherapy on established PONV. In order to obtain the widest range of studies we set no date of publication or language limits.
Types of participants
We considered all studies that included participants (both adult and paediatric, paediatric being children aged less than 18 years of age) having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, either as hospital inpatients or in day or ambulatory facilities, who were given aromatherapy treatments for management of existing PONV. For the purposes of this review we considered postoperative to be the period from day of surgery to discharge from hospital or, in the case of day hospital patients, up to the fifth postdischarge day.
We excluded studies of non‐surgical participants (medical, oncology). We also excluded studies in which aromatherapy was used solely to prevent postoperative nausea and vomiting.
Types of interventions
Interventions of interest were those where aromatherapy products were used by any delivery method (for example direct inhalation, diffusion, massage or compress) to treat symptoms of established postoperative nausea and vomiting, compared to a placebo or with standard antiemetic treatments. Aromatherapy was defined as the inhalation of the vapours of any substance for the purposes of a therapeutic benefit.
Types of outcome measures
Primary outcomes
-
Severity of nausea or vomiting, or both, post‐initiation of treatment as measured by a validated scale or medical or nursing observation
-
Duration of nausea or vomiting, or both, post‐initiation of treatment as measured by patient report or medical or nursing observation
Secondary outcomes
-
Use of pharmacological antiemetics
-
Any adverse reactions or events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness (Price 2007))
-
Patient satisfaction with treatment as measured by a validated scale
Search methods for identification of studies
Electronic searches
For the initial review we searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2011, Issue 3); MEDLINE (via Ovid) (1966 to 2 August 2011); Embase (1966 to 2 August 2011); CINAHL (EBSCOhost) (1982 to 2 August 2011); CAM on PubMed (1966 to 2 August 2011); Meditext (1995 to 2 August 2011); LILACS (1982 to 2 August 2011); and ISI Web of Science (1985 to 2 August 2011) (Hines 2012).
We conducted searches for this update on all the previous databases in March 2017 for the period 1 January 2011 to 2 March 2017.
We developed a specific strategy for each database. We based each search strategy on that developed for MEDLINE (see Appendix 2 for details). We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy, phases one and two, as contained in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
Searching other resources
We checked the reference lists of relevant articles and attempted to contact relevant trial authors to identify any additional or ongoing studies.
We also searched for relevant trials on specific sites:
-
Clinical Trial Results at www.clinicaltrialresults.org/ (March, 2017);
-
Open Grey at www.opengrey.eu/ (grey literature) (March, 2017);
-
Grey Literature Report at www.greylit.org/ (grey literature) (March, 2017);
-
Australian Clinical Trials Registry www.anzctr.org.au/Default.aspx (March, 2017);
-
Science.gov at www.science.gov/ (grey literature) (March, 2017);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) apps.who.int/trialsearch/Default.aspx
We did not apply language restrictions.
Data collection and analysis
Selection of studies
Two review authors (SH and ES) independently scanned the titles and abstracts of reports identified by the described variety of search strategies. We retrieved and evaluated potentially relevant studies, chosen by at least one author, in full‐text versions. We retrieved and translated any articles that appeared relevant but were not published in full in English. Three authors (SH, AC and ES) independently assessed the congruence of trials with the review's inclusion criteria using a checklist that was designed in advance for that purpose (Appendix 3).
Data extraction and management
Two review authors (SH and ES) independently extracted data using a tool developed and piloted by the authors (Appendix 4). We used Plot Digitizer software version 2.6.6 (Huwalt 2014) to extract some data that had been reported graphically in the included studies. Where necessary we contacted study authors to request missing data or details of methods. We dealt with trials with more than two arms either by combining intervention or placebo groups where appropriate, or excluding groups if appropriate to the specific comparison being performed.
Assessment of risk of bias in included studies
We assessed the risk of bias using the Cochrane tool provided in the Review Manager 5 (RevMan 5) software (RevMan 2014), described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were adjudicated by the third author (AC). We used the following five criteria to assess risk of bias for each individual study: random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.
Measures of treatment effect
Because of the subjective nature of nausea, measures of treatment effect were largely limited to patient‐reported effects, measured by various scales including visual analogue scales (VAS), verbal numerical rating scales (VRNS) and descriptive ordinal scales (DOS). We included other measures of effect, such as number of vomiting episodes or retching, and the use of pharmacological 'rescue' antiemetics. We used risk ratio (RR) with 95% confidence interval (95% CI) to measure treatment effect for dichotomous outcome measures and standardized mean differences (SMDs) with 95% CI for continuous outcomes.
Unit of analysis issues
For cross‐over trials, had sufficient data been available we planned to use a paired t‐test to analyse participant data. Had we included any cluster‐randomized trials, we would have meta‐analysed effect estimates and standard errors using the generic inverse‐variance method in RevMan 5. For studies using scales that could be standardized (e.g. converting 100 mm scale to 10 cm scale) to enable data pooling, standardization was done.
Dealing with missing data
Where necessary, we contacted authors of included studies regarding missing study information. We were able to contact some study authors to retrieve missing data, such as details about randomization, statistical detail and standard deviations (SDs), however others did not reply or were not contactable. Where we found that data were missing and the study authors were not contactable, where possible we calculated missing statistics (such as SDs) from other quoted statistics (such as frequencies, standard errors or CIs) (Altman 2005). If missing data remained, we performed an available case analysis, excluding data where outcome information was unavailable.
Assessment of heterogeneity
We assessed statistical heterogeneity through the use of the Chi₂ test, as well as by reviewing the I₂ statistic (Higgins 2003). If either the Chi₂ test resulted in a P value less than 0.10 or the I₂ statistic was greater than 40%, we further investigated the reasons for heterogeneity (Deeks 2011). Wherever appropriate we analysed studies with diverse interventions separately.
Assessment of reporting biases
Due to the small number of studies included in the meta‐analyses, we considered it inappropriate to generate funnel plots to assess reporting biases for all meta‐analyses (Egger 1997). We did consider studies from a wide range of locations, languages and publications, as well as unpublished work, which we believe has reduced the likelihood of reporting biases affecting our findings (Sterne 2011).
Data synthesis
We entered all trials included in the systematic review into RevMan 5 (RevMan 2014) and combined data quantitatively, where possible, although there was significant diversity of outcome measurement scales and time points, which limited the amount of data that could be pooled. We calculated pooled estimates using the random‐effects model with the Mantel‐Haenszel method as we observed small numbers of events (Borenstein 2010). We determined the levels of heterogeneity by the I₂ statistic (Deeks 2011; Higgins 2003).
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses where data were available, as described by Deeks and colleagues (Deeks 2001) and as recommended in Section 8.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to compare:
-
adults and children;
-
different types of surgery (e.g. orthopaedic and gynaecologic surgery);
-
types of aromatherapy delivery methods (e.g. inhalation, massage, ingestion);
-
trial quality (e.g. RCT, CCT).
Due to the limited data available, we were unable to perform any subgroup analyses.
Sensitivity analysis
In the 2012 review we had concerns about the risk of bias due to confounding in Merritt 2002 and we performed a sensitivity analysis and reported findings both with and without the results of this study (Hines 2012). On further consideration of this study and considering that while aggressive antiemetic prophylaxis may have had an effect on the overall study results in reducing the severity of nausea in the whole study population, that effect was likely to be similar between the groups and therefore not likely to have caused a difference between the intervention and control groups, and so we have deleted the sensitivity analysis in the 2017 update.
Summary of findings
We used the GRADE approach to summarize and interpret our findings (Langendam 2013). We used GRADEPro GDT software (GRADEpro GDT 2015) to import data from RevMan 5 (RevMan 2014) and create 'Summary of findings' tables.'Summary of findings' tables display the key results of the review by outcome, adjusted for the quality of the evidence. We downgraded the evidence from the included studies by one grade for serious, and two grades for very serious threats to study validity in terms of high risk of bias, indirect evidence from outcome reporting in the studies, serious inconsistency between the pooled studies, imprecision of effect estimates or detected publication bias. We synthesized the following outcomes in the 'Summary of findings' tables: severity of nausea at the end of treatment (primary outcome) duration of nausea (primary outcome), and use of rescue antiemetics (secondary outcome), adverse events and patient satisfaction.
Results
Description of studies
The studies were randomized controlled trials (RCTs) or controlled clinical trials (CCTs) conducted on postoperative adult and paediatric patients in postanaesthesia care units (PACU) and same‐day surgery units (SDSU). The intervention groups were given aromatherapy treatments to treat complaints of PONV. The control groups were treated with either a saline, sham aromatherapy, or controlled breathing control condition, or standard antiemetic drugs. The time points at which data were collected by each study varied from 2 minutes, 5 minutes, 15 minutes, 30 minutes and various combinations of these for total periods of 5 minutes to discharge from PACU or SDSU.
Results of the search
We conducted searches in a wide range of databases and sources: CENTRAL; MEDLINE; Embase; CINAHL; CAM on PubMed; Meditext; LILACS; Web of Science; Current Controlled Trials (2012); Clinical Study Results (2012); SIGLE (2012); New York Library of Medicine Grey Literature Report (2012); National Institute of Clinical Studies (2012); Google Scholar (English, German, Spanish) (2012); Conference Proceedings of the National Association for Holistic Aromatherapy; Clinical Trial Results at www.clinicaltrialresults.org/ (March, 2017); Open Grey at www.opengrey.eu/ (grey literature) (March, 2017); Grey Literature Report at www.greylit.org/ (grey literature) (March, 2017); Australian Clinical Trials Registry www.anzctr.org.au/Default.aspx (March, 2017); Science.gov at www.science.gov/ (grey literature) (March, 2017); World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) apps.who.int/trialsearch/Default.aspx (March, 2017) and reference lists.
In 2012, of the 1386 articles we identified, we deemed 44 relevant enough to be retrieved for further evaluation. After appraisal of the full version of each study, we found nine studies that met the criteria for inclusion in the review (Hines 2012).
In 2017, we identified 612 potentially relevant studies for the period 2011 to 2017 and retrieved 16 full‐text articles, nine of which met the criteria for inclusion in the review, although two are ongoing studies without results, so we included seven completed studies in the analysis. For further details see Figure 1.

Study flow diagram
Included studies
We included 16 studies, seven identified in the 2017 update and nine from the original 2012 review (Hines 2012), comprised of 11 RCTs (Anderson 2004; Cotton 2007; Hodge 2014; Hunt 2013; Kamalipour 2002; Kiberd 2016; Lane 2012; Pellegrini 2009; Sites 2014; Wang 1999; Winston 2003) and five CCTs (Cronin 2015; Ferruggiari 2012; Langevin 1997; Merritt 2002; Tate 1997) with a total of 1036 participants. The mean age and range data for all participants were not available for all studies. See Characteristics of included studies for further details.
Excluded studies
The 2012 review excluded 35 studies for not meeting the inclusion criteria, either by study design (not RCT or CCT) or by treatment objectives (prevention of PONV not treatment) (Hines 2012). See Characteristics of excluded studies for details.
In the 2017 update we retrieved 16 studies and excluded seven for not meeting the inclusion criteria, either by study design (not RCT/CCT) or by study outcomes (prevention of PONV not treatment). Therefore the total number of excluded studies in the updated review is 42.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified two registered trials that met the inclusion criteria for this review; however there are no results for these studies yet (NCT02189980; NCT02732379).
Risk of bias in included studies
We assessed the risk of bias in terms of allocation sequence generation, blinding, incomplete reporting of outcome data, and selective reporting. Risk of bias was variable across all included studies with a range of risks from low to high. For details of the risk of bias assessment, see Figure 2 and Figure 3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies

Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Allocation
Methods of allocation varied across the included studies. Nine studies explicitly stated the method of randomization: Wang 1999 utilized a 'random number table'; Cotton 2007, Pellegrini 2009; Sites 2014; and Winston 2003 utilized a 'computer generated random numbers table'; Hodge 2014, and Hunt 2013 used 'computer generated random number sequences'; Lane 2012 used "blocked systematic random assignment" and Anderson 2004 used a "random number generator". For Kamalipour 2002 the treatment and control groups were "randomly selected" but the authors did not state what method of randomization was used. In Langevin 1997, which used a cross‐over clinical trial design, the test agents were administered in a "random sequence" but again the method of randomization was not stated. In Kiberd 2016 randomization was done using a "block 6" method, which is not further described. The studies by Merritt 2002; Ferruggiari 2012 and Cronin 2015 were not adequately randomized (only Merritt 2002 being explicitly described as a CCT by its authors): in Merritt 2002 assignment to the treatment and control groups was alternated by day; in Ferruggiari 2012 research staff "randomly selected" the treatment from a box; while allocation in Cronin 2015 was based on the calendar month of admission with assignment to the experimental group in even‐numbered months and to the control in odd‐numbered months. The participants in Tate 1997 were "randomly allocated" to wards that had been assigned to the separate treatments, the control and placebo arms of the study, with no details provided about the randomization method.
Allocation concealment appeared to have been undertaken for six studies (Anderson 2004; Cotton 2007; Kiberd 2016; Lane 2012; Pellegrini 2009; Winston 2003) generally by the use of an independent or external allocator. The remaining ten studies did not report data on whether allocation was concealed.
Blinding
Blinding was explicitly done in Merritt 2002; Tate 1997; and Wang 1999. Three included studies (Anderson 2004; Langevin 1997; Wang 1999) also blinded assessors. While several other studies attempted to blind participants or assessors, or both, to the treatment allocation, the nature of the intervention and the difficulty of concealing strong odours meant that unblinding may have occurred. Kiberd 2016 reported that nursing staff and research assistants became unblinded to intervention allocation due to leakage of the treatment aroma. In seven studies (Anderson 2004; Cotton 2007; Hodge 2014; Kamalipour 2002; Langevin 1997; Pellegrini 2009; Sites 2014) the completeness of blinding was unclear. In six studies, blinding was clearly not done (Cronin 2015; Ferruggiari 2012; Hunt 2013; Kiberd 2016; Lane 2012; Winston 2003).
Incomplete outcome data
Data appeared to have been reported for all participants and outcomes in 10 studies (Anderson 2004; Cotton 2007; Ferruggiari 2012; Hunt 2013; Kamalipour 2002; Kiberd 2016; Pellegrini 2009; Sites 2014; Wang 1999; Winston 2003), however it was unclear whether this had occurred in five studies (Cronin 2015; Hodge 2014; Langevin 1997; Merritt 2002; Tate 1997). There appeared to be a large amount of missing data affecting the results in one study (Lane 2012).
Selective reporting
Most studies (Anderson 2004; Cotton 2007; Cronin 2015; Ferruggiari 2012; Hodge 2014; Hunt 2013; Kiberd 2016; Lane 2012; Langevin 1997; Pellegrini 2009; Wang 1999) were at low risk of selective reporting, but for three studies the risk was unclear (Kamalipour 2002; Merritt 2002; Winston 2003). We assessed two included studies as being at high risk of selective reporting (Sites 2014; Tate 1997).
Other potential sources of bias
Other potential sources of bias were evident in two studies. Due to the design of the study by Tate 1997, it was possible there was some demand characteristic effect (an effect where participants interpret the purpose of the study and modify their behaviour or reporting accordingly (Orne 1962)) on patient self‐reporting of results. The authors of Merritt 2002 reported that their study was probably confounded by the aggressive preoperative antiemetic prophylaxis given to 104 out of the 111 participants enrolled into the study, although it seems unlikely this would have had an effect on the direction of the results in favour of the intervention given that almost all participants in both groups received prophylaxis. Four studies appeared free of other potential sources of bias (Cotton 2007; Pellegrini 2009; Wang 1999; Winston 2003). It was unclear from the minimal data reported in Langevin 1997 and Kamalipour 2002 whether there were any other potential sources of bias. The aromatherapy inhalers used in Kiberd 2016 were supplied by the manufacturer, however the study authors state that the manufacturer had no other input into the study.
Effects of interventions
See: Summary of findings for the main comparison Aromatherapy compared to placebo for treatment of postoperative nausea and vomiting; Summary of findings 2 Peppermint compared to placebo for treatment of postoperative nausea and vomiting; Summary of findings 3 Isopropyl alcohol compared to standard treatment for postoperative nausea and vomiting; Summary of findings 4 Isopropyl alcohol compared to saline for treatment of postoperative nausea and vomiting
There were a variety of comparisons used by the included studies. Isopropyl alcohol vapour inhalation was the most commonly used experimental substance with 10 studies evaluating its effectiveness. Several studies used multiple comparisons, for example: peppermint oil versus isopropyl alcohol versus saline, or ginger versus an essential oil mix versus isopropyl alcohol versus saline placebo. Where studies used multiple comparison groups, only one intervention and one comparison group from those studies are used in any single meta‐analysis to avoid double‐counting of participants. Two studies evaluated controlled breathing to treat PONV. All included studies measured nausea as a chief outcome. Seven studies also reported data on the number of participants requiring rescue antiemetics for unresolved nausea. The diversity of comparisons, time points and measurement scales limited the data that could be pooled. We converted some data to standardized scales and measures to enable meta‐analyses.
Comparison: aromatherapy versus placebo
Primary outcome: severity of nausea
Eight studies overall reported data on the severity of nausea, but the variety of measurement scales and time points used limited the advisability of conducting meta‐analyses; however after standardization of scale data some pooling was possible. Six studies (Anderson 2004 (peppermint and saline groups only); Ferruggiari 2012 (peppermint and saline groups only); Hodge 2014; Lane 2012 (peppermint and water groups only); Merritt 2002; Sites 2014) with 241 participants compared aromatherapy to placebo (saline, water or controlled breathing) and measured nausea severity at greater than three minutes post‐treatment. Aromatherapies used were peppermint (Anderson 2004; Ferruggiari 2012; Lane 2012), an essential oil blend of lavender, peppermint, ginger and spearmint (Hodge 2014), and isopropyl alcohol (Merritt 2002). The GRADE assessment of study quality was low. No difference was found between the groups receiving aromatherapy and those receiving an inert placebo (SMD ‐0.22, 95% CI ‐0.63 to 0.18, P value = 0.28). These studies were moderately methodologically heterogeneous (I₂ statistic = 52%). (Analysis 1.1) (summary of findings Table for the main comparison).
Hunt 2013 conducted a four‐group comparison of ginger oil, saline, isopropyl alcohol and an essential oil blend of ginger, spearmint, peppermint and cardamom with 301 participants. While nausea severity was measured, it was reported as odds of greater improvement in nausea relief. Across the study arms, three of the four comparisons showed evidence of an effect: the essential oil blend was more effective than saline (OR 2.70, 95% CI 1.78, 4.56, P value < 0.001), or isopropyl alcohol (OR 2.13, 95% CI 1.50 to 3.17, P value < 0.001) and ginger was more effective than saline (OR 1.86, 95% CI 1.22 to 3.00, P value = 0.002).
Kiberd 2016 compared QueaseEase™ aromatherapy blend to placebo in 39 children having elective outpatient surgery and found only small, non‐significant effects on nausea and no difference in vomiting.
Primary outcome: duration of nausea
An overall comparison of aromatherapy to placebo for the number of participants free of nausea at the end of the treatment period included four studies with 193 participants (Kamalipour 2002; Langevin 1997; Sites 2014; Wang 1999) and found little or no effect for aromatherapy (RR 3.25, 95% CI 0.31 to 34.33, P value = 0.33, very low‐quality evidence) with a high degree of heterogeneity between the studies (I₂ statistic = 97%) and subgroup analyses were not possible due to the small number of studies (Analysis 1.2).
Secondary outcome: use of rescue antiemetics
Ten studies with 695 participants trialled aromatherapy interventions and reported on the use of rescue antiemetics (Anderson 2004; Cotton 2007; Cronin 2015; Hunt 2013; Kamalipour 2002; Kiberd 2016; Langevin 1997; Merritt 2002; Sites 2014; Winston 2003). Studies used peppermint (Anderson 2004; Sites 2014), essential oil blend or ginger (Hunt 2013); essential oil blend (Kiberd 2016) and isopropyl alcohol (Anderson 2004; Cotton 2007; Cronin 2015; Hunt 2013; Kamalipour 2002; Langevin 1997; Merritt 2002; Winston 2003) as the active interventions. Of these studies, seven studies with 609 participants compared aromatherapy interventions with placebo and reported data suitable for meta‐analysis (Anderson 2004; Cronin 2015; Hunt 2013; Kamalipour 2002; Kiberd 2016; Langevin 1997; Sites 2014). Fewer instances of rescue antiemetics were required by participants who received aromatherapy (RR 0.60, 95% CI 0.37 to 0.97, P value = 0.04) although heterogeneity was high (79%), likely due to the variety of substances trialled, and the GRADE assessment of study quality was low Analysis 1.3.
Kiberd 2016 (39 participants) found no difference between the QueaseEase™ aromatherapy and standard treatment groups in terms of use of rescue antiemetics (P value = 0.75, Eta 0.08).
Secondary outcome: adverse reactions
No data on adverse reactions to the experimental substances were reported by any of the included studies. No studies added in the 2017 update reported adverse reactions.
Secondary outcome: patient satisfaction with treatment
Two studies with 127 participants measured patient satisfaction with treatment.
Anderson 2004 measured patient satisfaction on a VAS (0 mm extremely dissatisfied, 100 mm fully satisfied). Participants (n = 33) across all three groups reported high levels of satisfaction with their treatment: isopropyl alcohol 90.3 (SD 14.9); peppermint oil 86.3 (SD 32.3); saline 83.7 (SD 25.6). Hodge 2014 (94 participants) also measured satisfaction on a scale of 0‐10 and reported a mean satisfaction of 6.9 in the group receiving essential oil blend aromatherapy, and 7.1 in the water placebo group (no further data reported).
The results from all studies reporting on this outcome are collated in Table 1.
| Study | Design | Intervention/comparison | Measure | Satisfied |
| RCT | IPA/Saline/Peppermint | 100 mm VAS (0 mm extremely dissatisfied; 100 mm fully satisfied) | IPA: 90.3 (SD: 14.9) peppermint: 86.3 (SD: 32.3) saline: 83.7 (SD: 25.6) | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/38 Comparison: 34/34 | |
| RCT | IPA/Promethazine | 5‐point DOS (1 = totally unsatisfied, 5 = totally satisfied) | Both groups reported median score 4 | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/50 Comparison: 30/50 |
DOS: descriptive ordinal scale; IPA: isopropyl alcohol; RCT: randomized controlled trial; SD: standard deviation; VAS: visual analogue scale
Comparison: peppermint versus placebo
Primary outcome: severity of nausea
Four studies (Anderson 2004; Ferruggiari 2012; Lane 2012; Sites 2014) with 115 participants compared peppermint aromatherapy to placebo (saline, water or controlled breathing) and measured nausea severity at five minutes post initial treatment. Moderately high heterogeneity (I₂ statistic = 66%) was probably due to clinical and methodological differences between the studies. The use of peppermint may lead to little or no difference in the severity of nausea (SMD ‐0.18, 95% CI ‐0.86 to 0.49, P value = 0.59) (Analysis 2.1). Tate 1997 compared peppermint oil to a peppermint essence placebo and a standard treatment control group but only reported average daily nausea scores on a 0 to 4 descriptive ordinal scale, which we were not able to include in the meta‐analysis. On the operative day the standard treatment group's mean daily nausea score was 0.97, the peppermint essence placebo group's was 1.61, and the peppermint oil group's was 0.5 (no SD reported), which the study authors report as a significant difference between the groups (P value = 0.02). The GRADE assessment of study quality was low (summary of findings Table 2).
Primary outcome: duration of nausea
No studies reported data on this outcome for this comparison.
Secondary outcome: use of rescue antiemetics
No studies reported data on this outcome for this comparison.
Secondary outcome: adverse reactions
No data on adverse reactions to the experimental substances were reported by any of the included studies. No studies added in the 2017 update reported adverse reactions.
Comparison: isopropyl alcohol versus standard treatment
Primary outcome: severity of nausea
Merritt 2002 compared isopropyl alcohol and standard antiemetic drugs in 39 adult participants, measuring nausea on a 0 to 10 descriptive ordinal scale (DOS) and found 52.4% (n = 11) of the experimental group had their nausea relieved after the first treatment, compared to 72.2% (n = 13) in the standard treatment group. There was no significant difference between the groups at post‐test (isopropyl alcohol mean 2.7 (SD 3.02), standard treatment mean 1.94 (SD 2.48).
Primary outcome: duration of nausea
Three studies with 176 participants (Cotton 2007; Pellegrini 2009 (PACU subgroup); Winston 2003) compared isopropyl alcohol to standard antiemetics and reported time in minutes to a 50% reduction in nausea scores. Heterogeneity between these studies was very low (I₂ statistic = 0%) and the pooled result was significant (SMD ‐1.10 minutes, 95% CI ‐1.43 to ‐0.78, P value < 0.001) indicating that aromatherapy using isopropyl alcohol has a significantly faster effect than the comparison drugs, ondansetron and promethazine (Analysis 3.1) (summary of findings Table 3). According to the GRADE analysis, if 1000 patients with PONV were given a placebo, 297 would be nausea‐free by the end of the treatment period, whereas if 1000 patients with PONV received aromatherapy, 695 would be nausea‐free at the end of the treatment period. The GRADE level of evidence was moderate.
Secondary outcome: use of rescue antiemetics
Four studies with a total of 215 participants compared isopropyl alcohol to standard treatment and reported the number of participants in each group who required rescue antiemetics (Cotton 2007; Merritt 2002; Pellegrini 2009; Winston 2003), which showed an effect when pooled (RR 0.67, 95% CI 0.46 to 0.98, P value = 0.04) (Analysis 3.2) (summary of findings Table 3).
Secondary outcome: adverse reactions
No data on adverse reactions to the experimental substances were reported by any of the included studies. No studies added in the 2017 update reported adverse reactions.
Secondary outcome: patient satisfaction with treatment
Four studies measured patient satisfaction with treatment. No new studies added in 2017 measured patient satisfaction.
Cotton 2007 (72 participants, comparing isopropyl alcohol to ondansetron) used a four‐point ordinal scale on which the participants were asked to rate their postoperative experience as poor, fair, good or excellent; participants in both the treatment and control groups reported their experience as good or excellent, resulting in no difference between the groups (P value > 0.05).
Winston 2003 (41 participants) also measured patient satisfaction using a four‐point ordinal scale (0 = poor; 1 = fair; 2 = good and 3 = excellent). For the ondansetron group: 0 = 1 participant (3%); 1 = 2 participants (6%); 2 = 17 participants (52%); and 3 = 13 participants (39%). For the isopropyl alcohol group, the satisfaction numbers were: 0 = 0 participants; 1 = 0 participants; 2 = 18 participants (47%), and 3 = 20 participants (53%). The study authors stated that, although these findings were not statistically significant, they nonetheless regarded them as clinically significant (unreported data supplied via email). We collapsed results from Cotton 2007 and Winston 2003 into dichotomous data (good or excellent interpreted as satisfied) and combined them in Analysis 3.3 (summary of findings Table 3).
Participants also reported high levels of satisfaction with their treatment regardless of allocation in Pellegrini 2009 (63 participants), with a median score of 4 on a 5‐point ordinal scale (1, totally dissatisfied; 2, somewhat dissatisfied; 3, somewhat satisfied; 4, satisfied; 5, totally satisfied).
Comparison: isopropyl alcohol versus placebo
Primary outcome: severity of nausea
Two studies (Anderson 2004; Cronin 2015) used isopropyl alcohol as an intervention and compared it to either controlled breathing or saline placebo (Anderson 2004 used a three‐group design comparing isopropyl alcohol, peppermint and saline) and reported data on nausea severity. We were unable to carry out any meta‐analyses for these studies due to differing measures and data reporting. Anderson 2004 compared isopropyl alcohol and saline and reported means and SDs for baseline, two and five minutes, reporting an overall decrease in nausea scores, which, while significant in comparison to baseline, did not differ between the groups at five minutes. Cronin 2015 trialled isopropyl alcohol with and without controlled breathing and reported means without SDs and reported similarly that while the nausea severity decreased significantly for all groups between baseline and five minutes, there was no significant difference between the control and intervention groups at five minutes.
Primary outcome: duration of nausea
Wang 1999 compared isopropyl alcohol and saline in a population of 39 children having elective outpatient surgery under general anaesthesia. Wang 1999 found that while isopropyl alcohol may have an effect on postoperative nausea at 20 minutes post‐treatment (P value = 0.05), this effect could not be sustained at 60 minutes (RR 2.85, 95% CI 0.32 to 25.07, P value = 0.35). No effect on postoperative vomiting was demonstrated at 20 minutes or 60 minutes (RR 1.27, 95% CI 0.33 to 4.93).
Secondary outcome: use of rescue antiemetics
Four studies of adult patients (Anderson 2004; Hunt 2013; Kamalipour 2002; Langevin 1997), with a total of 291 participants, compared isopropyl alcohol and saline and measured the number of participants who required rescue antiemetics. We combined these studies. Meta‐analysis showed no evidence of an effect (RR 0.39, 95% CI 0.12 to 1.24, P value = 0.11, very low‐quality evidence) although heterogeneity was again very high (I₂ statistic = 92%) (Analysis 4.1). Subgroup analyses were not possible due to the small number of studies (summary of findings Table 4).
One study of 39 paediatric patients having day surgical procedures (Wang 1999) also compared isopropyl alcohol and saline and measured the number of participants requiring rescue antiemetics. For participants with nausea only, 60% of those in the placebo (saline) group required rescue antiemetics compared to 9% of those in the isopropyl alcohol group (RR 0.15, 95% CI 0.02 to 1.05). For participants with vomiting, 89% of the saline group required rescue antiemetics compared to 67% of the isopropyl alcohol group (RR 0.75, 95% CI 0.23 to 1.12).
Secondary outcome: adverse reactions
No data on adverse reactions to the experimental substances were reported by any of the included studies. No studies added in the 2017 update reported adverse reactions.
Secondary outcome: patient satisfaction with treatment
No studies reported data on this outcome for this comparison.
Discussion
Summary of main results
This review was able to include studies of isopropyl alcohol, peppermint oil, ginger oil, essential oil blends of peppermint, spearmint, ginger and cardamom, or peppermint, spearmint, ginger and lavender aromatherapy interventions compared to water, saline or controlled breathing placebo, ondansetron, promethazine, or other unspecified 'standard antiemetic' treatments. All aromatherapy was delivered via direct inhalation of vapours. There were 979 adult and 39 paediatric participants in the 16 included studies. The majority of participants were women. Studies were conducted in both inpatient and day surgery settings. Outcomes of interest to this review measured by the included studies were severity of nausea, duration of nausea reported as time to reduction in nausea, the use of 'rescue' antiemetics, and patient satisfaction. No studies reported data on adverse effects. Study quality was moderate to very low.
Sixteen studies (Anderson 2004; Cotton 2007; Cronin 2015; Ferruggiari 2012; Hodge 2014; Hunt 2013; Kamalipour 2002; Kiberd 2016; Lane 2012; Langevin 1997; Merritt 2002; Pellegrini 2009; Sites 2014; Tate 1997; Wang 1999; Winston 2003) compared aromatherapies of various types to placebo and reported data on the severity and duration of nausea, use of rescue antiemetics and patient satisfaction. While there was little or no difference between the groups in terms of nausea severity, there were more participants who were nausea‐free at the end of treatment, and fewer participants who received aromatherapy required antiemetics to treat nausea.
Isopropyl alcohol was tested in several studies, both against standard pharmacological treatments and against other aromatherapies and placebo, in both adults and children. In comparison to saline placebo, isopropyl alcohol appears effective in reducing the number of patients requiring rescue antiemetics (Anderson 2004; Hunt 2013; Kamalipour 2002; Langevin 1997) and in providing short‐term relief of symptoms in children (Wang 1999). In three studies (Cotton 2007; Pellegrini 2009, Winston 2003), isopropyl alcohol provided a significantly faster time to 50% relief of symptoms than ondansetron and promethazine. When compared to standard antiemetic drugs, participants receiving isopropyl alcohol to treat their nausea required fewer instances of rescue antiemetics (Cotton 2007; Merritt 2002; Pellegrini 2009; Winston 2003). There were no data suitable to be pooled for a comparison of isopropyl alcohol to standard treatment for the outcome of nausea severity.
The updated 2017 searches introduced a greater variety of treatment substances into the review. Five included studies trialled peppermint aromatherapy as a treatment for PONV (Anderson 2004; Ferruggiari 2012; Lane 2012; Sites 2014; Tate 1997). Three included studies (Hodge 2014; Hunt 2013; Kiberd 2016) used blends of four essential oils as treatments for nausea: peppermint, spearmint, ginger and lavender in Hodge 2014 and Kiberd 2016 and peppermint, spearmint, ginger and cardamom in Hunt 2013. Peppermint, when compared to placebo, may lead to little or no difference in nausea severity.
Patient satisfaction with aromatherapy treatment appeared high in studies that measured this outcome (Anderson 2004; Cotton 2007; Pellegrini 2009; Winston 2003), with participants reporting high levels of satisfaction with their experience. However it should be noted that all participants in these studies (treatment and comparison groups) reported high levels of satisfaction, possibly indicating that increased attention to the care of PONV improved patient satisfaction with their care.
The findings are further summarized in 'Summary of findings' tables for aromatherapy versus placebo (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4).
Overall completeness and applicability of evidence
It seems likely that further studies of all aromatherapy products to treat PONV could provide different results from those described here if greater rigour was applied in the study methods. Due to the strong odours involved, aromatherapy is inherently a difficult intervention to conceal from participants, research staff and those assessing outcomes; however several included studies attempted no blinding at all. Unlike the previous iteration of this review, some larger, well‐conducted studies of peppermint oil or other aromatherapies have now been included, which has changed the evidence significantly. The evidence base for aromatherapy to treat PONV is still incomplete, with only two studies of children meeting the inclusion criteria and many aromatherapy treatments incompletely investigated or tested. While there appears to be no evidence of adverse reactions from the use of the included interventions, it is unclear from the included studies whether data were collected on any possible adverse reactions experienced by participants. In the context of current postoperative practice, there is a place for adjunct therapies to treat PONV and while aromatherapy is a simple and inexpensive treatment that seems to be more effective than placebo in terms of some outcomes, there is currently no evidence to suggest that it can replace pharmacological antiemetics.
Of additional concern are the early time points utilized by all included studies except Tate 1997, which did measure PONV at 24 and 48 hours but only reported average daily scores for each group. Apfel 2002 recommends that study authors measure PONV for early (up to two hours) and late (to 24 hours) outcomes. The data that we were able to include in this review remain incomplete for effects longer than 60 minutes.
Due to the many risk factors for and influences on PONV, such as type of anaesthesia, narcotic medication intake, sex, and type of surgery, it was a concern that there were differences between groups that might account for some of the effect. Examination of the demographic and procedural data, however, shows that control and experimental groups were very similar and that confounding due to risk factors was unlikely.
It should be remembered that we have not included any evidence of effectiveness for aromatherapy in the prevention of PONV and that all results apply only to treatment of an existing complaint.
Quality of the evidence
The included studies were comprised of 12 RCTs and four CCTs, with total of 1036 participants. The overall quality of the retrieved evidence was low, with incomplete reporting and unavailable data hampering pooling on some important outcomes. Due to the age of some studies or non‐contactability of the study authors, further data were not available in some cases. The 16 included studies measured the effectiveness of a range of commonly used aromatherapy interventions for this condition in settings appropriate to its use, that is, post‐anaesthesia care units and same‐day surgery units. Additionally, the high level of inconsistency in some of the pooled results reduces the level of confidence in those results. Imprecision, as a result of wide confidence intervals and small numbers of participants in some included studies also reduces the quality of the evidence, however indirectness and publication bias were less of a concern.
Potential biases in the review process
It is possible there are studies that were not identified in the searches or reference list checks done for this review, but it seems unlikely as search alerts running since the first version of this review was conducted identified no studies not also found with the search strategies. We have reported all the relevant data on the outcomes of interest to this review and attempted to contact five study authors for the newly added studies to obtain clarifications on methods or data not reported in the publication. Four of the five author groups contacted supplied the requested information. The new searches did not identify any non‐English‐language studies, unlike the initial searches in 2010, and this may indicate a flaw in the search strategies or simply a lack of new research. The inclusion of meta‐analyses with high heterogeneity, such as those in Analysis 1.2 (I2 = 97%) and Analysis 4.1 (I2 = 97%), may increase the risk of bias, however these analyses combine the results of multiple aromatherapy types and research designs, which are likely the source of heterogeneity.
Agreements and disagreements with other studies or reviews
A systematic review of the effectiveness of noninvasive complementary therapies for reducing PONV in women having abdominal laparoscopic hysterectomy (Hewitt 2009) found, similarly to this review, that there was no strong evidence to support the use of aromatherapy for PONV. We have been unable to find any other systematic reviews of aromatherapy for treating PONV.

Study flow diagram
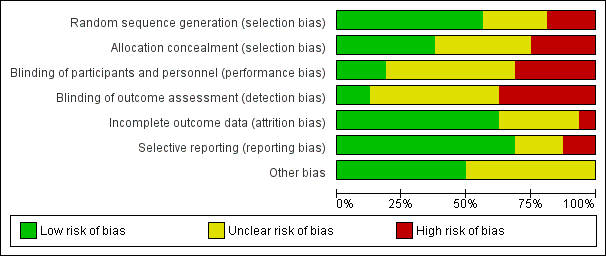
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
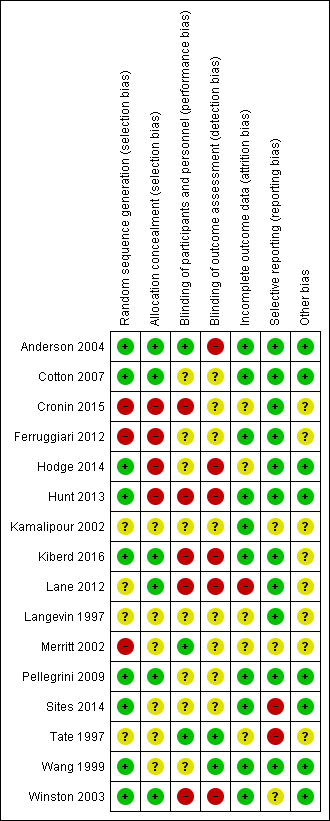
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
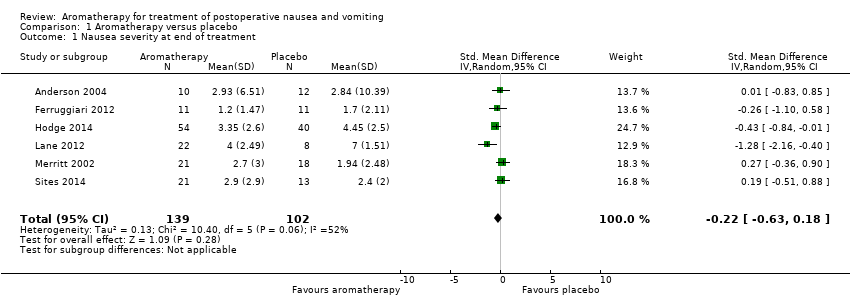
Comparison 1 Aromatherapy versus placebo, Outcome 1 Nausea severity at end of treatment.
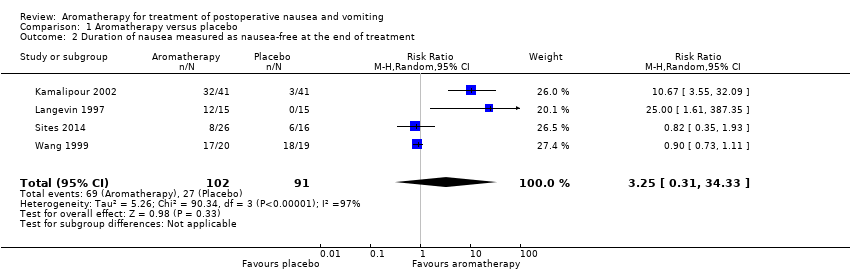
Comparison 1 Aromatherapy versus placebo, Outcome 2 Duration of nausea measured as nausea‐free at the end of treatment.

Comparison 1 Aromatherapy versus placebo, Outcome 3 Proportion requiring rescue antiemetics.

Comparison 2 Peppermint versus placebo, Outcome 1 Nausea severity at 5 minutes post‐initial treatment.
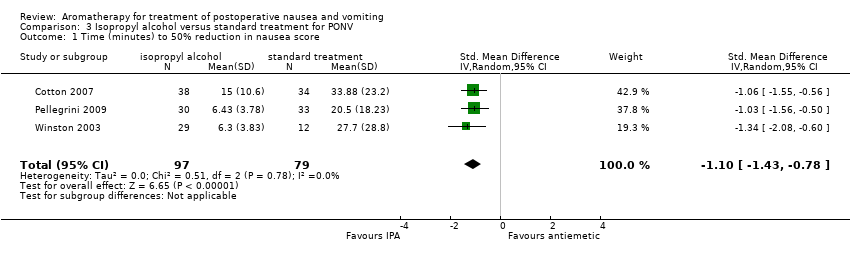
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 1 Time (minutes) to 50% reduction in nausea score.
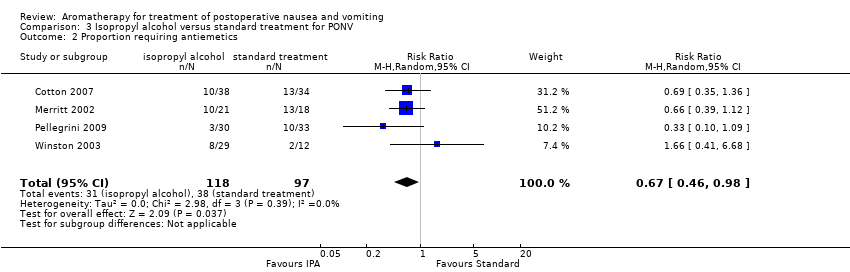
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 2 Proportion requiring antiemetics.
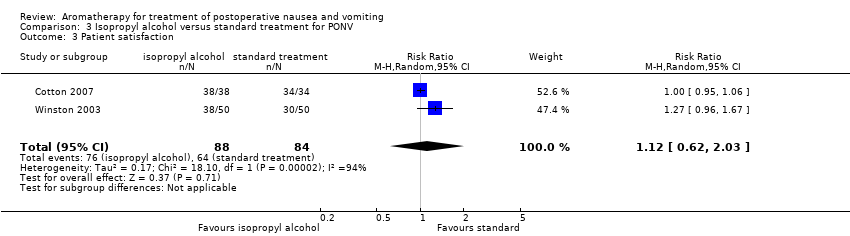
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 3 Patient satisfaction.

Comparison 4 Isopropyl alcohol versus saline, Outcome 1 Proportion requiring rescue antiemetics.
| Aromatherapy compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, either as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with aromatherapy | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.22 SD lower | ‐ | 241 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | Study population | RR 3.25 | 193 | ⊕⊝⊝⊝ | ||
| 30 per 100 | 96 per 100 | |||||
| Proportion requiring rescue antiemetics | Study population | RR 0.60 | 609 | ⊕⊕⊝⊝ | ||
| 68 per 100 | 41 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias across all studies due to study designs, downgraded one level. | ||||||
| Peppermint compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with peppermint | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.18 SD lower | ‐ | 115 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias in included studies due to study designs, downgraded one level. | ||||||
| Isopropyl alcohol compared to standard treatment for postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard treatment for PONV | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (measured as nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | The mean time to 50% reduction in nausea score was 20.5 minutes | SMD 1.10 SD lower | ‐ | 176 | ⊕⊕⊕⊝ | Risk in placebo group based |
| Use of rescue antiemetics | Study population | RR 0.67 | 215 | ⊕⊕⊕⊝ | ||
| 39 per 100 | 26 per 100 | |||||
| Patient satisfaction with treatment Measured by a validated scale | Study population | RR 1.12 | 172 | ⊕⊝⊝⊝ | ||
| 76 per 100 | 85 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No or unclear blinding in all included studies, downgraded one level. | ||||||
| Isopropyl alcohol compared to saline for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with saline | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | Study population | RR 0.39 | 291 | ⊕⊝⊝⊝ | ||
| 90 per 100 | 35 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Poor reporting in Kamalipour 2002 and Langevin 1997 affect confidence in results, downgraded one level. | ||||||
| Study | Design | Intervention/comparison | Measure | Satisfied |
| RCT | IPA/Saline/Peppermint | 100 mm VAS (0 mm extremely dissatisfied; 100 mm fully satisfied) | IPA: 90.3 (SD: 14.9) peppermint: 86.3 (SD: 32.3) saline: 83.7 (SD: 25.6) | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/38 Comparison: 34/34 | |
| RCT | IPA/Promethazine | 5‐point DOS (1 = totally unsatisfied, 5 = totally satisfied) | Both groups reported median score 4 | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/50 Comparison: 30/50 | |
| DOS: descriptive ordinal scale; IPA: isopropyl alcohol; RCT: randomized controlled trial; SD: standard deviation; VAS: visual analogue scale | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea severity at end of treatment Show forest plot | 6 | 241 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.63, 0.18] |
| 2 Duration of nausea measured as nausea‐free at the end of treatment Show forest plot | 4 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.31, 34.33] |
| 3 Proportion requiring rescue antiemetics Show forest plot | 7 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.37, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea severity at 5 minutes post‐initial treatment Show forest plot | 4 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.86, 0.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time (minutes) to 50% reduction in nausea score Show forest plot | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.43, ‐0.78] |
| 2 Proportion requiring antiemetics Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.98] |
| 3 Patient satisfaction Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.62, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue antiemetics Show forest plot | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.24] |

