Aromatherapie zur Behandlung von postoperativer Übelkeit und Erbrechen
Appendices
Appendix 1. Peppermint oil
Peppermint oil (Mentha piperita) is one of the oldest European herbs used for medicinal purposes. It is a hybrid species of spearmint (Mentha spicata) and water mint (Mentha aquatica) (Price 2007). The essential oil is derived by steam distillation of the fresh aerial parts of the flowering plant (Lis‐Balchin 2006). Peppermint oil is listed in the European Pharmacopeia, British Pharmacopoeia, and United States Pharmacopeia. The active ingredients of the peppermint essential oil (0.4% to 5%) are menthol (35% to 45%) and menthone (10% to 30%) (Lis‐Balchin 2006).
One possible mechanism of action of peppermint oil in the gastrointestinal system is inhibition of muscular contractions induced by serotonin and substance P (Hills 1991). Early studies (1969) showed that direct administration of peppermint oil to the stomach (27 patients) caused relaxation of the lower oesophageal sphincter (Sigmund 1969). Subsequent studies have shown that administration (dose of 0.1 mL peppermint oil in 20 mL of saline) to the sigmoid colon in five participants produced increased intraluminal pressure, abdominal cramps, and the urge to defecate and urinate, suggesting widespread stimulation of smooth muscle (Rogers 1988). In another study, peppermint oil injected into the colon (20 participants ) was shown to relieve colon spasms (Leicester 1982).
Peppermint oil has also been shown to accelerate the gastric emptying rate in dyspeptic patients as well as reduce the pain intensity (Dalvi 1991; May 1996). In a double‐blind study, it was shown that the incidence of postoperative nausea in 18 gynaecological patients was significantly reduced in those that inhaled the peppermint oil (Tate 1997). In another randomized double blind study, a liquid herbal extract containing peppermint oil as the principal ingredient was found to relieve the symptoms of pain, nausea, belching, and heartburn (Westphal 1996).
Appendix 2. Search strategies
1 Search strategy for CENTRAL, in the Cochrane Library
#1 MeSH descriptor Holistic Health explode all trees
#2 MeSH descriptor Aromatherapy explode all trees
#3 MeSH descriptor Medicine, Traditional explode all trees
#4 MeSH descriptor Naturopathy explode all trees
#5 MeSH descriptor Phytotherapy explode all trees
#6 MeSH descriptor Plants, Medicinal explode all trees
#7 MeSH descriptor Ginger explode all trees
#8 MeSH descriptor Mentha piperita explode all trees
#9 (Aromatherapy or "Holistic Health" or "Medicine, Traditional" or Naturopathy or Phytotherapy or "Plants, Medicinal" or Ginger or “Mentha piperita”):ti,ab
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Postoperative Nausea and Vomiting explode all trees
#12 MeSH descriptor Postoperative Care explode all trees
#13 MeSH descriptor Recovery Room explode all trees
#14 MeSH descriptor Anesthesia Recovery Period explode all trees
#15 (postoperative* or post surg* or surgical or recovery) and (vomit* or nausea* or sick* or PONV)
#16 (#11 OR #12 OR #13 OR #14 OR #15)
#17 (#10 AND #16)
2. Search Strategy for MEDLINE ( Ovid SP)
1. exp Aromatherapy/ or exp Plants, Medicinal/ or exp Mentha piperita/ or exp Ginger/ or exp Complementary Therapies/ or exp Naturopathy/ or exp Phytotherapy/ or Holistic Health/ or (aromatherap* or ((plant* or traditional or complementary) adj3 medicin*) or ginger or peppermint or isopropyl alcohol or (holistic adj3 health) or naturopath* or phytotherap* or (mentha adj3 piperita)).mp.
2. exp "Postoperative Nausea and Vomiting"/ or exp Anesthesia Recovery Period/ or (postoperative adj3 (care or nausea or vomit*)).mp. or (recovery adj3 (room or an?esthesia or period)).mp. or PONV
3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
4. 1 and 2 and 3
3 Search strategy for Embase (Ovid SP)
1. exp aromatherapy/ or exp alternative medicine/ or exp medicinal plant/ or exp Mentha piperita/ or exp peppermint/ or exp ginger/ or exp phytotherapy/ or (aromatherap* or ((plant* or traditional or complementary) adj3 medicin*) or ginger or peppermint or isopropyl alcohol or (holistic adj3 health) or naturopath* or phytotherap* or (mentha adj3 piperita)).mp.
2. exp "postoperative nausea and vomiting"/ or exp anesthetic recovery/ or postoperative care/ or (postoperative adj3 (care or nausea or vomit*)).mp. or (recovery adj3 (room or an?esthesia or period)).mp. or PONV
3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh.
4. 1 and 2 and 3
4 Search strategy for CINAHL (EBSCOhost)
S1 (MH "Aromatherapy") or (MH "Holistic Health") or (MH "Medicine, Traditional+") or (MH "Medicine, Oriental Traditional+") or (MH "Medicine, Chinese Traditional+") or (MH "Medicine, Latin American Traditional") or (MH "Medicine, African Traditional") or (MH "Australian Traditional Medicine Society") or (MH "Medicine, Native American") or (MH "Traditional Healers") or (MH "Medicine, Arabic") or (MH "Naturopathy") or (MH "Medicine, Herbal+") or (MH "Plants, Medicinal+") or (MH "Medicine, Herbal+") or (MH "Ginger") or (MH "Peppermint") or ((aromatherap* or complementary or ginger or peppermint or isopropyl alcohol) and ((traditional or natural or alternat*) and (therap* or medicine or treatment*)))
S2 (MH "Nausea and Vomiting+") or (MH "Nausea") or (MH "Postoperative Care+") or (MH "Post Anesthesia Care Units") or (MH "Anesthesia Recovery") or ((postoperative* or post surg* or surgical or recovery) and (vomit* or nausea* or sick* or PONV))
S3 (MH "Clinical Trials") or (random* or multicenter or prospective) or ((single or double or triple or treble) and (mask* or blind*))
S4 S1 AND S2 AND S3
5 Search strategy for CAM on PubMed (1966 to 2010)
| 1 | Search aromatherapy Limits: Complementary Medicine |
| 2 | Search peppermint Limits: Complementary Medicine |
| 3 | Search ginger Limits: Complementary Medicine |
| 4 | Search 1 OR 2 OR 3 Limits: Complementary Medicine |
| 5 | Search postoperative nausea vomiting Limits: Complementary Medicine |
| 6 | Search postoperative care Limits: Complementary Medicine |
| 7 | Search 5 OR 6 Limits: Complementary Medicine |
| 8 | Search 4 AND 7 Limits: Complementary Medicine |
6 Search strategy for Meditext (Informit 1995 to 2010) (now Informit Health Collection from January 2010)
| 1. | (aromatherapy OR natural medicine OR traditional medicine OR phytotherapy OR medicinal plant OR holistic health OR ginger OR peppermint) |
| 2. | ((postoperative nausea and vomiting) OR postoperative care OR recovery room OR post‐anesthesia recovery period OR PONV) |
| 3. | 1 AND 2 |
7 Search strategy for LILACS database
(mentha piperita OR gengiber offinale OR peppermint OR ginger OR aromatherap$ OR terap$ herb$ OR medic$ herb$ OR complement$ medic$ OR (essential AND oil))
8 Search strategy for ISI Web of Science
#1. TS=((nausea or vomiting) SAME postoperativ*)
#2. TS=(aromatherap* or complementary or ginger or peppermint or isopropyl alcohol ) AND TS=((traditional or natural or alternat*) and (therap* or medicine or treatment*))
#3. #1 AND #2
Appendix 3. Verification of Study Eligibility Form
Aromatherapy for PONV
VERIFICATION OF STUDY ELIGILIBILITY
| AUTHOR AND YEAR |
| JOURNAL |
| TITLE |
| NAME/CODE OF REVIEWER |
|
Setting: Acute hospital or surgical day facility Yes No |
|
Population: Adults or children having surgical procedures under anaesthesia Yes No
|
|
Intervention:Experimental group patients are receiving aromatherapy to treat PONV Yes No
|
|
Study Design: RCT or CCT Yes No
|
|
IF YOU HAVE NOT ANSWERED YES TO ALL OF THE ABOVE QUESTIONS, YOU SHOULD EXCLUDE THE STUDY. IF YOU ANSWERED YES TO ALL, PLEASE CONTINUE. |
|
Language: Does the study require translation before it can be appraised? Yes No
If yes, please arrange for translation before proceeding |
|
PLEASE RECORD ALL STUDY DETAILS AS PER THE DATA MANAGEMENT FLOW SHEET
|
Appendix 4. Data Extraction Form
| AUTHOR AND YEAR | |||||
| JOURNAL/SOURCE | |||||
| TITLE INITIALS OF REVIEWER: | |||||
| STUDY METHOD RCT ? Quasi RCT ? CCT ? | |||||
| PARTICIPANT | Group | Group | Group | Group | |
| Number in each group |
|
|
| ||
| Mean age and range |
|
|
|
| |
| Gender |
|
|
|
| |
| Population |
|
|
|
| |
| Setting |
|
|
|
| |
| Procedure/s |
|
|
|
| |
| Participants excluded in selection criteria |
|
|
| ||
| Participants who left study and reasons why |
|
|
|
| |
| INTERVENTION | Group | Group | Group | Group | |
| Aromatherapy type |
|
|
|
| |
| Method of administration |
|
|
|
| |
| Dose (if stated) |
|
|
|
| |
| Times administered |
|
|
|
| |
| Cost (if stated) |
|
|
|
| |
| Administered by? |
|
|
|
| |
| Control |
|
|
|
| |
| OUTCOMES | Group | Group | Group | Group | |
| Nausea (severity score?) |
|
|
|
| |
| Vomiting (severity score?) |
|
|
|
| |
| Adverse reactions |
|
|
|
| |
| Cost |
|
|
|
| |
| Rescue antiemetics used |
|
|
|
| |
| Author’s Conclusion | |||||

Study flow diagram
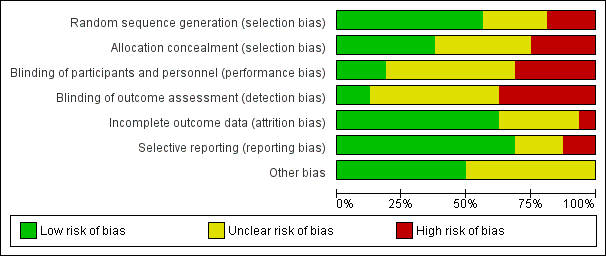
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
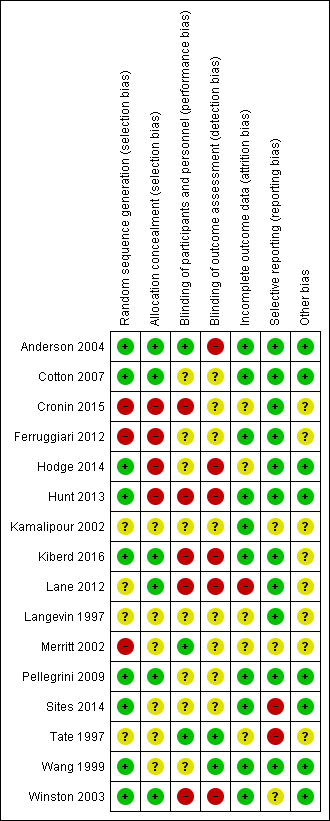
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
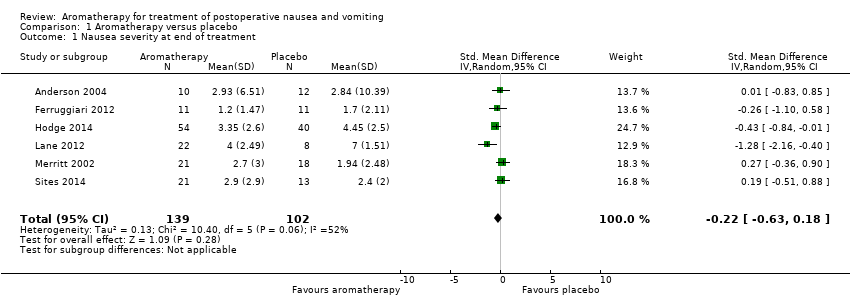
Comparison 1 Aromatherapy versus placebo, Outcome 1 Nausea severity at end of treatment.
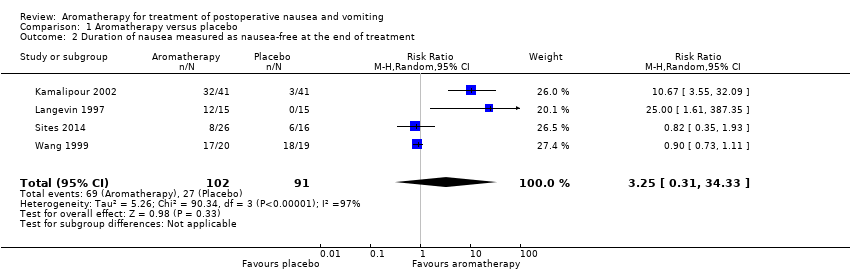
Comparison 1 Aromatherapy versus placebo, Outcome 2 Duration of nausea measured as nausea‐free at the end of treatment.

Comparison 1 Aromatherapy versus placebo, Outcome 3 Proportion requiring rescue antiemetics.

Comparison 2 Peppermint versus placebo, Outcome 1 Nausea severity at 5 minutes post‐initial treatment.
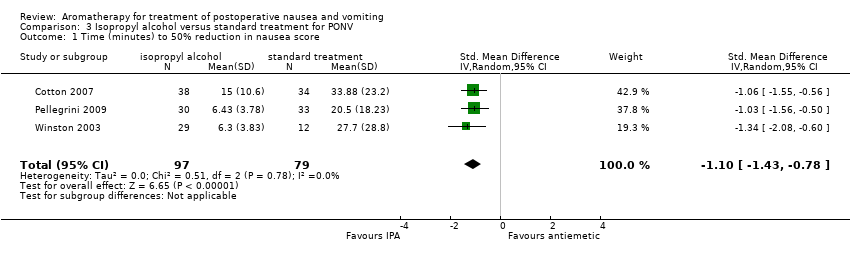
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 1 Time (minutes) to 50% reduction in nausea score.
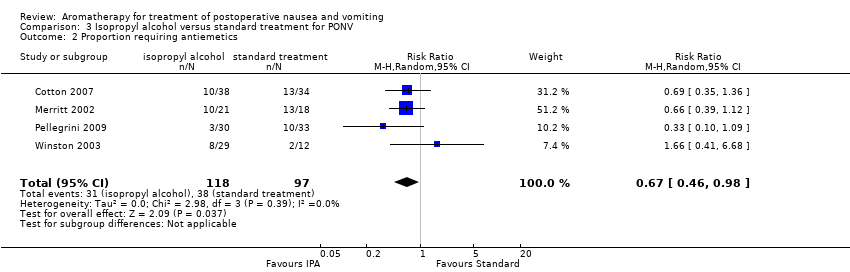
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 2 Proportion requiring antiemetics.
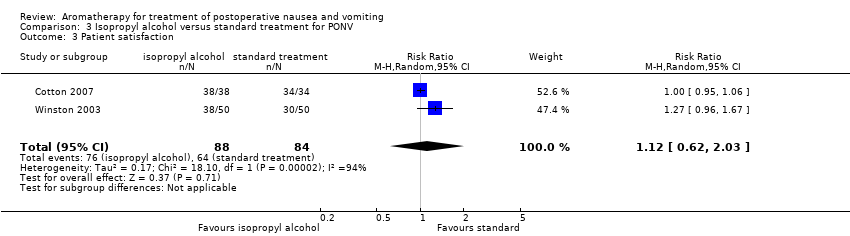
Comparison 3 Isopropyl alcohol versus standard treatment for PONV, Outcome 3 Patient satisfaction.

Comparison 4 Isopropyl alcohol versus saline, Outcome 1 Proportion requiring rescue antiemetics.
| Aromatherapy compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, either as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with aromatherapy | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.22 SD lower | ‐ | 241 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | Study population | RR 3.25 | 193 | ⊕⊝⊝⊝ | ||
| 30 per 100 | 96 per 100 | |||||
| Proportion requiring rescue antiemetics | Study population | RR 0.60 | 609 | ⊕⊕⊝⊝ | ||
| 68 per 100 | 41 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias across all studies due to study designs, downgraded one level. | ||||||
| Peppermint compared to placebo for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with peppermint | |||||
| Nausea severity | The mean nausea severity was 2.8 (SD = 10.39) | SMD 0.18 SD lower | ‐ | 115 | ⊕⊕⊝⊝ | Risk in placebo group based on control group in Anderson 2004 |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias in included studies due to study designs, downgraded one level. | ||||||
| Isopropyl alcohol compared to standard treatment for postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard treatment for PONV | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (measured as nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | The mean time to 50% reduction in nausea score was 20.5 minutes | SMD 1.10 SD lower | ‐ | 176 | ⊕⊕⊕⊝ | Risk in placebo group based |
| Use of rescue antiemetics | Study population | RR 0.67 | 215 | ⊕⊕⊕⊝ | ||
| 39 per 100 | 26 per 100 | |||||
| Patient satisfaction with treatment Measured by a validated scale | Study population | RR 1.12 | 172 | ⊕⊝⊝⊝ | ||
| 76 per 100 | 85 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No or unclear blinding in all included studies, downgraded one level. | ||||||
| Isopropyl alcohol compared to saline for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: adults and children having any type of surgical procedure under general anaesthesia, regional anaesthesia or sedation, as hospital inpatients or outpatients, with existing PONV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with saline | Risk with isopropyl alcohol | |||||
| Nausea severity Measured by a validated scale or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Nausea duration (nausea‐free at end of treatment) Measured by participant self‐report or medical or nursing observation | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Use of rescue antiemetics | Study population | RR 0.39 | 291 | ⊕⊝⊝⊝ | ||
| 90 per 100 | 35 per 100 | |||||
| Adverse events (common reactions to aromatherapy include skin rashes, dyspnoea, headache, cardiac arrhythmias, hypotension, hypertension or dizziness) | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| Patient satisfaction with treatment Measured by a validated scale | See comment | ‐ | ‐ | ‐ | The studies reporting this comparison did not report this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Poor reporting in Kamalipour 2002 and Langevin 1997 affect confidence in results, downgraded one level. | ||||||
| Study | Design | Intervention/comparison | Measure | Satisfied |
| RCT | IPA/Saline/Peppermint | 100 mm VAS (0 mm extremely dissatisfied; 100 mm fully satisfied) | IPA: 90.3 (SD: 14.9) peppermint: 86.3 (SD: 32.3) saline: 83.7 (SD: 25.6) | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/38 Comparison: 34/34 | |
| RCT | IPA/Promethazine | 5‐point DOS (1 = totally unsatisfied, 5 = totally satisfied) | Both groups reported median score 4 | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/50 Comparison: 30/50 | |
| DOS: descriptive ordinal scale; IPA: isopropyl alcohol; RCT: randomized controlled trial; SD: standard deviation; VAS: visual analogue scale | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea severity at end of treatment Show forest plot | 6 | 241 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.63, 0.18] |
| 2 Duration of nausea measured as nausea‐free at the end of treatment Show forest plot | 4 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.31, 34.33] |
| 3 Proportion requiring rescue antiemetics Show forest plot | 7 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.37, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea severity at 5 minutes post‐initial treatment Show forest plot | 4 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.86, 0.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time (minutes) to 50% reduction in nausea score Show forest plot | 3 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.43, ‐0.78] |
| 2 Proportion requiring antiemetics Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.98] |
| 3 Patient satisfaction Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.62, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue antiemetics Show forest plot | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.24] |

