Aromaterapia para el tratamiento de las náuseas y los vómitos posoperatorios
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized controlled trial of peppermint oil, isopropyl alcohol or normal saline aromatherapy to treat postoperative nausea and vomiting. Setting: Postanaesthesia care unit (PACU) acute hospital, USA. | |
| Participants | 33 patients aged 18 years+ having surgery under general or regional anaesthesia, or deep IV sedation, who reported nausea in postanaesthesia care unit. Treatment groups did not differ in the percentage having general anaesthesia, the type of surgery, age or gender distribution. Exclusions: patients who were unable to give informed consent; patients who did not require anaesthesia services. | |
| Interventions | On the patient's spontaneous report of postoperative nausea, they were instructed to take three slow deep breaths to inhale the vapours from a pre‐prepared gauze pad soaked with either peppermint oil, isopropyl alcohol or normal saline placebo held directly under their nostrils. After 2 minutes the patient was asked to rate their nausea by VAS and given the choice to continue aromatherapy or have standard IV anti‐emetics. At 5 minutes post the initial treatment, the patient was again asked to rate their nausea and if they would like to continue aromatherapy or have standard IV anti‐emetics. | |
| Outcomes | 1. Severity of nausea as measured on 100 mm VAS at 2 minutes and 5 minutes after treatment. Visual analogue scale from 'no nausea' to 'worst possible nausea'. 2. Choosing to use 'rescue' anti‐emetics. 3. Satisfaction with management of nausea, as measured by 100 mm VAS with range from 0 = extremely dissatisfied to 100 = fully satisfied. | |
| Notes | Possible lack of accuracy with some participants self‐recording data in PACU if they had poor or blurred vision. Authors Lynn Anderson and Dr Jeffrey Gross emailed to request further information on group sizes, which was supplied by Dr Gross. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "group assignments were made in a randomized, double‐blind fashion" Comment: probably done. Nurses administering treatment were unaware of contents of each package of treatment materials. Patients who had consented to participate entered study when they spontaneously reported nausea. |
| Allocation concealment (selection bias) | Low risk | "A random number generator determined the contents of each serially numbered bag." "...prepared by an individual not otherwise involved in the study..." Data "analysed by investigator unaware of treatment allocation". Comment: probably done |
| Blinding (performance bias and detection bias) | Unclear risk | Staff administering treatment blinded by use of "lightly scented" surgical masks. However patients were self‐reporting subjective assessment of nausea and were not blinded. Comment: Due to the strong aroma of the peppermint oil, it would be impossible to blind the patients receiving this to their allocation once treatment commenced. Probably not done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: outcomes reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Comment: results reported for all stated outcomes, however original study protocol not available. |
| Other bias | Low risk | Comment: study appears to be free of other sources of bias. |
| Methods | Prospective randomized study of isopropyl alcohol inhalation as compared to IV ondansetron for PONV. Replication of study: Winston 2003. Setting: PACU/same day surgery unit, USA. | |
| Participants | 100 women aged 18‐65 who were scheduled for laparoscopic same‐day surgery (ASA physical status I, II or III). Exclusions: patients who had recent upper respiratory tract infections, inability or impaired ability to breathe through the nose, or history of hypersensitivity to IPA, 5HT3 antagonists, promethazine or any other anaesthesia protocol medication, had used an anti‐emetic within 24 hours of surgery, were pregnant or breastfeeding, had history of inner ear pathology, motion sickness or migraine headaches or were taking disulfram, cefoperazone, or metronidazole. | |
| Interventions | Comparison of inhaled isopropyl alcohol to intravenous ondansetron for treatment of PONV. Ondansetron (control) group: nausea treated with ondansetron 4mg IV every 15 minutes to a maximum 8mg dose. Time, dose and VNRS score recorded. IPA (experimental) group: nausea treated by holding a folded alcohol pad approximately 1/2 inch from the participant's nares and instructing them to take 3 deep breaths in and out through the nose. Treatments given every 5 minutes up to a total of 3 administrations. Breakthrough PONV was treated with promethazine suppositories for both groups. Participants were also given supplies of IPA and promethazine to use as needed at home after discharge and asked to record any occurrences of PONV with a data collection tool provided by the researchers. | |
| Outcomes | Time to reduction in nausea score as measured by Verbal Numeric Rating Scale (VRNS) (range 0‐10 where 0 = no nausea and 10 = worst imaginable nausea). Collected for baseline at preop, then immediately postop in PACU and at any time the participant complained of nausea. Additionally, participants who complained of nausea were assessed every 5 minutes following treatment for 30 minutes and then every 15 minutes until discharge from PACU. Participants also reported data on PONV for the 24 hours post‐discharge as well rating their anaesthesia experience overall. | |
| Notes | Author, Joseph Pellegrini contacted for further data. Some was provided however due to data corruption problems not all requested data was available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patient was randomly assigned to the control group or the experimental group by using a computer‐generated random numbers program." Comment: done. |
| Allocation concealment (selection bias) | Low risk | "Block randomization was used for all of the studies using a computer generated randomization program done by an independent party (myself) who was not involved in the data collection" (emailed author response) Comment: done. |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: no information given regarding blinding. Does not appear to have been done. |
| Incomplete outcome data (attrition bias) | Low risk | 28 participants "disenrolled due to protocol violations": 12 from control group who were given IPA postoperatively; 6 from experimental group given other anti‐emetics in PACU before IPA; and 10 who lost their IPA or promethazine following discharge to home. Comment: probably done. |
| Selective reporting (reporting bias) | Unclear risk | Comment: original study protocol unavailable. Results reported for all stated outcomes. |
| Other bias | Low risk | Comment: study appears to be free of other sources of bias. |
| Methods | Randomized controlled trial of ISO versus normal saline placebo for treatment of PONV. Setting: postoperative care unit, acute hospital, Iran. | |
| Participants | 82 consecutive patients randomized into experimental and control groups. No age data or demographic except 48 female/34 male. | |
| Interventions | 2 sniffs of ISO (treatment) or 2 sniffs normal saline (control) (on reporting symptoms) and re‐treated at 5 minutes if necessary. Patients who did not respond the 2nd time received metoclopramide injection. | |
| Outcomes | Response to treatment/cessation of symptoms, recurrence of symptoms, use of rescue anti‐emetics. | |
| Notes | Attempted to contact author, Dr H Kamalipour, via email however no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly divided into two groups." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no data. |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: no data. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: data reported for all stated outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: brief report with little detail. |
| Other bias | Unclear risk | Comment: unable to ascertain from details reported. |
| Methods | Double‐blinded cross‐over clinical trial/pilot study. Setting: acute hospital, USA. | |
| Participants | 15 consecutive patients in PACU who complained of nausea or vomiting after elective surgery. | |
| Interventions | Either 0.5 ml saline or 0.5 ml isopropyl alcohol on a cotton ball (according to random sequence) was held under participants' noses and the participant was instructed to sniff twice. If symptoms recurred, the test agents were re‐administered in random sequence. When neither test agent was effective, standard anti‐emetics were given and the PONV assessed every 5 minutes until participant left PACU. | |
| Outcomes | Severity of PONV as assessed with VAS. VAS range from 0 = none to 10 = vomiting. Treatment failure attributed to the last agent given. | |
| Notes | No demographic data supplied in brief report. Letter sent to author, Dr Paul Langevin, to ask for more data, no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the test agents were readministered in the randomized sequence" Comment: no information on how this sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information reported on who conducted the allocation and how. |
| Blinding (performance bias and detection bias) | Unclear risk | "We designed a randomized double‐blinded study..." "Nurses who administered the test therapy were blinded to group assignment by applying an ISO‐soaked Band‐Aid under their noses while another person applied the test agent to a cotton ball, which was attached to a sponge stick." Comment: participants would not have been blinded to the treatment due to the distinctive odour of the isopropyl alcohol. Unclear where the 'double‐blinding' occurred. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: original study protocol not available. |
| Selective reporting (reporting bias) | Low risk | Comment: data reported for all participants, no apparent losses to follow‐up. |
| Other bias | Unclear risk | Comment: minimal data reported in this publication. |
| Methods | Controlled clinical trial of isopropyl alcohol inhalation for treatment of PONV. Setting: acute hospital, USA. | |
| Participants | 111 adults having surgery (40 with nausea were evaluated for study). Age range: 19‐80 years; mean age = 43. Types of surgery included intra‐abdominal (29.7%), orthopaedic/extremity (23.4%), perineal (19.8%) neuro‐skeletal (10.8%), extra‐thoracic (6.3%) eyes/ears/nose/throat (6.3%), neck (3.6%). Of 40 patients evaluated for study, 21 received IPA and 18 were controls. 1 patient entered into the study had their PONV resolve spontaneously. Inclusion criteria were (a) requirements for general anaesthesia, (b) ability to breathe through nose before and after procedure, (c) minimum of 18 years of age, (d) American Society of Anesthesiologists (ASA) physical status of I, II, or III, and (e) ability to read and write English. Exclusion criteria were (a) allergy to IPA, (b) alcohol abuse, (c) no recent history of nausea or vomiting within the last 8 hours, (d) no recent intake of cefoperazone, Antabuse, or metronidazole, (e) ability to communicate in recovery room, (f) regional anaesthesia, | |
| Interventions | Isopropyl alcohol inhalation for treatment of PONV. "If nausea or vomiting was present in control participants, an appropriate anti‐emetic was given. Experimental participants were given IPA via nasal inhalation using standard hospital alcohol pads. The participant was instructed to take three deep sniffs with the pad one inch from the nose. This was repeated every five minutes for three doses or until nausea and vomiting was relieved. If nausea and vomiting continued after three doses of IPA, then an intravenous drug was given." | |
| Outcomes | Severity of PONV as measured by a descriptive ordinal scale (DOS) from "0 to 10, with 0 being no nausea or vomiting and 10 being the worst nausea and vomiting they could imagine." Cost of treatment in USD. | |
| Notes | Anti‐emetic prophylaxis was given to patients in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Group assignment was alternated by day: experimental one day and control the next." Comment: study is controlled clinical trial. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocators and caregivers appear to have been aware of the allocation. |
| Blinding (performance bias and detection bias) | Low risk | "Participants were blinded to which treatment they were to receive." Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: original study protocol unavailable. Stated outcomes were all addressed in report. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no apparent loss to follow‐up. No P values reported for main findings of pre and post‐test DOS, though P value for cost differences reported. |
| Other bias | Unclear risk | "Only 40 of the 111 participants recruited had PONV. This is explained by aggressive prophylactic treatment at the study facility where only 7 (6.3%) of 111 participants did not receive prophylactic medication and none of these 7 participants had PONV. Additionally, the researchers speculate that pain may have been a confounding factor in accurate assessment on the DOS." Comment: several possible confounders. |
| Methods | Randomized controlled trial comparing 70% isopropyl alcohol inhalation to promethazine to treat breakthrough nausea in surgical patients at high risk of PONV. Setting: day hospital, USA. | |
| Participants | 85 surgical patients scheduled for general anaesthesia of more than 60 minutes’ duration and having 2 of the 4 individual risk Excluded: recent upper respiratory infection; documented allergy to IPA, ondansetron, promethazine, or metoclopramide; anti‐emetic or psychoactive drug use within 24 hours; inability to breathe through the nose; pregnancy; history of inner ear pathology; and/or taking disulfiram, cefoperazone, or metronidazole. | |
| Interventions | Control group: 12.5 to 25 mg IV promethazine for complaints of PONV in the postanaesthesia care unit (PACU) and same‐day surgery unit (SDSU) and by promethazine suppository self‐administration following discharge to home. Experimental group: administration of inhaled 70% IPA. | |
| Outcomes | Nausea, measured by Verbal Numeric Rating Scale (VNRS) (0‐10, 0 = no nausea 10 = worst imaginable nausea). Incidence of nausea events in PACU, SDSU or at home (number). Doses of promethazine required as rescue anti‐emetic (number). Promethazine requirements in PACU, SDSU or at home (mg). Time in minutes to 50% reduction of nausea scores. Participant satisfaction. | |
| Notes | All participants received anti‐emetic prophylaxis prior to surgery. Author J Pellegrini emailed to request numeric data for results published in graph form. Data received. Other clarifications requested and some were received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All subjects were then randomly assigned using a computer‐generated random numbers process into a control or an experimental group." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | "Block randomization was used for all of the studies using a computer generated randomization program done by an independent party (myself) who was not involved in the data collection." (emailed author response) Comment: probably done. |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: no data on blinding. It appears that participants and assessors were aware of group allocations during study. |
| Incomplete outcome data (attrition bias) | Low risk | "A total of 96 subjects were enrolled, but 11 subjects were withdrawn, leaving a total of 85 subjects (IPA group, 42; promethazine group, 43) whose data would be included in the final analysis. Reasons for withdrawal included 4 subjects who received additional anti‐emetics intraoperatively (2 in each group), 1 subject inadvertently enrolled despite being scheduled for a nasal surgical procedure (IPA group), and 6 subjects who required postoperative inpatient hospitalization for reasons unrelated to PONV (3 in each group)." Comment: probably done. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes stated in the article have data reported, however original study protocol is not available. |
| Other bias | Low risk | Comment: no other sources of bias apparent. |
| Methods | Three‐arm controlled clinical trial of peppermint oil inhalations, peppermint essence inhalations (placebo) and no treatment (control) to treat PONV in women. Setting: acute hospital, UK. | |
| Participants | 18 women undergoing major gynaecological surgery. Mean weight group 1: 152lb; group 2: 139.5lb; group 3: 144.2lb. Mean height group 1: 64.2in; group 2: 62.5in; group 3: 64.3in. Mean age group 1: 54 years; group 2: 43.2 years; group 3: 45.5 years. Participants were assessed as having no significant differences in personal characteristics, past medical history or preoperative anxiety levels. There were no statistically significant differences in preoperative fasting times, anaesthetic and recovery times or postoperative fasting times. Five of the experimental group had intra‐abdominal surgery, compared with three in each of the other two groups. | |
| Interventions | Participants were given bottles of their assigned substance postoperatively and instructed to inhale the vapours from the bottle whenever they felt nauseous. | |
| Outcomes | Self‐reported nausea as measured by VAS of 0‐4 where 0 = "not experiencing any nausea" and 4 = "about to vomit" reported as the average score per person per day. Cost of treatment in GBP. Patient satisfaction with treatment, reported narratively. | |
| Notes | Participants may or may not have received standard anti‐emetics in PACU. Author Sylvina Tate supplied some extra data on group allocation methods. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were assigned to one of three groups." Comment: author states that participants were "randomly assigned" to ward areas. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information reported regarding concealment. |
| Blinding (performance bias and detection bias) | Low risk | Comment: use of peppermint essence as placebo blinded experimental and placebo group patients to treatment allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no mention of patients lost to follow‐up, however group numbers are not reported. (Group numbers clarified by author via email). |
| Selective reporting (reporting bias) | High risk | Comment: trialists did not provide measure of statistical significance or measures of variance for daily average nausea scores, even though they state 'statistically significant difference in the amount of self‐reported nausea between the placebo and experimental groups. |
| Other bias | Unclear risk | Comment: due to study design, entirely possible there was some demand‐characteristic effect on patient self‐reporting of results. However, experimental group received 'on average, slightly less' postoperative anti‐emetics and more postoperative opioids than placebo group, which would tend to indicate evidence of an effect. |
| Methods | Double‐blind randomized controlled study of isopropyl alcohol as a treatment for PONV. "When any episode of vomiting or nausea occurred, patients were randomized, using a random number table to receive a cotton ball soaked with ISO or saline placed under the patient’s nose by the nursing staff. The patient was instructed to sniff twice by a nurse who was blind to group assignment. It should be emphasized that the nursing staffs were instructed not to smell the content of cotton ball and to hold it away from themselves when administering to patient. If the severity of nausea or vomiting improved after a single treatment, a VAS assessment of nausea was obtained every 5 minutes until the patient was discharged or PONV symptoms recurred. Improvement of nausea was defined as a decrease of at least 40% in initial VAS score, and improvement of vomiting was defined as no further episodes of vomiting. If, after treatment, severity of nausea did not improve or retching/vomiting persisted, a second treatment with the same agent was given. Treatment sequences were repeated for a maximum of three times in a 15‐minute period. When severity of either nausea or vomiting failed to improve despite three treatments, intravenous (IV) ondansetron 0.1 mg/kg (maximum 4 mg) was administered. If symptoms persisted, a second dose of ondansetron was administered. For patients who failed to improved after two ondansetron doses (maximum dose: 8mg), other IV ant‐emetic medications (i.e., 200 mg/kg of metoclopramide; 10 mg/kg droperidol) were given." Setting: acute paediatric day surgery centre. | |
| Participants | 91 children aged 6‐16 years having surgery under general anaesthesia. ASA physical status I and II. Of these, 39 developed PONV and were enrolled into treatment or control groups. Treament n = 20. Control n = 19. No significant differences in demographic data across groups. Exclusions: children with a history of chronic illness or developmental delay. | |
| Interventions | Inhalations of isopropyl alcohol or saline placebo. Intervention repeated up to three times. IV ondansetron was used as 'rescue therapy' if PONV continued. | |
| Outcomes | 1. Severity of nausea and vomiting as measured by 100 mm VAS with a range of 0 = no nausea to 100 = extreme nausea. 2. Use of rescue anti‐emetics as measured by drug and number of doses. | |
| Notes | Study author, Dr Shu‐Ming Wang contacted for any further data, however due to the age of the study there was none available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "If any episode of vomiting or nausea occurred, patients were randomized, using a random number table to receive a cotton ball soaked with ISO or saline placed under the patient’s nose by the nursing staff." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no data on who conducted the allocation and any degree of separation from the conduct of the study. |
| Blinding (performance bias and detection bias) | Low risk | "The patient was instructed to sniff twice by a nurse who was blind to group assignment. It should be emphasized that the nursing staffs were instructed not to smell the content of cotton ball and to hold it away from themselves when administering to patient." Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: data reported for all participants. No apparent losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: original study protocol not available. All stated outcomes reported. |
| Other bias | Low risk | Comment: no other sources of bias apparent. |
| Methods | Randomized controlled trial of isopropyl alcohol for treatment of PONV. Participants were randomized to receive either isopropyl alcohol inhalations, or 4mg ondansetron. Setting: same day surgery centre, USA. | |
| Participants | 100 women aged 18‐65 years who were scheduled for diagnostic laparoscopy, operative laparoscopy or laparoscopic bilateral tubal occlusion (ASA physical status I, II or III) in a day surgery unit. Exclusions: inability or impaired ability to breathe through the nose, or history of sensitivity to IPA or ondansetron, had used an anti‐emetic within 24 hours of surgery, pregnant or breastfeeding, reported existing nausea, history of significant PONV resistant to anti‐emetics, using disulfram or had a history of alcoholism. | |
| Interventions | Comparison of inhaled 70% isopropyl alcohol to ondansetron for treatment of PONV. Ondansetron (control) group: at first request for treatment participants in this group received IV ondansetron 4mg, repeated once in 15 minutes if required. 70% IPA (experimental) group: a standard alcohol prep pad was held under the participant's nose and she was instructed to take 3 consecutive deep breaths through the nose. Nausea score collected for baseline at preop, then immediately postop in PACU and at any time the participant complained of nausea. Additionally, participants who complained of nausea were assessed every 5 minutes following treatment for 30 minutes and then every 15 minutes until discharge from PACU. | |
| Outcomes | 1. Nausea score as measured by Verbal Numeric Rating Scale (VRNS) (range 0‐10 where 0 = no nausea and 10 = worst imaginable nausea). 2. Number of emetic events, defined as episodes of nausea or vomiting more than one minute apart. 3. Time to reduction of PONV in minutes. 4. Cost. 5. Patient satisfaction with anaesthesia care. | |
| Notes | This study was replicated by Cotton 2007 with the number and frequency of IPA inhalations increased. Author J Pellegrini provided additional data via email. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "subjects were randomly assigned to receive inhaled 70% IPA (experimental group) or IV ondansetron (control group) for the treatment of PON" "despite the use of block randomization". Comment: author states via email that randomization was conducted using a computer generated random numbers table. |
| Allocation concealment (selection bias) | Low risk | "Block randomization was used for all of the studies using a computer generated randomization program done by an independent party (myself) who was not involved in the data collection." Comment: probably done. |
| Blinding (performance bias and detection bias) | High risk | "...this did not allow us to blind the study intervention." Comment: it appears that no blinding of participants or caregivers was done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: it appears that data was reported for all participants, no evidence of exclusions or attrition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: original study protocol unavailable. Despite stating collection of data on patient satisfaction with anaesthetic experience, no results for this were reported, however this data was made available by an author via email. |
| Other bias | Low risk | Comment: no other sources of bias apparent. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. Not aromatherapy. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not PONV. | |
| Not PONV. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. | |
| Prevention of PONV, not treatment. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. | |
| Not RCT/CCT. | |
| Prevention of PONV, not treatment. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 4 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| Analysis 1.1  Comparison 1 Isopropyl alcohol versus standard treatment for PONV, Outcome 1 Proportion requiring rescue anti‐emetics. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 3 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.13] |
| Analysis 2.1  Comparison 2 Isopropyl alcohol versus standard treatment for PON: sensitivity analysis, Outcome 1 Proportion requiring rescue anti‐emetics. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 3 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.13] |
| Analysis 3.1  Comparison 3 Isopropyl alcohol versus standard treatment for PON, Outcome 1 Proportion requiring rescue anti‐emetics. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.00] |
| Analysis 4.1  Comparison 4 Isopropyl alcohol versus saline, Outcome 1 Proportion requiring rescue anti‐emetics. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patient satisfaction Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.62, 2.03] |
| Analysis 5.1  Comparison 5 Aromatherapy versus standard anti‐emetics, Outcome 1 Patient satisfaction. | ||||

Results of searches
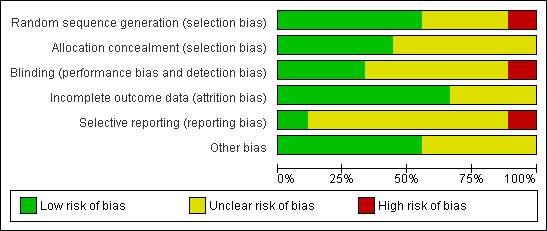
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
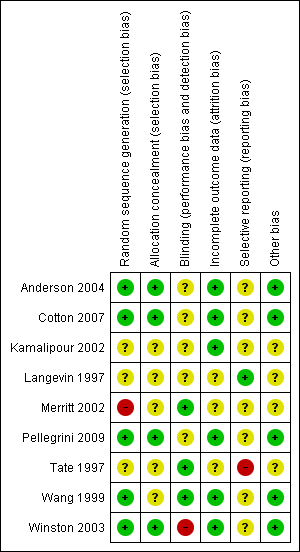
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
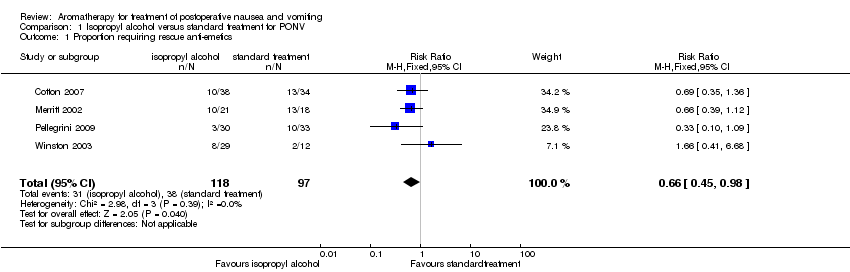
Comparison 1 Isopropyl alcohol versus standard treatment for PONV, Outcome 1 Proportion requiring rescue anti‐emetics.
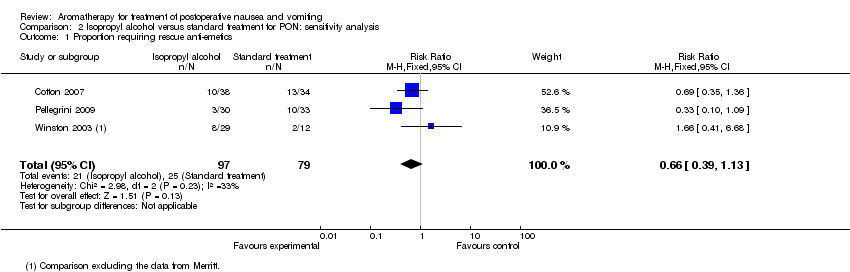
Comparison 2 Isopropyl alcohol versus standard treatment for PON: sensitivity analysis, Outcome 1 Proportion requiring rescue anti‐emetics.

Comparison 3 Isopropyl alcohol versus standard treatment for PON, Outcome 1 Proportion requiring rescue anti‐emetics.

Comparison 4 Isopropyl alcohol versus saline, Outcome 1 Proportion requiring rescue anti‐emetics.
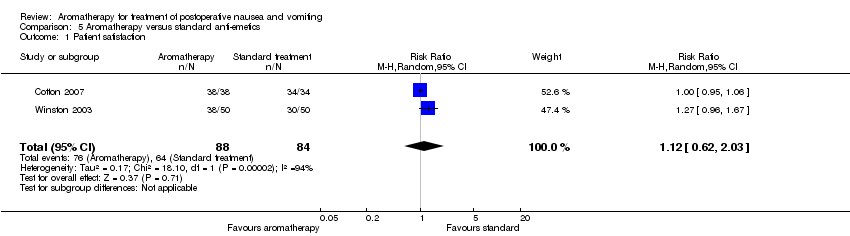
Comparison 5 Aromatherapy versus standard anti‐emetics, Outcome 1 Patient satisfaction.
| Isopropyl alcohol compared to standard treatment for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: patients with treatment of postoperative nausea and vomiting | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard treatment | Isopropyl alcohol | |||||
| Requirement for rescue anti‐emetics | Study population1 | RR 0.66 | 215 | ⊕⊕⊝⊝ | ||
| 392 per 1000 | 259 per 1000 | |||||
| Medium risk population1 | ||||||
| 275 per 1000 | 182 per 1000 | |||||
| Adverse effects4 | See comment | See comment | Not estimable | 0 | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Calculated using control group results. | ||||||
| Isopropyl alcohol compared to saline for treatment of postoperative nausea and vomiting | ||||||
| Patient or population: patients with treatment of postoperative nausea and vomiting | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| saline | Isopropyl alcohol | |||||
| Requirement for rescue anti‐emetics1,2 | Study population3 | RR 0.23 | 135 | ⊕⊕⊝⊝ | ||
| 868 per 1000 | 200 per 1000 | |||||
| Low risk population3 | ||||||
| 100 per 1000 | 23 per 1000 | |||||
| Adverse effects6 | See comment | See comment | Not estimable | 0 | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Participants enrolled into study on complaint of nausea and/or vomiting. | ||||||
| Study | Design | Intervention/Control | Outcome | Findings |
| RCT | IPA/ondansetron | Time to 50% reduction in nausea (VNRS1) | IPA: mean 15.00 (SD:10.6mins) Ondansetron: mean 33.88 (SD: 23.2mins) | |
| RCT | IPA/saline | Percentage "response"2 to treatment within 5 minutes | IPA: 78% Saline: 7.3% | |
| CCT | IPA/saline | Percent with complete relief of nausea in 5 minutes | IPA: 80% Saline: 0% | |
| RCT | IPA/Promethazine | Mean time to 50% reduction in nausea scores (VNRS1) | IPA: (mean +/‐ SD) PACU3: 6.43 +/‐ 3.78 minutes SDSU4: 8.33 +/‐ 4.82 minutes HOME5: 16.58 +/‐ 6.9 minutes Promethazine: (mean +/‐ SD) PACU3: 20.5 +/‐ 18.236 minutes SDSU4: 23.3 +/‐ 18.86 minutes HOME5: 26.67 +/‐ 12.5 minutes | |
| RCT | IPA/ondansetron | Mean time to 50% reduction of VNRS1 | IPA: 6.3 minutes Ondansetron: 27.7 minutes | |
| 1VRNS: Verbal Numeric Rating Scale. 2Meaning of response not defined by study authors. 3PACU: Postanaesthesia Care Unit. 4SDSU: Same Day Surgery Unit. 5Home: Participant's residence post‐discharge. | ||||
| Study | Design | Intervention/Control | Outcome | Findings |
| CCT | IPA/standard anti‐emetics | Decrease in mean nausea score (DOS1) 0‐10 (0 = no nausea, 10 = worst nausea and vomiting imaginable) | IPA: Mean DOS1 score Pre‐treatment: 5.71 Post‐treatment: 2.7 Standard treatment: Pre‐treatment: 6.11 Post‐treatment: 1.94 | |
| CCT | Peppermint oil/peppermint essence/standard treatment | Mean daily nausea scores (DOS1) 0‐4 (0 = no nausea, 4 = about to vomit) | Standard treatment: mean daily nausea score = 0.975 Peppermint essence mean daily nausea score (placebo): 1.61 Peppermint oil mean daily nausea score: 0.5 | |
| RCT | IPA/saline | Percentage of participants with decrease in nausea after 3 treatments (VAS) 0‐100 (0 = no nausea, 100 = extreme nausea) | IPA: 91% Saline: 40% | |
| 1DOS: Descriptive Ordinal Scale. | ||||
| Study | Design | Intervention/Comparison | Measure | Satisfied |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/38 Comparison: 34/34 | |
| RCT | IPA/ondansetron | 4‐point DOS (poor, fair, good, excellent) | Good or excellent: Intervention: 38/50 Comparison: 30/50 | |
| RCT | IPA/Promethazine | 5‐point DOS (1 = totally unsatisfied, 5 = totally satisfied) | Both groups report median score 4 | |
| RCT | IPA/Saline/Peppermint | 100mm VAS (0 mm extremely dissatisfied; 100 mm fully satisfied)
| IPA: 90.3 (SD: 14.9) peppermint: 86.3 (SD: 32.3) saline: 83.7 (SD: 25.6)
|
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 4 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 3 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 3 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion requiring rescue anti‐emetics Show forest plot | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patient satisfaction Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.62, 2.03] |

