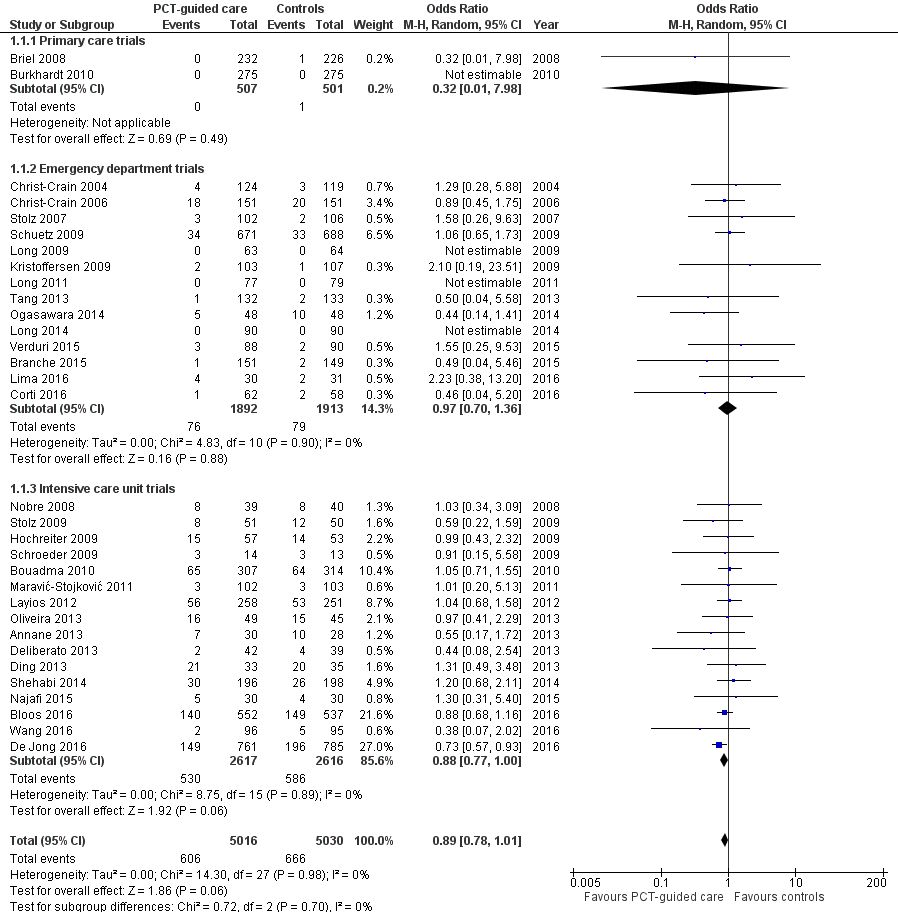
| Study ID | Country | Setting, type of trial | Clinical diagnosis | Type of PCTalgorithm and PCTcut‐offs used (µg/L) | N: ARI participants (study total) | Primary endpoint | Follow‐up time | Reasons for exclusion of patients |
| Annane 2013 | France | ICU, multicentre | Severe sepsis without overt source of infection and negative blood culture | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 5.0) | 0 (62) | Participants on AB on day 5 post randomisation | Hospital stay | 62 non‐ARI patients (4 of them with post randomisation consent withdrawal) |
| Bloos 2016 | Germany | ICU, multicentre | Severe sepsis or septic shock | Discontinuation at day 4, 7, and 10; R against AB: < 1.0 or > 50% drop to previous value | 219 (1180) | 28‐day mortality | 3 months | 91 post randomisation exclusions (informed consent not obtainable), 870 not ARI patients |
| Bouadma 2010 | France | ICU, multicentre | Suspected bacterial infections during ICU stay without prior AB (> 24 h) | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 394 (630) | All‐cause mortality | 2 months | 9 post randomisation exclusions (8 withdrew consent, 1 randomised twice); 227 non‐ARI patients |
| Branche 2015 | USA | ED, medical ward, single centre | Lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 265 (300) | Antibiotic exposure and safety | 3 months | 35 non‐ARI patients |
| Briel 2008 | Switzerland | Primary care, multicentre | Upper and lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 458 (458) | Days with restricted activities | 1 month | No exclusions |
| Burkhardt 2010 | Germany | Primary care, multicentre | Upper and lower ARI | Initiation; R against AB: < 0.25; R for AB: > 0.25 | 550 (571) | Days with restricted activities | 1 month | 21 post randomisation exclusions (2 withdrew consent, 1 due to loss of sample, 15 with autoimmune, inflammatory, or systemic disease, 2 with advanced liver disease, 1 with prior use of antibiotics) |
| Christ‐Crain 2004 | Switzerland | ED, single centre | Lower ARI with X‐ray confirmation | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 219 (243) | AB use | 2 weeks | 24 non‐ARI patients |
| Christ‐Crain 2006 | Switzerland | ED, medical ward, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 286 (302) | AB use | 6 weeks | 16 non‐ARI patients |
| Corti 2016 | Denmark | ED, single centre | AECOPD | Initiation and duration; R against AB < 0.25 (0.15)/80% decrease; R for AB > 0.25 | 120 (120) | AB use | 28 days | No exclusions |
| De Jong 2016 | Netherlands | ICU, multicentre | Critically ill patients with presumed infection | Duration; R against AB: < 0.5 or > 80% drop | 994 (1575) | AB use | 1 year | 29 post randomisation exclusions (25 protocol violations, 4 withdrew informed consent), 552 non‐ARI patients |
| Deliberato 2013 | Brazil | ICU, single centre | Septic patients with proven bacterial infection | Duration; R against AB: < 0.5 or > 90% drop | 66 (81) | AB use | ICU discharge or 14 days' post randomisation | 15 non‐ARI patients |
| Ding 2013 | China | ICU, single centre | Acute exacerbation of pulmonary fibrosis | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 0 (78) | AB use | 1 month | 10 post randomisation exclusions (7 lost to follow‐up, 3 withdrew informed consent), 68 data not shared |
| Hochreiter 2009 | Germany | Surgical ICU, single centre | Suspected bacterial infections and > 1 SIRS criteria | Duration; R against AB: < 1 or > 65% drop over 3 d | 43 (110) | AB use | Hospital stay | 67 non‐ARI patients |
| Kristoffersen 2009 | Denmark | ED, medical ward, multicentre | Lower ARI without X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 (> 0.5) | 210 (223) | AB use | Hospital stay | 13 post randomisation exclusions (3 no PCT testing, 6 not meeting inclusion criteria, 4 withdrew informed consent) |
| Layios 2012 | Belgium | ICU, single centre | Suspected infection | Initiation; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 160 (509) | AB use | 1 month | 120 no PCT measurements, 10 missing data, 219 non‐ARI patients |
| Lima 2016 | Brazil | ED, medical ward, single centre | Febrile neutropenia | Duration; R against AB: < 0.5 for 2 days or > 90% drop than highest measured concentration | 0 (62) | AB use | 28 days | 1 post randomisation exclusion (withdrew informed consent), 62 non‐ARI patients |
| Long 2009 | China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 127 (149) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent |
| Long 2011 | China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 156 (172) | AB use | 1 month | 16 post randomisation exclusions (6 lost to follow‐up, 7 withdrew consent, 3 with final diagnosis other than CAP) |
| Long 2014 | China | ED, single centre | Severe acute exacerbation of asthma | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 180 (180) | AB use | 1 year | No exclusions |
| Maravić‐Stojković 2011 | Serbia | ICU surgical, single centre | Infection after open heart surgery | Initiation; R for AB: > 0.5 | 5 (205) | AB use, AB cost | Hospital stay | 200 non‐ARI patients |
| Najafi 2015 | Iran | ICU, single centre | SIRS without apparent source of infection | Initiation; R for AB: > 2 | 0 (60) | AB use | Hospital stay | 60 patient data not shared |
| Nobre 2008 | Switzerland | ICU, single centre | Suspected severe sepsis or septic shock | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 52 (79) | AB use | 1 month | 27 non‐ARI patients |
| Ogasawara 2014 | Japan | Medical ward, single centre | Aspiration pneumonia | Predefined duration; AB for 3 d: < 0.5; AB for 5 d: 0.5 to 1.0; AB for 7 d: > 1 | 0 (105) | Relapse and 30‐day mortality | 1 month | 9 post randomisation exclusions (2 withdrew consent, 7 others), 96 data not shared |
| Oliveira 2013 | Brazil | ICU, multicentre | Severe sepsis or septic shock | Discontinuation; initial < 1.0: R against AB: 0.1 at day 4; initial > 1.0: R against: > 90% drop | 58 (97) | AB use | 28 days or hospital discharge | 3 post randomisation exclusions (2 withdrew consent, 1 technical problems), 36 patients with a final diagnosis other than ARI |
| Schroeder 2009 | Germany | Surgical ICU, single centre | Severe sepsis following abdominal surgery | Duration; R against AB: < 1 or > 65% drop over 3 d | 8 (27) | AB use | Hospital stay | 19 non‐ARI patients |
| Schuetz 2009 | Switzerland | ED, medical ward, multicentre | Lower ARI with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 1304 (1381) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent, 55 non‐ARI patients |
| Shehabi 2014 | Australia | ICU, multicentre | Suspected sepsis, undifferentiated infections | Duration; R against AB: < 0.25 (< 0.1) or > 90% drop | 156 (400) | AB use | 3 months | 6 post randomisation exclusions (6 withdrew consent), 238 non‐ARI patients |
| Stolz 2007 | Switzerland | ED, medical ward, single centre | Exacerbated COPD | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 208 (226) | AB use | 2 to 3 weeks | 18 post randomisation exclusions (absence of COPD) |
| Stolz 2009 | Switzerland, USA | ICU, multicentre | VAP when intubated > 48 h | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 101 (101) | AB‐free days alive | 1 month | No exclusions |
| Tang 2013 | China | ED, single centre | Exacerbation of asthma | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 0 (265) | AB use | 6 weeks | 10 post randomisation exclusions (5 lost to follow‐up, 3 died, 2 withdrew consent), 255 data not shared |
| Verduri 2015 | Italy | ED, medical ward, multicentre | AECOPD | Initiation; R against AB:< 0.1; R for AB: > 0.25 | 178 (183) | Number of exacerbations | 6 months | 5 post randomisation exclusions (5 lost to follow‐up because they did not meet the inclusion criteria) |
| Wang 2016 | China | ICU, single centre | AECOPD | All participants had initial PCT < 0.1; AB group treated with AB for at least 3 days, control group no AB in the first 10 days | 191 (194) | Treatment success within 10 days | 30 days | 3 post randomisation exclusions (3 with pneumonia according to CT scan) |