Procalcitonina para iniciar o interrumpir los antibióticos en las infecciones respiratorias agudas
Resumen
Antecedentes
Las infecciones respiratorias agudas (IRA) comprenden un grupo amplio y heterogéneo de infecciones, como las bacterianas, las virales y por otras etiologías. En los años recientes, la procalcitonina (PCT), un marcador sanguíneo para las infecciones bacterianas, ha surgido como una herramienta prometedora para mejorar las decisiones acerca del tratamiento con antibióticos (antibioticoterapia guiada por la PCT). Varios ensayos controlados aleatorizados (ECA) han demostrado la factibilidad del uso de procalcitonina para iniciar e interrumpir los antibióticos en diferentes poblaciones de pacientes con IRA y diferentes contextos que varían desde la atención primaria hasta los servicios de urgencias, las salas del hospital y las unidades de cuidados intensivos. Sin embargo, no está claro el efecto del uso de procalcitonina en los resultados clínicos. Esta es una actualización de una revisión Cochrane y un metanálisis de datos de los participantes individuales publicados por primera vez en 2012, que fueron diseñados para considerar la seguridad del control de antibióticos guiado por la PCT.
Objetivos
El objetivo de esta revisión sistemática basada en datos de pacientes individuales fue evaluar la seguridad y la eficacia del uso de procalcitonina para iniciar o interrumpir los antibióticos en una gran diversidad de pacientes con IRA de gravedad variable y de diferentes contextos clínicos.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), que contiene el registro especializado del Grupo Cochrane de Infecciones Respiratorias Agudas (Cochrane Acute Respiratory Infections Group's Specialised Register), MEDLINE y en Embase en febrero 2017 para identificar ensayos adecuados. También se hicieron búsquedas en ClinicalTrials.gov para identificar ensayos en curso en abril 2017.
Criterios de selección
Se incluyeron ECA de participantes adultos con IRA que recibieron un tratamiento con antibióticos sobre la base de un algoritmo de procalcitonina (algoritmo de control de antibióticos guiado por la PCT) o la atención habitual. Se excluyeron los ensayos cuando se concentraban exclusivamente en los niños o cuando utilizaban procalcitonina para una finalidad diferente a guiar la iniciación y la duración del tratamiento con antibióticos.
Obtención y análisis de los datos
De forma independiente, dos equipos de revisores evaluaron la metodología y extrajeron los datos de los estudios primarios. Las variables de evaluación primarias fueron la mortalidad por todas las causas y el fracaso del tratamiento a los 30 días, para los cuales las definiciones se unificaron entre los ensayos. Las variables de evaluación secundarias fueron la administración de antibióticos, los efectos secundarios relacionados con los antibióticos, y la duración de la estancia hospitalaria. Los odds ratios (OR) y los intervalos de confianza (IC) del 95% se calcularon mediante la regresión logística jerárquica multivariable ajustada para la edad, el sexo y el diagnóstico clínico mediante un modelo de efectos fijos. Los diferentes ensayos se agregaron como efectos aleatorios en el modelo. Se realizaron los análisis de sensibilidad estratificados por contexto clínico y tipo de IRA. También se realizó un metanálisis de datos agregados.
Resultados principales
De 32 ECA aptos que incluían 18 ensayos nuevos para esta actualización de 2017 se obtuvieron datos de participantes individuales de 26 ensayos con 6708 participantes, los cuales se incluyeron en el metanálisis principal de datos de los participantes individuales. No se obtuvieron datos de los participantes individuales para cuatro ensayos, y dos ensayos no incluyeron a personas con IRA confirmada. Según GRADE, la calidad de la evidencia fue alta para los resultados de la mortalidad y la exposición a los antibióticos, y la calidad fue moderada para los resultados del fracaso del tratamiento y los efectos secundarios relacionados con los antibióticos.
Variables de evaluación primarias: hubo 286 muertes en 3336 participantes que recibieron tratamiento guiado por la procalcitonina (8,6%) en comparación con 336 de 3372 controles (10,0%), lo cual da lugar a una mortalidad significativamente baja asociada con el tratamiento guiado por la procalcitonina (OR ajustado 0,83; IC del 95%: 0,70 a 0,99; P = 0,037). No fue posible calcular la mortalidad en los ensayos de atención primaria debido a que sólo se informó una muerte en un participante del grupo de control. El fracaso del tratamiento no fue significativamente inferior en los participantes que recibieron tratamiento guiado por la procalcitonina (23,0% versus 24,9% en el grupo de control, OR ajustado 0,90; IC del 95%: 0,80 a 1,01; P = 0,068). Los resultados fueron similares entre los subgrupos por contexto clínico y tipo de infección respiratoria, sin evidencia sobre la modificación del efecto (P para la interacción > 0,05). Variables de evaluación secundarias: la procalcitonina como guía se asoció con una reducción de 2,4 días en la exposición a los antibióticos (5,7 versus 8,1 días, IC del 95%: ‐2,71 a ‐2,15; p < 0,001) y un riesgo menor de efectos secundarios relacionados con los antibióticos (16,3% versus 22,1%, OR ajustado 0,68; IC del 95%: 0,57 a 0,82; P < 0,001). La duración de la estancia hospitalaria y la estancia en la unidad de cuidados intensivos fueron similares en ambos grupos. Un análisis de sensibilidad de datos agregados basado en los 32 ensayos elegibles mostró resultados similares.
Conclusiones de los autores
Este metanálisis actualizado de los datos individuales de los participantes de 12 países muestra que el uso de procalcitonina para guiar la iniciación y la duración del tratamiento con antibióticos resulta en un riesgo menor de mortalidad, un menor consumo de antibióticos y un riesgo menor de efectos secundarios relacionados con los antibióticos. Los resultados fueron similares para diferentes contextos clínicos y tipos de IRA, lo cual apoya el uso de la procalcitonina en el contexto del control de antibióticos en los pacientes con IRA. Se necesita investigación futura de alta calidad para confirmar los resultados en los pacientes con inmunosupresión y en los pacientes con infecciones no respiratorias.
PICO
Resumen en términos sencillos
Pruebas de los niveles de procalcitonina sanguínea para decidir cuándo iniciar e interrumpir el tratamiento con antibióticos en adultos con infecciones respiratorias agudas
Pregunta de la revisión
¿Cuáles son los efectos del uso de la procalcitonina para iniciar o interrumpir los antibióticos en los pacientes con infecciones respiratorias agudas en comparación con la atención habitual en cuanto a la mortalidad y el fracaso del tratamiento?
Antecedentes
El uso de antibióticos innecesarios en pacientes con infecciones respiratorias agudas contribuye significativamente al aumento de la resistencia bacteriana, los costes médicos y el riesgo de eventos adversos relacionados con el fármaco. La procalcitonina como marcador sanguíneo aumenta en las infecciones bacterianas y disminuye cuando los pacientes se recuperan de la infección. La procalcitonina puede medirse en la sangre de los pacientes por medio de diferentes pruebas comercialmente disponibles con un tiempo de respuesta de alrededor de una a dos horas, y apoyar la toma de decisiones clínicas acerca de la iniciación y la interrupción del tratamiento con antibióticos.
Fecha de la búsqueda
Se realizaron búsquedas electrónicas el 10 febrero 2017. Se realizaron búsquedas de ensayos en curso el 12 de abril de 2017.
Características de los estudios
Todos los ensayos incluidos asignaron al azar a los participantes con infecciones respiratorias agudas para recibir antibióticos basado en los niveles de procalcitonina (grupo “guiado por la procalcitonina”) o los mismos fueron asignados a un grupo de control. Los ensayos se realizaron en la atención primaria, el servicio de urgencias y las salas médicas, y la unidad de cuidados intensivos. Los participantes incluidos presentaban infecciones agudas de las vías respiratorias superiores o inferiores, como neumonía, bronquitis, exacerbaciones de la enfermedad pulmonar obstructiva crónica y otras.
Fuentes de financiación de los estudios
Todos los estudios fueron ensayos iniciados por el investigador. La mitad de los ensayos fueron financiados por organismos nacionales o no informaron el financiamiento, y la mitad de los ensayos recibieron financiamiento de la industria del biomarcador (p.ej., Thermo Fisher Scientific).
Resultados clave
Se estudiaron 6708 participantes de 26 ensayos en 12 países. La mortalidad a los 30 días fue significativamente inferior en los participantes que recibieron tratamiento guiado por la procalcitonina en comparación con los participantes del control (286 muertes en 3336 participantes bajo tratamiento guiado por la procalcitonina (8,6%) versus 336 muertes en 3372 controles (10,0%)). No hubo diferencias significativas con respecto a los fracasos del tratamiento. Los resultados fueron similares para los diferentes contextos clínicos (atención primaria, servicio de urgencias, unidad de cuidados intensivos) y los diferentes tipos de infección respiratoria. Con respecto a la exposición a los antibióticos, los participantes del grupo guiado por la procalcitonina tuvieron una reducción de 2,4 días en la exposición a los antibióticos y una reducción de los efectos secundarios relacionados con los antibióticos (16,3% versus 22,1%).
Calidad de la evidencia
La calidad de la evidencia fue alta para la mortalidad y la exposición a los antibióticos. La mayoría de los ensayos no utilizó el cegamiento, sin embargo, no se cree que la mortalidad haya sido sesgada por dicha limitación. La calidad de la evidencia fue moderada para el fracaso del tratamiento y los efectos secundarios relacionados con los antibióticos debido a que las definiciones para estas variables principales de evaluación en los ensayos no fueron idénticas.
Authors' conclusions
Summary of findings
| Procalcitonin algorithm compared to standard care for guiding antibiotic therapy in acute respiratory tract infections | ||||||
| Patient or population: people with acute respiratory tract infections | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | PCTalgorithm | |||||
| Mortality | Study population | OR 0.83 | 6708 | ⊕⊕⊕⊕ | ||
| 100 per 1000 | 86 per 1000 (76 to 95) | |||||
| Treatment failure | Study population | OR 0.90 | 6708 | ⊕⊕⊕⊝ | ||
| 249 per 1000 | 230 per 1000 (216 to 245) | |||||
| Antibiotic‐related side effects Follow‐up: 30 days | Study population 221 per 1000 | 163 per 1000 (145 to 182) | OR 0.68 | 3034 | ⊕⊕⊕⊝ | |
| Antibiotic exposure | The mean antibiotic exposure in the control groups was | The mean antibiotic exposure in the intervention groups was | ‐ | 6708 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No downgrading for serious concerns. Still, there is some concern about unconcealed allocation in several trials in the emergency department and intensive care settings. There is also some concern about low adherence with the PCT algorithm in the intervention group. We consider unblinded outcome assessment as not relevant for the outcome of death. | ||||||
Background
Acute respiratory infections (ARIs) account for over 10% of global disease burden and are the most common reason for antibiotic therapy in primary care and hospital settings (Evans 2002; Gonzales 1997; Zaas 2014).
Description of the condition
Acute respiratory infections comprise a heterogeneous group of infections including bacterial, viral, and other aetiologies. As many as 75% of all antibiotic doses are prescribed for ARIs, despite their mainly viral cause (Doan 2014; Evans 2002). Early initiation of adequate antibiotic therapy is the cornerstone in the treatment of bacterial ARIs and is associated with improved clinical outcomes (Hoare 2006; Kumar 2006; Kumar 2009; Liberati 2009b; Spurling 2010). However, overuse of antibiotics by overprescription in outpatients with bronchitis (Arnold 2005), for instance, and prolonged duration of antibiotic therapy in people with bacterial ARIs in the hospital and intensive care unit (ICU) settings is associated with increased resistance to common bacteria, high costs, and adverse drug reactions (Gonzales 1997; Goossens 2005; Lawrence 2009; Zaas 2014).
Description of the intervention
The presence of a diagnostic 'gold standard' or reference standard represents the best available method for establishing the presence or absence of a disease. Optimally, a morphological verification such as histopathology or, in the case of ARIs, growth of typical pathogens in blood cultures or sputum cultures can be obtained to establish the 'correct' diagnosis. Regrettably, the use of blood cultures as the assumed gold standard in ARIs lacks sensitivity, specificity, or both, with only around 10% of people with pneumonia having positive cultures and some of them being false positives (Muller 2010). In this diagnostic uncertainty, surrogate biomarker to estimate the likelihood for the presence of a bacterial infection and to grade disease severity are of great interest (Schuetz 2015). In such a circumstance, two fundamentally different concepts are employed. One concept tends to ignore potential dilemmas in the accuracy of the alleged gold standard but assumes a well‐defined illness, which is represented by the assumption drawn following a diagnostic test or a clinical diagnosis. The second concept discards alleged gold standards and focuses on patient outcomes. In the case of ARIs, the clinical benefit of a diagnostic biomarker, such as procalcitonin (PCT), can be measured by clinical outcomes of randomised intervention studies, assuming that if the person recovered without antibiotics then there was no relevant bacterial illness.
In recent years, PCT has emerged as a promising marker for the diagnosis of bacterial infections because higher levels are found in severe bacterial infections but remain fairly low in viral infections and non‐specific inflammatory diseases (Muller 2000; Muller 2001; Muller 2010). Procalcitonin is released in multiple tissues in response to bacterial infections via a direct stimulation of cytokines, such as interleukin (IL)‐1β, tumour necrosis factor (TNF)‐ɑ, and IL‐6. Conversely, PCT production is blocked by interferon gamma, a cytokine released in response to viral infections (Muller 2000). Hence, PCT may be used to support clinical decision making for the initiation and discontinuation of antibiotic therapy in different types of infections and indications (Sager 2017; Schuetz 2016). Randomised controlled trials (RCTs) have demonstrated the feasibility of such a strategy in different ARI patient populations and different settings ranging from primary care to emergency departments and hospital wards to medical and surgical ICUs (Bloos 2016; Branche 2015; Corti 2016; De Jong 2016; Deliberato 2013; Layios 2012; Long 2014; Maravić‐Stojković 2011; Oliveira 2013; Shehabi 2014; Verduri 2015; Wang 2016).
How the intervention might work
Procalcitonin levels correlate with the risk of relevant bacterial infections and decrease upon recovery. Procalcitonin testing may therefore help physicians decide in which patients antibiotics are needed and when it is safe to stop treatment (Kutz 2015). The use of PCT in clinical protocols may thus decrease antibiotic consumption in two ways: by preventing unnecessary antibiotic prescriptions and by limiting durations of antibiotic treatment (Sager 2017; Schuetz 2011a).
Why it is important to do this review
While several RCTs have evaluated PCT‐guided antibiotic treatment, most individual trials included participants with different types of respiratory and non‐respiratory infections and lacked the statistical power to assess the risk for mortality and severe infectious disease complications associated with PCT‐guided decision making. Previous meta‐analyses of RCTs investigating the effect of PCT algorithms on antibiotic use focused on the critical care setting, people with suspicion of bacterial infections, and people with sepsis and respiratory infections (Heyland 2011; Hoeboer 2015; Tang 2009; Wacker 2013). However, these meta‐analyses used aggregated data and were not able to investigate the effects of PCT on different ARI diagnoses and on outcomes other than mortality. A previous meta‐analysis based on individual participant data published in the Cochrane Library did not find a significant difference in clinical outcomes, but confidence intervals remained relatively wide (Schuetz 2012). Safety of using PCT for antibiotic decision making remained thus unproven.
Objectives
The aim of this systematic review based on individual participant data was to assess the safety and efficacy of using procalcitonin for starting or stopping antibiotics over a large range of patients with varying severity of ARIs and from different clinical settings.
Methods
Criteria for considering studies for this review
Types of studies
Prospective RCTs comparing a strategy to initiate or discontinue antibiotic therapy based on PCT levels with a control arm without PCT measurements were eligible for inclusion. Participants were randomised to receive antibiotics either based on PCT levels ('PCT‐guided' group) or a control group without knowledge of PCT levels, including antibiotic management based on usual care or guidelines. We did not include non‐randomised studies.
Types of participants
We included adult participants with clinical diagnoses of ARIs: either a lower ARI including community‐acquired pneumonia (CAP), hospital‐acquired pneumonia (HAP), ventilator‐associated pneumonia (VAP), acute bronchitis, exacerbation of asthma, or exacerbation of chronic obstructive pulmonary disease (COPD); or an upper ARI including common cold, rhino‐sinusitis, pharyngitis, tonsillitis, or otitis media. We also included people with sepsis and suspected ARIs in the analyses. We excluded trials if they focused exclusively on children or used PCT to escalate antibiotic therapy. We made no exclusions based on language of reports or clinical setting. We included trials from primary care, emergency departments, and medical and surgical ICUs.
Types of interventions
Strategies to initiate or discontinue antibiotic therapy based on PCT levels compared with usual care were eligible.
Types of outcome measures
We defined primary and secondary outcomes to a follow‐up time of 30 days. For trials with shorter follow‐up periods, we used the available information (i.e. until hospital discharge). We excluded all trials with different follow‐up times for mortality in a sensitivity analysis.
Primary outcomes
-
All‐cause mortality following randomisation up to a follow‐up time of 30 days.
-
Setting‐specific treatment failure within 30 days of inclusion.
For the primary care setting, we defined treatment failure as death, hospitalisation, ARI‐specific complications (e.g. empyema for lower ARIs, meningitis for upper ARIs), recurrent or worsening infection, and still having ARI‐associated discomfort at 30 days. For the emergency department setting, we defined treatment failure as death, ICU admission, rehospitalisation after index hospital discharge, ARI‐associated complications (e.g. empyema or acute respiratory distress syndrome for lower ARIs), and recurrent or worsening infection within 30 days of follow‐up. For the medical and surgical ICU setting, we defined treatment failure as death within 30 days of follow‐up and recurrent or worsening infection.
Secondary outcomes
-
Antibiotic use (initiation of antibiotics, duration of antibiotics, and total exposure to antibiotics (total amount of antibiotic days divided by total number of participants)).
-
Length of hospital stay for hospitalised participants.
-
Length of ICU stay for critically ill participants.
-
Number of days with restricted activities within 14 days after randomisation for primary care participants.
-
Antibiotic‐related side effects.
Search methods for identification of studies
We updated the search strategy for this review in February 2017 in collaboration with the Cochrane Acute Respiratory Infections Group's Information Specialist. We performed data collection based on the protocol of a previous meta‐analysis of individual participant data published in the Cochrane Library (Schuetz 2008).
Electronic searches
We updated the searches for this review in February 2017, running the search across all databases from the date of inception to 10 February 2017. We screened all new references identified by the search. We searched the following databases for published studies:
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1), part of the Cochrane Library, which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, www.cochranelibrary.com/ (accessed 10 February 2017) (Appendix 1);
-
MEDLINE Ovid (1966 to 10 February 2017) (Appendix 2);
-
Embase.com (1980 to 10 February 2017) (Appendix 3).
We used the search strategy in Appendix 4 to conduct searches for the 2012 version of this review (Schuetz 2012).
We also searched for ongoing and completed trials in the following trial register:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/; searched 12 April 2017).
We did not apply any language or publication restrictions.
Searching other resources
We contacted experts for further eligible trials.
Data collection and analysis
We requested individual participant data from the investigators of all included trials. We checked all provided data against published reports, and if needed, corrected any discrepancies.
We prepared this review update according to PRISMA guidelines and the PRISMA‐IPD guideline (Liberati 2009a; Stewart 2015).
Selection of studies
At least two review authors (RS, YW, PS) independently assessed trial eligibility based on titles, abstracts, full‐text reports, and further information from investigators as needed.
Data extraction and management
We checked data from each trial against reported results and resolved any queries with the principal investigator, trial data manager, or statistician. The mortality and adverse outcome rates from trials included in this review may differ slightly from previous reports because we treated data in a consistent manner across all trials.
Assessment of risk of bias in included studies
Two review authors (RS, YW) assessed the methodological quality of each included study using the Cochrane 'Risk of bias' tool and resolved any disagreements by discussion (Higgins 2011). Methodological criteria included: adequate sequence generation and concealment of treatment allocation; blinding of participants, physicians and clinical outcome assessment; whether the study was free of selective reporting; and the proportion of participants lost to follow‐up. We documented the proportion of participants in the PCT group that adhered to the PCT algorithm used in each study, defining adherence to the PCT algorithm of lower than 70% as high risk of bias, and, if not reported, as unclear risk. Due to the study design of the included studies, physicians were aware of the participants' study group because in the intervention group physicians used the PCT result for decision making about antibiotic treatment, while in the control group no PCT result was communicated to the physicians. Blinding of physicians was therefore not feasible, resulting in an unclear risk for performance bias in all studies.
We assessed the quality of evidence at the outcome level using the GRADE approach (GRADEpro GDT 2014).
Measures of treatment effect
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) using multivariable hierarchical logistic regression for the co‐primary endpoints of mortality from any cause and treatment failure (Thompson 2001; Turner 2000). We fitted corresponding linear and logistic regression models for continuous and binary secondary endpoints, respectively. We calculated Kaplan‐Meier curves for time to death for graphical display.
We used Stata version 12.1 (College Station, TX) for statistical analyses (Stata 12.1).
Unit of analysis issues
The unit of our primary analysis was the individual study participant. We analysed all participants in the study group to which they were randomised. We calculated summary estimates using aggregated data from individual trials as a sensitivity analysis.
Dealing with missing data
We received the full data sets from all trials included in the individual participant data analysis (n = 26) with all available follow‐up information (if recorded in the trials).
We assumed in our main analysis that participants lost to follow‐up did not experience an event. We explored if a complete‐case analysis (excluding participants lost to follow‐up) or an analysis assuming that participants lost to follow‐up experienced an event would change the results for the primary outcomes of mortality and treatment failure in sensitivity analyses. We checked all individual participant data against the published results but did not find significant differences that warranted further exploration.
Assessment of heterogeneity
We performed prespecified analyses stratified by clinical setting (i.e. primary care, emergency department, ICU) and ARI diagnosis (CAP, COPD, bronchitis, VAP) to investigate the consistency of results across our heterogeneous patient populations in terms of disease severity. We formally tested for potential subgroup effects by adding the clinical setting and ARI diagnosis in turn to the regression model together with the corresponding interaction term with the PCT group as a fixed‐effect model. We assessed heterogeneity by estimating the I2 statistic (the percentage of total variance across trials that is due to heterogeneity rather than chance) in meta‐analyses using aggregated data and by testing for heterogeneity using the Cochran Q test (Higgins 2003).
Assessment of reporting biases
We assessed reporting bias by attempting to identify if the study was included in a trial registry, a protocol was available, and if the methods section provided a list of outcomes. We compared listed outcomes from those sources to outcomes reported in the published papers.
Data synthesis
We used multivariable hierarchical logistic regression to combine participant data from the trials (Thompson 2001; Turner 2000). Apart from the group variable indicating the use of a PCT algorithm, we included important prognostic factors such as participant age and ARI diagnosis as an additional fixed effect; to account for within‐ and between‐trial variability, we added a categorical trial variable to the model as a random effect. In meta‐analyses with aggregated trial data we calculated summary ORs using a random‐effects model and the Mantel‐Haenszel facility of Review Manager 5 (RevMan 2014).
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: all‐cause mortality at 30 days, setting‐specific treatment failure at 30 days, total exposure to antibiotics, and antibiotic‐related side effects (summary of findings Table for the main comparison). The results reported in this table correspond to the main IPD analysis and are slightly different from the aggregate data analysis. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We performed prespecified analyses stratified by clinical setting and ARI diagnosis and formally tested for potential subgroup effects by adding an interaction term into the statistical model.
Sensitivity analysis
We performed prespecified sensitivity analyses based on the main quality indicators: allocation concealment, blinded outcome assessment, adherence to the PCT algorithm (we defined low adherence to PCT algorithms as < 70%), and follow‐up time for mortality other than one month. We also performed an aggregate data meta‐analysis using all trials with potentially eligible participants.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
After removal of duplicates, we identified 998 records that we further assessed based on title and abstracts, excluding 919 records. We obtained 79 full‐text study reports, and following assessment excluded 39 that did not meet our inclusion criteria. Eight studies were ongoing trials. From 32 eligible RCTs (9909 participants) including 18 new trials for this 2017 update, we obtained individual participant data from 26 trials including 6708 participants, which were included in the main individual participant data meta‐analysis (see Figure 1). We did not obtain individual participant data for four trials, and two trials did not include participants with confirmed ARIs. The sensitivity aggregate analysis includes all 32 trials.
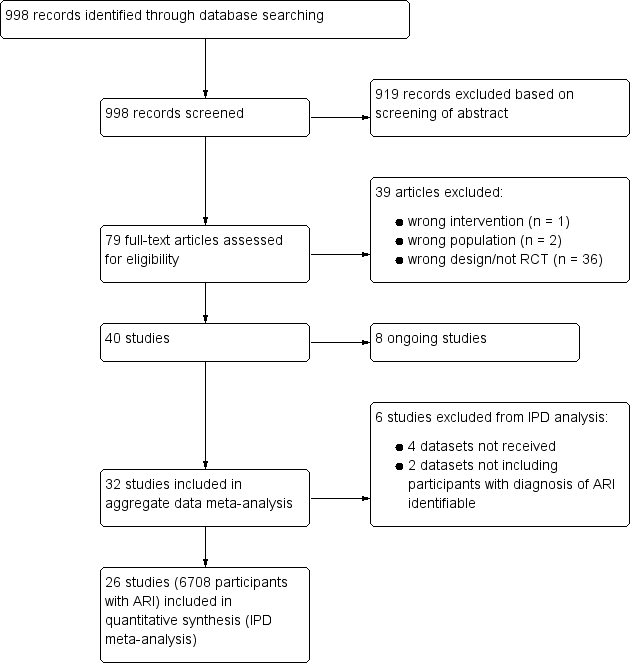
Study flow diagram.
Abbreviations: ARI: acute respiratory infection; IPD: individual participant data; RCT: randomised controlled trial
Included studies
We included a total of 26 studies involving 6708 participants in the main individual participant data meta‐analysis. Study characteristics are presented in Table 1.
| Study ID | Country | Setting, type of trial | Clinical diagnosis | Type of PCTalgorithm and PCTcut‐offs used (µg/L) | N: ARI participants (study total) | Primary endpoint | Follow‐up time | Reasons for exclusion of patients |
| France | ICU, multicentre | Severe sepsis without overt source of infection and negative blood culture | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 5.0) | 0 (62) | Participants on AB on day 5 post randomisation | Hospital stay | 62 non‐ARI patients (4 of them with post randomisation consent withdrawal) | |
| Germany | ICU, multicentre | Severe sepsis or septic shock | Discontinuation at day 4, 7, and 10; R against AB: < 1.0 or > 50% drop to previous value | 219 (1180) | 28‐day mortality | 3 months | 91 post randomisation exclusions (informed consent not obtainable), 870 not ARI patients | |
| France | ICU, multicentre | Suspected bacterial infections during ICU stay without prior AB (> 24 h) | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 394 (630) | All‐cause mortality | 2 months | 9 post randomisation exclusions (8 withdrew consent, 1 randomised twice); 227 non‐ARI patients | |
| USA | ED, medical ward, single centre | Lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 265 (300) | Antibiotic exposure and safety | 3 months | 35 non‐ARI patients | |
| Switzerland | Primary care, multicentre | Upper and lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 458 (458) | Days with restricted activities | 1 month | No exclusions | |
| Germany | Primary care, multicentre | Upper and lower ARI | Initiation; R against AB: < 0.25; R for AB: > 0.25 | 550 (571) | Days with restricted activities | 1 month | 21 post randomisation exclusions (2 withdrew consent, 1 due to loss of sample, 15 with autoimmune, inflammatory, or systemic disease, 2 with advanced liver disease, 1 with prior use of antibiotics) | |
| Switzerland | ED, single centre | Lower ARI with X‐ray confirmation | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 219 (243) | AB use | 2 weeks | 24 non‐ARI patients | |
| Switzerland | ED, medical ward, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 286 (302) | AB use | 6 weeks | 16 non‐ARI patients | |
| Denmark | ED, single centre | AECOPD | Initiation and duration; R against AB < 0.25 (0.15)/80% decrease; R for AB > 0.25 | 120 (120) | AB use | 28 days | No exclusions | |
| Netherlands | ICU, multicentre | Critically ill patients with presumed infection | Duration; R against AB: < 0.5 or > 80% drop | 994 (1575) | AB use | 1 year | 29 post randomisation exclusions (25 protocol violations, 4 withdrew informed consent), 552 non‐ARI patients | |
| Brazil | ICU, single centre | Septic patients with proven bacterial infection | Duration; R against AB: < 0.5 or > 90% drop | 66 (81) | AB use | ICU discharge or 14 days' post randomisation | 15 non‐ARI patients | |
| China | ICU, single centre | Acute exacerbation of pulmonary fibrosis | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 0 (78) | AB use | 1 month | 10 post randomisation exclusions (7 lost to follow‐up, 3 withdrew informed consent), 68 data not shared | |
| Germany | Surgical ICU, single centre | Suspected bacterial infections and > 1 SIRS criteria | Duration; R against AB: < 1 or > 65% drop over 3 d | 43 (110) | AB use | Hospital stay | 67 non‐ARI patients | |
| Denmark | ED, medical ward, multicentre | Lower ARI without X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 (> 0.5) | 210 (223) | AB use | Hospital stay | 13 post randomisation exclusions (3 no PCT testing, 6 not meeting inclusion criteria, 4 withdrew informed consent) | |
| Belgium | ICU, single centre | Suspected infection | Initiation; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 160 (509) | AB use | 1 month | 120 no PCT measurements, 10 missing data, 219 non‐ARI patients | |
| Brazil | ED, medical ward, single centre | Febrile neutropenia | Duration; R against AB: < 0.5 for 2 days or > 90% drop than highest measured concentration | 0 (62) | AB use | 28 days | 1 post randomisation exclusion (withdrew informed consent), 62 non‐ARI patients | |
| China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 127 (149) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent | |
| China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 156 (172) | AB use | 1 month | 16 post randomisation exclusions (6 lost to follow‐up, 7 withdrew consent, 3 with final diagnosis other than CAP) | |
| China | ED, single centre | Severe acute exacerbation of asthma | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 180 (180) | AB use | 1 year | No exclusions | |
| Serbia | ICU surgical, single centre | Infection after open heart surgery | Initiation; R for AB: > 0.5 | 5 (205) | AB use, AB cost | Hospital stay | 200 non‐ARI patients | |
| Iran | ICU, single centre | SIRS without apparent source of infection | Initiation; R for AB: > 2 | 0 (60) | AB use | Hospital stay | 60 patient data not shared | |
| Switzerland | ICU, single centre | Suspected severe sepsis or septic shock | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 52 (79) | AB use | 1 month | 27 non‐ARI patients | |
| Japan | Medical ward, single centre | Aspiration pneumonia | Predefined duration; AB for 3 d: < 0.5; AB for 5 d: 0.5 to 1.0; AB for 7 d: > 1 | 0 (105) | Relapse and 30‐day mortality | 1 month | 9 post randomisation exclusions (2 withdrew consent, 7 others), 96 data not shared | |
| Brazil | ICU, multicentre | Severe sepsis or septic shock | Discontinuation; initial < 1.0: R against AB: 0.1 at day 4; initial > 1.0: R against: > 90% drop | 58 (97) | AB use | 28 days or hospital discharge | 3 post randomisation exclusions (2 withdrew consent, 1 technical problems), 36 patients with a final diagnosis other than ARI | |
| Germany | Surgical ICU, single centre | Severe sepsis following abdominal surgery | Duration; R against AB: < 1 or > 65% drop over 3 d | 8 (27) | AB use | Hospital stay | 19 non‐ARI patients | |
| Switzerland | ED, medical ward, multicentre | Lower ARI with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 1304 (1381) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent, 55 non‐ARI patients | |
| Australia | ICU, multicentre | Suspected sepsis, undifferentiated infections | Duration; R against AB: < 0.25 (< 0.1) or > 90% drop | 156 (400) | AB use | 3 months | 6 post randomisation exclusions (6 withdrew consent), 238 non‐ARI patients | |
| Switzerland | ED, medical ward, single centre | Exacerbated COPD | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 208 (226) | AB use | 2 to 3 weeks | 18 post randomisation exclusions (absence of COPD) | |
| Switzerland, USA | ICU, multicentre | VAP when intubated > 48 h | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 101 (101) | AB‐free days alive | 1 month | No exclusions | |
| China | ED, single centre | Exacerbation of asthma | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 0 (265) | AB use | 6 weeks | 10 post randomisation exclusions (5 lost to follow‐up, 3 died, 2 withdrew consent), 255 data not shared | |
| Italy | ED, medical ward, multicentre | AECOPD | Initiation; R against AB:< 0.1; R for AB: > 0.25 | 178 (183) | Number of exacerbations | 6 months | 5 post randomisation exclusions (5 lost to follow‐up because they did not meet the inclusion criteria) | |
| China | ICU, single centre | AECOPD | All participants had initial PCT < 0.1; AB group treated with AB for at least 3 days, control group no AB in the first 10 days | 191 (194) | Treatment success within 10 days | 30 days | 3 post randomisation exclusions (3 with pneumonia according to CT scan) |
AB: antibiotic
AECOPD: acute exacerbation of chronic obstructive pulmonary disease
ARI: acute respiratory infection
CAP: community‐acquired pneumonia
COPD: chronic obstructive pulmonary disease
CT: computed tomography
d: days
ED: emergency department
h: hours
ICU: intensive care unit
PCT: procalcitonin
R: recommendation for or against antibiotics
SIRS: systemic inflammatory response syndrome
VAP: ventilator‐associated pneumonia
Participants
Baseline characteristics of included participants were similar in the PCT and control groups with respect to important prognostic features (Table 2). Most participants were recruited either in the emergency department or ICU setting, and CAP was the most frequent ARI diagnosis, reported in more than 40% of participants.
| Parameter | Control (n = 3372) | PCT group (n = 3336) |
| Demographics | ||
| Age (year), mean (SD) | 61.2 ± 18.4 | 60.7 ± 18.8 |
| Male gender, n (%) | 1910 (56.6%) | 1898 (56.9%) |
| Clinical setting, no (%) | ||
| Primary care | 501 (14.9%) | 507 (15.2%) |
| Emergency department | 1638 (48.6%) | 1615 (48.4%) |
| Intensive care unit | 1233 (36.6%) | 1214 (36.4%) |
| Primary diagnosis | ||
| Total upper ARI, n (%) | 280 (8.3%) | 292 (8.8%) |
| Common cold | 156 (4.6%) | 149 (4.5%) |
| Rhino‐sinusitis, otitis | 67 (2.0%) | 73 (2.2%) |
| Pharyngitis, tonsillitis | 46 (1.4%) | 61 (1.8%) |
| Total lower ARI, n (%) | 3092 (91.7%) | 3044 (91.2%) |
| Community‐acquired pneumonia | 1468 (43.5%) | 1442 (43.2%) |
| Hospital‐acquired pneumonia | 262 (7.8%) | 243 (7.3%) |
| Ventilator‐associated pneumonia | 186 (5.5%) | 194 (5.8%) |
| Acute bronchitis | 287 (8.5%) | 257 (7.7%) |
| Exacerbation of COPD | 631 (18.7%) | 621 (18.6%) |
| Exacerbation of asthma | 127 (3.8%) | 143 (4.3%) |
| Other lower ARI | 131 (3.9%) | 144 (4.3%) |
| Procalcitonin upon enrolment | ||
| PCT< 0.1 ug/L | 921 (35.6%) | 981 (30.9%) |
| PCT 0.1 to 0.25 ug/L | 521 (20.1%) | 608 (19.2%) |
| PCT > 0.25 to 0.5 ug/L | 308 (11.9%) | 383 (12.1%) |
| PCT > 0.5 to 2.0 ug/L | 358 (13.8%) | 520 (16.4%) |
| PCT > 2.0 ug/L | 482 (18.6%) | 679 (21.4%) |
ARI: acute respiratory infection
COPD: chronic obstructive pulmonary disease
PCT: procalcitonin
SD: standard deviation
Settings
Trials were conducted in 12 countries: Switzerland, Germany, France, Italy, USA, China, Denmark, Netherlands, Brazil, Belgium, Australia, and Serbia. Trials were conducted in different clinical settings including primary care, emergency departments and medical wards, and ICU. There were two primary care trials with upper and lower respiratory infection patients; 11 emergency department and medical ward trials with lower ARI patients; and 13 ICU trials with mostly septic patients due to infections of the lower respiratory tract.
Interventions
Procalcitonin algorithms used in the different trials were similar in concept and recommended initiation and/or continuation of antibiotic therapy based on similar PCT cut‐off levels (reviewed in Schuetz 2011a). However, there were differences: some trials in primary care and the emergency department used only a single PCT measurement on admission to guide initiation of antibiotics, while the other trials (predominantly in hospitalised patients with severe infections) used repeated measurements for guiding the duration of treatment. One trial used a point‐of‐care device (Corti 2016). Adherence to algorithms varied, ranging from 44% to 100% (Table 3).
| Study ID | Allocation concealment | Blinded | Follow‐up for mortality | Adherence to PCT algorithm in PCT group | Follow‐up for mortality |
| Yes (central randomisation) | No | 58/58 (100%) | 63% adherence | LOS | |
| Yes (central randomisation) | No | 1045/1089 (96%) | 49.6% adherence | 28 days and 90 days | |
| Yes (central randomisation) | Yes | 393/394 (100%) | 47% adherence | 28 days and 60 days | |
| Yes (central randomisation using blocks of 4) | No | 250/300 (83.3%) | 64% adherence | 1 month and 3 months | |
| Yes (central randomisation) | Yes | 454/458 (99%) | 85% adherence | 28 days | |
| Yes (central randomisation) | Yes | 546/550 (99%) | 87% adherence | 28 days | |
| No (alternating weeks) | No | 230/243 (95%) | 83% adherence | 10 to 14 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 300/302 (99%) | 87% adherence | 56 days | |
| Yes (randomisation algorithm was concealed to treating clinicians and participants) | No | 120/120 (100%) | 61.1% adherence | 28 days | |
| Yes (central randomisation) | No | 1546/1546 (100%) | 44% adherence | 28 days and 1 year | |
| Yes (opaque, sealed envelopes) | No | 81/81 (100%) | 52% adherence | LOS | |
| Yes (central randomisation) | No | 68/78 (87.2%) | Not reported | 30 days | |
| No (unconcealed drawing of lots) | No | 43/43 (100% until discharge) | Not reported | LOS | |
| Yes (central randomisation) | No | 210/210 (100% until discharge) | 59% adherence | LOS | |
| Not reported | No | 509/509 (100%) | Not reported | Intensive care unit LOS | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 61/62 (98.4%) | 73.3% adherence | 28 days and 90 days | |
| No (odd and even patient ID numbers) | No | 127/127 (100%) | Not reported | Not reported | |
| No (odd and even patient ID numbers) | No | 156/156 (100%) | Not reported | 28 days | |
| Yes (central randomisation) | No | 169/180 (93.9%) | 96.6% adherence | LOS and 1 year | |
| Yes (central randomisation) | No | 205/205 (100%) | Not reported | 30 days and LOS | |
| Yes (central randomisation) | No | 30/30 (100%) | Not reported | LOS | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 52/52 (100%) | 81% adherence | 28 days and LOS | |
| Not reported | No | 96/96 (100%) | Not reported | 30 days | |
| Yes (central randomisation) | No | 94/94 (100%) | 86.2% adherence | 28 days | |
| No (unconcealed drawing of lots) | No | 8/8 (100% until discharge) | Not reported | LOS | |
| Yes (central randomisation) | Yes | 1358/1359 (100%) | 91% adherence | 28 days | |
| Yes (central randomisation) | Yes | 394/394 (100%) | Not reported | LOS and 90 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 208/208 (100%) | Not reported | 6 months | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 101/101 (100%) | Not reported | 28 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 258/265 (97.4%) | Not reported | 6 weeks | |
| Yes (central randomisation) | No | 178/178 (100%) | Not reported | 6 months | |
| Yes (those responsible for allocation concealment were not involved in the measurement of results) | No | 191/191 (100%) | 82.3% adherence (17 participants in the control group received AB) | 30 days |
LOS: length of stay
PCT: procalcitonin
Comparators
In control group participants, PCT was not used to guide treatment decisions, but this decision was up to the treating physician team. In some trials, physicians were asked to follow antibiotic guidelines for control group participants (Briel 2008; Schuetz 2009). In one trial, the control group was guided with C‐reactive protein levels (Oliveira 2013).
Funding sources
All studies were investigator‐initiated trials. Half of the trials were funded by national agencies or did not report funding; the other half of the trials received funding from the biomarker industry (e.g. Thermo Fisher Scientific).
Excluded studies
We excluded a total of 39 studies due to wrong intervention (n = 1), wrong population (n = 2), and wrong design (not RCT) (n = 36). A total of nine studies reported as ongoing in the 2012 review were now available for assessment; we included four of these studies in this current update (Annane 2013; Bloos 2016; De Jong 2016; Lima 2016), and did not include five studies due to wrong population (paediatrics).
Ongoing studies
Our searches of the trial register identified seven ongoing studies that we will assess for inclusion for the next review update (Ongoing studies). These studies focus on the utility of PCT in people with pneumonitis (NCT02862314), pulmonary embolism (NCT02261610), lower respiratory infection (NCT02130986), heart failure (NCT02787603), and intraoperative positive‐end expiratory pressure optimisation (NCT02931409). Two trials are antibiotic efficacy trials (NCT02332577; NCT02440828).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 2 and Figure 3. The risk of bias was mostly low for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting; unclear for blinding of personnel in all studies; and mostly high for blinding of outcome assessment.
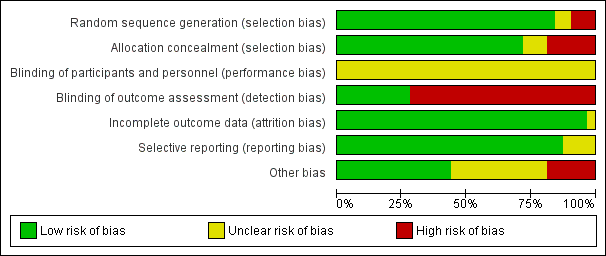
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
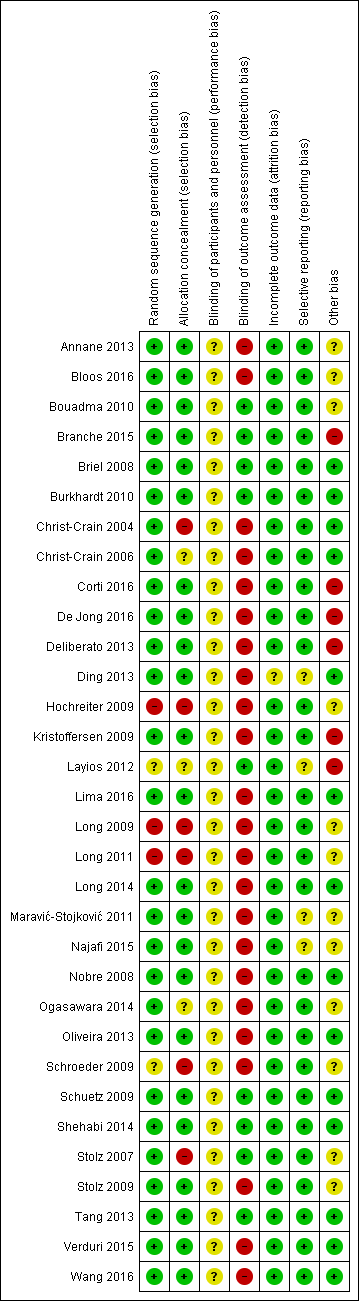
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies randomised participants to intervention (PCT testing) or control groups. A total of 25 trials with mainly computer‐generated lists and centralised randomisation were at low risk of selection bias. Seven trials were at high or unclear risk of selection bias. Risk for selection bias with regard to random sequence generation was due to weekly allocation (Christ‐Crain 2004), unnumbered envelopes (Christ‐Crain 2006; Stolz 2007), use of odd and even patient identification numbers (Long 2009; Long 2011), and unconcealed drawing of lots (Hochreiter 2009; Schroeder 2009).
Blinding
None of the included trials blinded physicians to group allocation because PCT was used for decision making in the intervention group, thus all trials had unclear risk for blinding of participants and personnel.
All trials used blinded outcome assessment (Briel 2008; Bouadma 2010; Branche 2015; Layios 2012; Schuetz 2009; Shehabi 2014; Stolz 2007; Tang 2013), employing blinded telephone interviews to assess vital status and other outcomes.
Incomplete outcome data
The included trials had a high follow‐up for mortality with few participants lost to follow‐up (Table 3). In seven trials, outcome assessment was done after hospital or ICU discharge (Deliberato 2013; Hochreiter 2009; Kristoffersen 2009; Layios 2012; Long 2009; Schroeder 2009; Shehabi 2014). One trial had a high number of post randomisation exclusions (six in the intervention arm versus four in the control group) and thus had an unclear risk of bias (Ding 2013).
Selective reporting
No reporting bias was found when study protocols and final results were compared. However, we did not find registration numbers for four trials (Ding 2013; Layios 2012; Maravić‐Stojković 2011; Najafi 2015), which we considered to be at unclear risk of bias. We found no evidence of reporting bias by visual inspection of funnel plots (Figure 4).
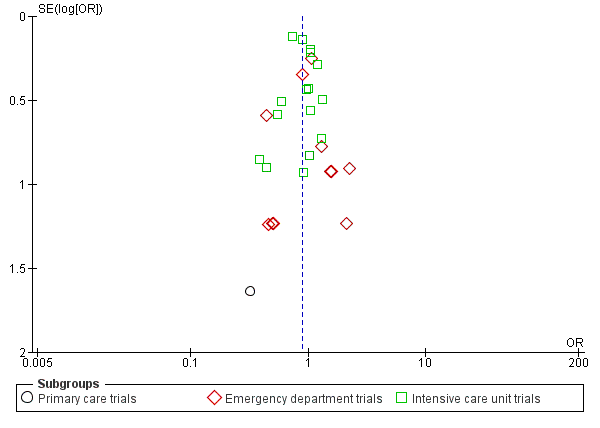
Funnel plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.
Other potential sources of bias
Another potential source of bias relates to low adherence to the PCT algorithms, particularly for safety endpoints. Overall, adherence varied, ranging from 44% to 100% (Table 3).
With regard to funding, 16 trials reported no industry funding (six did not report any funding, 10 reported public funding), and in 16 trials Thermo Fisher, the producer of the PCT assay, funded or co funded the studies by providing free‐of‐charge PCT kits or additional research funds, or both.
Effects of interventions
Primary outcomes
1. All‐cause mortality following randomisation up to a follow‐up time of 30 days
There were 286 deaths in 3336 PCT‐guided participants (8.6%) compared to 336 in 3372 controls (10.0%) resulting in a significantly lower mortality associated with PCT‐guided therapy (adjusted odds ratio (OR) 0.83, 95% confidence interval (CI) 0.70 to 0.99, P = 0.037) (Table 4). This effect was consistent across clinical settings (P for interaction > 0.05), although mortality could not be estimated in primary care trials because only one death was reported in a control group participant. The effect on mortality was also consistent among different ARI diagnoses (CAP, COPD, bronchitis, VAP) (P for interaction > 0.05). As a further sensitivity analysis and to investigate heterogeneity among trials, we also calculated an aggregate data meta‐analysis based on the aggregate results of all 32 potentially eligible trials (thus not limited to ARI participants only). In this analysis, the results proved robust, although the mortality estimate did not reach statistical significance (OR 0.89, 95% CI 0.78 to 1.01; Analysis 1.1; Figure 5). There was no evidence of heterogeneity (I2 = 0%).
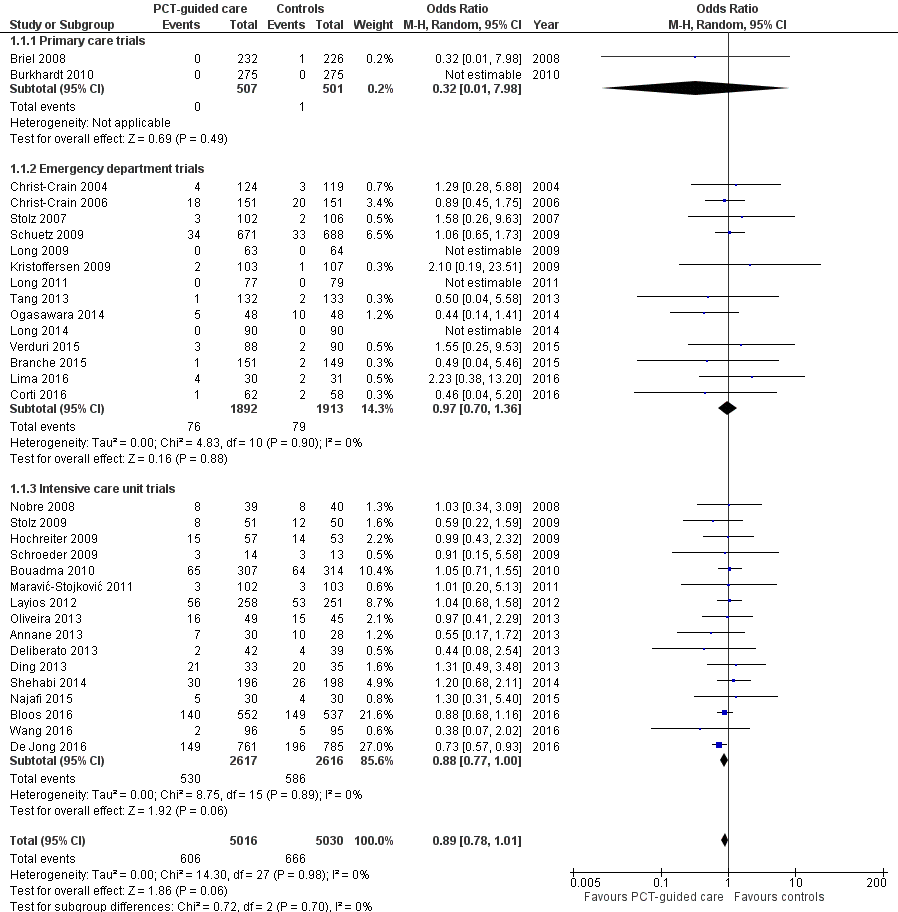
Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.
| Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction | |
| Overall | 3372 | 3336 | ||
| 30 days mortality, n (%) | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Treatment failure, n (%) | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Length of ICU stay, mean (±SD) | 13.3 ± 16.0 | 13.7 ± 17.2 | 0.39 (‐0.81 to 1.58), P = 0.524 | NA |
| Length of hospital stay, mean (±SD) | 13.7 ± 20.6 | 13.4 ± 18.4 | ‐0.19 (‐0.96 to 0.58), P = 0.626 | NA |
| Antibiotic‐related side effects, n (%) | 336 (22.1%) | 247 (16.3%) | 0.68 (0.57 to 0.82), P < 0.001 | NA |
| According to setting | ||||
| Primary care | 501 | 507 | ||
| 30 days mortality, n (%) | 1 (0.2%) | 0 (0.0%) | NA | NA |
| Treatment failure, n (%) | 164 (32.7%) | 159 (31.4%) | 0.96 (0.73 to 1.25), P = 0.751 | 0.715 |
| Days with restricted activities, mean (±SD) | 8.9 ± 4.2 | 8.9 ± 4.1 | 0.07 (‐0.44 to 0.59), P = 0.777 | NA |
| Antibiotic‐related side effects, n (%) | 128 (25.7%) | 102 (20.2%) | 0.65 (0.46 to 0.91), P = 0.012 | 0.596 |
| Emergency department | 1638 | 1615 | ||
| 30 days mortality, n (%) | 62 (3.8%) | 57 (3.5%) | 0.91 (0.63 to 1.33), P = 0.635 | 0.546 |
| Treatment failure, n (%) | 292 (17.8%) | 259 (16.0%) | 0.87 (0.72 to 1.05), P = 0.141 | 0.807 |
| Length of hospital stay, mean (±SD) | 8.2 ± 10.5 | 8.1 ± 7.5 | ‐0.14 (‐0.73 to 0.44), P = 0.631 | 0.684 |
| Antibiotic‐related side effects, n (%) | 208 (20.3%) | 145 (14.4%) | 0.66 (0.52 to 0.83), P = 0.001 | 0.596 |
| Intensive care unit | 1233 | 1214 | ||
| 30 days mortality, n (%) | 273 (22.3%) | 229 (19.0%) | 0.84 (0.69 to 1.02), P = 0.081 | 0.619 |
| Length of ICU stay, mean (±SD) | 14.8 ± 16.2 | 15.3 ± 17.5 | 0.56 (‐0.82 to 1.93), P = 0.427 | 0.849 |
| Length of hospital stay, mean (±SD) | 26.3 ± 26.9 | 25.8 ± 23.9 | ‐0.33 (‐2.28 to 1.62), P = 0.739 | 0.641 |
| According to diagnosis | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| 30 days mortality, n (%) | 206 (14.1%) | 175 (12.2%) | 0.82 (0.66 to 1.03), P = 0.083 | 0.958 |
| Treatment failure, n (%) | 385 (26.2%) | 317 (22.0%) | 0.78 (0.66 to 0.93), P = 0.005 | 0.052 |
| Length of ICU stay, mean (±SD) | 10.5 ± 10.3 | 11.9 ± 13.3 | 1.45 (0.15 to 2.75), P = 0.029 | 0.119 |
| Length of hospital stay, mean (±SD) | 13.3 ± 15.7 | 13.9 ± 16.1 | 0.74 (‐0.25 to 1.73), P = 0.143 | 0.094 |
| Antibiotic‐related side effects, n (%) | 186 (27.7%) | 127 (19.1%) | 0.62 (0.48 to 0.80), P < 0.001 | 0.227 |
| Exacerbation of COPD | 631 | 621 | ||
| 30 days mortality, n (%) | 24 (3.8%) | 19 (3.1%) | 0.8 (0.43 to 1.48), P = 0.472 | 0.847 |
| Treatment failure, n (%) | 110 (17.4%) | 104 (16.7%) | 0.94 (0.70 to 1.27), P = 0.704 | 0.676 |
| Length of hospital stay, mean (±SD) | 9.3 ± 13.9 | 8.4 ± 7.2 | ‐0.60 (‐1.84 to 0.64), P = 0.342 | 0.658 |
| Antibiotic‐related side effects, n (%) | 30 (10.9%) | 29 (10.5%) | 0.93 (0.53 to 1.63), P = 0.805 | 0.198 |
| Acute bronchitis | 287 | 257 | ||
| 30 days mortality, n (%) | 0 (0.0%) | 2 (0.8%) | NA | NA |
| Treatment failure, n (%) | 55 (19.2%) | 52 (20.2%) | 1.11 (0.72 to 1.70), P = 0.643 | 0.4 |
| Length of hospital stay, mean (±SD) | 2.6 ± 5.7 | 2.2 ± 4.7 | ‐0.21 (‐0.90 to 0.48), P = 0.556 | 0.97 |
| Antibiotic‐related side effects, n (%) | 54 (21.6%) | 39 (17.3%) | 0.77 (0.49 to 1.22), P = 0.263 | 0.657 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| 30 days mortality, n (%) | 29 (15.6%) | 23 (12.0%) | 0.75 (0.41 to 1.39), P = 0.366 | 0.644 |
| Treatment failure, n (%) | 51 (27.4%) | 44 (22.7%) | 0.78 (0.48 to 1.28), P = 0.332 | 0.522 |
| Length of ICU stay, mean (±SD) | 23.5 ± 20.5 | 21.8 ± 19.1 | ‐1.74 (‐5.64 to 2.17), P = 0.383 | 0.441 |
| Length of hospital stay, mean (±SD) | 33.8 ± 27.6 | 32.0 ± 23.1 | ‐2.14 (‐7.04 to 2.75), P = 0.391 | 0.448 |
Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals
ARI: acute respiratory infection
CI: confidence interval
COPD: chronic obstructive pulmonary disease
ICU: intensive care unit
NA: not applicable
OR: odds ratio
PCT: procalcitonin
SD: standard deviation
2. Setting‐specific treatment failure within 30 days of inclusion
Treatment failure was not significantly lower in PCT‐guided participants (23.0% versus 24.9%, adjusted OR 0.90, 95% CI 0.80 to 1.01, P = 0.068). These results were similar among subgroups by clinical setting and type of respiratory infection (P for interaction > 0.05). With an OR of 0.90 (95% CI 0.81 to 0.99), treatment failure was significantly lower in PCT group participants in an aggregate data meta‐analysis based on all 32 potentially eligible trials (thus relying on the original definition of treatment failure as used in the trials). There was no evidence of heterogeneity (I2 = 0%) (Figure 6). We also performed several predefined sensitivity analyses, which showed no evidence for interactions (see summary in Table 5).
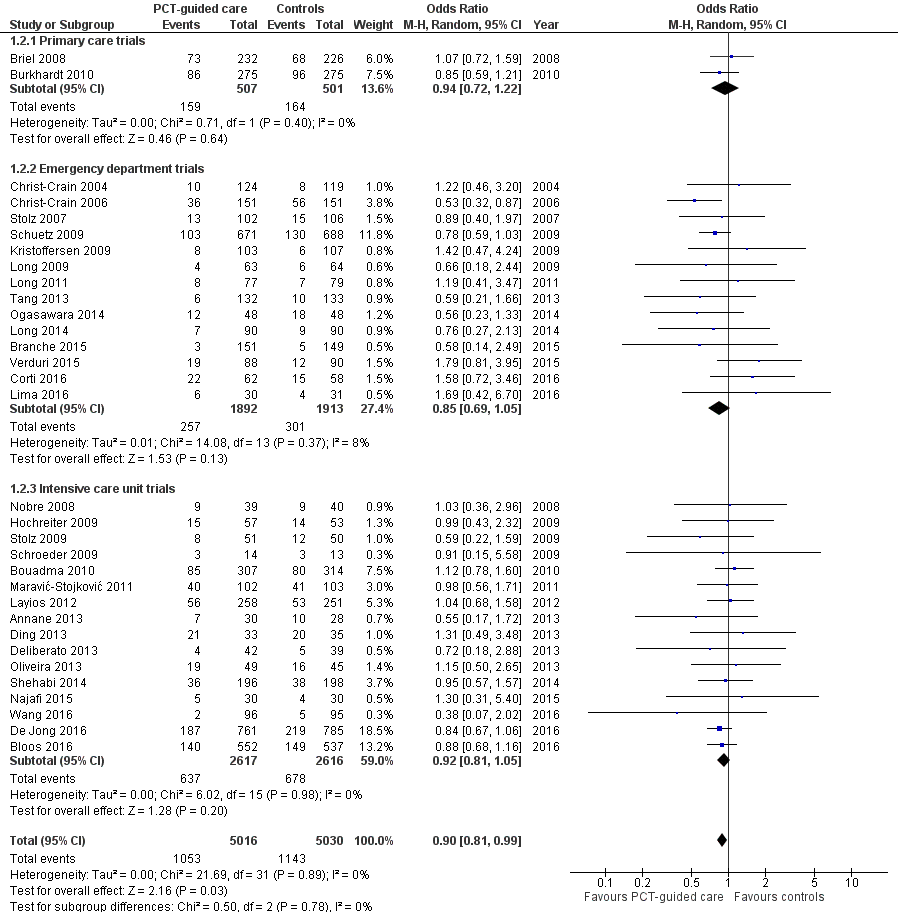
Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.2 Treatment failure at 30 days.
| Mortality | ||||
| Main analysis | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Adherence | ||||
| High adherence | 82 (4.5%) | 75 (4.1%) | 0.88 (0.63 to 1.22), P = 0.434 | 0.617 |
| Low adherence (< 70% or not reporting) | 254 (16.4%) | 211 (14.0%) | 0.83 (0.67 to 1.02), P = 0.073 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 305 (10.6%) | 250 (8.8%) | 0.80 (0.67 to 0.97), P = 0.021 | 0.229 |
| High risk for allocation concealment bias (or not reporting) | 31 (6.5%) | 36 (7.3%) | 1.12 (0.66 to 1.91), P = 0.672 | |
| Blinding | ||||
| Blinded outcome assessment | 113 (6.5%) | 102 (5.9%) | 0.85 (0.64 to 1.13), P = 0.259 | 0.537 |
| No blinded outcome assessment | 223 (13.8%) | 184 (11.5%) | 0.81 (0.65 to 1.01), P = 0.062 | |
| Follow‐up | ||||
| Follow up for mortality at 1 month | 275 (10.7%) | 224 (8.9%) | 0.81 (0.67 to 0.99), P = 0.039 | 0.325 |
| Follow up for mortality < 1 month | 61 (7.6%) | 62 (7.6%) | 0.94 (0.64 to 1.38), P = 0.756 | |
| Treatment failure | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Adherence | ||||
| High adherence | 419 (23.1%) | 381 (21.0%) | 0.89 (0.76 to 1.04), P = 0.148 | 0.752 |
| Low adherence (< 70% or not reporting) | 422 (27.1%) | 387 (25.5%) | 0.92 (0.77 to 1.08), P = 0.301 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 776 (26.8%) | 699 (24.6%) | 0.89 (0.79 to 1.01), P = 0.069 | 0.486 |
| High risk for allocation concealment bias (or not reporting) | 65 (13.6%) | 69 (13.8%) | 0.99 (0.68 to 1.44), P = 0.939 | |
| Blinding | ||||
| Blinded outcome assessment | 412 (23.5%) | 367 (21.1%) | 0.88 (0.75 to 1.03), P = 0.117 | 0.574 |
| No blinded outcome assessment | 429 (26.5%) | 401 (25.1%) | 0.94 (0.79 to 1.11), P = 0.438 | |
CI: confidence interval
NA: not applicable
OR: odds ratio
PCT: procalcitonin
Secondary outcomes
1. Antibiotic use (initiation of antibiotics, duration of antibiotics, and total exposure to antibiotics (total amount of antibiotic days divided by total number of participants))
Procalcitonin guidance was associated with a reduction in total antibiotic exposure (mean 8.1 days compared to 5.7 days, regression coefficient ‐2.43 days (95% CI ‐2.71 to ‐2.15), P < 0.001). Also, duration of antibiotic treatment in treated participants was shorter (mean 9.4 days compared to 8.0 days, adjusted coefficient ‐1.83 days (95% CI ‐2.15 to ‐1.5), P < 0.001) (Table 6).
| Parameter | Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction |
| Overall | 3372 | 3336 | ||
| Initiation of antibiotics, n (%) | 2894 (86.3%) | 2351 (71.5%) | 0.27 (0.24 to 0.32), P < 0.001 | |
| Duration of antibiotics (days), mean (±SD) | 9.4 ± 6.2 | 8.0 ± 6.5 | ‐1.83 (‐2.15 to ‐1.50), P < 0.001 | |
| Total exposure of antibiotics (days), mean (±SD) | 8.1 ± 6.6 | 5.7 ± 6.6 | ‐2.43 (‐2.71 to ‐2.15), P < 0.001 | |
| Setting‐specific outcomes | ||||
| Primary care | 501 | 507 | ||
| Initiation of antibiotics, n (%) | 316 (63.1%) | 116 (22.9%) | 0.13 (0.09 to 0.18), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.3 ± 2.5 | 7.0 ± 2.8 | ‐0.52 (‐1.07 to 0.04), P = 0.068 | 0.064 |
| Total exposure of antibiotics (days), mean (±SD) | 4.6 ± 4.1 | 1.6 ± 3.2 | ‐3.02 (‐3.45 to ‐2.58), P < 0.001 | 0.101 |
| Emergency department | 1638 | 1615 | ||
| Initiation of antibiotics, n (%) | 1354 (83.2%) | 1119 (71.3%) | 0.49 (0.41 to 0.58), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.8 ± 5.4 | 7.3 ± 5.1 | ‐2.45 (‐2.86 to ‐2.05), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 8.2 ± 6.2 | 5.2 ± 5.4 | ‐3.02 (‐3.41 to ‐2.62), P < 0.001 | < 0.001 |
| Intensive care unit | 1233 | 1214 | ||
| Initiation of antibiotics, n (%) | 1224 (99.8%) | 1116 (91.9%) | 0.02 (0.01 to 0.05), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.8 ± 7.8 | ‐1.23 (‐1.82 to ‐0.65), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.1 ± 7.9 | ‐1.44 (‐1.99 to ‐0.88), P < 0.001 | < 0.001 |
| Disease‐specific outcomes | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| Initiation of antibiotics, n (%) | 1455 (99.4%) | 1340 (92.9%) | 0.08 (0.04 to 0.15), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 10.5 ± 6.2 | 8.0 ± 5.7 | ‐2.45 (‐2.87 to ‐2.02), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 10.4 ± 6.2 | 7.5 ± 5.9 | ‐2.94 (‐3.38 to ‐2.50), P < 0.001 | 0.004 |
| Exacerbation of COPD | 631 | 621 | ||
| Initiation of antibiotics, n (%) | 453 (71.8%) | 266 (42.8%) | 0.29 (0.23 to 0.36), P < 0.001 | 0.017 |
| Duration of antibiotics (days), mean (±SD) | 7.4 ± 5.3 | 7.2 ± 6.7 | ‐1.15 (‐2.00 to ‐0.31), P = 0.007 | 0.003 |
| Total exposure of antibiotics (days), mean (±SD) | 5.3 ± 5.6 | 3.1 ± 5.6 | ‐2.22 (‐2.83 to ‐1.60), P < 0.001 | 0.506 |
| Acute bronchitis | 287 | 257 | ||
| Initiation of antibiotics, n (%) | 189 (65.9%) | 68 (26.5%) | 0.18 (0.12 to 0.26), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.1 ± 3.0 | 6.4 ± 3.5 | ‐0.35 (‐1.15 to 0.45), P = 0.393 | 0.359 |
| Total exposure of antibiotics (days), mean (±SD) | 4.7 ± 4.2 | 1.7 ± 3.3 | ‐2.95 (‐3.59 to ‐2.31), P < 0.001 | 0.33 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| Initiation of antibiotics, n (%) | 186 (100.0%) | 193 (99.5%) | NA | NA |
| Duration of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.22 (‐3.80 to ‐0.65), P = 0.006 | 0.253 |
| Total exposure of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.45 (‐4.09 to ‐0.82), P = 0.003 | 0.786 |
Note: Duration refers to the total days of antibiotic therapy in participants in whom antibiotics were initiated. Total exposure refers to the total days of antibiotic therapy in all randomised participants.
Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals
ARI: acute respiratory infection
CI: confidence interval
COPD: chronic obstructive pulmonary disease
NA: not applicable
OR: odds ratio
PCT: procalcitonin
SD: standard deviation
2. Length of hospital stay for hospitalised participants
However, the effect on antibiotic consumption differed according to clinical setting. In the primary care setting, lower antibiotic exposure was mainly due to lower initial prescription rates (P < 0.001 for interaction between primary care setting and PCT group on antibiotic prescriptions). Similarly, lower antibiotic exposure due to lower prescription rates was found in selected infections such as acute bronchitis (adjusted OR 0.18, 95% CI 0.12 to 0.26; P for interaction < 0.001). Lower antibiotic prescription rates (adjusted OR 0.49, 95% CI 0.41 to 0.58) and shorter duration of antibiotic therapy in participants with initiation of antibiotic (adjusted coefficient ‐2.45 days, 95% CI ‐2.86 to ‐2.05) contributed to the lower overall exposure in the emergency department setting.
Length of hospital stay and ICU stay were similar in both groups with no evidence for different effects in subgroups (P for interaction > 0.05).
3. Length of ICU stay for critically ill participants
For the ICU setting, the lower exposure was mainly explained by shorter treatment durations (adjusted difference in days ‐1.23, 95% CI ‐1.82 to ‐0.65). Similarly, for CAP, the lower exposure was mainly explained by shorter durations (adjusted difference in days ‐2.45, 95% CI ‐2.87 to ‐2.02).
4. Number of days with restricted activities within 14 days after randomisation for primary care participants
For studies conducted in the primary care setting, there was no difference in days with restricted activities of daily living between PCT and control group participants (days, 8.9 ± 4.2 versus 8.9 ± 4.1, regression coefficient 0.07 (95% CI ‐0.44 to 0.59), P = 0.777).
5. Antibiotic‐related side effects
There was also a significant reduction in antibiotic‐related side effects (16.3% versus 22.1%, adjusted OR 0.68, 95% CI 0.57 to 0.82, P < 0.001). This outcome was only assessed in some of the primary care and emergency department trials (n = 6), and not in ICU trials. There was no evidence for subgroup effects (P for interaction > 0.05).
Discussion
Summary of main results
This updated systematic review and meta‐analysis included 32 trials, of which 26 trials were used for the main individual participant data analysis. Trials were conducted in 12 countries and included different clinical settings and types of respiratory infections. There was mostly low risk for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting. There was unclear risk in all studies for blinding of personnel, and mostly high risk for blinding of outcome assessment. The results indicate a significant reduction in mortality (high‐quality evidence according to GRADE, summary of findings Table for the main comparison) and non‐significant result for treatment failure (moderate‐quality evidence according to GRADE, summary of findings Table for the main comparison) when PCT was used to guide initiation and duration of antibiotic treatment in ARI participants compared to control participants. Additionally, antibiotic consumption and side effects from antibiotics were significantly reduced across different clinical settings and types of ARIs. There was no effect on length of hospital stay and ICU length of stay. Results were similar in subgroup and sensitivity analyses including an aggregate data analysis with all 32 potentially eligible trials. Limitations include incomplete individual participant data, with four research groups not agreeing to the sharing of individual participant data; incomplete follow‐up information in some of the trials where no outcome assessment was done after 30 days of enrolment; differences in definitions of treatment failure among trials; and exclusion of some patient populations such as immunosuppressed people. Still, results from this updated individual participant data meta‐analysis support the use of PCT in the context of antibiotic stewardship in people with ARIs.
Overall completeness and applicability of evidence
The strengths of our review include an explicit study protocol, a comprehensive search to retrieve all relevant trials, access to individual participant‐level data from all but four of the included trials, and standardised outcome definitions across trials, thereby overcoming limitations of meta‐analyses using aggregated data. To minimise the risk of data‐driven associations, we prespecified a limited number of prognostic factors and subgroup variables for our statistical model. We allowed for potential clustering effects by using random‐effects models for included trials. Our results proved robust in sensitivity analyses focusing on high‐quality trials and on participants with complete follow‐up data.
The accuracy of PCT for diagnosing bacterial infections has been called into question by previous meta‐analyses of observational studies, which demonstrated mixed results (Jones 2007; Simmonds 2005; Tang 2007; Uzzan 2006). However, a more recent meta‐analysis using positive culture as the reference method found moderate to high discrimination of systematic inflammatory response syndrome and sepsis (Wacker 2013). Since there are no available gold standards for the diagnosis of the clinical conditions included in our analysis, most studies used clinical consensus criteria, which may differ among studies. Rather than relying on these imperfect diagnostic criteria, we were able to assess the value of PCT algorithms by means of RCTs measuring clinically relevant, participant‐level outcomes.
Despite these merits, this review has several limitations. We limited our analysis to adults with ARIs who were mostly immunocompetent, and excluded some pathogens (i.e. Legionella or Pseudomonas infections). The results of these trials may therefore not be generalised to people who are immunocompromised, with specific pathogens or infections other than ARIs, or children. Previous RCTs have shown that PCT guidance also reduces antibiotic exposure in a neonatal sepsis population but not in children with fever without a source (Manzano 2010). We found several ongoing RCTs in children evaluating PCT algorithms that should shed further light on the benefits and harms of PCT use for children. The included trials compared the PCT strategy to a control group where antibiotic therapy was guided based on 'usual practice' or based on current guideline recommendations. The magnitude of antibiotic reduction obviously correlates strongly with antibiotic prescription patterns, and in regions of low antibiotic prescription the PCT strategy may have smaller effects.
Quality of the evidence
Characteristics of the individual trials are presented in Table 1. Most trials had a follow‐up of one month, with two trials assessing outcome after 14 to 21 days and several trials following participants until hospital discharge (or ICU discharge) only. Procalcitonin algorithms used in the different trials were similar in concept and recommended initiation and/or continuation of antibiotic therapy based on similar PCT cut‐off levels (Table 1). However, there were differences: some trials in primary care and the emergency department used only a single PCT measurement on admission to guide initiation of antibiotics (Burkhardt 2010; Christ‐Crain 2004), while the other trials (predominantly in hospitalised participants with severe infections) used repeated measurements for guiding the duration of treatment. Adherence to algorithms was variable. In terms of methodological quality, trials had concealed allocation, but in several trials blinded outcome assessment was not done. All trials achieved complete or near‐complete follow‐up for mortality. None of the trials blinded participants or physicians to group allocation. The overall quality of the evidence according to GRADE was moderate to high (summary of findings Table for the main comparison).
Potential biases in the review process
Due to the differences in patient populations included in this analysis, which ranged from primary care to the ICU, we adapted the definition of treatment failure to clinical settings by including setting‐specific components in this composite outcome. This may challenge the clinical interpretation in the overall analysis.
Agreements and disagreements with other studies or reviews
While mortality did not differ significantly in our initial meta‐analysis (adjusted OR 0.94, 95% CI 0.71 to 1.23) (Schuetz 2012), we found a significantly lower mortality rate in PCT‐guided participants in this update. This result was robust in subgroup analyses and in our sensitivity analysis. Also, when considering all trials in the aggregate data analysis, mortality tended to be reduced, although not significantly (OR 0.89, 95% CI 0.78 to 1.01). Importantly, the largest‐yet ICU trial from the Netherlands has reported a significantly lower mortality in PCT‐guided participants (De Jong 2016).
Two of the included individual trials reported reduced length of stay, particularly within the ICU. Yet, despite a marked reduction in the duration of antibiotic therapy across trials and settings, there was no difference in length of ICU and hospital stay between the two groups in our comprehensive analysis. One might expect that clinically stable patients with discontinued intravenous antibiotics could be safely discharged unless there are extenuating circumstances. Perceived needs by physicians to further monitor these patients in the unit or inability to transfer patients to other inpatient or aftercare locations may partly explain this finding.
There is ongoing controversy about the diagnostic performance of PCT and other blood markers to correctly identify patients with a bacterial infection. In fact, several observational studies have questioned the added value of PCT in addition to clinical signs, such as a primary care study authored by van Vugt and colleagues reporting no additional benefit of PCT to a clinical assessment (van Vugt 2013). Importantly, in the context of respiratory infections, diagnostic studies are limited by the lack of a reference standard, with blood cultures only detecting a minority of cases (e.g. only 10% to 20% of patients with clinically and radiologically confirmed CAP have positive blood cultures) (Muller 2010; Wacker 2013). Interventional research, such as the trials included in the current analysis, do not rely on a reference standard but compare resource use (e.g. antibiotics) and clinical outcomes in people with and without use of the diagnostic marker. For the primary care setting, PCT had a very strong effect on antibiotic consumption (reduction of antibiotic exposure by 70%, from 4.6 to 1.6 days) without compromising disease resolution and patient safety. Of note, we were not able to assess the effect of PCT on mortality due to the very low risk situation with only one non‐survivor (control group) among the 1008 included participants.
The available evidence from RCTs, as summarised in this report, supports the use of PCT for de‐escalation of antibiotic therapy for people with ARIs. The same may not be true for escalation of antibiotic therapy when PCT levels increase as demonstrated in a recent large sepsis trial (Jensen 2011), where PCT‐guided escalation of diagnostic procedures and antimicrobial therapy in the ICU did not improve survival and led to organ‐related harm and prolonged ICU stays.

Study flow diagram.
Abbreviations: ARI: acute respiratory infection; IPD: individual participant data; RCT: randomised controlled trial

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.
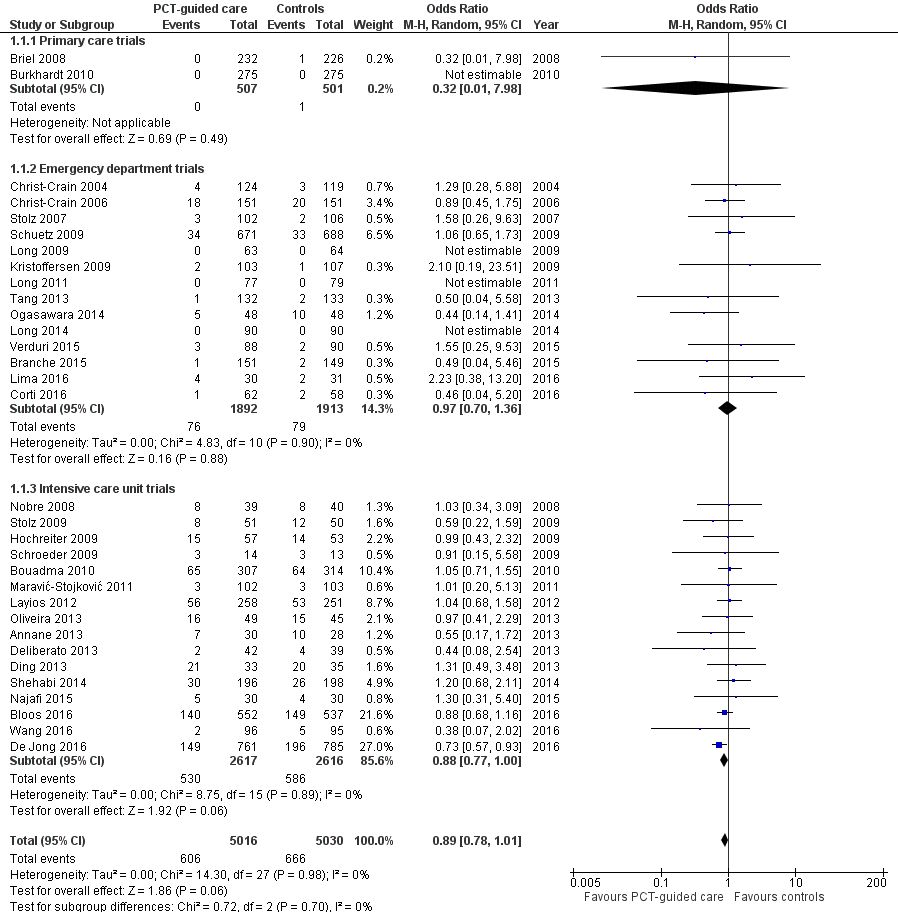
Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.

Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.2 Treatment failure at 30 days.

Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 1 Mortality at 30 days.

Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 2 Treatment failure at 30 days.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 1 Mortality at 30 days stratified by adherence.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 2 Treatment failure at 30 days stratified by adherence.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 3 Mortality at 30 days stratified by allocation concealment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 4 Treatment failure at 30 days stratified by allocation concealment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 5 Mortality at 30 days stratified by blinded outcome assessment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 6 Treatment failure at 30 days stratified by blinded outcome assessment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 7 Mortality at 30 days stratified by follow up.
| Procalcitonin algorithm compared to standard care for guiding antibiotic therapy in acute respiratory tract infections | ||||||
| Patient or population: people with acute respiratory tract infections | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | PCTalgorithm | |||||
| Mortality | Study population | OR 0.83 | 6708 | ⊕⊕⊕⊕ | ||
| 100 per 1000 | 86 per 1000 (76 to 95) | |||||
| Treatment failure | Study population | OR 0.90 | 6708 | ⊕⊕⊕⊝ | ||
| 249 per 1000 | 230 per 1000 (216 to 245) | |||||
| Antibiotic‐related side effects Follow‐up: 30 days | Study population 221 per 1000 | 163 per 1000 (145 to 182) | OR 0.68 | 3034 | ⊕⊕⊕⊝ | |
| Antibiotic exposure | The mean antibiotic exposure in the control groups was | The mean antibiotic exposure in the intervention groups was | ‐ | 6708 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No downgrading for serious concerns. Still, there is some concern about unconcealed allocation in several trials in the emergency department and intensive care settings. There is also some concern about low adherence with the PCT algorithm in the intervention group. We consider unblinded outcome assessment as not relevant for the outcome of death. | ||||||
| Study ID | Country | Setting, type of trial | Clinical diagnosis | Type of PCTalgorithm and PCTcut‐offs used (µg/L) | N: ARI participants (study total) | Primary endpoint | Follow‐up time | Reasons for exclusion of patients |
| France | ICU, multicentre | Severe sepsis without overt source of infection and negative blood culture | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 5.0) | 0 (62) | Participants on AB on day 5 post randomisation | Hospital stay | 62 non‐ARI patients (4 of them with post randomisation consent withdrawal) | |
| Germany | ICU, multicentre | Severe sepsis or septic shock | Discontinuation at day 4, 7, and 10; R against AB: < 1.0 or > 50% drop to previous value | 219 (1180) | 28‐day mortality | 3 months | 91 post randomisation exclusions (informed consent not obtainable), 870 not ARI patients | |
| France | ICU, multicentre | Suspected bacterial infections during ICU stay without prior AB (> 24 h) | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 394 (630) | All‐cause mortality | 2 months | 9 post randomisation exclusions (8 withdrew consent, 1 randomised twice); 227 non‐ARI patients | |
| USA | ED, medical ward, single centre | Lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 265 (300) | Antibiotic exposure and safety | 3 months | 35 non‐ARI patients | |
| Switzerland | Primary care, multicentre | Upper and lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 458 (458) | Days with restricted activities | 1 month | No exclusions | |
| Germany | Primary care, multicentre | Upper and lower ARI | Initiation; R against AB: < 0.25; R for AB: > 0.25 | 550 (571) | Days with restricted activities | 1 month | 21 post randomisation exclusions (2 withdrew consent, 1 due to loss of sample, 15 with autoimmune, inflammatory, or systemic disease, 2 with advanced liver disease, 1 with prior use of antibiotics) | |
| Switzerland | ED, single centre | Lower ARI with X‐ray confirmation | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 219 (243) | AB use | 2 weeks | 24 non‐ARI patients | |
| Switzerland | ED, medical ward, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 286 (302) | AB use | 6 weeks | 16 non‐ARI patients | |
| Denmark | ED, single centre | AECOPD | Initiation and duration; R against AB < 0.25 (0.15)/80% decrease; R for AB > 0.25 | 120 (120) | AB use | 28 days | No exclusions | |
| Netherlands | ICU, multicentre | Critically ill patients with presumed infection | Duration; R against AB: < 0.5 or > 80% drop | 994 (1575) | AB use | 1 year | 29 post randomisation exclusions (25 protocol violations, 4 withdrew informed consent), 552 non‐ARI patients | |
| Brazil | ICU, single centre | Septic patients with proven bacterial infection | Duration; R against AB: < 0.5 or > 90% drop | 66 (81) | AB use | ICU discharge or 14 days' post randomisation | 15 non‐ARI patients | |
| China | ICU, single centre | Acute exacerbation of pulmonary fibrosis | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 0 (78) | AB use | 1 month | 10 post randomisation exclusions (7 lost to follow‐up, 3 withdrew informed consent), 68 data not shared | |
| Germany | Surgical ICU, single centre | Suspected bacterial infections and > 1 SIRS criteria | Duration; R against AB: < 1 or > 65% drop over 3 d | 43 (110) | AB use | Hospital stay | 67 non‐ARI patients | |
| Denmark | ED, medical ward, multicentre | Lower ARI without X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 (> 0.5) | 210 (223) | AB use | Hospital stay | 13 post randomisation exclusions (3 no PCT testing, 6 not meeting inclusion criteria, 4 withdrew informed consent) | |
| Belgium | ICU, single centre | Suspected infection | Initiation; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 160 (509) | AB use | 1 month | 120 no PCT measurements, 10 missing data, 219 non‐ARI patients | |
| Brazil | ED, medical ward, single centre | Febrile neutropenia | Duration; R against AB: < 0.5 for 2 days or > 90% drop than highest measured concentration | 0 (62) | AB use | 28 days | 1 post randomisation exclusion (withdrew informed consent), 62 non‐ARI patients | |
| China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 127 (149) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent | |
| China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 156 (172) | AB use | 1 month | 16 post randomisation exclusions (6 lost to follow‐up, 7 withdrew consent, 3 with final diagnosis other than CAP) | |
| China | ED, single centre | Severe acute exacerbation of asthma | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 180 (180) | AB use | 1 year | No exclusions | |
| Serbia | ICU surgical, single centre | Infection after open heart surgery | Initiation; R for AB: > 0.5 | 5 (205) | AB use, AB cost | Hospital stay | 200 non‐ARI patients | |
| Iran | ICU, single centre | SIRS without apparent source of infection | Initiation; R for AB: > 2 | 0 (60) | AB use | Hospital stay | 60 patient data not shared | |
| Switzerland | ICU, single centre | Suspected severe sepsis or septic shock | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 52 (79) | AB use | 1 month | 27 non‐ARI patients | |
| Japan | Medical ward, single centre | Aspiration pneumonia | Predefined duration; AB for 3 d: < 0.5; AB for 5 d: 0.5 to 1.0; AB for 7 d: > 1 | 0 (105) | Relapse and 30‐day mortality | 1 month | 9 post randomisation exclusions (2 withdrew consent, 7 others), 96 data not shared | |
| Brazil | ICU, multicentre | Severe sepsis or septic shock | Discontinuation; initial < 1.0: R against AB: 0.1 at day 4; initial > 1.0: R against: > 90% drop | 58 (97) | AB use | 28 days or hospital discharge | 3 post randomisation exclusions (2 withdrew consent, 1 technical problems), 36 patients with a final diagnosis other than ARI | |
| Germany | Surgical ICU, single centre | Severe sepsis following abdominal surgery | Duration; R against AB: < 1 or > 65% drop over 3 d | 8 (27) | AB use | Hospital stay | 19 non‐ARI patients | |
| Switzerland | ED, medical ward, multicentre | Lower ARI with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 1304 (1381) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent, 55 non‐ARI patients | |
| Australia | ICU, multicentre | Suspected sepsis, undifferentiated infections | Duration; R against AB: < 0.25 (< 0.1) or > 90% drop | 156 (400) | AB use | 3 months | 6 post randomisation exclusions (6 withdrew consent), 238 non‐ARI patients | |
| Switzerland | ED, medical ward, single centre | Exacerbated COPD | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 208 (226) | AB use | 2 to 3 weeks | 18 post randomisation exclusions (absence of COPD) | |
| Switzerland, USA | ICU, multicentre | VAP when intubated > 48 h | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 101 (101) | AB‐free days alive | 1 month | No exclusions | |
| China | ED, single centre | Exacerbation of asthma | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 0 (265) | AB use | 6 weeks | 10 post randomisation exclusions (5 lost to follow‐up, 3 died, 2 withdrew consent), 255 data not shared | |
| Italy | ED, medical ward, multicentre | AECOPD | Initiation; R against AB:< 0.1; R for AB: > 0.25 | 178 (183) | Number of exacerbations | 6 months | 5 post randomisation exclusions (5 lost to follow‐up because they did not meet the inclusion criteria) | |
| China | ICU, single centre | AECOPD | All participants had initial PCT < 0.1; AB group treated with AB for at least 3 days, control group no AB in the first 10 days | 191 (194) | Treatment success within 10 days | 30 days | 3 post randomisation exclusions (3 with pneumonia according to CT scan) | |
| AB: antibiotic | ||||||||
| Parameter | Control (n = 3372) | PCT group (n = 3336) |
| Demographics | ||
| Age (year), mean (SD) | 61.2 ± 18.4 | 60.7 ± 18.8 |
| Male gender, n (%) | 1910 (56.6%) | 1898 (56.9%) |
| Clinical setting, no (%) | ||
| Primary care | 501 (14.9%) | 507 (15.2%) |
| Emergency department | 1638 (48.6%) | 1615 (48.4%) |
| Intensive care unit | 1233 (36.6%) | 1214 (36.4%) |
| Primary diagnosis | ||
| Total upper ARI, n (%) | 280 (8.3%) | 292 (8.8%) |
| Common cold | 156 (4.6%) | 149 (4.5%) |
| Rhino‐sinusitis, otitis | 67 (2.0%) | 73 (2.2%) |
| Pharyngitis, tonsillitis | 46 (1.4%) | 61 (1.8%) |
| Total lower ARI, n (%) | 3092 (91.7%) | 3044 (91.2%) |
| Community‐acquired pneumonia | 1468 (43.5%) | 1442 (43.2%) |
| Hospital‐acquired pneumonia | 262 (7.8%) | 243 (7.3%) |
| Ventilator‐associated pneumonia | 186 (5.5%) | 194 (5.8%) |
| Acute bronchitis | 287 (8.5%) | 257 (7.7%) |
| Exacerbation of COPD | 631 (18.7%) | 621 (18.6%) |
| Exacerbation of asthma | 127 (3.8%) | 143 (4.3%) |
| Other lower ARI | 131 (3.9%) | 144 (4.3%) |
| Procalcitonin upon enrolment | ||
| PCT< 0.1 ug/L | 921 (35.6%) | 981 (30.9%) |
| PCT 0.1 to 0.25 ug/L | 521 (20.1%) | 608 (19.2%) |
| PCT > 0.25 to 0.5 ug/L | 308 (11.9%) | 383 (12.1%) |
| PCT > 0.5 to 2.0 ug/L | 358 (13.8%) | 520 (16.4%) |
| PCT > 2.0 ug/L | 482 (18.6%) | 679 (21.4%) |
| ARI: acute respiratory infection | ||
| Study ID | Allocation concealment | Blinded | Follow‐up for mortality | Adherence to PCT algorithm in PCT group | Follow‐up for mortality |
| Yes (central randomisation) | No | 58/58 (100%) | 63% adherence | LOS | |
| Yes (central randomisation) | No | 1045/1089 (96%) | 49.6% adherence | 28 days and 90 days | |
| Yes (central randomisation) | Yes | 393/394 (100%) | 47% adherence | 28 days and 60 days | |
| Yes (central randomisation using blocks of 4) | No | 250/300 (83.3%) | 64% adherence | 1 month and 3 months | |
| Yes (central randomisation) | Yes | 454/458 (99%) | 85% adherence | 28 days | |
| Yes (central randomisation) | Yes | 546/550 (99%) | 87% adherence | 28 days | |
| No (alternating weeks) | No | 230/243 (95%) | 83% adherence | 10 to 14 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 300/302 (99%) | 87% adherence | 56 days | |
| Yes (randomisation algorithm was concealed to treating clinicians and participants) | No | 120/120 (100%) | 61.1% adherence | 28 days | |
| Yes (central randomisation) | No | 1546/1546 (100%) | 44% adherence | 28 days and 1 year | |
| Yes (opaque, sealed envelopes) | No | 81/81 (100%) | 52% adherence | LOS | |
| Yes (central randomisation) | No | 68/78 (87.2%) | Not reported | 30 days | |
| No (unconcealed drawing of lots) | No | 43/43 (100% until discharge) | Not reported | LOS | |
| Yes (central randomisation) | No | 210/210 (100% until discharge) | 59% adherence | LOS | |
| Not reported | No | 509/509 (100%) | Not reported | Intensive care unit LOS | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 61/62 (98.4%) | 73.3% adherence | 28 days and 90 days | |
| No (odd and even patient ID numbers) | No | 127/127 (100%) | Not reported | Not reported | |
| No (odd and even patient ID numbers) | No | 156/156 (100%) | Not reported | 28 days | |
| Yes (central randomisation) | No | 169/180 (93.9%) | 96.6% adherence | LOS and 1 year | |
| Yes (central randomisation) | No | 205/205 (100%) | Not reported | 30 days and LOS | |
| Yes (central randomisation) | No | 30/30 (100%) | Not reported | LOS | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 52/52 (100%) | 81% adherence | 28 days and LOS | |
| Not reported | No | 96/96 (100%) | Not reported | 30 days | |
| Yes (central randomisation) | No | 94/94 (100%) | 86.2% adherence | 28 days | |
| No (unconcealed drawing of lots) | No | 8/8 (100% until discharge) | Not reported | LOS | |
| Yes (central randomisation) | Yes | 1358/1359 (100%) | 91% adherence | 28 days | |
| Yes (central randomisation) | Yes | 394/394 (100%) | Not reported | LOS and 90 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 208/208 (100%) | Not reported | 6 months | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 101/101 (100%) | Not reported | 28 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 258/265 (97.4%) | Not reported | 6 weeks | |
| Yes (central randomisation) | No | 178/178 (100%) | Not reported | 6 months | |
| Yes (those responsible for allocation concealment were not involved in the measurement of results) | No | 191/191 (100%) | 82.3% adherence (17 participants in the control group received AB) | 30 days | |
| LOS: length of stay | |||||
| Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction | |
| Overall | 3372 | 3336 | ||
| 30 days mortality, n (%) | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Treatment failure, n (%) | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Length of ICU stay, mean (±SD) | 13.3 ± 16.0 | 13.7 ± 17.2 | 0.39 (‐0.81 to 1.58), P = 0.524 | NA |
| Length of hospital stay, mean (±SD) | 13.7 ± 20.6 | 13.4 ± 18.4 | ‐0.19 (‐0.96 to 0.58), P = 0.626 | NA |
| Antibiotic‐related side effects, n (%) | 336 (22.1%) | 247 (16.3%) | 0.68 (0.57 to 0.82), P < 0.001 | NA |
| According to setting | ||||
| Primary care | 501 | 507 | ||
| 30 days mortality, n (%) | 1 (0.2%) | 0 (0.0%) | NA | NA |
| Treatment failure, n (%) | 164 (32.7%) | 159 (31.4%) | 0.96 (0.73 to 1.25), P = 0.751 | 0.715 |
| Days with restricted activities, mean (±SD) | 8.9 ± 4.2 | 8.9 ± 4.1 | 0.07 (‐0.44 to 0.59), P = 0.777 | NA |
| Antibiotic‐related side effects, n (%) | 128 (25.7%) | 102 (20.2%) | 0.65 (0.46 to 0.91), P = 0.012 | 0.596 |
| Emergency department | 1638 | 1615 | ||
| 30 days mortality, n (%) | 62 (3.8%) | 57 (3.5%) | 0.91 (0.63 to 1.33), P = 0.635 | 0.546 |
| Treatment failure, n (%) | 292 (17.8%) | 259 (16.0%) | 0.87 (0.72 to 1.05), P = 0.141 | 0.807 |
| Length of hospital stay, mean (±SD) | 8.2 ± 10.5 | 8.1 ± 7.5 | ‐0.14 (‐0.73 to 0.44), P = 0.631 | 0.684 |
| Antibiotic‐related side effects, n (%) | 208 (20.3%) | 145 (14.4%) | 0.66 (0.52 to 0.83), P = 0.001 | 0.596 |
| Intensive care unit | 1233 | 1214 | ||
| 30 days mortality, n (%) | 273 (22.3%) | 229 (19.0%) | 0.84 (0.69 to 1.02), P = 0.081 | 0.619 |
| Length of ICU stay, mean (±SD) | 14.8 ± 16.2 | 15.3 ± 17.5 | 0.56 (‐0.82 to 1.93), P = 0.427 | 0.849 |
| Length of hospital stay, mean (±SD) | 26.3 ± 26.9 | 25.8 ± 23.9 | ‐0.33 (‐2.28 to 1.62), P = 0.739 | 0.641 |
| According to diagnosis | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| 30 days mortality, n (%) | 206 (14.1%) | 175 (12.2%) | 0.82 (0.66 to 1.03), P = 0.083 | 0.958 |
| Treatment failure, n (%) | 385 (26.2%) | 317 (22.0%) | 0.78 (0.66 to 0.93), P = 0.005 | 0.052 |
| Length of ICU stay, mean (±SD) | 10.5 ± 10.3 | 11.9 ± 13.3 | 1.45 (0.15 to 2.75), P = 0.029 | 0.119 |
| Length of hospital stay, mean (±SD) | 13.3 ± 15.7 | 13.9 ± 16.1 | 0.74 (‐0.25 to 1.73), P = 0.143 | 0.094 |
| Antibiotic‐related side effects, n (%) | 186 (27.7%) | 127 (19.1%) | 0.62 (0.48 to 0.80), P < 0.001 | 0.227 |
| Exacerbation of COPD | 631 | 621 | ||
| 30 days mortality, n (%) | 24 (3.8%) | 19 (3.1%) | 0.8 (0.43 to 1.48), P = 0.472 | 0.847 |
| Treatment failure, n (%) | 110 (17.4%) | 104 (16.7%) | 0.94 (0.70 to 1.27), P = 0.704 | 0.676 |
| Length of hospital stay, mean (±SD) | 9.3 ± 13.9 | 8.4 ± 7.2 | ‐0.60 (‐1.84 to 0.64), P = 0.342 | 0.658 |
| Antibiotic‐related side effects, n (%) | 30 (10.9%) | 29 (10.5%) | 0.93 (0.53 to 1.63), P = 0.805 | 0.198 |
| Acute bronchitis | 287 | 257 | ||
| 30 days mortality, n (%) | 0 (0.0%) | 2 (0.8%) | NA | NA |
| Treatment failure, n (%) | 55 (19.2%) | 52 (20.2%) | 1.11 (0.72 to 1.70), P = 0.643 | 0.4 |
| Length of hospital stay, mean (±SD) | 2.6 ± 5.7 | 2.2 ± 4.7 | ‐0.21 (‐0.90 to 0.48), P = 0.556 | 0.97 |
| Antibiotic‐related side effects, n (%) | 54 (21.6%) | 39 (17.3%) | 0.77 (0.49 to 1.22), P = 0.263 | 0.657 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| 30 days mortality, n (%) | 29 (15.6%) | 23 (12.0%) | 0.75 (0.41 to 1.39), P = 0.366 | 0.644 |
| Treatment failure, n (%) | 51 (27.4%) | 44 (22.7%) | 0.78 (0.48 to 1.28), P = 0.332 | 0.522 |
| Length of ICU stay, mean (±SD) | 23.5 ± 20.5 | 21.8 ± 19.1 | ‐1.74 (‐5.64 to 2.17), P = 0.383 | 0.441 |
| Length of hospital stay, mean (±SD) | 33.8 ± 27.6 | 32.0 ± 23.1 | ‐2.14 (‐7.04 to 2.75), P = 0.391 | 0.448 |
| Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals ARI: acute respiratory infection | ||||
| Mortality | ||||
| Main analysis | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Adherence | ||||
| High adherence | 82 (4.5%) | 75 (4.1%) | 0.88 (0.63 to 1.22), P = 0.434 | 0.617 |
| Low adherence (< 70% or not reporting) | 254 (16.4%) | 211 (14.0%) | 0.83 (0.67 to 1.02), P = 0.073 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 305 (10.6%) | 250 (8.8%) | 0.80 (0.67 to 0.97), P = 0.021 | 0.229 |
| High risk for allocation concealment bias (or not reporting) | 31 (6.5%) | 36 (7.3%) | 1.12 (0.66 to 1.91), P = 0.672 | |
| Blinding | ||||
| Blinded outcome assessment | 113 (6.5%) | 102 (5.9%) | 0.85 (0.64 to 1.13), P = 0.259 | 0.537 |
| No blinded outcome assessment | 223 (13.8%) | 184 (11.5%) | 0.81 (0.65 to 1.01), P = 0.062 | |
| Follow‐up | ||||
| Follow up for mortality at 1 month | 275 (10.7%) | 224 (8.9%) | 0.81 (0.67 to 0.99), P = 0.039 | 0.325 |
| Follow up for mortality < 1 month | 61 (7.6%) | 62 (7.6%) | 0.94 (0.64 to 1.38), P = 0.756 | |
| Treatment failure | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Adherence | ||||
| High adherence | 419 (23.1%) | 381 (21.0%) | 0.89 (0.76 to 1.04), P = 0.148 | 0.752 |
| Low adherence (< 70% or not reporting) | 422 (27.1%) | 387 (25.5%) | 0.92 (0.77 to 1.08), P = 0.301 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 776 (26.8%) | 699 (24.6%) | 0.89 (0.79 to 1.01), P = 0.069 | 0.486 |
| High risk for allocation concealment bias (or not reporting) | 65 (13.6%) | 69 (13.8%) | 0.99 (0.68 to 1.44), P = 0.939 | |
| Blinding | ||||
| Blinded outcome assessment | 412 (23.5%) | 367 (21.1%) | 0.88 (0.75 to 1.03), P = 0.117 | 0.574 |
| No blinded outcome assessment | 429 (26.5%) | 401 (25.1%) | 0.94 (0.79 to 1.11), P = 0.438 | |
| CI: confidence interval | ||||
| Parameter | Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction |
| Overall | 3372 | 3336 | ||
| Initiation of antibiotics, n (%) | 2894 (86.3%) | 2351 (71.5%) | 0.27 (0.24 to 0.32), P < 0.001 | |
| Duration of antibiotics (days), mean (±SD) | 9.4 ± 6.2 | 8.0 ± 6.5 | ‐1.83 (‐2.15 to ‐1.50), P < 0.001 | |
| Total exposure of antibiotics (days), mean (±SD) | 8.1 ± 6.6 | 5.7 ± 6.6 | ‐2.43 (‐2.71 to ‐2.15), P < 0.001 | |
| Setting‐specific outcomes | ||||
| Primary care | 501 | 507 | ||
| Initiation of antibiotics, n (%) | 316 (63.1%) | 116 (22.9%) | 0.13 (0.09 to 0.18), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.3 ± 2.5 | 7.0 ± 2.8 | ‐0.52 (‐1.07 to 0.04), P = 0.068 | 0.064 |
| Total exposure of antibiotics (days), mean (±SD) | 4.6 ± 4.1 | 1.6 ± 3.2 | ‐3.02 (‐3.45 to ‐2.58), P < 0.001 | 0.101 |
| Emergency department | 1638 | 1615 | ||
| Initiation of antibiotics, n (%) | 1354 (83.2%) | 1119 (71.3%) | 0.49 (0.41 to 0.58), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.8 ± 5.4 | 7.3 ± 5.1 | ‐2.45 (‐2.86 to ‐2.05), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 8.2 ± 6.2 | 5.2 ± 5.4 | ‐3.02 (‐3.41 to ‐2.62), P < 0.001 | < 0.001 |
| Intensive care unit | 1233 | 1214 | ||
| Initiation of antibiotics, n (%) | 1224 (99.8%) | 1116 (91.9%) | 0.02 (0.01 to 0.05), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.8 ± 7.8 | ‐1.23 (‐1.82 to ‐0.65), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.1 ± 7.9 | ‐1.44 (‐1.99 to ‐0.88), P < 0.001 | < 0.001 |
| Disease‐specific outcomes | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| Initiation of antibiotics, n (%) | 1455 (99.4%) | 1340 (92.9%) | 0.08 (0.04 to 0.15), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 10.5 ± 6.2 | 8.0 ± 5.7 | ‐2.45 (‐2.87 to ‐2.02), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 10.4 ± 6.2 | 7.5 ± 5.9 | ‐2.94 (‐3.38 to ‐2.50), P < 0.001 | 0.004 |
| Exacerbation of COPD | 631 | 621 | ||
| Initiation of antibiotics, n (%) | 453 (71.8%) | 266 (42.8%) | 0.29 (0.23 to 0.36), P < 0.001 | 0.017 |
| Duration of antibiotics (days), mean (±SD) | 7.4 ± 5.3 | 7.2 ± 6.7 | ‐1.15 (‐2.00 to ‐0.31), P = 0.007 | 0.003 |
| Total exposure of antibiotics (days), mean (±SD) | 5.3 ± 5.6 | 3.1 ± 5.6 | ‐2.22 (‐2.83 to ‐1.60), P < 0.001 | 0.506 |
| Acute bronchitis | 287 | 257 | ||
| Initiation of antibiotics, n (%) | 189 (65.9%) | 68 (26.5%) | 0.18 (0.12 to 0.26), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.1 ± 3.0 | 6.4 ± 3.5 | ‐0.35 (‐1.15 to 0.45), P = 0.393 | 0.359 |
| Total exposure of antibiotics (days), mean (±SD) | 4.7 ± 4.2 | 1.7 ± 3.3 | ‐2.95 (‐3.59 to ‐2.31), P < 0.001 | 0.33 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| Initiation of antibiotics, n (%) | 186 (100.0%) | 193 (99.5%) | NA | NA |
| Duration of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.22 (‐3.80 to ‐0.65), P = 0.006 | 0.253 |
| Total exposure of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.45 (‐4.09 to ‐0.82), P = 0.003 | 0.786 |
| Note: Duration refers to the total days of antibiotic therapy in participants in whom antibiotics were initiated. Total exposure refers to the total days of antibiotic therapy in all randomised participants. Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals ARI: acute respiratory infection | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 30 days Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.98] |
| 1.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.36] |
| 1.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 1.00] |
| 2 Treatment failure at 30 days Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 2.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 2.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 2.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 30 days stratified by adherence Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.81, 1.37] |
| 1.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.97] |
| 2 Treatment failure at 30 days stratified by adherence Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 2.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.02] |
| 2.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 3 Mortality at 30 days stratified by allocation concealment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 3.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.01] |
| 3.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 4 Treatment failure at 30 days stratified by allocation concealment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 4.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| 4.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.64, 1.04] |
| 5 Mortality at 30 days stratified by blinded outcome assessment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| 5.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.32] |
| 5.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.95] |
| 6 Treatment failure at 30 days stratified by blinded outcome assessment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 6.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.06] |
| 6.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 7 Mortality at 30 days stratified by follow up Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| 7.1 Trials with 1 month follow up for mortality | 18 | 7337 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 7.2 Trials with different follow up for mortality | 14 | 2709 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.30] |

