Deferasirox para el tratamiento de la sobrecarga de hierro en pacientes con síndrome mielodisplásico
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007461.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 octubre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hematología
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Joerg Meerpohl: conceived, designed and coordinated the review. He performed data collection and data management, as well as analysis and interpretation of the data. He wrote the review and approved the final version.
Lisa Schell: performed data collection and data management (search update). She conducted the literature searches in 2014, was involved with writing the review update and approval of the final version.
Gerta Ruecker: provided statistical advice and methodological support. She gave general advice on the review and approved the final version.
Nigel Fleeman: was co‐author of the HTA report by McLeod (McLeod 2009), gave general advice on the review and approved the final version.
Edith Motschall: gave advice on the search strategy and conducted the literature searches in 2010.
Charlotte Niemeyer: interpreted the data, provided clinical expertise and general advice on the review and approved the final version.
Dirk Bassler: performed data collection and data management. He analysed and interpreted the data, was involved with writing the review and approved the final version.
Sources of support
Internal sources
-
German Cochrane Centre, Freiburg, Germany.
External sources
-
No sources of support supplied
Declarations of interest
Joerg Meerpohl enrolled two adolescents with thalassaemia and one with Diamond‐Blackfan anaemia in a post‐marketing surveillance study on deferasirox and participated once in a Novartis advisory board meeting on paediatric iron overload over five years ago. The other review authors have no known conflicts of interest.
Acknowledgements
We thank the peer reviewers for their valuable comments which helped us to improve the protocol and review. We also thank the editorial team, especially Nicole Skoetz, for their support in preparing the protocol and this review. Christina Reese helped with the literature searches and retrieval of full articles. Claire McLeod gave valuable input at the protocol stage.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Oct 28 | Deferasirox for managing iron overload in people with myelodysplastic syndrome | Review | Joerg J Meerpohl, Lisa K Schell, Gerta Rücker, Nigel Fleeman, Edith Motschall, Charlotte M Niemeyer, Dirk Bassler | |
| 2010 Nov 10 | Deferasirox for managing iron overload in people with myelodysplastic syndrome | Review | Joerg J Meerpohl, Gerd Antes, Gerta Rücker, Nigel Fleeman, Edith Motschall, Charlotte M Niemeyer, Dirk Bassler | |
| 2008 Oct 08 | Deferasirox for managing iron overload in patients with myelodysplastic syndrome | Protocol | Joerg J Meerpohl, Gerd Antes, Gerta Rücker, Claire McLeod, Nigel Fleeman, Charlotte Niemeyer, Dirk Bassler | |
Differences between protocol and review
There are no deviations from the protocol first published in The Cochrane Library (Meerpohl 2008).
Methods not implemented
Since we did not find any trials that met our inclusion criteria, we did not implement the following aspects of the protocol.
Data extraction and management
Aside from details relating to the quality of the included studies, we planned to extract two groups of data:
-
Study characteristics ‐ place of publication, date of publication, population characteristics, setting, detailed nature of intervention, detailed nature of comparator and detailed nature of outcomes. A key purpose of these data is to define unexpected clinical heterogeneity in included studies independently from analysis of results.
-
Results of included studies with respect to each of the main outcomes indicated in the review question. We intended to carefully record reasons why an included study does not contribute data on a particular outcome and to consider the possibility of selective reporting of results on particular outcomes.
We planned to undertake data extraction by authors independently (JM, LS), using a data extraction form developed by the review authors. We planned to resolve disagreements by consensus. Missing data was requested from the original investigators. Either JM or LS were to transcribe data from the data extraction form into Review Manager 2014 and DB would verify all data entry for discrepancies.
Assessment of risk of bias in included studies
We did not perform an assessment of risk of bias because of insufficient data. We planned that JM and LS would assess every trial independently using a simple form following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We would have assessed the following domains as either 'Yes' (i.e. low risk of bias), 'Unclear' (i.e. uncertain risk of bias) or 'No' (i.e. high risk of bias):
-
Sequence generation
-
Allocation concealment
-
Blinding (of participants, personnel and outcome assessors)
-
Incomplete outcome data
-
Selective outcome reporting
-
Other sources of bias
We planned to discuss any inconsistencies between the review authors in the interpretation of risk of bias and their significance to the selected trials and to resolve any differences with a third author (DB). No study was to be automatically excluded as a result of a rating of 'Unclear' or 'No'. We intended that we would present the evaluation of the risk of bias in included studies in tabular form in the 'Results' section of the review.
Measures of treatment effect
We planned to analyse extracted data using the most up‐to‐date version of RevMan available at the time of analysis (Review Manager 2014).
We planned to extract hazard ratios with their 95% confidence intervals (CIs) for the time‐to‐event data mortality and end‐organ damage (see below). If hazard ratios were not given, we would have used indirect estimation methods described by Parmar 1998 and Williamson 2002 to calculate them.
If we were neither able to extract these data from the study reports nor able to receive the necessary information from the primary investigators, we would, as an alternative, have used the proportions of participants with the respective outcomes measured at three months, six months and then at six‐monthly intervals (i.e. 12 months, 18 months and so on) to be able to calculate relative risks. If outcome data were recorded at other time periods, then we would have given consideration to examining these as well.
We planned to express results for binary outcomes as relative risk (RR) with 95% CIs as measure of uncertainty. We planned to express continuous outcomes as mean difference (MD) with 95% CIs as measure of uncertainty.
Unit of analysis issues
When conducting a meta‐analysis combining results from cross‐over studies we planned to use the methods recommended by Elbourne 2002. For combining parallel and cross‐over trials, we would use methods described by Curtin (Curtin 2002a; Curtin 2002b; Curtin 2002c).
For some outcomes, a possible perception of the comparison might be whether deferasirox is equivalent to standard treatment with deferoxamine. Therefore, as secondary analysis, we planned to consider per‐protocol analysis, as is often used for equivalence studies, for our primary outcome as well as for the groups one to five of our secondary outcomes.
For time‐to‐event data, we would have considered a relative difference in hazard ratios of less than 10% equivalent. For relative risks, we would have defined non‐inferiority as a relative risk difference of less than 10% in treatment failures compared to standard therapy. For the continuous outcomes of "measures of iron overload and iron excretion" as well as "costs" we would also have considered a relative difference of 10% as equivalent. Resulting CIs would have been discussed with respect to methods suggested by Witte 2004.
In principle it is conceivable that the evidence found might be suitable for indirect comparisons or multiple‐treatment meta‐analysis. If so, we planned to apply these concepts according to methods discussed by Glenny 2005 and Salanti 2008, respectively, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), once they are supported by Review Manager 2014.
Dealing with missing data
We planned to follow the general recommendations for dealing with missing data in Cochrane Reviews (Higgins 2011b):
-
Whenever possible we planned to contact the original investigators to request missing data.
-
We would have clearly stated the assumptions of any methods used to cope with missing data (e.g. imputation of missing data and accounting for the fact that these were imputed with uncertainty).
-
We would have performed sensitivity analyses to assess how sensitive results are to reasonable changes in the assumptions that are made.
-
We would have addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We planned to assess statistical heterogeneity using the I2 statistic (Higgins 2002; Higgins 2003). This measure describes the percentage of total variation across studies that is caused by heterogeneity rather than by chance (Higgins 2003). The values of I2 lie between 0% and 100%. We planned to use a simplified categorisation of heterogeneity with the following categories: low (I2 less than 30%), moderate (I2 between 30% and 60%) and high (I2 more than 60%) (Deeks 2011).
In future review updates, if we detect moderate or high heterogeneity we intend to explore clinical heterogeneity by examining differences between groups as detailed below.
Assessment of reporting biases
If we include more than 10 trials, we will use funnel plots to graphically assess the likelihood of publication bias. We will take care in translating the results of included studies into recommendations for action by involving all review authors in drawing conclusions.
Data synthesis
We planned to conduct meta‐analyses of pooled data from all contributing trials using a fixed‐effect model for the primary analysis. Since no trials met our inclusion criteria, we did not perform meta‐analyses.
For future updates of the review we will use both a fixed‐effect and a random‐effects model and report results from both models.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for the different prognostic subtypes of MDS according to the IPSS (Greenberg 1997), as well as to the morphological MDS subtypes according to the WHO classification (Harris 1999; Vardiman 2002). We planned to assess clinical heterogeneity by examining differences due to baseline measures of iron overload, dose of intervention, concomitant use of growth factors (erythropoietin, G‐CSF) and age. In addition, we planned to assess heterogeneity regarding study characteristics as described in the paragraph on Data collection and analysis and Assessment of risk of bias in included studies.
Sensitivity analysis
We did not perform sensitivity analyses based on assessment of risk of bias and publication status (unpublished and published studies) as we could not include any trials in this review. However, for future updates we plan to investigate the robustness of our results through a sensitivity analysis on the basis of the methodological quality of the included trials by defining the following groups: low risk of bias (successful blinding of all patients, people involved in treatment and care and outcome assessors; adequate allocation concealment; and loss to follow‐up of less than 20%); high risk of bias (no blinding; inadequate allocation concealment; and loss to follow‐up of more than 20%); unclear risk of bias (rating of unclear risk of bias in at least one of these three categories).
'Summary of findings' table
We will develop a 'Summary of findings' table according to the GRADE methodology (Balshem 2011; Guyatt 2011). We plan to present information on the following outcomes: mortality; cardiac failure; endocrine disease; iron status (assessed by magnetic resonance imaging (MRI) or superconducting quantum interference device (SQUID)), if not available, ferritin; two most relevant or severe AEs; discontinuations.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
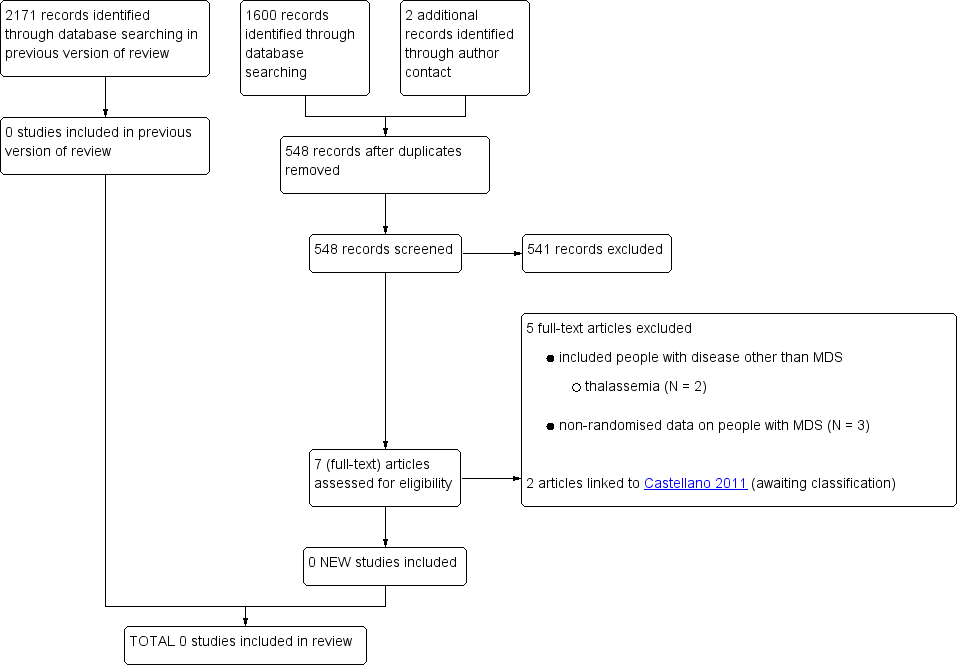
Study flow diagram (update search performed in April 2014),

