Mensajes por telefonía móvil para facilitar el autocuidado de las enfermedades crónicas
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT (3 arms, study duration 12 months) | |
| Participants | Paediatric patients (aged 8 to 18 years) with Type 1 Diabetes Mellitus receiving conventional insulin therapy attending a clinic in Tayside, Scotland. A total of 92 patients were randomised, of which 89 received their allocated interventions and data were analysed for 90 patients (Group 1 n = 27; Group 2 n = 32; Group 3 n = 31). | |
| Interventions | Participants were randomly assigned to one of three groups: 1) Conventional Insulin Therapy (CIT); 2) CIT with SweetTalk intervention; or 3) Intensive Insulin Therapy (IIT) with SweetTalk intervention. We excluded the third arm from this review. Sweet Talk is an automated, scheduled text‐messaging system designed to offer regular support to patients with diabetes to optimise their self‐management and diabetes control. Patients contract personal diabetes self‐management goals during the diabetes consultation and, based on these goals and patients' age, sex and diabetes regimen, SweetTalk schedules the automated delivery of a series of appropriately‐tailored text messages, including a weekly reminder of the goal set in clinic, and a daily message providing tips, information or reminders to reinforce this goal. In addition, patients receive occasional text newsletters regarding topical diabetes issues. | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcome measures were determined at baseline and at the end of the study (12 months). | |
| Notes | Mobile phones and ongoing technical support for the study were provided by Orange. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated allocation sequence was used to assign participants to one of three groups. |
| Allocation concealment (selection bias) | Low risk | Allocation is said to have been concealed. |
| Incomplete outcome data (attrition bias) | Unclear risk | At the end of the study (12 months) 4/27 patients were missing from Group 1 (3 discontinued therapy for clinical reasons, 1 withdrew), 6/33 missing from Group 2 (5 discontinued therapy for clinical reasons, 1 moved away), and 5/29 missing from Group 3 (5 discontinued therapy for clinical reasons). The number of patients who discontinued the intervention is comparable in all 3 groups and relatively small. Unlikely to influence results. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Low risk | Intervention and control groups were comparable at baseline; no other sources of bias were identified. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible due to nature of the intervention. Blinding of researchers was not discussed, but likely not done. Unlikely to influence outcome measures. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment. |
| Methods | RCT (2 arms, study duration 3 months) | |
| Participants | Diabetes patients (aged 12 to 25 yrs) on insulin treatment (n = 40). | |
| Interventions | The Computerized Automated Reminder Diabetes System (CARDS) includes a web‐based module and a messaging/reminder module designed to run autonomously. Participants log into the system via a secure website where they can customize their schedule for reminder messages, and view, edit, and print their blood glucose (BG) diaries. Participants can opt to receive two daily factoids: one related to diabetes education/nutrition and one with trivia. At a pre‐set time, CARDS sends a reminder to check the BG either by cell phone text message (intervention) or by email (control). After a user submits a BG value, regardless of the result, (s)he receives positive feedback. If the submitted BG value is out of range, CARDS provides a warning to take appropriate action according to the healthcare team's recommendations, and then recheck the BG. | |
| Outcomes | Primary outcomes: Number of BG results submitted. Secondary outcomes: HbA1c (%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomized to receive reminders either via cell phone text messaging or by e‐mail". No further information on the method or randomisation was presented. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment. |
| Incomplete outcome data (attrition bias) | Low risk | Data presented for all patients randomised. Presumably no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Low risk | There were no significant differences between the email (control) and cell phone (intervention) groups at baseline; no other sources of bias were identified. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible due to nature of the intervention. Blinding of researchers was not discussed, but likely not done. Unlikely to influence outcome measures. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment. |
| Methods | RCT (2 arms, study duration 24 weeks, cluster randomisation) | |
| Participants | Ambulatory hypertension (HT) patients (aged over 18 yrs) whose HT was not well uncontrolled with monotherapy, and who were eligible for treatment with a combination of a single‐dose angiotensin II antagonist and a diuretic (n=67). Excluded were patients: a) on treatment with 2 or more antihypertensive drugs; b) with secondary HT; c) with known contra‐indications for any of the antihypertensive drugs to be used; d) whose clinical condition might have interfered with the study; e) who were participating in other research studies; f) who lived with a person who was being treated with the same antihypertensive drug; or g) who were unable to give their informed consent. | |
| Interventions | Patients in the intervention group were subscribed to an SMS alerting system programmed to generate random messages. The aim of the messages was to provide information on HT, promote compliance, and good health and dietary habits, and remind patients to take their medication. Two messages were sent per week on randomly chosen weekdays during the 6‐month study period. Receipt of the messages was free to participants in the study and independent of their telephone service operator. | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Researchers were randomised to 1 of the 2 groups with a random number table. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment. |
| Incomplete outcome data (attrition bias) | Unclear risk | After 24 weeks data for 3/36 patients were missing from the control group and 2/36 missing from the intervention group due to lack of record of the number of tablets consumed. The reasons for loss to follow‐up are similar in both groups and unlikely to affect the results. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Low risk | Intervention and control groups were comparable at baseline; no other sources of bias were identified. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible due to nature of the intervention. Blinding of researchers was not discussed, but likely not done. Unlikely to influence outcome measures. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment. |
| Methods | RCT (2 arms, study duration 16 weeks) | |
| Participants | Patients with moderate persistent asthma for at least 6 months and being treated with inhaled corticosteroids and long acting beta agonist at a general hospital clinic in Zagreb, Croatia (n=16). | |
| Interventions | Patients in the intervention group were instructed to send their Peak Expiratory Flow (PEF) results daily via text message to a mobile telephone connected to a computer running the Asthma Center 0.90 Software. The software automatically computed maximal, minimal, and mean PEF, PEF variability, and compliance. Patients also received weekly instructions by text message from an asthma specialist on adjustments of therapy and recommended follow‐up based on the PEF values received by text message. Patients in both the intervention and control groups were treated according to GINA guidelines and kept paper asthma diaries. | |
| Outcomes | Pulmonary Function Test results (Forced Expiratory Volume in the first second (FEV1), PEF variability, Forced Vital Capacity); compliance with PEF measurements; asthma symptoms (cough, night symptoms, wheezing, limitation of activity); daily consumption of inhaled medicine and; cost to patient and provider. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by computer into either the SMS study group or the control group. Although it is not explicitly mentioned, this suggests use of a random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment. |
| Incomplete outcome data (attrition bias) | Low risk | No patient withdrew from the study after enrolment. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Unclear risk | Intervention and control groups comparable at baseline. However, the study "is limited by the small number of patients and by the particulars of the population studied. The follow‐up period may not have been sufficiently long to reveal all significant differences between the groups." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was not blinded, but this, we believe, has not influenced the outcome. First, compliance was not significantly different in the two groups. Second, the patients in both groups were managed by the same current guidelines." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No control group | |
| No control group | |
| Combines mobile phone and PDA‐based data transmission (not SMS) with SMS based feedback | |
| No control group; qualitative study | |
| Combines mobile phone based (WAP, GPRS and SMS) and Internet‐based data transmission with SMS‐based feedback | |
| Combines SMS‐based data transmission with regular mobile phone conversation | |
| Combines mobile phone‐based data transmission (not SMS) with tailored feedback to patients via SMS | |
| No control group | |
| No outcome measures reported after initiation of the study | |
| Combines SMS‐based data transmission with regular mobile phone conversation | |
| No control group | |
| Combines PC and mobile phone‐based data transmission with Internet and SMS‐based recommendations | |
| Combines SMS with Internet‐based data input | |
| No control group; qualitative study | |
| Combines SMS‐based support with use of open pedometers | |
| Combines SMS with GPRS data transmission | |
| Combines SMS‐based questionnaire with email alerts and personal follow‐up; No control group | |
| Combines PC or mobile phone‐based data transmission with SMS and Internet‐based feedback | |
| Combines mobile phone‐based data transmission (not SMS) with SMS‐based feedback | |
| No control group |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Improving childhood asthma management through a telemedicine monitoring network |
| Methods | RCT (study duration 6 months) |
| Participants | Participants (aged 3 to 16 yrs) with established doctor diagnosis of episodic or persistent asthma who have had at least one admission to hospital or one episode of acute care in an emergency department or paediatric clinic or general practitioner for asthma requiring steroid rescue within the previous 12 months. |
| Interventions | Asthma monitoring via mobile phone using SMS |
| Outcomes | Primary outcomes: Health resource utilisation Secondary outcomes: School days missed (children) and days off work (parents); Use of medications; Health related Quality of Life (QOL) |
| Starting date | September 2006 |
| Contact information | Jackson, M, Department of Respiratory Medicine Royal Children's Hospital, Herston Rd, Herston, Brisbane QLD, Australia. [email protected] |
| Notes | Recruiting at the time of this review. |
| Trial name or title | Using a text‐message system to engage depressed adolescents in cognitive‐behavioral therapy homework. |
| Methods | RCT (study duration 2 month) |
| Participants | Participants (aged 13 to 17 yrs) with major depressive disorder |
| Interventions | Homework will be standardised through a primary tool (DTR) for participants to evaluate and respond in writing to their automatic thoughts. The text‐messaging system allows homework to be submitted directly through a cellular phone, includes text‐messaged homework reminder prompts, and collates all homework for therapists to review with participants during therapy sessions. This is assigned and reviewed weekly for 4 weeks. |
| Outcomes | Primary outcomes: Therapy homework compliance (% homework completed) Secondary outcomes: Self reported depressive symptoms (Mood Feeling Questionnaire) |
| Starting date | February 2009 |
| Contact information | Liang, HC. University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States. [email protected] |
| Notes | Recruiting at the time of this review. |
| Trial name or title | Effect of daily Short Message System (SMS) reminders on medication adherence to oral antipsychotics in patients with schizophrenia. |
| Methods | RCT (study duration 6 months) |
| Participants | Stabilised out‐patients (aged over 18 yrs) with a diagnosis of schizophrenia (DSM‐IV TR criteria) and on oral antipsychotic mono‐therapy. |
| Interventions | Daily SMS medication reminders |
| Outcomes | Self‐reported adherence (Morisky Green Questionnaire); Disease awareness (Scale to Assess Unawareness of Mental Disorder (SUMD) Insight Questionnaire); Clinical Global Impression‐Schizophrenia scale score; EQ‐5D score; Attitude towards compliance (DAI‐10). |
| Starting date | April 2009 |
| Contact information | Maurino, J and Diez, T. AstraZeneca Pharmaceuticals, Spain. [email protected] |
| Notes | Recruiting at the time of this review. |
| Trial name or title | Assessment of the health‐related effects of compliance optimization in asthma through use of SMS (Short Message System) ‐ a controlled trial. |
| Methods | RCT (study duration 90 days) |
| Participants | Participants (aged 18 to 45 yrs) with asthma. 244 participants enrolled |
| Interventions | SMS compliance and monitoring system for optimised asthma treatment |
| Outcomes | Asthma control; EQ‐5D score; Use of health services; Use of preventive medicine |
| Starting date | November 2007 |
| Contact information | Claus M ldrup, Associate Professor PhD, University of Copenhagen. [email protected] |
| Notes | Study completed May 2008 |
| Trial name or title | Telemedicine Influence in the Follow up of the Type 2 Diabetes Patient |
| Methods | RCT (study duration 12 months) |
| Participants | Participants (aged over 30 yrs) with a diagnosis of type 2 diabetes and on Self‐Monitoring Blood Glucose (SMBG) at least 6 months before |
| Interventions | Participants could send SMBG values to a web page via SMS. The healthcare provider could access this web page to check and, if necessary, return recommendations by SMS |
| Outcomes | HbA1c level |
| Starting date | October 2003 |
| Contact information | Rodríguez‐Idígoras, MI. Málaga Health Department, Junta de Andalucia, Spain. [email protected] |
| Notes | Study completed June 2005. Authors contacted: publication in preparation at the time of this review |
| Trial name or title | Reinforcement of adherence to prescription recommendations in diabetic patients using Short Message Service (SMS) ‐ a pilot study |
| Methods | RCT (study duration 12 months) |
| Participants | Participants (aged 30 to 65 yrs) with type 2 diabetes for a minimum period of 5 years and receiving oral hypoglycaemic agents and/or insulin. |
| Interventions | SMS reminders (once per 3 days) regarding the need for adherence to lifestyle modification and medication. |
| Outcomes | At baseline and at the end of the study, lipids, and renal function test will be done. A validated questionnaire will be used to assess physical activity, diet habits, adherence to drug prescriptions and frequency of monitoring of blood glucose. Body weight, blood pressure, biochemical variables, scores for diet and physical activity and compliance to drugs, will be compared. |
| Starting date | August 2008 |
| Contact information | Shetty, SA. India Diabetes Research Foundation (IDRF) and Dr. A. Ramachandran's Diabetes Hospitals. [email protected]; [email protected]. |
| Notes | Recruiting at the time of this review. |
| Trial name or title | Short Message Service (SMS) impact on patient compliance receiving long‐term lipid lowering therapy with statins. |
| Methods | RCT (study duration 12 months) |
| Participants | Participants (aged 18 to 80 yrs) discharged from the Intensive Cardiac Care Unit or the Internal Medicine Department following acute coronary syndrome (ACS) events such as unstable angina or acute myocardial infarction who will be prescribed a statin for the first time for preventing further coronary episodes. 120 participants enrolled. |
| Interventions | Daily SMS medication reminders |
| Outcomes | Primary outcomes: Number of patients who achieve target LDL goals Secondary outcomes: Reductions of total cholesterol, LDL, LDL/HDL and CRP; Increase of HDL; Readmissions due to ACS |
| Starting date | August 2006 |
| Contact information | Shotan, A. Hillel Yaffe medical center. [email protected] |
| Notes | Ongoing at the time of this review. |
| Trial name or title | A non‐interventional naturalistic project to investigate the effect of the use of SMS text service on treatment adherence in patients treated with Seroquel. |
| Methods | Prospective case study |
| Participants | Participants with schizophrenia or participants experiencing a manic episode associated with a bipolar disorder who were being treated with Quetiapine according to the Core Data Sheet and who were on a stable dosing regime. 128 participants enrolled |
| Interventions | Daily SMS text messages to enhance patient adherence with medication |
| Outcomes | Unknown |
| Starting date | September 2005 |
| Contact information | van Schayk, NPJT, AstraZeneca, The Netherlands |
| Notes | Study completed April 2008 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Diabetes ‐ Glycaemic control (HbA1c) Show forest plot | 2 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.77, 0.47] |
| Analysis 1.1  Comparison 1 Health outcomes, Outcome 1 Diabetes ‐ Glycaemic control (HbA1c). | ||||
| 2 Health outcomes, other (dichotomous measures) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Health outcomes, Outcome 2 Health outcomes, other (dichotomous measures). | ||||
| 2.1 Diabetes ‐ Complications: Diabetic ketoacidosis (DKA) | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.10, 3.12] |
| 2.2 Diabetes ‐ Complications: Severe hypoglycaemia | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.78] |
| 2.3 Hypertension ‐ Blood pressure not under control (no of cases)) | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.41, 1.29] |
| 3 Health outcomes, other (continuous measures, health outcomes improve with declining mean) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Health outcomes, Outcome 3 Health outcomes, other (continuous measures, health outcomes improve with declining mean). | ||||
| 3.1 Diabetes ‐ Body weight (BMI SDS) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.35, 0.51] |
| 3.2 Hypertension ‐ Systolic blood pressure (mmHg) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐4.37, 6.57] |
| 3.3 Hypertension ‐ Diastolic blood pressure (mmHg) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.84 [‐2.14, 5.82] |
| 3.4 Hypertension ‐ Body weight (in kgs) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐2.76 [‐8.17, 2.65] |
| 3.5 Asthma ‐ PEF variability (%) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐11.12 [‐19.56, ‐2.68] |
| 3.6 Asthma ‐ Symptoms | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.56, ‐0.17] |
| 4 Health outcomes, other (continuous measures, health outcomes improve with increasing mean) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Health outcomes, Outcome 4 Health outcomes, other (continuous measures, health outcomes improve with increasing mean). | ||||
| 4.1 Asthma ‐ Pulmonary function test (FEV1) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐15.91, 21.91] |
| 4.2 Asthma ‐ Forced vital capacity (%) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐16.33, 13.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knowledge and management of diabetes Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Capacity to self‐manage the condition, Outcome 1 Knowledge and management of diabetes. | ||||
| 1.1 Self‐efficacy for diabetes (SED) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 6.10 [0.45, 11.75] |
| 1.2 Diabetes social support interview (DSSI) | 1 | 236 | Mean Difference (IV, Random, 95% CI) | 4.39 [2.85, 5.92] |
| 1.3 Diabetes knowledge scale (DKS) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.60, 0.60] |
| 2 Treatment compliance Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Capacity to self‐manage the condition, Outcome 2 Treatment compliance. | ||||
| 2.1 Hypertension ‐ Compliance with medication at six months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 8.90 [0.18, 17.62] |
| 2.2 Asthma ‐ Compliance with PEF measurement | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 4.90 [‐14.82, 24.62] |
| 2.3 Diabetes adherence (Visual analogue score) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐2.58, 16.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||
| 1 Diabetes ‐ Clinic visit Show forest plot | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.22, 0.82] | ||||||||||||||||
| Analysis 3.1  Comparison 3 Health service utilisation, Outcome 1 Diabetes ‐ Clinic visit. | ||||||||||||||||||||
| 2 Diabetes ‐ Hotline contact Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.09, 1.08] | ||||||||||||||||
| Analysis 3.2  Comparison 3 Health service utilisation, Outcome 2 Diabetes ‐ Hotline contact. | ||||||||||||||||||||
| 3 Asthma ‐ Utilisation Show forest plot | Other data | No numeric data | ||||||||||||||||||
| Analysis 3.3
Comparison 3 Health service utilisation, Outcome 3 Asthma ‐ Utilisation. | ||||||||||||||||||||
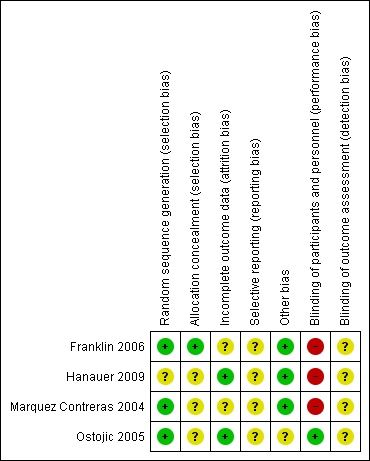
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Health outcomes, Outcome 1 Diabetes ‐ Glycaemic control (HbA1c).
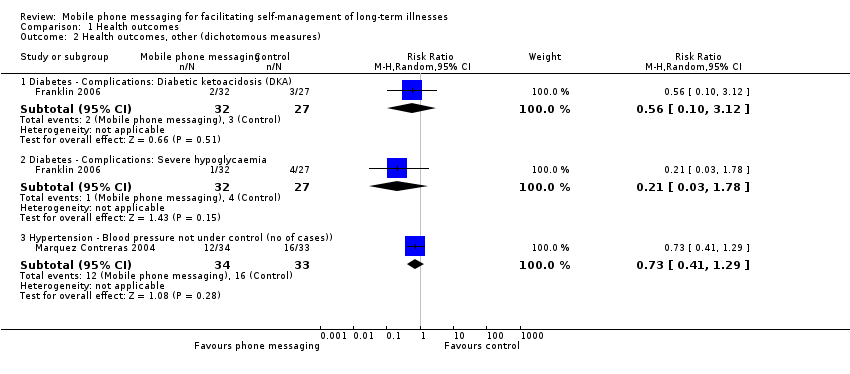
Comparison 1 Health outcomes, Outcome 2 Health outcomes, other (dichotomous measures).
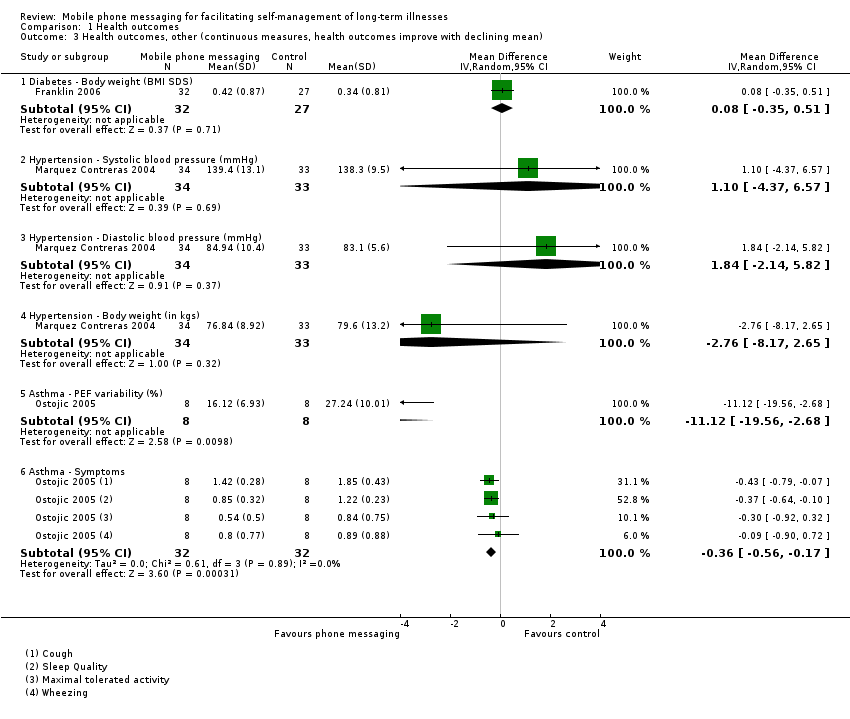
Comparison 1 Health outcomes, Outcome 3 Health outcomes, other (continuous measures, health outcomes improve with declining mean).

Comparison 1 Health outcomes, Outcome 4 Health outcomes, other (continuous measures, health outcomes improve with increasing mean).

Comparison 2 Capacity to self‐manage the condition, Outcome 1 Knowledge and management of diabetes.

Comparison 2 Capacity to self‐manage the condition, Outcome 2 Treatment compliance.

Comparison 3 Health service utilisation, Outcome 1 Diabetes ‐ Clinic visit.

Comparison 3 Health service utilisation, Outcome 2 Diabetes ‐ Hotline contact.
| Study | Outcome | Mobile phone (n=8) | Control (n=8) |
| Ostojic 2005 | Hospitalisations | 2 | 7 |
| Ostojic 2005 | Office visits | 21 | 15 |
Comparison 3 Health service utilisation, Outcome 3 Asthma ‐ Utilisation.
| Patient or population: Patients with long‐term illnesses Comparison: Usual care, or usual care with self‐management support delivered by email | |||
| Outcomes | Impact | No of Participants | Quality of the evidence |
| Health outcomes: Glycaemic control (HbA1c) | One study found no statistical difference on glycaemic control between groups receiving the intervention or usual care. The other study found mobile phone messaging no more effective than email reminders in achieving glycaemic control. Overall, mean pooled glycaemic control (HbA1C) for the control groups was 9.9 (SD 1.5). In the text messaging groups this was 0.15 units lower (0.77 lower to 0.47 higher). | 88 | ⊕⊕⊕⊝ |
| Health outcomes: Variety of measures | For diabetes and hypertension no statistically significant differences were found between the intervention and control groups on body mass index, weight or blood pressure. For asthma a significant improvement in the text messaging group was found for only 2 out of 4 outcome measures, that is peak expiratory flow variability and pooled symptom score. | 142 (3 studies) | ⊕⊕⊕⊝ |
| Capacity to self‐manage the condition: Management and knowledge of diabetes | Patients receiving text messaging support showed significantly improved scores on the Self‐Efficacy for Diabetes test and the Diabetes Social Support Interview. It did not, however, result in improved knowledge of diabetes. | 59 (1 study) | ⊕⊕⊕⊝
|
| Capacity to self‐manage the condition: Treatment compliance | Medication compliance in hypertension patients was 8.9% higher (0.18% higher to 17.62% higher) in the text messaging group as compared with the control group. There were no statistically significant effects on compliance with peak expiratory flow (PEF) measurement for asthma patients, or on self‐reported adherence in young people with diabetes. Text message prompts for diabetes patients initially also resulted in a higher number of blood glucose results (46.0) sent back than email prompts (23.5) did. | 142 (3 studies) | ⊕⊕⊕⊝ |
| Participants' evaluation of the intervention | Patients receiving mobile phone messaging support reported improvement in self‐management of diabetes, wanted to continue receiving messages, and preferred mobile phone messaging to email as a method to access the Computerised Automated Reminder Diabetes System. | 72 (2 studies) | ⊕⊝⊝⊝ |
| Health service utilisation | Diabetes patients receiving text messaging support made a comparable number of clinic visits and calls to an emergency hotline as patients without the support. For asthma patients, the total number of office visits was higher in the text messaging group, whereas the number of hospital admissions was higher for the control group. | 75 (2 studies) | ⊕⊝⊝⊝ |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1 Number of participants is low in both studies on diabetes. 2 All included trials have a low number of participants. 3 The number of participants is low in both included trials. The outcomes are not compared between the intervention and control groups. 4 Both included trials have a low number of participants. The reasons for clinic or clinic visits and hospitalisations were not known, so the causal link between the intervention and the outcome measures is not clear. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Diabetes ‐ Glycaemic control (HbA1c) Show forest plot | 2 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.77, 0.47] |
| 2 Health outcomes, other (dichotomous measures) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Diabetes ‐ Complications: Diabetic ketoacidosis (DKA) | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.10, 3.12] |
| 2.2 Diabetes ‐ Complications: Severe hypoglycaemia | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.78] |
| 2.3 Hypertension ‐ Blood pressure not under control (no of cases)) | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.41, 1.29] |
| 3 Health outcomes, other (continuous measures, health outcomes improve with declining mean) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Diabetes ‐ Body weight (BMI SDS) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.35, 0.51] |
| 3.2 Hypertension ‐ Systolic blood pressure (mmHg) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐4.37, 6.57] |
| 3.3 Hypertension ‐ Diastolic blood pressure (mmHg) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.84 [‐2.14, 5.82] |
| 3.4 Hypertension ‐ Body weight (in kgs) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐2.76 [‐8.17, 2.65] |
| 3.5 Asthma ‐ PEF variability (%) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐11.12 [‐19.56, ‐2.68] |
| 3.6 Asthma ‐ Symptoms | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.56, ‐0.17] |
| 4 Health outcomes, other (continuous measures, health outcomes improve with increasing mean) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Asthma ‐ Pulmonary function test (FEV1) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐15.91, 21.91] |
| 4.2 Asthma ‐ Forced vital capacity (%) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐16.33, 13.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knowledge and management of diabetes Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Self‐efficacy for diabetes (SED) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 6.10 [0.45, 11.75] |
| 1.2 Diabetes social support interview (DSSI) | 1 | 236 | Mean Difference (IV, Random, 95% CI) | 4.39 [2.85, 5.92] |
| 1.3 Diabetes knowledge scale (DKS) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.60, 0.60] |
| 2 Treatment compliance Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Hypertension ‐ Compliance with medication at six months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 8.90 [0.18, 17.62] |
| 2.2 Asthma ‐ Compliance with PEF measurement | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 4.90 [‐14.82, 24.62] |
| 2.3 Diabetes adherence (Visual analogue score) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐2.58, 16.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Diabetes ‐ Clinic visit Show forest plot | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.22, 0.82] |
| 2 Diabetes ‐ Hotline contact Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.09, 1.08] |
| 3 Asthma ‐ Utilisation Show forest plot | Other data | No numeric data | ||

