Terapias psicológicas para el tratamiento del dolor crónico (excluida la cefalea) en adultos
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT; 2 arms;assessed at pretreatment, 3 months follow‐up, 1 year follow‐up | |
| Participants | 3 month follow‐up n = 286 Start of treatment n = 293 Sex: 160 F, 133 M Mean age = 40.5 (SD 4.5) Source = patients referred for inpatient rehabilitation Diagnosis = chronic low back pain Mean years of pain = not given (minimum 6 months) | |
| Interventions | "progressive intervention of intensive physical training and psychosocial activation AKSELI" "control: less intensive physical training and passive physical therapies" | |
| Outcomes | Primary pain outcome: none Primary disability outcome: none Primary mood outcome: BDI Catastrophising outcome: none 1. lumbar flexion‐extension 2. lateral flexion 3. trunk rotation 4. hamstring tightness 5. number of sit‐ups 6. number of arch‐ups 7. static strength of back muscles 8. number of squats 9. Million index of pain and disability mean of 14 items rated 0 to 100 10. low back pain capacity 1 to 3 11. leisure activities physical intensity 0 to 10 12. number of visits to doctors (12‐month follow‐up) 13. number of physical therapy outpatient visits (12‐month follow‐up) 14. WHO occupational handicap 0 to 5 15. sick days 16. Beck Depression Inventory 17. Symptom Check List 18. Multidimensional Health Locus of Control 19. Social Adjustment Scale 20. Karolinska Scales of Personality | |
| Notes | Excluded from 2009 review for marginal psychological content; included in 2012 update No data Yates quality scale: total quality = 16/35, design quality = 13/26, treatment quality = 3/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “patients stratified according to age … and sex and randomly divided into intervention and control groups” |
| Allocation concealment (selection bias) | High risk | No information but post‐randomisation exclusion of patients “not fit” for intervention group |
| Incomplete outcome data (attrition bias) | High risk | Attrition implied not reported; no reporting of differences |
| Selective reporting (reporting bias) | High risk | Many outcomes not reported |
| Blinding of outcome assessment (detection bias) | High risk | Self report and examination by physiatrist and physiotherapist at baseline and follow‐up. No statement about blinding. |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 6 months | |
| Participants | End of treatment n = 42 Start of treatment n = 45 Sex: 12 F, 33 M Mean age = 39.9 (SD 8.9) Source = pain and rehabilitation clinic Diagnosis = chronic low back pain Mean years of pain = not given | |
| Interventions | "Psychology based programme: multicomponent CBT" "Standard inpatient rehabilitation" | |
| Outcomes | Primary pain outcome: MPQ PRI Primary disability outcome: WHYMPI pain interference Primary mood outcome: WHYMPI distress Catastrophising outcome: none 1. Primary aerobic impairment 2. Self efficacy 3. West Haven Yale Multidimensional Pain Inventory (WHYMPI) self control 4. West Haven Yale Multidimensional Pain Inventory (WHYMPI) pain interference 5. West Haven Yale Multidimensional Pain Inventory (WHYMPI) mood 6. Disability 7. Melzack Pain Questionnaire Pain Response Index (MPQ PRI) | |
| Notes | CBT versus TAU, post‐treatment and follow‐up: analyses 3.1, 3.2, 3,3, 4.1, 4.2, 4.3 Yates quality scale: total quality = 15/35, design quality = 11/26, treatment quality = 4/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Abstract: “Forty‐five low back pain patients were randomly assigned”; no details in Methods |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Inadequately reported |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 6 months | |
| Participants | End of treatment n = 76 Start of treatment n = 94 Sex: 57 F, 19 M Mean age = 49.3 (SD 9.7) Source = pain or rehabilitation clinic Diagnosis = chronic low back pain Mean years of pain = 10.8 | |
| Interventions | "CBT added to medical treatment" "Medical treatment" | |
| Outcomes | Primary pain outcome: NRS 0 to 10 pain Primary disability outcome: disability in physical function from Dusseldorf Disability Scale Primary mood outcome: none Catastrophising outcome: PRSS catastrophising 1. Pain Intensity Numerical Rating Scale (0 to 10) 2. Control over pain Numerical Rating Scale (0 to 10) 3. Days per week pain‐free 4. Days per week pain medication use 5. Use of cognitive strategies (self report) 6. Use of avoidance behaviour (self report) 7. Pleasant activities (self report) 8. Social support (self report) 9. Philosophical beliefs (self report) 10. Catastrophising (bespoke scale) 11. Active coping (bespoke scale) 12. Disability in social relationships from Dusseldorf Disability Scale 13. Disability in social roles from Dusseldorf Disability Scale 14. Disability in physical function from Dusseldorf Disability Scale 15. Disability in mental performance from Dusseldorf Disability Scale 16. Disability in physical performance from Dusseldorf Disability Scale | |
| Notes | CBT versus TAU, post‐treatment: analyses 3.1, 3.2 Yates quality scale: total quality = 18/35, design quality = 12/26, treatment quality = 6/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Through assignment of random numbers, patients were allocated to an experimental treatment or a control group.” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reported; 1 difference found between dropouts and completers |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; assessed at pretreatment and post‐treatment | |
| Participants | End of treatment n = 94 Start of treatment n = 143 Sex: 79 F, 64 M Mean age = 45.2 (SD 9.2) Source = referrals to Pain Management Service after medical treatment completed Diagnosis = chronic non‐cancer pain Mean years of pain = median 4.0 | |
| Interventions | "Graded exposure in vivo and outpatient multidisciplinary chronic pain management group program" "outpatient multidisciplinary chronic pain management group program" "Waiting list control" | |
| Outcomes | Primary pain outcome: pain VAS Primary disability outcome: Pain Disability Index Primary mood outcome: DASS depression Catastrophising outcome: none 1. Pain VAS 2. Tampa Scale for Kinesiophobia: fear of movement/re/injury 3. Pain Self‐Efficacy Questionnaire (PSEQ) 4. Pain Disability Index (PDI) 5. Depression, Anxiety & Stress Scale (DASS): depression and anxiety scores only 6. Activity level: performance over 2 weeks of 10 usually avoided activities 7. 6‐minute walk test | |
| Notes | Chronic pain management programme with graded exposure versus waiting list control December 2009 search Data obtained from author: analyses 3.1, 3.2, 3.3 Yates quality scale: total quality = 23/35, design quality = 15/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generation |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Attrition fully reported; no differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Examination by physiotherapist and self report: no blinding reported |
| Methods | RCT; 4 arms; assessed at pre‐treatment, post‐treatment, 3 months, 1 year, 2 years | |
| Participants | End of treatment n = 109 Start of treatment n = 119 Sex: 108 F, 11 M Mean age = 44 (SD 10) Source = mainly community Diagnosis = fibromyalgia Mean years of pain = 11.5 | |
| Interventions | "Biofeedback + relaxation + exercise" "Biofeedback + relaxation" "Exercise" "Education attentional control" | |
| Outcomes | Primary pain outcome: no data available Primary disability outcome: no data available Primary mood outcome: no data available Catastrophising outcome: no data available Arthritis Impact Measurement Scale: Physical Activity subscale (AIMS) Symptom Checklist (SCL‐90R) distress Center for Epidemiologic Studies Depression Scale (CES‐D) Arthritis Self‐Efficacy Scale Sleep rating 0 to 12 Tender Point Index Myalgic score Physician's VAS rating of disease severity Keefe & Block Pain Behaviour: observation | |
| Notes | No data Yates quality scale: total quality = 20/35, design quality = 15/26, treatment quality = 5/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned" |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition partially reported and did not differ across groups; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Subjects examined by physician unaware of treatment conditions and with no other contact with subjects |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 4 months, 12 months | |
| Participants | End of treatment n = 55 Start of treatment n = 60 Sex: 60 F, 0 M Mean age = 49.6 (SD 7.0) Source = not stated Diagnosis = fibromyalgia Mean years of pain = 12.4 | |
| Interventions | "Interactional School of Fibromyalgia" "Control" | |
| Outcomes | Primary pain outcome: MPI pain severity Primary disability outcome: MPI interference with daily activity Primary mood outcome: none Catastrophising outcome: none 1. VAS pain (pain diary) 2. MPI pain severity 3. MPI pain interference daily activity 4. MPI control over pain 5. MPI mood 6. MPI family and social support 7. VAS suffering (pain diary) 8. VAS ability to do daily activity (pain diary) | |
| Notes | December 2009 search No data Yates quality scale: total quality = 13/35, design quality = 10/26, treatment quality = 3/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Attrition partially reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at post‐treatment and 6‐month follow‐up | |
| Participants | End of treatment: n = 62 Start of treatment: n = 65 Sex: 33 F, 29 M Age: mean = 39.4 (SD 11.1) Mean years of pain = 2.1 (SD 2.5) Source = outpatient rehabilitation unit Diagnosis = pain (neck and shoulder) after whiplash injury | |
| Interventions | CBT rehabilitation plus EMG biofeedback versus CBT rehabilitation | |
| Outcomes | Primary pain outcome: no data Primary disability outcome: Canadian Occupational Performance Measure Primary mood outcome: none Catastrophising outcome: none Canadian Occupational Performance Measure Multi‐dimensional Pain Inventory (Swedish) | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.2 and 2.2 2011 search Yates quality scale: total quality = 21/35, design quality = 17/26, treatment quality = 4/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomization was performed by casting a die after the participant’s acceptance: odd numbers for treatment group ....” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Attrition fully reported |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Therapists conducted assessments: statement that study not blinded |
| Methods | RCT; 2 arms; assessed at pretreatment, post‐treatment, 6‐month follow‐up, 12‐month follow‐up | |
| Participants | End of treatment n = 218 Start of treatment n = 256 Sex: 210 F, 46 M Mean age = 81.8 (SD 6.5) Source = residential retirement facilities Diagnosis = pain more than 3 months; average last week > 2/10: mixed sites, largest legs and feet Mean years of pain = not given | |
| Interventions | "pain self‐management training group (SMG) intervention" "education only control condition" | |
| Outcomes | Primary pain outcome: BPI pain Primary disability outcome: RMDQ Primary mood outcome: Geriatric Depression Scale Catastrophising outcome: CSQ catastrophising 1. Roland & Morris Disability Questionnaire 2. Brief Pain Inventory: pain 3. Brief Pain Inventory: interference with activity 4. Geriatric Depression Scale 5. Arthritis Self‐Efficacy Scale 6. CSQ catastrophising 7. Chronic Pain Coping Inventory: guarding 8. Chronic Pain Coping Inventory: resting 9. Chronic Pain Coping Inventory: asking for assistance 10. Chronic Pain Coping Inventory: relaxation 11. Chronic Pain Coping Inventory: task persistence 12. Chronic Pain Coping Inventory: exercise/stretch 13. Chronic Pain Coping Inventory: seeking support 14. Chronic Pain Coping Inventory: coping self statements 15. Chronic Pain Coping Inventory: pacing 16. Medication use: record | |
| Notes | December 2009 search: 1.1, 1.2, 1.3, 1.4, 2.1, 2.2, 2.3, 2.4 Yates quality scale: total quality = 30/35, design quality = 21/26, treatment quality = 9/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by (retirement) facility, by statistician using random number generator |
| Allocation concealment (selection bias) | Low risk | By independent statistician |
| Incomplete outcome data (attrition bias) | Low risk | Fully reported attrition |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 6 months follow‐up | |
| Participants | End of treatment n = 59 Start of treatment n = 64 Sex: 42 F, 17 M Mean age = 54.1 (SD 11.4) Source = rheumatology clinic Diagnosis = rheumatoid arthritis Mean years of pain = 3.1 | |
| Interventions | "Tailor made CBT" "Treatment as usual" | |
| Outcomes | Primary pain outcome: IRGL Pain Primary disability outcome: IRGL Functional Disability (Composite Z score) Primary mood outcome: BDI depression Catastrophising outcome: Illness Cognitions ‐ Helplessness Disease Activity Invloed van Reuma op Gezondheid en Leefwijze (IRGL): Functional Disability Invloed van Reuma op Gezondheid en Leefwijze (IRGL): Pain Invloed van Reuma op Gezondheid en Leefwijze (IRGL): Anxiety Invloed van Reuma op Gezondheid en Leefwijze (IRGL): Perceived support Social network Illness Cognitions: Helplessness Illness Cognitions: Acceptance Active Coping with Pain Passive Coping with pain Active Coping with Stress Passive Coping with Stress Fatigue Beck Depression Inventory Negative Mood (ZwartSpooren) Medication compliance | |
| Notes | CBT versus TAU, post‐treatment and follow‐up: analyses 3.1, 3.2, 3.3, 4.1, 4.2, 4.3 Yates quality scale: total quality = 25/35, design quality = 18/26, treatment quality = 7/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random numbers |
| Allocation concealment (selection bias) | Low risk | “previously determined” |
| Incomplete outcome data (attrition bias) | Low risk | Attrition fully reported |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 3 months | |
| Participants | End of treatment n = 51 Start of treatment n = 60 Sex: 60 F, 0 M Mean age = 45.7 (SD 2.3) Source = Rheumatology outpatients Diagnosis = fibromyalgia Mean years of pain = 3.6 | |
| Interventions | "Cognitive behavioral therapy" "Routine medical visits" | |
| Outcomes | Primary pain outcome: VAS Primary disability outcome: FIQ (no data for SF‐36) Primary mood outcome: BDI Catastrophising outcome: none 1. Visual analogue scale for pain 2. Verbal improvement scale (5 categories) 3. Fibromyalgia Impact Questionnaire (FIQ) 4. SF‐36 physical capacity (function) 5. SF‐36 physical aspects (role) 6. SF‐36 pain 7. SF‐36 general health 8. SF‐36 vitality 9. SF‐36 social aspects 10. SF‐36 emotional aspects 11. SF‐36 mental health 12. Beck Depression Inventory (BDI) 13. Spielberger State‐Trait Anxiety Inventory (STAI State) 14. Number of paracetamol tablets | |
| Notes | December 2009 search: analyses 3.1, 3.2, 3.3 Yates quality scale: total quality = 16/35, design quality = 14/26, treatment quality = 2/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized by drawing lots" |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition partially reported; statement that dropouts were not different |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Evaluation by clinician blind to treatment allocation |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 1 year | |
| Participants | End of treatment n = 158 Start of treatment n = 176 Sex: 109 F, 83 M (at start of treatment) Mean age = 52.5 (SD 12.4) Source = mixed community and volunteer Diagnosis = shoulder pain Mean years of pain = not given | |
| Interventions | "Graded exercise" "Primary care TAU" | |
| Outcomes | Primary pain outcome: NRS Primary disability outcome: Shoulder Disability Questionnaire Primary mood outcome: none Catastrophising outcomes: PCCL catastrophising Shoulder disability questionnaire Shoulder pain Pain intensity NRS Quality of life Fear avoidance Kinesiophobia (2 items) Pain Coping and Cognition List: catastrophising Pain Coping and Cognition List: coping General Practitioner visits Physician visits Physiotherapy visits Number of drug prescriptions Number of days work absence Total cost of health care (Euros) | |
| Notes | BT versus TAU: analyses 7.1, 7.2, 7.4, 8.2 Yates quality scale: total quality = 26/35, design quality = 20/26, treatment quality = 6/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation according to random number table |
| Allocation concealment (selection bias) | Low risk | Random number table generated by person not involved in study; opaque sealed envelopes; “Blinding for patients .... of allocated treatment was not possible” but treatment preferences elicited and shown to have no effect on outcome |
| Incomplete outcome data (attrition bias) | Low risk | Attrition fully reported; dropouts different in pain characteristics but not outcome measures at baseline |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Researchers not involved in randomisation collect data |
| Methods | RCT; 3 arms: CBT + biofeedback; CBT; waiting list control, post‐treatment (WLC assigned to treatment so no WLC at 6‐month follow‐up) | |
| Participants | End of treatment: n = 116 Start of treatment: n = 128 Sex: 77 F, 39 M Mean age: 48.8 (SD 11.7) Source = medical referrals (86%) or response to newspaper advert (14%) Diagnosis = chronic back pain Mean years of pain: 8.1 (SD 8.7) | |
| Interventions | "CBT with biofeedback" "CBT" "waiting list control" | |
| Outcomes | Primary pain outcome: 0 to 10 NRS pain intensity Primary disability outcome: PDI Primary mood outcome: BDI Catastrophising outcome: none Pain intensity 0 to 10 NRS Pain average of 4x daily diary for 1 week Pain Disability Index Beck Depression Inventory Coping Strategies Scale from FESV Health‐Related Life Satisfaction Scale Global treatment change Treatment satisfaction (Adverse events noted from pain intensity and global treatment change) Health care use: doctor visits for pain | |
| Notes | Combined (CBT + biofeedback and CBT) versus WLC: analyses 3.1, 3.2, 3.3 2011 update search Yates quality scale: total quality 24/35, design quality 17/26, design quality 15/26 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random number generation |
| Allocation concealment (selection bias) | Unclear risk | “coordinated by the first author” before study |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; statement that dropout data will be reported elsewhere |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; assessed pre‐treatment, post‐treatment, 6/9 months | |
| Participants | End of treatment n = 80 Start of treatment n = 92 Sex: 87 F, 5 M (at start of treatment) Mean age = 47.3 (SD 10.4) Source = volunteers Diagnosis = SLE Mean years of pain = 11 | |
| Interventions | "CBT with biofeedback" "Symptom monitoring and support" "Treatment as usual" | |
| Outcomes | Primary pain outcome: AIMS2 pain 0 to 10 Primary disability outcome: SF36 physical function (reversed) Primary mood outcome: CES‐D Depression Catastrophising outcome: perceived stress Arthritis Impact Measurement Scale (AIMS) 2: pain Multidimensional Pain Inventory: interference Center for Epidemiologic Studies Depression Scale (CES‐D) Arthritis Self‐Efficacy Perceived stress Short Form 36 physical function Fatigue severity Global self assessment Disease activity systemic lupus activity measure‐revised (SLAM‐R) Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.1, 1.2, 1.3, 2.1, 2.2, 2.3 CBT versus TAU, post‐treatment and follow‐up: analyses 3.1, 3.2, 3.3, 4.1, 4.2, 4.3 Yates quality scale: total quality = 25/35, design quality = 20/26, treatment quality = 5/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “assigned randomly, based on a software‐generated randomization plan” |
| Allocation concealment (selection bias) | High risk | Not reported, but equal credibility of treatments rated by participants |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Rheumatologist and researcher assessors masked to treatment assignment |
| Methods | RCT; 2 arms; assessed pre‐treatment, 1 year | |
| Participants | End of treatment n = 387 Start of treatment n = 469 Sex: 298 F, 171 M Mean age = 43 (SD 10.6) Source = National Insurance system contact Diagnosis = mixed chronic pain Mean years of pain = not given | |
| Interventions | "Cognitive behaviour therapy" "Treatment as usual" | |
| Outcomes | Primary pain outcome: VAS pain Primary disability outcome: none Primary mood outcome: HSCL distress Catastrophising outcome: none Visual analogue scale pain (in afternoon) Physical training Hopkins Checklist (HSCL) Distress (Norwegian version) Attribution style Work satisfaction Ergonomic performance Subjective health rating | |
| Notes | CBT versus TAU post‐treatment: analyses 4.1, 4.3 Yates quality scale: total quality = 12/35, design quality = 10/26, treatment quality = 2/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated at random by cards in sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation sequence by someone not involved in study |
| Incomplete outcome data (attrition bias) | High risk | Attrition partially reported; no test for differences |
| Selective reporting (reporting bias) | High risk | Not fully reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessment by physiotherapists who tried to remain blind to treatment |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 1 year | |
| Participants | End of treatment n = 121 Start of treatment n = 127 Sex: 97 F, 30 M Mean age = 50.5 (SD 10.6) Source = rheumatology clinic Diagnosis = rheumatoid arthritis (hand) Mean years of pain = 1.6 | |
| Interventions | "Joint protection arthritis education" "Standard arthritis education" | |
| Outcomes | Primary pain outcome: none available Primary disability outcome: AIMS2 activities of daily living Primary mood outcome: none available Catastrophising outcome: RAI helplessness Adherence to joint protection Hand pain visual analogue scale Overall pain visual analogue scale Tender count (28 joints) Swollen joint count (28 joints) Early morning stiffness Grip strength Hand joint alignment Arthritis Impact Measurement Scale (AIMS) 2: ADL Arthritis Impact Measurement Scale (AIMS) 2: upper limb function Arthritis Impact Measurement Scale (AIMS) 2: lower limb function Arthritis Impact Measurement Scale (AIMS) 2: current health status Arthritis Self Efficacy (pain) Arthritis Self Efficacy (other) Rheumatoid attitude index (helplessness) Rheumatoid attitude index (internality) Satisfaction with health | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.2, 2.2 Yates quality scale: total quality = 18/35, design quality = 15/26, treatment quality = 3/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “allocated randomly” |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared in advance |
| Incomplete outcome data (attrition bias) | High risk | Attrition partially reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Independent assessor |
| Methods | RCT; 2 arms; assessed pre‐treatment, post‐treatment, 6 months, 18 months | |
| Participants | End of treatment n = 59 Start of treatment n = 63 Sex: 63 F, 0 M (at start of treatment) Mean age = 43.4 (SD 8.4) Source = pain or rehabilitation clinic Diagnosis = non‐specific back or neck pain Mean years of pain = 4.2 | |
| Interventions | "Woman‐specific CBT" "Regular CBT" | |
| Outcomes | Primary pain outcome: VAS pain intensity Primary disability outcome: Disability Rating Index Primary mood outcome: BDI depression Catastrophising outcome: RAI helplessness Pain intensity visual analogue scale Beck Depression Inventory (BDI) Anxiety visual analogue scale Disability Rating Index Health perception numerical rating scale Coping Strategies Questionnaire (CSQ) Rheumatoid attitudes index (helplessness) | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.1, 1.2, 1.3, 2.1, 2.2, 2.3 Yates quality scale: total quality = 16/35, design quality = 13/26, treatment quality =3/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomisation using random numbers table |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Attrition partially reported; no test for differences |
| Selective reporting (reporting bias) | High risk | Partially reported |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blind to treatment condition |
| Methods | RCT; 4 arms; assessed at pre‐treatment, post‐treatment, 6 months, 18 months, 3 years | |
| Participants | End of treatment n = 186 Start of treatment n = 214 Sex: 117 F, 93 M Mean age = 43.3 (SD 10.4) Source = pain or rehabilitation clinic Diagnosis = mixed (mostly chronic low back pain) Mean years of pain = 2.7 | |
| Interventions | "CBT" "Behavioural medicine rehabilitation" "Behaviourally orientated physical therapy" (BT) "Treatment as usual" | |
| Outcomes | Primary pain outcome: SF36 pain (reversed) Primary disability outcome: SF36 physical function (reversed) Primary mood outcome: SF36 mental health (reversed) Catastrophising outcomes: none Short Form 36 Pain Short Form 36 Physical Function Short Form 36 Mental Health | |
| Notes | CBT versus TAU, post‐treatment and follow‐up (6 months): analyses 3.1, 3.2, 3.3, 4.1, 4.2, 4.3 BT versus TAU, post‐treatment and follow‐up (6 months): analyses 7.1, 7.2, 7.3, 8.1, 8.2, 8.3 Baseline N used as N unavailable for post‐treatment and follow‐up results Yates quality scale: total quality = 27/35, design quality = 20/26, treatment quality =7/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Shuffled sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes; procedure by researchers blind to participant screening |
| Incomplete outcome data (attrition bias) | High risk | Partially reported; differential attrition; no test of differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Data gathered by research team |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 6 months, 1 year, 2 years | |
| Participants | End of treatment n = 120 Start of treatment n = 132 Sex: 120 F, 12 M (start of treatment) Mean age = 46.3 (SD 7.5) Source = community Diagnosis = chronic low back pain Mean years of pain = 1.3 | |
| Interventions | "semi‐intensive multidisciplinary rehabilitation" "individual physiotherapy" | |
| Outcomes | Primary pain outcome: pain intensity 0 to 10 Primary disability outcome: Oswestry Disability Index 0 to 100 Primary mood outcome: (DEPS) depression 0 to 30 Catastrophising outcome: none Low back pain intensity 0 to 10 Sciatic pain intensity 0 to 10 Oswestry Disability Index 0 to 100 Subjective work capacity 0 to 10 Recent sick leave due to back pain Beliefs re working (2‐year follow‐up) 0 to 10 The Depression Scale (DEPS) 0 to 30 Health care consumption 12 months | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.1, 1.2, 1.3, 2.1, 2.2, 2.3 Yates quality scale: total quality = 23/35, design quality = 20/26, treatment quality = 3/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes; numbers generated by independent statistician |
| Incomplete outcome data (attrition bias) | Unclear risk | Fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT. 3 arms; assessed pre‐treatment, post‐treatment, 6 months | |
| Participants | End of treatment n = 94 Start of treatment n = 99 Sex: 71 F, 28 M Mean age = 64.0 (SD 11.5) Source = rheumatology clinic Diagnosis = osteoarthritis of the knee Mean years of pain = 12.0 | |
| Interventions | "coping skills training" "arthritis education" "standard care" | |
| Outcomes | Primary pain outcome: AIMS pain Primary disability outcome: AIMS physical disability Primary mood outcome: AIMS psychological disability Catastrophising outcome: none Arthritis Impact Measurement Scale (AIMS): pain Arthritis Impact Measurement Scale (AIMS): psychological disability Arthritis Impact Measurement Scale (AIMS): physical disability Pain behaviour (Keefe & Block) observation Coping Strategy Questionnaire Medication use | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.1, 1.2, 1.3, 2.1, 2.2, 2.3 CBT versus TAU, post‐treatment and follow‐up: analyses 3.1, 3.2, 3.3, 4.1, 4.2, 4.3 Yates quality scale: total quality = 26/35, design quality = 18/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly assigned (using a random number table)” |
| Allocation concealment (selection bias) | High risk | Not reported (but equal credibility of treatments rated by participants) |
| Incomplete outcome data (attrition bias) | Low risk | Fully reported |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; assessed at pre‐treatment, post‐treatment, 6 months, 1 year | |
| Participants | End of treatment n = 82 Start of treatment n = 88 Sex: 54 F, 34 M Mean age = 62.6 (SD 10.1) Source = volunteer Diagnosis = osteoarthritis of knee Mean years of pain = 10.7 | |
| Interventions | "spouse‐assisted coping skills training" "coping skills training" "spouse‐supported arthritis education" | |
| Outcomes | Primary pain outcome: AIMS pain Primary disability outcome: AIMS physical disability Primary mood outcome: AIMS mental disability Catastrophising outcome: none Arthritis Impact Measurement Scale (AIMS): pain Arthritis Impact Measurement Scale (AIMS): physical Arthritis Impact Measurement Scale (AIMS): psychological Coping Strategies Questionnaire: coping Coping Strategies: pain control Pain behaviour (Keefe & Block) observation | |
| Notes | CBT versus active, post‐treatment: analyses 1.1, 1.2, 1.3 Yates quality scale: total quality = 25/35, design quality = 17/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported (but equal credibility of treatments rated by participants) |
| Incomplete outcome data (attrition bias) | Unclear risk | Fully reported; no differential attrition but no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; assessed at pre‐treatment, post‐treatment, 6 months, 1 year | |
| Participants | End of treatment n = 133 Start of treatment n = 148 Sex: 94 F, 54 M Mean age = 30.8 (SD 9.1) Source = pain or rehabilitation clinic Diagnosis = chronic low back pain Mean years of pain = 9.8 | |
| Interventions | "operant + cognitive coping skills" "operant + group discussion" "waiting list" | |
| Outcomes | Primary pain outcome: no data available Primary disability outcome: no data available Primary mood outcome: no data available Catastrophising outcome: none (all reduced by factor analysis to 3 scores: motoric, coping control, negative affect) Pain Behaviour Scale Checklist for Interpersonal Pain Behaviour Behavioural approach test (walking distance) Multi dimensional Locus of Control Pain Cognition Checklist Coping Strategies Questionnaire Nijmegen Hyperventilation Questionnaire Visual analogue scale: pain McGill Pain Questionnaire: pain | |
| Notes | No data Yates quality scale: total quality = 28/35, design quality = 20/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “independent researcher blindly drew [numbers assigned randomly to patients] and assigned to one of three conditions” |
| Allocation concealment (selection bias) | Low risk | independent researcher |
| Incomplete outcome data (attrition bias) | Low risk | Fully reported |
| Selective reporting (reporting bias) | Unclear risk | Reported as factor scores |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor unaware of treatment condition |
| Methods | RCT; 3 arms; assessed at pre‐treatment, post‐treatment, 6 months | |
| Participants | End of treatment n = 52 Start of treatment n = 58 Sex: 52 F, 25 M (from the 77 who agreed to participate) Mean age = 57.0 (SD 12.7) Source = rheumatology clinics Diagnosis = rheumatoid arthritis Mean years of pain = 15.6 | |
| Interventions | "cognitive behavioural therapy" "occupational therapy" "waiting list" | |
| Outcomes | Primary pain outcome: IRGL pain Primary disability outcome: IRGL function (Reversed) Primary mood outcome: IRGL depression Catastrophising outcome: none Invloed van Reuma op Gezondheid en Leefwijze (IRGL): function Invloed van Reuma op Gezondheid en Leefwijze (IRGL): self care Invloed van Reuma op Gezondheid en Leefwijze (IRGL): pain Invloed van Reuma op Gezondheid en Leefwijze (IRGL): anxiety Invloed van Reuma op Gezondheid en Leefwijze (IRGL): depression Invloed van Reuma op Gezondheid en Leefwijze (IRGL): potential support Invloed van Reuma op Gezondheid en Leefwijze (IRGL): actual support Invloed van Reuma op Gezondheid en Leefwijze (IRGL): mutual visits | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.1, 1.2, 1.3, 2.1, 2.2, 2.3 CBT versus TAU, post‐treatment and follow‐up: N < 20 Yates quality scale: total quality = 21/35, design quality = 14/26, treatment quality = 7/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Fully reported; several differences between dropouts and completers |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at 2 pre‐treatment occasions, post‐treatment, 6‐month follow‐up, 12‐month follow‐up | |
| Participants | End of treatment n = 77 Start of treatment n = 85 Sex: 41 F, 44 M Mean age = 45.3 (SD 9.5) Source = rehabilitation clinics, occupational health, pain department Diagnosis = back pain (and at least moderate fear on TSK) Mean years of pain = 9 | |
| Interventions | "Exposure in vivo" "Operant graded activity" | |
| Outcomes | Primary pain outcome: MPQ pain intensity Primary disability outcome: Quebec Back Pain Disability Scale (Dutch version) Primary mood outcome: none Catastrophising outcome: PCS 1. Quebec Back Pain Disability Scale (Dutch version) 2. Patient Specific Complaints: VAS 0 to 100 of difficulty with 3 activities 3. Perceived harmfulness of activities (PHODA) 4. Pain Catastrophizing Scale: catastrophising 5. Daily activity: actimeter 6. Pain: mean of VAS 0 to 100 scales for current, worst and least pain | |
| Notes | December 2009 search Exposure in vivo versus operant graded activity: analyses 5.1, 5.2, 5.4, 6.1, 6.2, 6.4 Yates quality scale: total quality = 32/35, design quality = 24/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “predetermined and computer‐generated randomization schedule” |
| Allocation concealment (selection bias) | Low risk | Sealed envelope; research assistant only could access randomization schedule |
| Incomplete outcome data (attrition bias) | Low risk | Fully reported |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Electronic administration of assessments |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 18‐month follow‐up | |
| Participants | End of treatment n = 123 Start of treatment n = 125 Sex: 68 F, 57 M Mean age = 42.6 (SD not given) Source = primary care Diagnosis = non‐specific back or neck pain Mean years of pain = not given but had to be sick listed for more than 6 weeks up to 2 years; mean over 7 months sick listed | |
| Interventions | "Cognitive‐behavioural rehabilitation" "Primary care" | |
| Outcomes | Primary pain outcome: none Primary disability outcome: none Primary mood outcome: none Catastrophising outcome: none 1. Sick listed days 2. Healthcare visits | |
| Notes | December 2009 search No data available Yates quality scale: total quality = 18/35, design quality = 16/26, treatment quality = 2/6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Randomisation generated by independent statistician; in opaque envelopes |
| Incomplete outcome data (attrition bias) | Unclear risk | Fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Assessors not blind to treatment condition, except for sick listing outcome |
| Methods | RCT; 2 arms; CBT + standard treatment; standard treatment; post‐treatment | |
| Participants | End of treatment: n = 54 Start of treatment: n = 54 Sex: 46 F; 8 M Mean age: 41.0 (SD 11.0) Source = dental clinics and dentists (15%); newspaper and web adverts (85%) Diagnosis = temporomandibular disorder Mean years of pain: 5.6 (SD 5.4) | |
| Interventions | CBT + standard treatment; standard treatment (splint, diet, NSAIDs) | |
| Outcomes | Primary pain outcome: MPI 0 to 6 Primary disability outcome: interference MPI 0 to 6 Primary mood outcome: CES‐D Catastrophising outcome: data not available Pain Intensity MPI 0 to 6 CES‐D Depression Interference with activity MPI 0 to 6 2 items modified from Catastrophising Sub‐Scale CSQ Several times daily sampling of pain, control, affect, coping, catastrophising | |
| Notes | CBT versus TAU: analyses 3.1, 3.2, 3.3 2011 update search Yates quality scale: total quality 14/35, design quality 11/26, treatment quality 3/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised urn randomisation |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Attrition not reported |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed pre‐treatment, 6 months follow‐up | |
| Participants | End of treatment n = 245 Start of treatment n = 353 Sex: 264 F, 89 M Mean age = 52.1 (SD 9.6) Source = pain or rehabilitation clinic Diagnosis = mixed chronic pain, many chronic low back pain Mean years of pain = 9.6 | |
| Interventions | "Cognitive behaviour therapy" "minimal home study" | |
| Outcomes | Primary pain outcome: MPI pain severity Primary disability outcome: MPI pain interference Primary mood outcome: MPI affective distress Catastrophising outcome: none 11‐point box scale: pain severity Pain discomfort scale: pain distress Multidimensional Pain Inventory: pain severity Multidimensional Pain Inventory: affective distress Multidimensional Pain Inventory: self control Multidimensional Pain Inventory: interference Multidimensional Pain Inventory: social support and spouse behaviour subscales | |
| Notes | CBT versus active, follow‐up: analyses 2.1, 2.2, 2.3 Yates quality scale: total quality = 11/35, design quality = 9/26, treatment quality = 2/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized using a computer‐generated random number list" |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No attrition during treatment, only at follow‐up; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 4 arms; assessed at pre‐treatment, post‐treatment | |
| Participants | End of treatment n = 94 Start of treatment n = 94 Sex: 77 F, 7 M Mean age = 35.8 (SD 9.9) Source = pain or rehabilitation clinic and volunteer Diagnosis = temporomandibular joint disorder Mean years of pain = 7.0 | |
| Interventions | "Biofeedback" (BT) "Cognitive behavioural skills training" (CBT) "Cognitive behavioural skills training + biofeedback" "no treatment control" | |
| Outcomes | Primary pain outcome: CPI pain index Primary disability outcome: none available Primary mood outcome: none available Catastrophising outcomes: none Characteristic Pain Index (CPI) pain severity 0 to 100 Graded Chronic Pain Score Profile of Mood States total | |
| Notes | CBT versus TAU, post‐treatment: analysis 3.1 BT versus TAU, post‐treatment: analysis 7.1 Yates quality scale: total quality = 19/35, design quality = 12/26, treatment quality = 7/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "patients were assigned to group in a semi‐random fashion using the urn method of random assignment" |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Attrition not reported |
| Selective reporting (reporting bias) | High risk | Partially reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 6 months | |
| Participants | End of treatment n = 71 Start of treatment n = 96 Sex: 63 F, 8 M (at follow‐up) Mean age = 53.1 (SD no given) Source = pain or rehabilitation clinic, support groups Diagnosis = fibromyalgia Mean years of pain = 11.1 | |
| Interventions | "behavioural treatment" "education" | |
| Outcomes | Primary pain outcome: not available Primary disability outcome: quality of well being Primary mood outcome: CES‐D Depression Catastrophising outcome: RAI helplessness Pain index: composite of Fibromyalgia Impact Questionnaire pain scale, MPQ PRI, number of body areas, and flare index Pain Behavior Checklist self reported pain behaviour Pain behaviour (Keefe & Block) observation Center for Epidemiologic Studies Depression Scale (CES‐D) Rheumatology Attitudes Index helplessness subscale Pain Management Inventory active and passive coping Quality of Well being Scale QWB: structured interview on functional impairment Quality of Social Support Scale Myalgia score, nurse rated on examination | |
| Notes | BT versus active, post‐treatment and follow‐up: analyses 5.2, 5.3, 6.2, 6.3 Yates quality scale: total quality = 21/35, design quality = 15/26, treatment quality = 6/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In blocks, “randomly assigned, using a random numbers table” |
| Allocation concealment (selection bias) | High risk | Not reported, though credibility ratings equal across treatments |
| Incomplete outcome data (attrition bias) | Low risk | Attrition fully reported; differential attrition across groups; no differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; assessed at pre‐treatment, 6 months, 1 year | |
| Participants | End of treatment n = 83 Start of treatment n = not given Sex: 3 F, 80 M Mean age = 60.6 (SD 7.7) Source = hospital Diagnosis = rheumatoid arthritis Mean years of pain = 11.4 | |
| Interventions | "cognitive behavioural pain management group" "attention placebo group" "control group" (TAU) | |
| Outcomes | Primary pain outcome: no data available Primary disability outcome: no data available Primary mood outcome: no data available Catastrophising outcome: none Visual analogue scale pain McGill Pain Questionnaire pain dimensions Coping Strategies Questionnaire Arthritis Impact Measurement Scale (AIMS) Beck Depression Inventory Symptom Checklist‐90R psychological symptoms Hassles Scale Ways of Coping Questionnaire Arthritis Helplessness Index Disease status measures including walking speed | |
| Notes | No data Yates quality scale: total quality = 17/35, design quality = 13/26, treatment quality = 4/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “using a table of random numbers, subjects were assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Attrition not reported |
| Selective reporting (reporting bias) | High risk | Partially reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 1 month | |
| Participants | End of treatment n = 69 Start of treatment n = 71 Sex: 49 F, 20 M Mean age = 52.7 (SD 14.4) Source = community Diagnosis = mixed chronic pain Mean years of pain = 10.0 | |
| Interventions | "Cognitive behaviour therapy" "waiting list" | |
| Outcomes | Primary pain outcome: pain diary Primary disability outcome: pain interference Primary mood outcome: none available Catastrophising outcome: none Pain diary 0 to 5: highest and lowest ratings Pain interference 0 to 5 Coping 0 to 5 Medication use | |
| Notes | CBT versus TAU, post‐treatment: analyses 3.1, 3.2 Yates quality scale: total quality = 13/35, design quality = 10/26, treatment quality = 3/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition reported; no test for differences |
| Selective reporting (reporting bias) | High risk | Partially reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; mindfulness‐based stress reduction, active relaxation control, waiting list; post‐treatment, 2‐month follow‐up | |
| Participants | End of treatment n = 148 Start of treatment n = 177 Sex: 177 F; 0 M Mean age = 52.5 (SD 9.6) Source = newspapers, GP and specialist referrals, patient self help groups Diagnosis = fibromyalgia Mean years of pain: 4.0 (SD 3.9) | |
| Interventions | Mindfulness‐based stress reduction; active control (relaxation, support and education); waiting list | |
| Outcomes | Primary pain outcome: Pain Perception Scale (sensory) Primary disability outcome: Fibromyalgia Impact Questionnaire Primary mood outcome: CES‐D Catatrophising outcome: none Pain Perception Scale (Sensory and Affective) Fibromyalgia Impact Questionnaire Depression: CES‐D Anxiety: Trait Sub‐scale STAI Pittsburgh Sleep Quality Index Health‐related Quality of Life Freiburg Mindfulness Inventory Physical symptoms: Giessen Complaint Questionnaire Ongoing therapies, medical visits and medication Medication diary Goal‐attainment scaling by interview | |
| Notes | Active relaxation control versus waiting list; analyses 7.1, 7.2, 7.3 2011 update search Yates quality scale: total quality = 31/35, design quality = 23/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomized in blocks by a computer algorithm” |
| Allocation concealment (selection bias) | Low risk | Patients and personnel blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded |
| Methods | RCT; 4 arms; assessed at pre‐treatment, post‐treatment, 1 year | |
| Participants | End of treatment n = 212 Start of treatment n = 223 Sex: 106 F, 117 M Mean age = 41.6 (SD 10.0) Source = pain or rehabilitation clinic Diagnosis = CLBP Mean years of pain = 4/6 | |
| Interventions | "Cognitive behavioural therapy + active physical treatment" "Cognitive behavioural therapy" "active physical treatment" "waiting list" | |
| Outcomes | Primary pain outcome: MPQ PRI (follow‐up only) Primary disability outcome: Roland & Morris Disability Scale Primary mood outcome: BDI Catastrophising outcome: process only Roland Morris Disability Questionnaire disability Difficulty with 3 most limited activities: 0 to 100 Visual analogue scale pain Beck Depression Inventory Pain Cognitions List: catastrophising, pain control subscales as process measures Follow‐up only MPQ PRI 6. 5‐minute walk 7. 50‐foot walk 8. timed stand‐to‐sits 9. extended reach 10. stair climb 11. lifting task | |
| Notes | 1‐year follow‐up Smeets 2008; December 2009 search CBT plus active PT versus active PT: analyses 1.1, 1.2, 1.3, 2.1, 2,2. 2.3 GA plus problem solving versus WLC: analyses 3.1, 3.2, 3.3 (waiting list not followed up) Yates quality scale: total quality = 28/35, design quality = 23/26, treatment quality = 5/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks by computer‐generated algorithm |
| Allocation concealment (selection bias) | Low risk | Generated by independent statistician; sealed envelopes |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment by blinded research assistants |
| Methods | RCT; 3 arms; assessed at pre‐treatment, post‐treatment, 6 months | |
| Participants | End of treatment n = 43 Start of treatment n = 57 Sex: 46 F, 11 M Mean age = 54.0 (SD 13.0) Source = rheumatology clinic Diagnosis = rheumatoid arthritis Mean years of pain not given | |
| Interventions | "group psychotherapy" "relaxation/assertion" "no treatment" | |
| Outcomes | Primary pain outcome: no data available Primary disability outcome: no data available Primary mood outcome: no data available Catastrophising outcome: none 4 aggregate outcome measures: Functional status, social adaptation, psychological adaptation, psychological symptoms Measures contributing to these: Arthritis Impact Measurement Scale (AIMS) Short Form 36 Rathus Assertive Behavior Scale Rosenberg Self‐Esteem Scale Hostility Inventory Wright’s Human Service Scale & Handicap Problems Inventory | |
| Notes | No data Yates quality scale: total quality = 10/35, design quality = 9/26, treatment quality = 1/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Attrition not reported |
| Selective reporting (reporting bias) | High risk | Partially reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; assessed at pre‐treatment, post‐treatment, 6 months, 15 months | |
| Participants | End of treatment n = 61 Start of treatment n = 83 Sex: 61 F, 0 M Mean age = 47.3 (SD 8.3) Source = hospital for rheumatic disorders Diagnosis = fibromyalgia Mean years of pain = 16.5 | |
| Interventions | "operant treatment" "standard physical treatment" | |
| Outcomes | Primary pain outcome: MPI pain Primary disability outcome: MPI interference Primary mood outcome: MPI affective distress Catastrophising outcome: none Diary pain intensity Multidimensional Pain Inventory: pain Multidimensional Pain Inventory: interference Multidimensional Pain Inventory: life control Multidimensional Pain Inventory: affective distress Multidimensional Pain Inventory: social support Multidimensional Pain Inventory: self efficacy Multidimensional Pain Inventory: punishing responses, solicitous responses, distracting responses Multidimensional Pain Inventory: total activities Doctor visits (from medical records) Hospital days (from medical records) Sleep hours diary Medication diary Tubingen pain behaviour scale | |
| Notes | BT versus TAU, post‐treatment and follow‐up: analyses 7.1, 7.2, 7.3, 8.1, 8.2, 8.3 Yates quality scale: total quality = 15/35, design quality = 11/26, treatment quality = 4/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; self help acceptance and commitment therapy, self help applied relaxation; post‐treatment: 6‐month and 12‐month follow‐up | |
| Participants | End of treatment: n = 64 Start of treatment: n = 98 Sex: 63 F; 35 M Source = pain clinic Diagnosis = mixed chronic pain Mean age: 46.0 (SD 12.3) Mean years of pain: not given (98% more than 1 year) | |
| Interventions | Self help acceptance and commitment therapy; self help applied relaxation | |
| Outcomes | Primary pain outcome: pain intensity 0 to 10 Primary disability outcome: OMPQ 5 items Primary mood outcome: Depression HADS Catastrophising outcome: none Pain intensity 0 to 10 Function: 5 items 0 to 10 from Orebro Musculoskeletal Pain Questionnaire (reverse direction) Depression HADS Anxiety HADS Satisfaction With Life Scale Chronic Pain Acceptance Questionnaire | |
| Notes | ACT versus active control: analyses 1.1, 1.2, 1.3, 2.1, 2.2, 2.3 2011 update search Yates quality scale: total quality 18/35, design quality 13/26, treatment quality 5/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized by drawing pieces of paper with type of intervention” |
| Allocation concealment (selection bias) | High risk | Not reported, but treatment credibility equal |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; assessed at pre‐treatment, post‐treatment, 6 months, 1 year | |
| Participants | End of treatment n = 53 Start of treatment n = 81 Sex: 30 F, 51 M Mean age = 46.0 (SD not given) Source = pain or rehabilitation clinic Diagnosis = CLBP Mean years of pain = 6.2 | |
| Interventions | "CBT" "operant behavior therapy" "waiting list" | |
| Outcomes | Primary pain outcome: MPQ PRI Primary disability outcome: SIP patient‐rated Primary mood outcome: none available Catastrophising outcome: CEQ Multidimensional Pain Questionnaire: Pain Response Index Sickness Impact Profile: patient‐rated Sickness Impact Profile: spouse‐rated Pain behaviour (Keefe & Block) observation Pain Behavior Checklist patient‐rated Pain Behavior Checklist spouse‐rated Cognitive Errors Questionnaire | |
| Notes | CBT versus TAU, post‐treatment (waiting list not followed up): analyses 3.1, 3.2 BT versus TAU, post‐treatment (waiting list not followed up): analyses 7.2 Yates quality scale: total quality = 23/35, design quality = 15/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Partially reported but full account of excluded measures |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT.; 2 arms; assessed at pre‐treatment, post‐treatment, 6 months, 1 year | |
| Participants | End of treatment n = 142 Start of treatment n = 158 Sex: 128 F, 30 M Mean age = 37.4 (SD 11.3) Source = pain or rehabilitation clinic Diagnosis = temporomandibular joint pain Mean years of pain = not given | |
| Interventions | "brief CBT: Pain Management Training" "education/attention control: Self care control" | |
| Outcomes | Primary pain outcome: Graded Chronic Pain Scale: Pain Intensity Primary disability outcome: none available Primary mood outcome: BDI depression Catastrophising outcome: PCS Graded Chronic Pain Scale: Activity Interference Graded Chronic Pain Scale: Pain Intensity Beck Depression Inventory (BDI) Mandibular Function Impairment Questionnaire (MFIQ) Survey of Pain Attitudes (SOPA) TMD self efficacy scale CSQ catastrophising subscale Pain Catastrophizing Scale rumination subscale Chronic Pain Coping Inventory (CPCI) task persistence, coping self statements, relaxation, rest | |
| Notes | CBT versus active, post‐treatment and follow‐up: analyses 1.1, 1.3, 2.1, 2.3 Yates quality scale: total quality = 27/35, design quality = 22/26, treatment quality = 5/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated by biostatistician |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes; independent personnel; treatment credibility unequal so used as covariate |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; CBT: WLC; post‐treatment: 6‐month follow‐up | |
| Participants | End of treatment: n = 152 Start of treatment: n = 158 Sex: 148 F, 10 M Mean age: 40.8 (SD 10.5) Mean years of pain: not given (< 5 years since diagnosis) Source = rheumatology clinics Diagnosis = fibromyalgia | |
| Interventions | Tailored CBT with exercise training; waiting list control | |
| Outcomes | Primary pain outcome: Pain IRGL Primary disability outcome: Mobility IRGL Primary mood outcome: Negative mood IRGL Catastrophising outcome: none Pain: 6 items of IRGL Disability: 7 mobility items of IRGL (reversed) Impact: Fibromyalgia Impact Questionnaire Negative mood: 6 items of IRGL Anxiety: 10 items of IRGL | |
| Notes | CBT versus WLC: analyses 3.1, 3.2, 3.3, 4.1, 4.2, 4.3 2011 update search Yates quality scale: total quality 24/35, design quality 15/26, treatment quality 9/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomized in clusters” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition reported; 2 differences between dropouts and completers |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 3 arms; assessed at pre‐treatment, post‐treatment, 6 months, 1 year | |
| Participants | End of treatment n = 122 Start of treatment n = 131 Sex: 110 F, 15 M Mean age = 44.0 (SD 9.4) Source = pain or rehabilitation clinic Diagnosis = fibromyalgia Mean years of pain = 10.2 | |
| Interventions | "cognitive + group discussion" "education + group discussion" "waiting list" | |
| Outcomes | Primary pain outcome: pain intensity score Primary disability outcome: none available Primary mood outcome: BDI depression Catastrophising outcome: none Composite scores from factor analysis: Pain intensity, pain coping, pain control, relaxation, catastrophising, pain behaviour, activity Measures contributing to factors: Multidimensional Pain Questionnaire: Pain Response Index Coping Strategies Questionnaire (CSQ) Beck Depression Inventory (BDI) (none available) Fear Survey Schedule Arthritis knowledge Maudsley Obsessive Compulsive Inventory Pain behaviour scale Multidimensional Pain Locus of Control Scale (MPCL) Walking distance, walking time, cycling time | |
| Notes | CBT versus active, post‐treatment: analyses 1.1, 1.3 Yates quality scale: total quality = 20/35, design quality = 16/26, treatment quality = 4/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly assigned” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reported |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | High risk | Not reported |
| Methods | RCT; 2 arms; acceptance and commitment therapy, CBT; post‐treatment and 6 month follow‐up | |
| Participants | End of treatment: n = 99 Start of treatment: n = 114 Sex: 58 F; 56 M Mean age: 54.9 (SD 12.5) Mean years of pain: 15 (SD 35.5) Source = primary care (40%); adverts and newspaper article (40%); pain support groups (10%); various (10%) Diagnosis = mixed chronic pain | |
| Interventions | ACT versus CBT | |
| Outcomes | Primary pain outcome: BPI pain severity Primary disability outcome: BPI interference Primary mood outcome: BDI Catastrophising outcome: none Pain severity: BPI Sub‐scale Disability: BPI Interference Sub‐scale (primary outcome) Disability: MPI General Activity Sub‐scale Depression: BDI‐II Anxiety: PASS Quality of life: SF‐12 physical and mental subscores Treatment Satisfaction Questionnaire | |
| Notes | ACT versus CBT: analyses 1.1, 1.2, 1.3, 2.1, 2.2, 2.3 2011 update search. Yates quality scale: total quality = 32/35, design quality = 24/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group randomisation generated by computer |
| Allocation concealment (selection bias) | Low risk | Staff member who accessed randomisation code had no contact with participants |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; several differences between dropouts and completers |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment staff blind to treatment condition |
| Methods | RCT; 3 arms; assessed at pre‐treatment, post‐treatment, 6 months, 1 year | |
| Participants | End of treatment n = 99 Start of treatment n = 121 Sex: 68 F, 53 M Mean age = 50.0 (SD 11.5) Source = pain clinic Diagnosis = mixed chronic pain, low back commonest Mean years of pain = 7.8 | |
| Interventions | "inpatient CBT" "outpatient CBT" "waiting list" | |
| Outcomes | Primary pain outcome: VAS pain Primary disability outcome: SIP patient‐rated Primary mood outcome: BDI depression Catastrophising outcome: CSQ catastrophising Visual analogue scale (VAS): pain intensity Visual analogue scale (VAS): pain distress Sickness Impact Profile (SIP): patient‐rated Beck Depression Inventory (BDI) State‐Trait Anxiety Inventory (STAI) Coping Strategies Questionnaire (CSQ): catastrophising Pain Self‐Efficacy Questionnaire (PSEQ) Pain Cognitions Questionnaire (PCQ) Walk distance Arm endurance Stair climb Stand ups Medication use Health care use | |
| Notes | CBT versus TAU, post‐treatment (waiting list not followed up): analyses 3.1, 3.2, 3.3 Yates quality scale: total quality = 22/35, design quality = 15/26, treatment quality = 7/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly assigned by throw of a die” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reported |
| Selective reporting (reporting bias) | High risk | Partially reported |
| Blinding of outcome assessment (detection bias) | Low risk | “interviewers and assistants blind to the patients’ treatment” |
| Methods | RCT; 3 arms; Assessed at pre‐treatment, post‐treatment, 6 months follow‐up | |
| Participants | Start of treatment N = 142 End of treatment N = 137 46 M, 97 F Mean age 62.1 men, 50.6 women Diagnosis = rheumatoid arthritis Mean years of rheumatoid arthritis 15.4 years men, 11.6 years women | |
| Interventions | "cognitive behavioral therapy for pain" "mindfulness meditation and emotion regulation therapy" "education‐only group" | |
| Outcomes | Primary pain outcome: pain diary 0 to 100 Primary disability outcome: none Primary mood outcome: PANAS negative affect Catastrophising outcome: CSQ catastrophising subscale rescored Pain once‐daily diary 0 to 100 Positive and Negative Affect Schedule (PANAS): provides positive affect and negative affect scores Depressive symptoms: sum of 6 items Pain coping efficacy (2 items, 1 to 5) CSQ catastrophising subscale Pain control 1 to 10 Disease Activity Score from examination of 28 joints by rheumatologist Interleukin IL‐6 | |
| Notes | December 2009 search Data obtained from author Used CBT for pain and education control group: 1.1, 1.3, 1.4, 2.1, 2.3, 2.4 Yates quality scale: total quality = 27/35, design quality = 19/26, treatment quality = 8/9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Not reported; treatment credibility measured but at end of treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition fully reported; no test for differences |
| Selective reporting (reporting bias) | Low risk | Fully reported |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment by staff not involved in treatment |
AIMS: Arthritis Impact Measurement Scale; BDI: Beck Depression Inventory; BT: behaviour therapy; CBT: cognitive behavioural therapy; CEQ: Cognitive Errors Questionnaire; CES‐D: Center for Epidemiologic Studies Depression Scale; CLBP: chronic low back pain; CSQ: Coping Strategies Questionnaire; DASS: Depression, Anxiety & Stress Scale; EMG: electromyograph; FESV: Pain‐Related Distress Questionnaire; FIQ: Fibromyalgia Impact Questionnaire; GA: graded activity; HADS: Hospital Anxiety and Depression Scale; HSCL: Hopkins Checklist; IRGL: Invloed van Reuma op Gezondheid en Leefwijze; MPQ PRI: Melzack Pain Questionnaire Pain Response Index; NRS: numerical rating scale; OMPQ: Orebro Musculoskeletal Pain Questionnaire; PANAS: Positive and Negative Affect Schedule; PCCL: Pain Coping and Cognition List; PCS: Pain Catastrophizing Scale; PDI: Pain Disability Index; PRSS: Pain‐Related Self‐Statements; PT: physical treatment; RAI: Rheumatoid Arthritis Index; RCT: randomised controlled trial; SD: standard deviation; SIP: Sickness Impact Profile; SLE: systemic lupus erythematosus; SOPA: Survey of Pain Attitudes; TAU: treatment as usual; TSK: Tampa Scale for Kinesiophobia; VAS: visual analogue scale; WHO: World Health Organization; WHYMPI: West Haven Yale Multidimensional Pain Inventory; WLC: waiting list control.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Insufficient psychotherapeutic content | |
| Hypnosis study | |
| Inadequate n: the number of patients in any treatment arm was less than 10 | |
| Not chronic pain | |
| Insufficient psychotherapeutic content | |
| N < 20 | |
| Insufficient psychotherapeutic content (participants could opt out of psychology and 71% did) | |
| Insufficient psychotherapeutic content | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| Internet trial | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| n < 10 | |
| Not all had chronic pain | |
| N < 20 | |
| No primary psychological treatment for pain or non‐psychological comparator | |
| No primary psychological treatment for pain or non‐psychological comparator | |
| N < 10 | |
| Not chronic pain | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| Intervention pre‐dental procedure: no outcome of psychology intervention available | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| No primary psychological treatment for pain or non‐psychological comparator | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| Not chronic pain | |
| Cross‐over trial and data on outcome collected after cross‐over | |
| Not clearly randomised | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| Trial plan not trial | |
| Insufficient psychotherapeutic content | |
| Not a treatment trial | |
| Insufficient psychotherapeutic content | |
| Hypnosis study | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| N < 10 | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| N < 20 | |
| N < 10 | |
| N < 10 | |
| Not chronic pain | |
| Not chronic pain | |
| N < 20 | |
| Internet trial | |
| Insufficient psychotherapeutic content (counselling) | |
| N < 20 | |
| N < 10 | |
| Insufficient psychotherapeutic content | |
| N < 20 | |
| Not chronic pain | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| N < 20 | |
| N < 10 | |
| N < 10 | |
| N < 20 | |
| Intervention for depression not pain | |
| N < 10 | |
| N < 20 | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| Insufficient psychotherapeutic content | |
| Not random allocation | |
| Insufficient psychotherapeutic content | |
| Not chronic pain | |
| Study of predictors not outcomes of intervention | |
| Insufficient psychotherapeutic content | |
| N < 20 | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| N < 10 | |
| N < 20 | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| Equivalence trial | |
| Not chronic pain | |
| No primary psychological treatment for pain or non‐psychological comparator | |
| N < 20 | |
| N < 20 | |
| Insufficient psychotherapeutic content | |
| N < 20 |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT; 2 arms; CT and “attention control” equivalent to treatment as usual |
| Participants | End of treatment: n = 30 Start of treatment: n = 30 Sex: 24 F; 6 M Mean age: 46 (range 38 to 57) Mean years of pain: not given Source: not given Diagnosis: resistant burning mouth syndrome |
| Interventions | Cognitive therapy; regular monitoring |
| Outcomes | Pain on 1 to 7 scale |
| Notes | Not identified by electronic searches but from references of another review |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 13 | 1258 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.24, 0.04] |
| Analysis 1.1  Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 12 | 1130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| Analysis 1.2  Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 13 | 1256 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.19, 0.09] |
| Analysis 1.3  Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 3 Mood. | ||||
| 4 Catastrophising Show forest plot | 6 | 735 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, 0.00] |
| Analysis 1.4  Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 4 Catastrophising. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 11 | 1261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.06] |
| Analysis 2.1  Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 12 | 1295 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.28, ‐0.02] |
| Analysis 2.2  Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 11 | 1261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.05] |
| Analysis 2.3  Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 3 Mood. | ||||
| 4 Catastrophising Show forest plot | 2 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.18, 0.29] |
| Analysis 2.4  Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 4 Catastrophising. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 16 | 1148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.37, ‐0.05] |
| Analysis 3.1  Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 15 | 1105 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.47, ‐0.04] |
| Analysis 3.2  Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 12 | 899 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.57, ‐0.18] |
| Analysis 3.3  Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 3 Mood. | ||||
| 4 Catastrophising Show forest plot | 5 | 308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.76, ‐0.31] |
| Analysis 3.4  Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 4 Catastrophising. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 7 | 635 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.25, 0.08] |
| Analysis 4.1  Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 6 | 450 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.51, 0.25] |
| Analysis 4.2  Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 7 | 637 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.51, ‐0.00] |
| Analysis 4.3  Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 3 Mood. | ||||
| 4 Catastrophising Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 4 Catastrophising. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Behavioural vs active control post‐treatment, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.58, 0.07] |
| Analysis 5.2  Comparison 5 Behavioural vs active control post‐treatment, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Behavioural vs active control post‐treatment, Outcome 3 Mood. | ||||
| 4 Catastrophising Show forest plot | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.60, 0.05] |
| Analysis 5.4  Comparison 5 Behavioural vs active control post‐treatment, Outcome 4 Catastrophising. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Behavioural vs active control follow‐up, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.50, 0.16] |
| Analysis 6.2  Comparison 6 Behavioural vs active control follow‐up, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Behavioural vs active control follow‐up, Outcome 3 Mood. | ||||
| 4 Catastrophising Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Behavioural vs active control follow‐up, Outcome 4 Catastrophising. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 5 | 484 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.79, 0.24] |
| Analysis 7.1  Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 5 | 504 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.98, 0.16] |
| Analysis 7.2  Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 3 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.42, 0.35] |
| Analysis 7.3  Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 3 Mood. | ||||
| 4 Catastrophising Show forest plot | 3 | 269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.43, ‐0.01] |
| Analysis 7.4  Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 4 Catastrophising. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.32, 0.26] |
| Analysis 8.1  Comparison 8 Behavioural vs treatment as usual follow‐up, Outcome 1 Pain. | ||||
| 2 Disability Show forest plot | 3 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.51, 0.44] |
| Analysis 8.2  Comparison 8 Behavioural vs treatment as usual follow‐up, Outcome 2 Disability. | ||||
| 3 Mood Show forest plot | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.07, 0.77] |
| Analysis 8.3  Comparison 8 Behavioural vs treatment as usual follow‐up, Outcome 3 Mood. | ||||
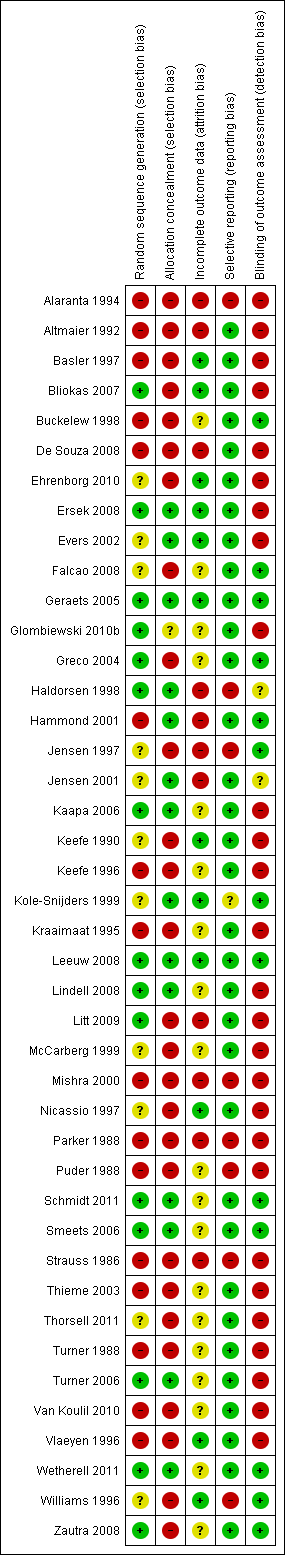
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 1 Pain.

Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 2 Disability.

Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 3 Mood.
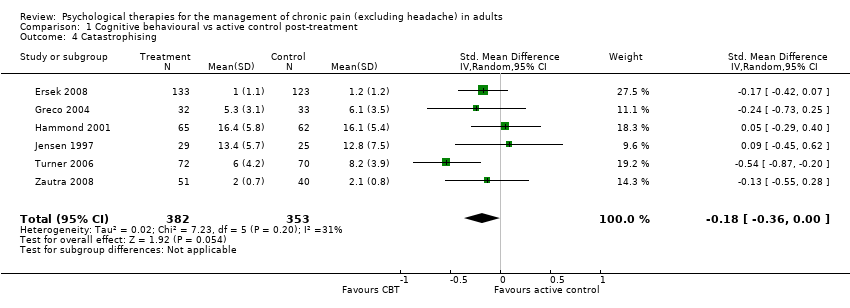
Comparison 1 Cognitive behavioural vs active control post‐treatment, Outcome 4 Catastrophising.

Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 1 Pain.
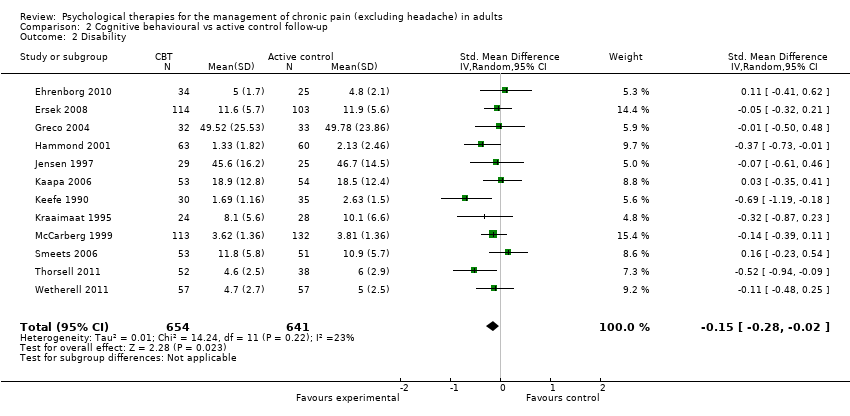
Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 2 Disability.
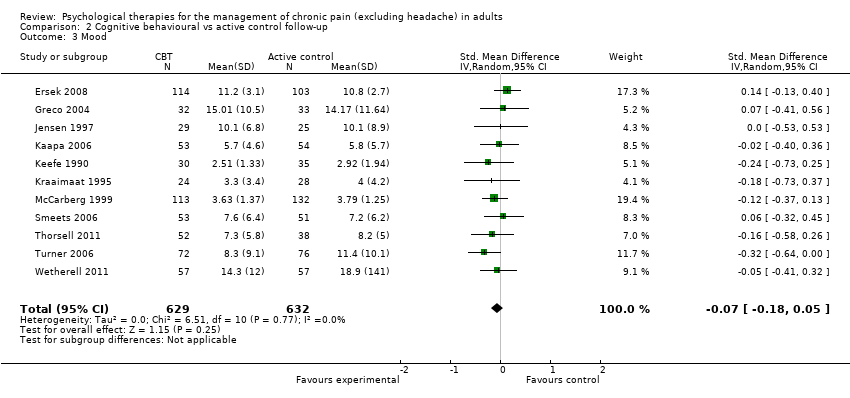
Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 3 Mood.

Comparison 2 Cognitive behavioural vs active control follow‐up, Outcome 4 Catastrophising.
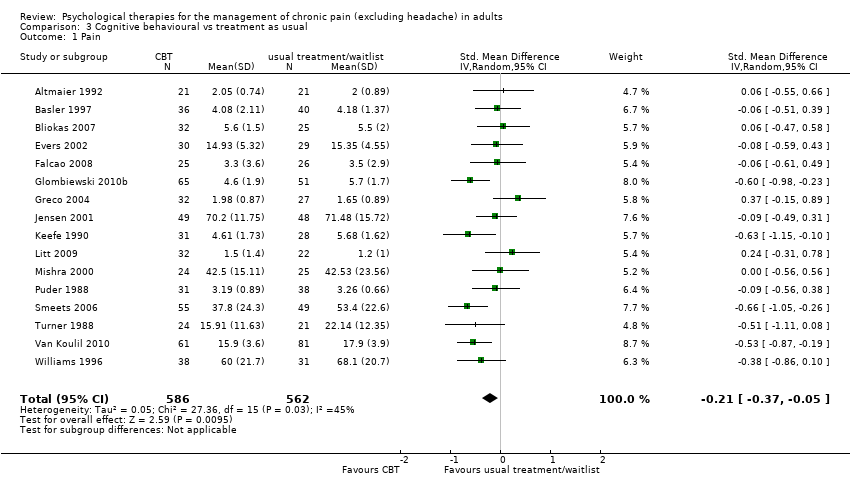
Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 1 Pain.
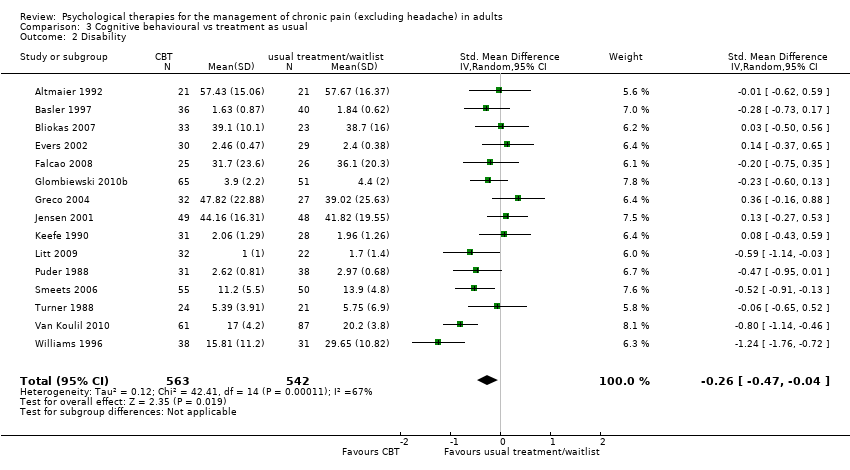
Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 2 Disability.

Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 3 Mood.

Comparison 3 Cognitive behavioural vs treatment as usual, Outcome 4 Catastrophising.
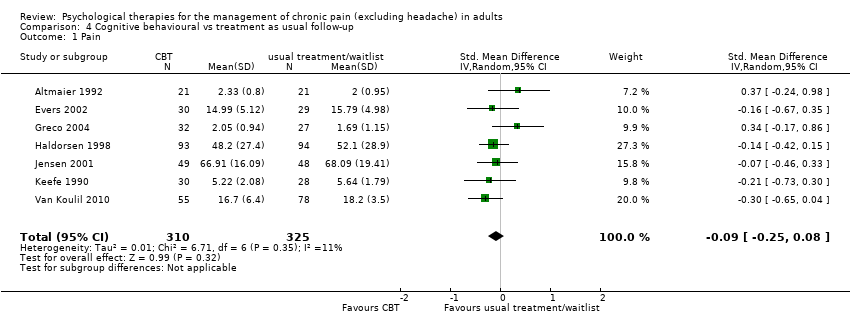
Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 1 Pain.
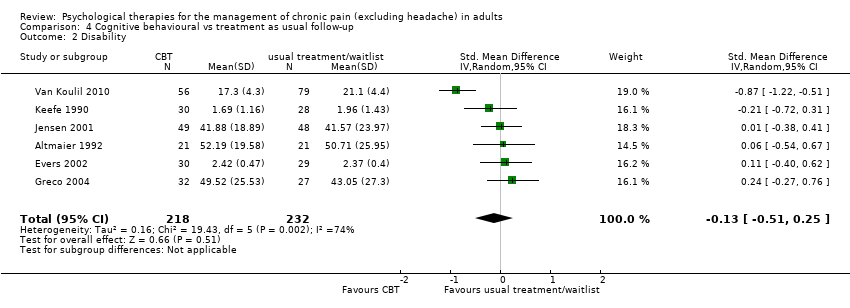
Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 2 Disability.

Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 3 Mood.
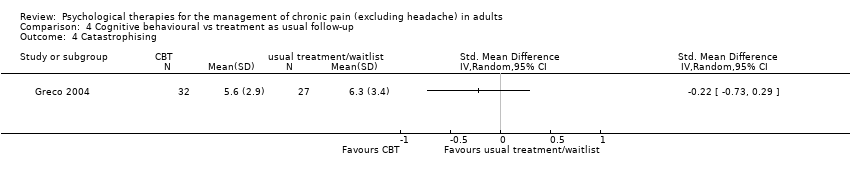
Comparison 4 Cognitive behavioural vs treatment as usual follow‐up, Outcome 4 Catastrophising.

Comparison 5 Behavioural vs active control post‐treatment, Outcome 1 Pain.

Comparison 5 Behavioural vs active control post‐treatment, Outcome 2 Disability.
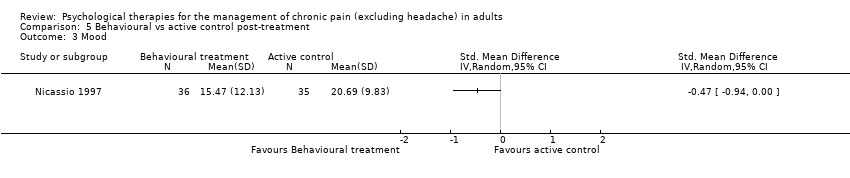
Comparison 5 Behavioural vs active control post‐treatment, Outcome 3 Mood.

Comparison 5 Behavioural vs active control post‐treatment, Outcome 4 Catastrophising.

Comparison 6 Behavioural vs active control follow‐up, Outcome 1 Pain.

Comparison 6 Behavioural vs active control follow‐up, Outcome 2 Disability.

Comparison 6 Behavioural vs active control follow‐up, Outcome 3 Mood.

Comparison 6 Behavioural vs active control follow‐up, Outcome 4 Catastrophising.
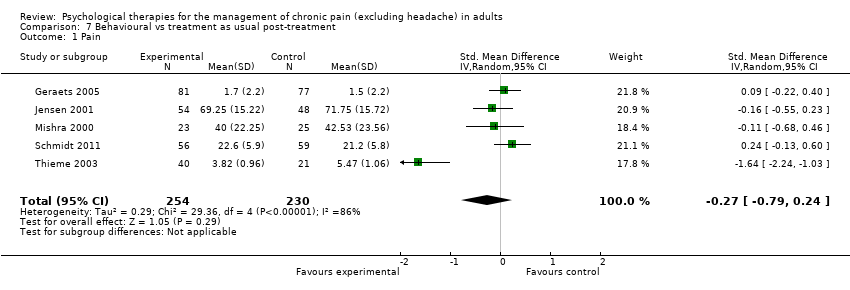
Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 1 Pain.

Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 2 Disability.
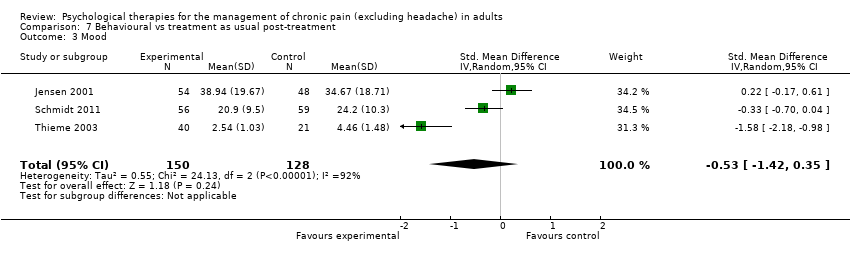
Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 3 Mood.

Comparison 7 Behavioural vs treatment as usual post‐treatment, Outcome 4 Catastrophising.

Comparison 8 Behavioural vs treatment as usual follow‐up, Outcome 1 Pain.

Comparison 8 Behavioural vs treatment as usual follow‐up, Outcome 2 Disability.
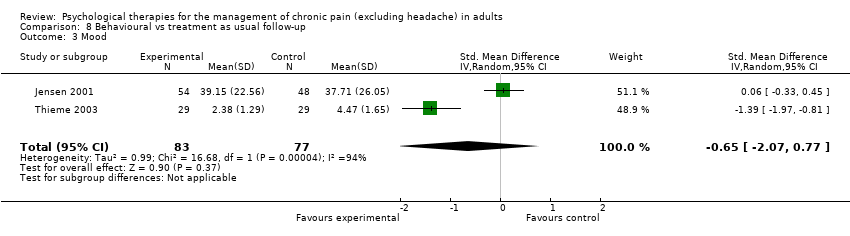
Comparison 8 Behavioural vs treatment as usual follow‐up, Outcome 3 Mood.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 13 | 1258 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.24, 0.04] |
| 2 Disability Show forest plot | 12 | 1130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| 3 Mood Show forest plot | 13 | 1256 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.19, 0.09] |
| 4 Catastrophising Show forest plot | 6 | 735 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, 0.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 11 | 1261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.06] |
| 2 Disability Show forest plot | 12 | 1295 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.28, ‐0.02] |
| 3 Mood Show forest plot | 11 | 1261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.05] |
| 4 Catastrophising Show forest plot | 2 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.18, 0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 16 | 1148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.37, ‐0.05] |
| 2 Disability Show forest plot | 15 | 1105 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.47, ‐0.04] |
| 3 Mood Show forest plot | 12 | 899 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.57, ‐0.18] |
| 4 Catastrophising Show forest plot | 5 | 308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.76, ‐0.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 7 | 635 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.25, 0.08] |
| 2 Disability Show forest plot | 6 | 450 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.51, 0.25] |
| 3 Mood Show forest plot | 7 | 637 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.51, ‐0.00] |
| 4 Catastrophising Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Disability Show forest plot | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.58, 0.07] |
| 3 Mood Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Catastrophising Show forest plot | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.60, 0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Disability Show forest plot | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.50, 0.16] |
| 3 Mood Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Catastrophising Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 5 | 484 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.79, 0.24] |
| 2 Disability Show forest plot | 5 | 504 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.98, 0.16] |
| 3 Mood Show forest plot | 3 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.42, 0.35] |
| 4 Catastrophising Show forest plot | 3 | 269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.43, ‐0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.32, 0.26] |
| 2 Disability Show forest plot | 3 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.51, 0.44] |
| 3 Mood Show forest plot | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.07, 0.77] |

