Topical rubefacients for acute and chronic pain in adults
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT DB crossover groups Duration 7 days in each phase | |
| Participants | Chronic osteoarthritis of the knee (mean 17 years duration, not secondary to other arthritis or acute trauma, confirmed by X‐ray) All patients had at least moderate pain N = 26 (one excluded from analysis due to unrelated medical problem) M = 24, F = 1 Mean age 62 years (range 35‐72) | |
| Interventions | Triethanolamine salicylate (10%) cream (Myoflex), n = 25 Placebo cream, n = 25 3.5 g x 4 daily to affected knee | |
| Outcomes | Preferred drug or placebo or neither based on: Pain 4 point scale Pain 11 point scale Patient assessed pain relief 5 point scale Physician assessed pain relief 5 point scale Physician assessed tenderness 4 point scale Patient preference Continuous measures of swelling, stiffness, and activity Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R1 DB2 W1 Oxford Pain Validity Scale: 10 Ineligible for inclusion if salicylates within two days before test period Eligible if on other drug treatment, if taking NSAIDs included only if stable on stated dose for preceding month No change in dose of existing drugs or new analgesics started during the study period No intra‐articular steroids within last 6 weeks No other treatment (heat, exercise, massage) during study period | |
| Methods | RCT DB parallel groups Duration 10 days | |
| Participants | Musculoskeletal pain (e.g. tendon, muscle, or ligament injury) Patients had moderate or mild pain N = 20 M = 8, F = 12 Age range 19‐86 years | |
| Interventions | Diethylamine salicylate (10%), myrtecaine (1%) cream (Algesal Suractive), n = 10 Placebo cream, n = 10 x 3 daily at the site of pain | |
| Outcomes | Rest pain 4 point scale Functional limitation 5 point scale Presence of: Spontaneous pain Swelling Heat Composite score based on above (20 points) Improvement in: Rest pain 4 point scale Composite score | |
| Notes | Oxford Quality Score: R1 DB2 W0 Oxford Pain Validity Scale: 10 Myrtecaine (Nopoxamine) is a local anaesthetic agent | |
| Methods | RCT DB parallel groups Duration 15 days Assessment on days 2, 3, 4, 8, 15, 29 | |
| Participants | Acute ankle sprain presenting within 48 h Injury severity rated moderate or severe N = 80 M = 63, F = 17 Mean age 27 years (range 18‐50) | |
| Interventions | Salicylic acid (2%), adrenal extract (1%), mucopolysaccharide polysulphate (.2%) ointment (Mobilat), n = 40 Placebo ointment, n = 40 10‐15 cm x 2 daily | |
| Outcomes | Pressure distribution on walking Swelling Ankle joint movement Rest pain 100 mm VAS Movement pain 100 mm VAS Adverse events | |
| Notes | Oxford Quality Score: R2 DB2 W1 Oxford Pain Validity Scale: 12 Suprarenal extract results in 0.02% corticosteroids | |
| Methods | RCT DB parallel groups Duration 11 days Assessment at on days 2, 4, 9, 11 | |
| Participants | Acute ankle or knee sprain within 24 h Patients had moderate or slight pain N = 156 M = 98, F = 58 Mean age 32 years (range 18‐65) | |
| Interventions | Salicylic acid (2%), mucopolysaccharide polysulphate (.2%) cream (Movelat), n = 78 Placebo cream, n = 78 10 cm x 2 daily | |
| Outcomes | Movement pain 100 mm VAS Rest pain 100 mm VAS Swelling Physician global assessment 4 point scale Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R1 DB2 W0 Oxford Pain Validity Scale: 10 No concomitant treatment allowed except max 1 g paracetamol x3 daily | |
| Methods | RCT DB active control crossover groups Duration 7 days in each phase 4 day washout between phases | |
| Participants | Chronic musculoskeletal disorders (extra‐articular, articular, and vertebral musculoskeletal illness, some sprains) N = 50 M = 25, F = 25 Average age 49 years | |
| Interventions | Diethylamine salicylate (10%), sodium heparin (50 IU/g), menthol (0.2%) gel (Dolo‐Menthoneurin), n = 25 Etofenamate (5%), n = 25 | |
| Outcomes | Spontaneous pain 4 point scale Tenderness 4 point scale Swelling 4 point scale Movement restriction 4 point scale After first phase: Patient global assessment 4 point scale Physician global assessment 4 point scale After second phase: Patient global assessment 3 point scale Physician global assessment 3 point scale Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R1 DB1 W1 Oxford Pain Validity Scale: 7 Etofenamate is an NSAID Adverse events reported for both phases combined | |
| Methods | RCT DB parallel groups Duration 14 days Assessment on days 3, 14 | |
| Participants | Acute mechanical low back pain N = 40 | |
| Interventions | Methylsalicylate (2.6%), ethylsalicylate (1.8%), glycol salicylate (.9%), salicylic acid (.9%), camphor (0.4%), menthol (5.5%), capsicum oleoresin (1.5%) ointment (Rado‐Salil), n = 20 Placebo ointment, n = 20 Frequency of application not stated | |
| Outcomes | Pain 100 mm VAS Duration of confinement to bed Muscular reflex contracture 5 point scale Spine mobility: Schober's index Finger‐floor distance Lumbar extension Patient global assessment 5 point scale Physician global assessment 5 point scale Number of rescue paracetamol (250 mg) tablets Amount of ointment used Adverse events | |
| Notes | Oxford Quality Score: R1 DB2 W0 Oxford Pain Validity Scale: 9 No analgesics, anti‐inflammatories, or physical treatments allowed other than rescue medication (max 45 x 250 mg paracetamol) | |
| Methods | RCT DB double dummy active control parallel groups Duration 7 days Daily assessment | |
| Participants | Chronic musculoskeletal pain (articular, e.g. osteoarthritis, and non‐articular, e.g. bursitis) for at least weeks (mean 3 years' duration, range weeks to 25 years) Baseline pain at least mild to moderate N = 40 M = 10, F = 30 Mean age 53 years (range 20‐81) | |
| Interventions | Triethanolamine salicylate (10%) cream (Aspercreme) + placebo tablets, n = 20 Aspirin (325 mg) tablets + placebo cream, n = 20 Cream applied to affected area and two tablets taken x 4 daily (mealtimes and bedtime) | |
| Outcomes | Pain relief 4 point scale Speed of pain relief Pain severity Patient global assessment of pain relief 4 point scale Physician global assessment of pain relief 4 point scale Combined physician and patient global assessment 4 point scale Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R1 DB2 W1 Oxford Pain Validity Scale: 9 One week washout of aspirin before trial All other anti‐inflammatories allowed during trial Excluded if pre‐existing high dose aspirin therapy | |
| Methods | RCT DB active control parallel group Duration 12 days Assessment on days 4, 8, 12 | |
| Participants | Slight articular and extra‐articular sports injuries in last 24 h N = 137 Average age 23 years (range 13‐59) | |
| Interventions | Benzydamine salicylate (6%) spray (Benzasal), n = 35 Fepradinol (6%) spray (Dalgen), n = 102 One spray x 4 daily | |
| Outcomes | Pain on passive movement 5 point scale Pain on active movement 5 point scale Inflammation 5 point scale Functional limitation 5 point scale Time to cure Physician global assessment 5 point scale Adverse events | |
| Notes | Oxford Quality Score: R1 DB1 W0 Oxford Pain Validity Scale: 7 Baseline scores for inflammation differed between the two groups Fepradinol is an NSAID | |
| Methods | RCT DB parallel groups Duration 7 days Assessment on days 3, 7 | |
| Participants | Sprained ankle Baseline pain slight to severe N = 42 M = 20, F = 22 Age range 15 to 60+ years | |
| Interventions | Salicylic acid (2%), adrenal extract (1%), mucopolysaccharide polysulphate (0.2%) gel (Movelat), n = 20 Placebo gel, n = 22 | |
| Outcomes | Relief of pain Time to return to normal activity Adverse events Composite score based on above plus ankle ROM, swelling Withdrawals | |
| Notes | Oxford Quality Score: R1 DB1 W0 Oxford Pain Validity Scale: 11 | |
| Methods | RCT DB parallel groups Duration 15 days Assessment on days 10, 15, 20 | |
| Participants | Temporomandibular disorders N = 52 M = 5, F = 47 | |
| Interventions | Methylsalicylate, copper and zinc pyrocarboxylate, lysine‐aspartic acid, herbal extracts cream (Theraflex‐TMJ), n = 26 Placebo cream, n = 26 1/4 to 1/2 teaspoon cream onto affected area x 2 daily (morning, bedtime) | |
| Outcomes | Spontaneous pain 10 cm VAS Adverse events | |
| Notes | Oxford Quality Score: R2 DB1 W0 Oxford Pain Validity Scale: 7 | |
| Methods | RCT DB parallel groups Duration 9 days Assessment on days 3, 7, 9 | |
| Participants | Sports injuries Baseline pain mild to severe N = 100 M = 49, F = 32 Average age 30 years (range 14‐58) | |
| Interventions | Escin 1%, diethylamine salicylate 5% (Reparil‐Gel), n = 50 Placebo gel, n = 50 Gel applied at least x 4 daily to affected area | |
| Outcomes | Spontaneous pain 4 point scale Loaded pain 4 point scale Movement pain 4 point scale Pain on pressure 4 point scale Tightness 4 point scale Temperature 4 point scale Haematoma 4 point scale Swelling 4 point scale Ratio of range of movement to unaffected limb Ratio of size to unaffected limb Patient global assessment 5 point scale Physician global assessment 5 point scale Improvement in spontaneous pain 3 point scale Improvement in movement pain 3 point scale Remission in spontaneous pain 3 point scale Remission in movement pain 3 point scale Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R1 DB2 W1 Oxford Pain Validity Score: 8 19 patients had no data and were not included | |
| Methods | RCT DB parallel groups Duration 14 days Assessment days 7, 14 | |
| Participants | Non‐articular rheumatic back pain N = 113 Average age 56 years | |
| Interventions | Glycol salicylate 10% gel (Phardol‐Mono), n = 54 Placebo gel, n = 59 5 cm x 3 or x 4 on affected area | |
| Outcomes | Drop‐out pain‐free at day 14 Drop‐out pain‐free at day 7 2 point reduction on 10 cm VAS at day 14 Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R1 DB2 W0 Oxford Pain Validity Score: 12 16 patients excluded due to high rheumatoid factor levels | |
| Methods | RCT DB parallel groups Duration 4 weeks Assessment weeks 2, 4 | |
| Participants | Osteoarthritis of the hip or knee N = 116 M = 52, F = 64 Mean age 61 years (range 19‐86) | |
| Interventions | Copper (0.4%) salicylate (4%) gel in vehicle (methanol 2%, camphor 1%, eucalyptus oil 1%), n = 58 Placebo vehicle gel, n = 58 1.5 g x 2 daily applied to inner forearm | |
| Outcomes | Rest pain 100 mm VAS Movement pain 100 mm VAS Patient rated efficacy 4 point scale Investigator rated 4 point scale Use of rescue medication Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R2 DB2 W1 Oxford Pain Validity Score: 11 Gel applied remote to site of injury 500 mg paracetamol rescue medication provided Excluded if: NSAIDs in last 7 days Corticosteroids in last 28 days Alterations in arthritis treatment in last 28 days | |
| Methods | RCT DB pseudo‐active control (assumed to be placebo in this review), parallel groups Duration 7 days Daily assessment One day washout if NSAIDs or other analgesia taken in last 24 h | |
| Participants | Acute low back pain in last 72 h Moderate to severe pain on movement N = 161 M = 87, F = 74 Mean age 41 years | |
| Interventions | Glycol salicylate (10%), methylnicotinate (1%), capsicum oleoresin (0.1%), histamine hydrochloride (0.1%) (Cremor Capsici Compositus FNA), n = 78 Comfrey (10%), poison ivy (5%), marsh Labrador tea (5%) gel (Spiroflor SRL), n = 83 3 g x3 daily applied to affected area | |
| Outcomes | 80% reduction in pain 100 mm VAS 100% reduction in pain 100 mm VAS Nights of disturbed sleep Absence from work Use of rescue analgesia Patient global assessment 6 point scale Physician global assessment 6 point scale Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R2 DB1 W1 Oxford Pain Validity Score: 12 Spiroflor SRL, while officially classified as 'homeopathic' in some countries, would be better considered as a herbal remedy because the active ingredients are not diluted to homeopathic levels 500 mg paracetamol rescue medication (max 8 x 500 mg tablets daily) Treatments were not identical in smell, colour, or consistency Protocol compliance was poor (mainly due to under/over dosing) Concentration of capsaicin is only 0.008% | |
| Methods | RCT DB pseudo‐active control (assumed to be placebo in this review), parallel groups Duration 14 days Assessment at days 3, 6, 9, 14 | |
| Participants | Musculoskeletal (knee, spinal or shoulder) disease N = 100 M = 48, F = 52 Average age 51 years | |
| Interventions | Ethylene glycol monosalicylate ester (10%), nonivamide (0.2%) in ointment base of sodium heparin (50 IU/g), methylsalicylate (0.1%) and essential oils (Enelbin‐Rheuma), n = 50 Salicylic acid (2%) in above ointment base n = 50 8‐10 cm of ointment on affected site x 3 or x 4 daily | |
| Outcomes | Restriction of movement 4 point scale Swelling 4 point scale Muscle tension 4 point scale Spontaneous pain 4 point scale Pain on pressure 4 point scale Movement pain 4 point scale Physician global assessment 4 point scale based on above scores Curative efficacy 4 point scale Withdrawals Adverse events | |
| Notes | Oxford Quality Score: R2 DB2 W1 Oxford Pain Validity Score: 10 | |
| Methods | RCT DB parallel groups Duration 15 days | |
| Participants | Musculoskeletal disease (e.g. osteoarthritis) and traumatic injury (e.g. sprains) Baseline pain none to intense N = 56 M = 20, F = 36 Mean age 54 years | |
| Interventions | Diethylamine salicylate (10%), myrtecaine (1%) cream (Algesal Suractive), n = 32 Placebo cream, n = 24 Application x 3 daily | |
| Outcomes | Improvement in global assessment 4 point scale (global assessment based on 18 point scale of basic pain, paroxysmal pain, swelling, functional limitation) Improvement in rest pain 4 point scale Improvement in paroxysmal pain 4 point scale Improvement in swelling 4 point scale Improvement in functional limitation 4 point scale | |
| Notes | Oxford Quality Score: R2 DB1 W0 Oxford Pain Validity Scale: 9 Myrtecaine (Nopoxamine) is a local anaesthetic agent Patients on anti‐inflammatories or analgesics excluded | |
DB ‐ double blind; F ‐ female; M ‐ male; N ‐ total number in study; n ‐ number in treatment arm; R ‐ randomised; RCT ‐ randomised controlled trial; W ‐ withdrawals
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not RCT | |
| Cannot extrapolate data | |
| Not rubefacient, not blinded | |
| Not RCT | |
| Not randomised | |
| Oral condition | |
| Too short duration | |
| Quasi‐randomised | |
| No usable data | |
| Study I is a republished version of Golden 1978 but no data could be extracted for either Study I or Study II | |
| Not RCT | |
| Oral condition, not RCT |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success (e.g. 50% reduction in pain) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Rubefacient versus placebo, Outcome 1 Clinical success (e.g. 50% reduction in pain). | ||||
| 1.1 Acute conditions | 4 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.51, 2.46] |
| 1.2 Chronic conditions | 6 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.22, 2.04] |
| 2 Adverse events Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Rubefacient versus placebo, Outcome 2 Adverse events. | ||||
| 2.1 Any adverse event | 11 | 984 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.19, 2.04] |
| 2.2 Local adverse events | 10 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.12, 4.12] |
| 3 Withdrawals Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Rubefacient versus placebo, Outcome 3 Withdrawals. | ||||
| 3.1 Lack of efficacy | 5 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.87] |
| 3.2 Adverse events | 7 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.19 [1.52, 11.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success (e.g. 50% reduction in pain) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Rubefacient versus active control, Outcome 1 Clinical success (e.g. 50% reduction in pain). | ||||
| 1.1 Acute | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Chronic | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Rubefacient versus active control, Outcome 2 Adverse events. | ||||
| 2.1 Any adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Local adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawals Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Rubefacient versus active control, Outcome 3 Withdrawals. | ||||
| 3.1 Lack of efficacy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
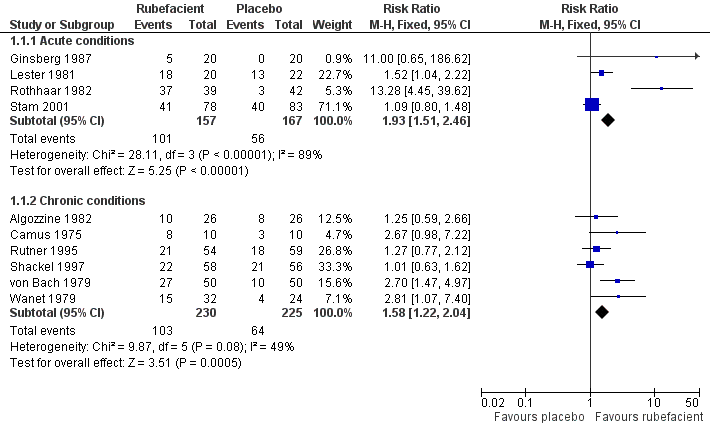
Forest plot of comparison: 1 Rubefacient versus placebo, outcome: 1.1 Clinical success (e.g. 50% reduction in pain).

Forest plot of comparison: 1 Rubefacient versus placebo, outcome: 1.4 Adverse events.
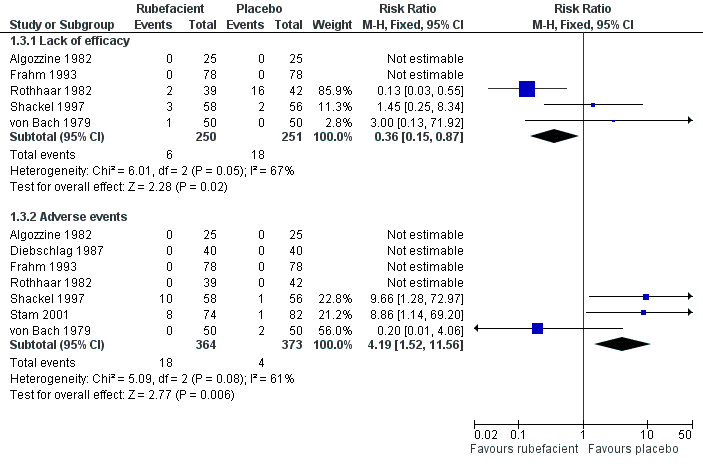
Forest plot of comparison: 1 Rubefacient versus placebo, outcome: 1.2 Withdrawals.
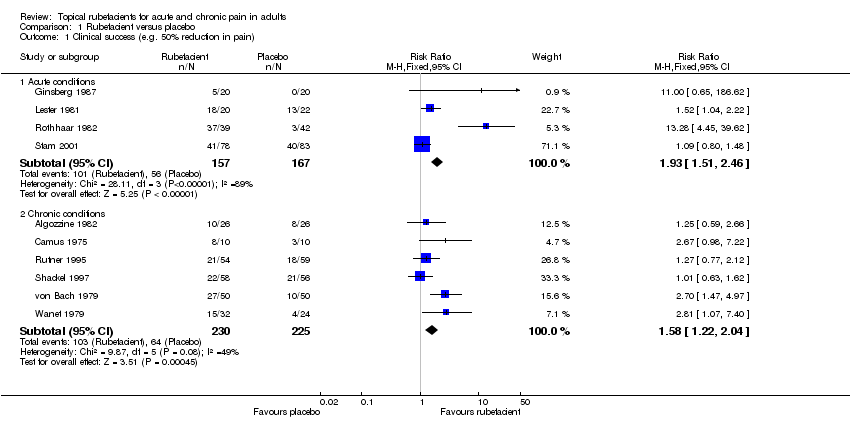
Comparison 1 Rubefacient versus placebo, Outcome 1 Clinical success (e.g. 50% reduction in pain).
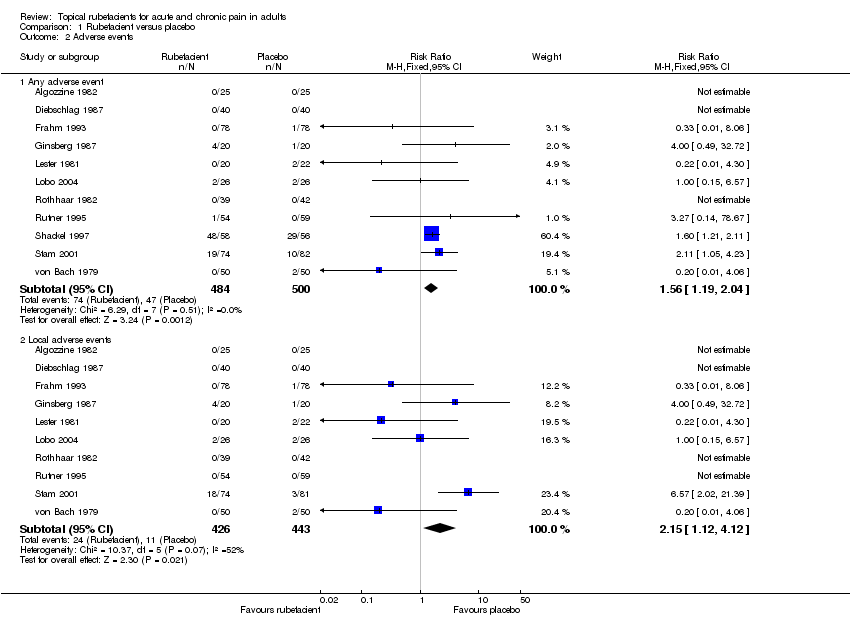
Comparison 1 Rubefacient versus placebo, Outcome 2 Adverse events.
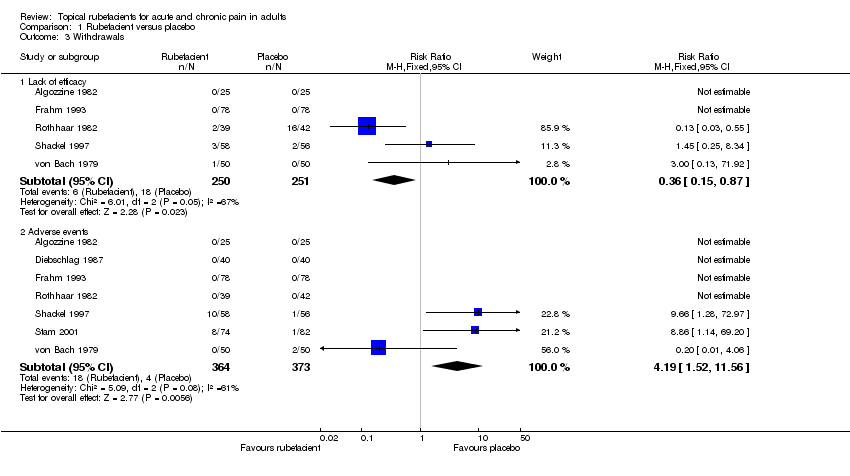
Comparison 1 Rubefacient versus placebo, Outcome 3 Withdrawals.
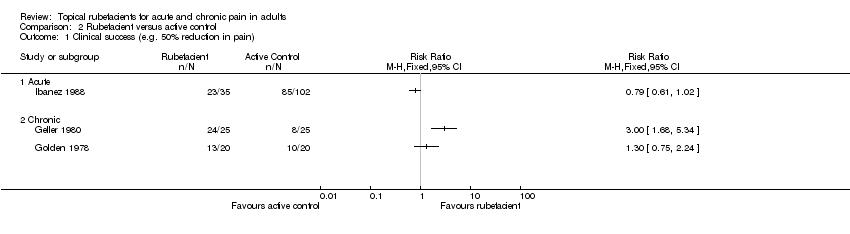
Comparison 2 Rubefacient versus active control, Outcome 1 Clinical success (e.g. 50% reduction in pain).
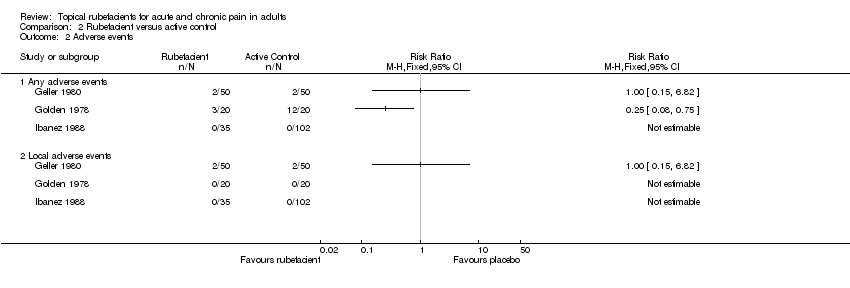
Comparison 2 Rubefacient versus active control, Outcome 2 Adverse events.

Comparison 2 Rubefacient versus active control, Outcome 3 Withdrawals.
| Analgesia | ||||
| Study ID | Treatment | Outcome measure | Success | Rescue Medication |
| Acute | ||||
| (1) Salicylate, adrenal extract, and mucopolysaccharide ointment (Mobilat) (2) Placebo ointment | Movement pain on 100 mm VAS at: (a) 8 days (b) 15 days | No dichotomous data (a) Significant difference in favour of (1) (b) Significant difference in favour of (1) | No data | |
| (1) Salicylate and mucopolysaccharide cream (Movelat) (2) Placebo cream | Movement pain on 100 mm VAS at: (a) 9 days (b) 11 days | No dichotomous data (a) Significant difference in favour of (1) (b) No significant difference | No data | |
| (1) Salicylate and capsicum oleoresin ointment (Rado‐Salil) (2) Placebo ointment | Patient global assessment ('excellent' or 'good') at: (a) 3 days (b) 14 days | (a) (1) 5/20 (2) 0/20 (b) (1) 10/20 (2) 2/20 | Total number of rescue tablets (250 mg paracetamol) used: (1) 24 (2) 36 | |
| (1) Salicylate spray (2) Fepradinol spray active control | 'Cure' at 12 days | (1) 23/35 (2) 85/102 | No data | |
| (1) Salicylate, adrenal extract, and mucopolysaccharide gel (Movelat) (2) Placebo gel | Relief of pain by 7 days | (1) 18/20 (2) 13/22 | No data | |
| (1) Salicylate gel (Reparil‐Gel) (2) Placebo gel | Patient global assessment ('very good' or 'good') at 9 days | (1) 37/39 (2) 3/42 | No data | |
| (1) Salicylate, nicotinate, capsicum oleoresin, and histamine gel (Cremor Capsici Compositus FNA) (2) Herbal gel (Spiroflor SRL) active control | 80% reduction in pain on 100 mm VAS at 7 days | (1) 41/78 (2) 40/83 | Number using rescue medication (paracetamol): (1) 65/82 (2) 56/75 | |
| Chronic | ||||
| (1) Salicylate cream (Myoflex) (2) Placebo cream | Pain relief score at 7 days favours (1) or (2) | (1) 10/26 (2) 8/26 | No data | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | Improvement in rest pain score at 10 days | (1) 8/10 (2) 3/10 | No data | |
| (1) Salicylate and heparin gel (Dolo‐Menthoneurin) (2) Etofenamate gel active control | Patient global score ('very good' or 'good') after phase 1 at 7 days | (1) 24/25 (2) 8/25 | No data | |
| (1) Salicylate cream (Aspercreme) + placebo tablets (2) Aspirin tablets + placebo cream active control | Patient global assessment of pain relief ('excellent' or 'good') at 7 days | (1) 13/20 (2) 10/20 | No data | |
| (1) Salicylate cream (Theraflex‐TMJ) | Spontaneous pain VAS (10 cm) at: (a) 15 days (b) 10 days | No dichotomous data (a) Significant difference in favour of (1) (b) No significant difference | No data | |
| (1) Salicylate gel (Phardol‐Mono) (2) Placebo gel | Drop‐out 'pain free' by day 14 | (1) 21/54 (2) 18/59 | No data | |
| (1) Salicylate gel (2) Placebo gel | Patient global assessment ('very good' or 'good') at 28 days | (1) 22/58 (2) 21/56 | Number using rescue medication (paracetamol): (1) 43/56 (2) 39/55 (1) 555 (2) 600 | |
| (1) Salicylate and nonivamide in heparin and salicylate ointment (Enelbin‐Rheuma) (2) Salicylate in heparin and salicylate ointment active control | Global assessment ('very good' or 'good') at 14 days | (1) 27/50 (2) 10/50 | No data | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | Rest pain score at 15 days | (1) 15/32 (2) 4/24 | No data | |
| Withdrawals and exclusions | Adverse events | |||||
| Study ID | Treatment | All withdrawals and exclusions | Lack of efficacy | Adverse events | All adverse events | Local adverse events |
| (1) Salicylate cream (Myoflex) (2) Placebo cream | 1/26 unrelated to study | (1) 0/25 (2) 0/25 | (1) 0/25 (2) 0/25 | (1) 0/25 (2) 0/25 | (1) 0/25 (1) 0/25 | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | No data | No data | No data | No data | No data | |
| (1) Salicylate, adrenal extract, and mucopolysaccharide ointment (Mobilat) (2) Placebo ointment | No data | No data | (1) 0/40 (2) 0/40 | (1) 0/40 (2) 0/40 | (1) 0/40 (2) 0/40 | |
| (1) Salicylate and mucopolysaccharide cream (Movelat) (2) Placebo cream | 7/16 violation of protocol | (1) 0/78 (2) 0/78 | (1) 0/78 (2) 0/78 | (1) 0/78 (2) 1/78 | (1) 0/78 (2) 1/78 | |
| (1) Salicylate and heparin gel (Dolo‐Menthoneurin) (2) Etofenamate gel active control | Phase 1: (1) 0/25 (2) 0/25 | Phase 1: (1) 0/25 (2) 0/25 | Phase 1: (1) 0/25 (2) 0/25 Phase 2: (1) 0/25 (2) 0/25 | Phases 1 and 2 combined: (1) 2/50 (2) 2/50 | Phases 1 and 2 combined: (1) 2/50 (2) 2/50 | |
| (1) Salicylate and capsicum oleoresin ointment (Rado‐Salil) (2) Placebo ointment | No data | No data | No data | (1) 4/20 (2) 1/20 | (1) 4/20 (2) 1/20 | |
| (1) Salicylate cream (Aspercreme) + placebo tablets (2) Aspirin tablets + placebo cream active control | (1) 1/20 (2) 8/20 | (1) 1/20 (2) 2/20 | (1) 0/20 (2) 6/20 | (1) 3/20 (2) 12/20 | (1) 0/20 (2) 0/20 | |
| (1) Salicylate spray (2) Fepradinol spray active control | No data | No data | (1) 0/35 (2) 0/102 | (1) 0/35 (2) 0/102 | (1) 0/35 (2) 0/102 | |
| (1) Salicylate, adrenal extract, and mucopolysaccharide gel (Movelat) (2) Placebo gel | 8/50 4 excluded due to fractures, 4 lost to follow‐up | No data | No data | (1) 0/20 (2) 2/22 | (1) 0/20 (2) 2/22 | |
| (1) Salicylate cream (Theraflex‐TMJ) | No data | No data | No data | (1) 2/26 (2) 2/26 | (1) 2/26 (2) 2/26 | |
| (1) Salicylate gel (Reparil‐Gel) (2) Placebo gel | (1) 13/50 11 with no data, rest lack of efficacy (2) 24/50 8 with no data, rest lack of efficacy | (1) 2/39 (2) 16/42 | (1) 0/39 (2) 0/42 | (1) 0/39 (2) 0/42 | (1) 0/39 (2) 0/42 | |
| (1) Salicylate gel (Phardol‐Mono) (2) Placebo gel | 7/136 lost to follow‐up | No data | No data | (1) 1/54 unrelated disc prolapse (2) 0/59 | (1) 0/54 (2) 0/59 | |
| (1) Salicylate gel (2) Placebo gel | (1) 15/58 14 withdrew during trial, 1 lost to follow‐up (2) 10/58 2 withdrew before treatment, 7 withdrew during trial, 1 lost to follow‐up | (1) 3/58 | (1) 10/58 | (1) 48/58 | Total number of adverse events: (1) 80 (2) 27 | |
| (1) Salicylate, nicotinate, capsicum oleoresin, and histamine gel (Cremor Capsici Compositus FNA) (2) Herbal gel (Spiroflor SRL) active control | (1) 4/78 lost to follow‐up (2) 2/83 1 death, 1 lost to follow‐up | No data | (1) 8/74 (2) 1/82 unrelated death | (1) 19/74 (2) 10/82 | (1) 18/74 (2) 3/81 | |
| (1) Salicylate and nonivamide in heparin and salicylate ointment (Enelbin‐Rheuma) (2) Salicylate in heparin and salicylate ointment active control | (1) 0/50 (2) 2/50 | (1) 1/50 (2) 0/50 | (1) 0/50 (2) 2/50 | (1) 0/50 (2) 2/50 | (1) 0/50 (2) 2/50 | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | No data | No data | No data | No data | No data | |
| Subgroup | Studies | Participants | Fixed‐effect RR (95% CI) | NNT (95% CI) |
| All acute studies | 4 | 324 | 1.9 (1.5 to 2.5) | 3.2 (2.4 to 4.9) |
| Excluding Lester 19811 | 3 | 282 | 2.0 (1.5 to 2.7) | 3.2 (2.4 to 5.0) |
| Excluding validity < 9 | 3 | 243 | 1.3 (1.01 to 1.7) | not calculated |
| Excluding quality < 3 or validity < 9 | 2 | 201 | 1.2 (0.90 to 1.7) | not calculated |
| Outcomes ≥ 7 days | 3 | 202 | 1.8 (1.4 to 2.4) | 3.1 (2.3 to 4.8) |
| Outcomes ≥ 7 days2 | 4 | 324 | 2.0 (1.5 to 2.5) | 3.1 (2.3 to 4.4) |
| 1. Quality score < 3, outcome measure unspecified 'improvement' 2. Including additional data at 14 days from Ginsberg 1987 | ||||
| Subgroup | Studies | Participants | Fixed‐effect RR (95% CI) | NNT (95% CI) |
| All chronic studies | 6 | 429* | 1.6 (1.2 to 2.0) | 6.2 (4.0 to 13) |
| Excluding von Bach 19791 | 5 | 329* | 1.4 (1.03 to 1.8) | 8.8 (4.7 to 70) |
| Excluding Shackel 19972 | 5 | 315* | 1.9 (1.4 to 2.5) | 4.6 (3.2 to 8.5) |
| Group size ≥ 40 | 3 | 327 | 1.5 (1.1 to 2.0) | 7.4 (4.2 to 31) |
| Outcome measure global assessment or categorical score | 4 | 290 | 1.8 (1.3 to 2.5) | 4.8 (3.2 to 10) |
| Duration ≥ 14 days | 4 | 383 | 1.6 (1.2 to 2.1) | 6.3 (4.0 to 16) |
| * Including 26 patients in a crossover trial (Algozzine 1982) 1. Lower dose salicylate control 2. Application to a site remote from pain | ||||
| Event rate (%) | ||||||
| Subgroup | Rubefacient | Placebo | Studies (with ≥ 1 event) | Participants (in studies with ≥ 1 event) | Fixed effect RR (95% CI) | NNH (95% CI) |
| Any adverse event | ||||||
| All studies | 74/484 (15%) | 47/500 (9%) | 8 | 773 | 1.6 (1.2 to 2.0) | 13 (7.9 to 43) |
| All studies excluding Shackel 19971 | 26/426 (6%) | 18/44 (4%) | 7 | 659 | 1.5 (0.88 to 2.6) | not calculated |
| All studies excluding Stam 20012 and von Bach 19793 | 55/360 (15%) | 35/368 (10%) | 6 | 517 | 1.5 (1.1 to 2.0) | 12 (6.8 to 67) |
| Acute studies only | 23/271 (8%) | 14/284 (5%) | 4 | 394 | 1.7 (0.96 to 3.2) | not calculated |
| Acute excluding Stam 20012 | 4/197 (2%) | 4/202 (2%) | 3 | 238 | 1.03 (0.30 to 3.5) | not calculated |
| Chronic studies only | 51/213 (24%) | 33/216 (15%) | 4 | 379 | 1.5 (1.1 to 2.0) | 10 (5.5 to 68) |
| Chronic excluding Shackel 19971 | 3/155 (2%) | 4/160 (3%) | 3 | 265 | 0.82 (0.23 to 2.9) | not calculated |
| Chronic excluding von Bach 19793 | 51/163 (31%) | 31/166 (19%) | 3 | 279 | 1.6 (1.2 to 2.1) | 6.7 (3.9 to 23) |
| Local adverse events | ||||||
| All studies | 24/426 (6%) | 11/443 (2%) | 6 | 545 | 2.2 (1.1 to 4.1) | 20 (11 to 140) |
| All studies excluding Stam 20012 and von Bach 19793 | 6/302 (2%) | 6/312 (2%) | 4 | 290 | 1.02 (0.37 to 2.8) | not calculated |
| Acute studies only | 22/271 (8%) | 7/283(2%) | 4 | 393 | 3.1 (1.4 to 6.7) | 13 (7.5 to 37) |
| Acute excluding Stam 20012 | 4/197 (2%) | 4/202 (2%) | 3 | 238 | 1.03 (0.30 to 3.5) | not calculated |
| Withdrawals due to adverse events | ||||||
| All studies | 18/364 (5%) | 4/373 (1%) | 3 | 370 | 4.2 (1.5 to 12) | 13 (7.9 to 35) |
| Chronic studies only | 10/133 (8%) | 3/131 (2%) | 2 | 214 | 2.9 (0.88 to 9.7) | not calculated |
| 1. High event rate 2. Herbal control 3. Lower dose salicylate control | ||||||
| Outcome | Fixed‐effect RR estimate (95% CI) | Random‐effects RR estimate (95% CI) |
| Acute efficacy | 1.9 (1.5 to 2.5) | 2.7 (1.05 to 7.0) |
| Chronic efficacy | 1.6 (1.2 to 2.0) | 1.6 (1.1 to 2.4) |
| Any adverse events | 1.6 (1.2 to 2.0) | 1.6 (1.3 to 2.1) |
| Local adverse events | 2.2 (1.1 to 4.1) | 1.3 (0.35 to 4.7) |
| All withdrawals | 0.85 (0.57 to 1.3) | 0.92 (0.41 to 2.1) |
| Withdrawal due to adverse events | 4.2 (1.5 to 12) | 3.4 (0.40 to 28) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success (e.g. 50% reduction in pain) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Acute conditions | 4 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.51, 2.46] |
| 1.2 Chronic conditions | 6 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.22, 2.04] |
| 2 Adverse events Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any adverse event | 11 | 984 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.19, 2.04] |
| 2.2 Local adverse events | 10 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.12, 4.12] |
| 3 Withdrawals Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Lack of efficacy | 5 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.87] |
| 3.2 Adverse events | 7 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.19 [1.52, 11.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success (e.g. 50% reduction in pain) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Acute | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Chronic | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Any adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Local adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawals Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Lack of efficacy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

