Drenaje retroperitoneal versus ningún drenaje después de la linfadenectomía pélvica para la prevención de formación de linfoquistes en pacientes con neoplasias ginecológicas
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT. Randomisation was determined using a computer‐based method. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. Weekly abdominal and pelvic ultrasound was performed on 110 of 137 women to detect lymphocyst formation. | |
| Participants | Women with FIGO stage I‐IV ovarian carcinoma (43 women), stage IB‐III cervical carcinoma (45 women), and stage I‐II endometrial carcinoma (49 women). The women underwent intensive surgical staging/tumour reductive surgery/second‐look laparotomy (ovarian carcinoma) or Piver type I/II radical hysterectomy (endometrial carcinoma) or Piver type III/IV radical hysterectomy (cervical carcinoma) plus bilateral pelvic or pelvic and para‐aortic lymphadenectomy. Study setting: the Catholic University of Rome and the Hospital San Carlo di Nancy, Rome, Italy | |
| Interventions | Women were randomised intraoperatively to the use of drains (68 women) or no drain (69 women). For those randomised to drains, 2 retroperitoneal low‐pressure closed‐suction drains were placed. For pelvic/para‐aortic lymphadenectomy, the first drain was placed at the insertion of the mesenteric artery down to the left external iliac artery, and the second drain at the level of the paracaval area from the point where the ovarian vein enters the cava down to the right external iliac artery. For pelvic lymphadenectomy, the cranial part of the drains was at the level of the ipsilateral common iliac vessels. The drains were removed when the loss was less than 50 mL in 24 hours. | |
| Outcomes | There was a significant increase in complications (43% versus 22%) in the drain group, mainly related to lymphocyst. The hospital stay was shorter in the group not drained. | |
| Notes | In all cases, the pelvic peritoneum and the peritoneum along the paracolic gutters were left open. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was stated that "Randomization was centralized and computer‐based." |
| Allocation concealment (selection bias) | Low risk | It was stated that "Randomization was centralized and computer‐based." The allocation concealment was probably adequate. |
| Blinding (performance bias and detection bias) | Low risk | Weekly ultrasound pelvic and abdominal examinations were performed; the ultrasound operator was unaware of the ongoing study. |
| Blinding (performance bias and detection bias) | Unclear risk | The blinding of participants, treatment providers, and outcome assessors was not documented. |
| Incomplete outcome data (attrition bias) | Low risk | It was stated that the weekly ultrasound examinations to detect lymphocyst formation were performed on 110 women operated on at the Catholic University (of 137 women in the study). |
| Selective reporting (reporting bias) | Unclear risk | The rate of asymptomatic lymphocyst formation was not specifically addressed. |
| Methods | Multicentre RCT (12 European cancer centres). Randomisation was determined using a computer‐based method. Stratification was performed per centre. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. Ultrasonography or CT scan was performed at 1 and 12 months postoperatively on all women to identify lymphocyst formation. | |
| Participants | Women with FIGO stage IA1‐IIA cervical carcinoma (198 women), endometrial carcinoma (35 women), and vaginal carcinoma (1 woman). All had radical hysterectomy and bilateral pelvic lymphadenectomy. Study setting: European cancer centres (the EORTC trial) | |
| Interventions | Women were randomised following surgery to either pelvic drainage (117 women) or no drainage (117 women). For those randomised to drains, 2 passive or active suction drains were placed in the retroperitoneal fossa and inserted via the vagina or the abdominal route, according to the institution's policy. The drains were removed when the loss was less than 50 mL in 24 hours. | |
| Outcomes | The study found no difference in the incidence of postoperative lymphocyst formation or postoperative complications between the 2 groups. The late (12 months) incidence of symptomatic lymphocysts was 5.9% in the drain group versus 0.9% in the no‐drain group (P = 0.06). | |
| Notes | In all cases, the vaginal cuff was primarily closed and the pelvic peritoneum was left open. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) | Unclear risk | The blinding of participants, treatment providers, and outcome assessors was not documented. |
| Incomplete outcome data (attrition bias) | Low risk | |
| Methods | RCT. Randomisation was determined using a random number table. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. An abdominal ultrasound scan was performed approximately 8 weeks after the surgery to identify any asymptomatic Iymphocysts. | |
| Participants | Women with FIGO stage IA‐IIB cervical carcinoma (95 women) and stage IIB endometrial carcinoma (5 women). All had Piver type II radical hysterectomy and bilateral pelvic lymphadenectomy. Study setting: Regional Department of Gynaecological Oncology, Gateshead, United Kingdom | |
| Interventions | Women were randomised immediately before abdominal closure to the use of drains (51 women) or no drain (49 women). For those randomised to drains, 2 suction drains were inserted, 1 through each iliac fossa, and placed alongside the site of node dissection. The drains were usually removed when the loss was less than 100 mL in 24 hours. | |
| Outcomes | The detection of lymphocysts by ultrasound and clinical examination in the drains group (15.6% and 5.9%, respectively) did not differ significantly from the group not drained (17.4% and 6.1%, respectively). Postoperative morbidity did not differ between the groups. | |
| Notes | In each case, the vaginal cuff edge was oversewn, leaving the vaginal vault open, and the pelvis was not reperitonised. Of the 100 women randomised, 8 defaulted their ultrasound scan: 5 were in the drained group and 3 were not. Of these 8, 2 (1 in each group) were assessed further; 1 had a magnetic resonance imaging scan and another had a laparotomy, both showing no evidence of lymphocysts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was determined using a random number table. |
| Allocation concealment (selection bias) | Low risk | The random allocation was done using numbered envelopes kept in the operating theatre. The allocation concealment was probably adequate, although the authors did not specifically mention whether or not the envelopes were sealed and opaque. |
| Blinding (performance bias and detection bias) | Unclear risk | An abdominal ultrasound scan was performed approximately 8 weeks after surgery to identify any asymptomatic lymphocysts. Also, the clinical detection of lymphocysts at subsequent review visits was documented. However, any attempt to blind outcome assessors was not mentioned. |
| Blinding (performance bias and detection bias) | Unclear risk | The blinding of participants, treatment providers, and outcome assessors was not documented. |
| Incomplete outcome data (attrition bias) | Low risk | It was stated that "Of the 100 women randomized, eight defaulted their ultrasound scan; five were in the drained group and three were not." Also, it was stated that "the scan was reported as unsatisfactory in one patient who had been drained." |
| Selective reporting (reporting bias) | Low risk | — |
| Methods | RCT. Randomisation was conducted by block randomisation. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. Transabdominal and transvaginal ultrasound were performed at 4, 8, and 12 weeks after surgery to detect lymphocyst formation. | |
| Participants | Women with FIGO stage IA2‐IIA cervical carcinoma (100 women). All had Piver type II/III radical hysterectomy and bilateral pelvic lymphadenectomy. Study setting: Chiang Mai University Hospital, Chiang Mai, Thailand | |
| Interventions | Women were randomised immediately before abdominal closure to the use of drains with closure of pelvic peritoneum (52 women) or no drain with pelvic peritoneum left open (48 women). For those randomised to drains, 2 low‐pressure closed‐suction drains were placed along the side of node dissection and brought out extraperitoneally through the anterior abdominal wall lateral to the rectus muscle margins. The drains were removed when the loss was less than 50 mL in 24 hours. | |
| Outcomes | Asymptomatic lymphocysts were sonographically detected at 4, 8, and 12 weeks postoperatively in 6.8%, 4.6%, and 7.7% of women, respectively, in the group not drained, whereas none were found in the drain group (P = 0.2). Postoperative morbidity did not differ significantly between the 2 groups. | |
| Notes | The vaginal vault was primarily closed in all cases. Of the 52 women in the drain group, 50, 46, and 44 women had ultrasound done at 4, 8, and 12 weeks after surgery, respectively, as scheduled. Of the 48 women in the no‐drain group, 44, 43, and 39 women underwent ultrasound examination at each scheduled visit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of block randomisation was used. |
| Allocation concealment (selection bias) | Low risk | The random allocation was done using sealed, numbered envelopes kept in the operating theatre. |
| Blinding (performance bias and detection bias) | Low risk | The operators of transabdominal and transvaginal ultrasound to detect lymphocyst formation were not aware of the group allocation. |
| Blinding (performance bias and detection bias) | High risk | The participants, treatment providers, and outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The number of participants who had postoperative ultrasound performed at each visit as scheduled was reported. Accordingly, the number of missing cases was known. |
| Selective reporting (reporting bias) | Low risk | — |
CT: computerised tomography
EORTC: European Organisation for Research and Treatment of Cancer
FIGO: International Federation of Gynecology and Obstetrics
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not an RCT | |
| RCT comparing closure of pelvic and parietal peritoneum (with placement of a T‐shape suction drain through the vagina) to no peritoneal closure (but the vagina closed and 2 abdominal drains placed) | |
| Not an RCT | |
| RCT comparing placement of a low‐pressure drain in the aortic area to no placement of para‐aortic drain after complete para‐aortic lymphadenectomy. However, most participants had suction drains placed in the pelvis. | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Studied the effect of the procedure on preventing vaginal shortening on lymphocyst formation. A closed‐suction drain was placed in each paravesical space for all participants. Not an RCT |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery Show forest plot | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.04, 13.35] |
| Analysis 1.1  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery. | ||||
| 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.26, 8.37] |
| Analysis 1.2  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery. | ||||
| 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery Show forest plot | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.30, 1.71] |
| Analysis 1.3  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery. | ||||
| 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.38] |
| Analysis 1.4  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery. | ||||
| 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.89, 2.45] |
| Analysis 1.5  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery. | ||||
| 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.12 [0.89, 56.97] |
| Analysis 1.6  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery. | ||||
| 7 Febrile morbidity Show forest plot | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.87, 3.55] |
| Analysis 1.7  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 7 Febrile morbidity. | ||||
| 8 Pelvic infection Show forest plot | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.62] |
| Analysis 1.8  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 8 Pelvic infection. | ||||
| 9 Wound infection Show forest plot | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.18] |
| Analysis 1.9  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 9 Wound infection. | ||||
| 10 Wound dehiscence Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 6.89] |
| Analysis 1.10  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 10 Wound dehiscence. | ||||
| 11 Fistula Show forest plot | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.34, 4.57] |
| Analysis 1.11  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 11 Fistula. | ||||
| 12 Bowel obstruction Show forest plot | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.12] |
| Analysis 1.12  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 12 Bowel obstruction. | ||||
| 13 Leg oedema Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.77] |
| Analysis 1.13  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 13 Leg oedema. | ||||
| 14 Deep venous thrombosis Show forest plot | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.38, 10.84] |
| Analysis 1.14  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 14 Deep venous thrombosis. | ||||
| 15 Symptomatic ascites Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.92] |
| Analysis 1.15  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 15 Symptomatic ascites. | ||||
| 16 Blood transfusion Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.21] |
| Analysis 1.16  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 16 Blood transfusion. | ||||
| 17 Duration of surgery (minute) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐15.35, 23.75] |
| Analysis 1.17  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 17 Duration of surgery (minute). | ||||
| 18 Return of bowel sounds (day) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.11, 0.39] |
| Analysis 1.18  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 18 Return of bowel sounds (day). | ||||
| 19 Hospital stay (day) Show forest plot | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.34, 0.74] |
| Analysis 1.19  Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 19 Hospital stay (day). | ||||

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

PRISMA flow diagram.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery.
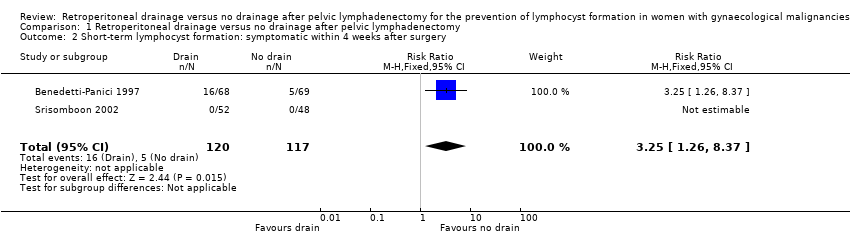
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery.
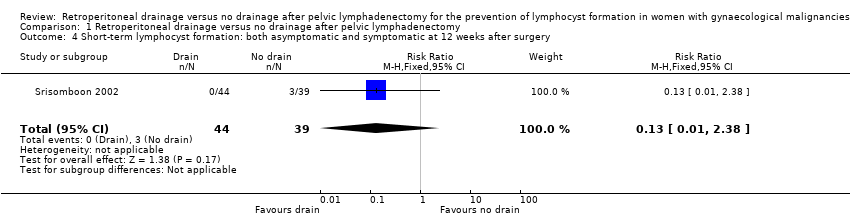
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery.
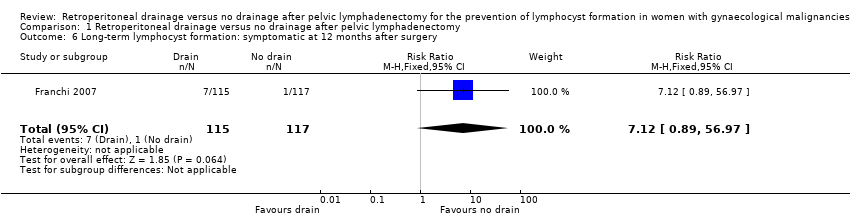
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery.
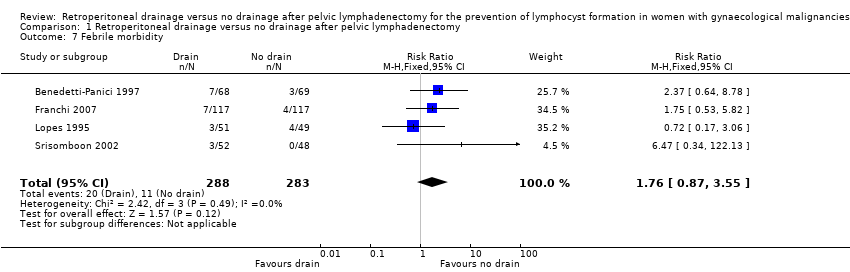
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 7 Febrile morbidity.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 8 Pelvic infection.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 9 Wound infection.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 10 Wound dehiscence.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 11 Fistula.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 12 Bowel obstruction.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 13 Leg oedema.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 14 Deep venous thrombosis.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 15 Symptomatic ascites.
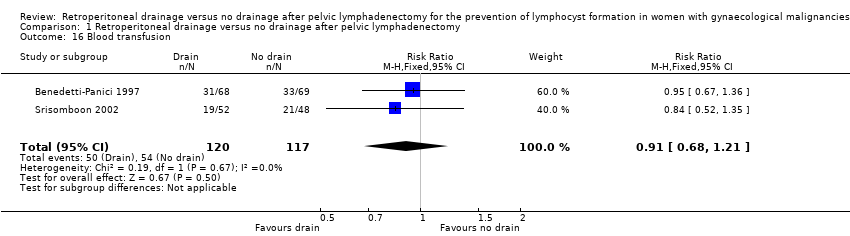
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 16 Blood transfusion.
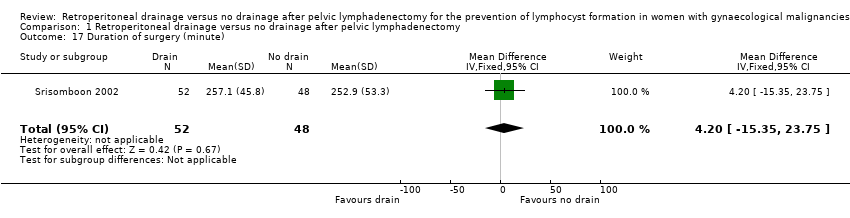
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 17 Duration of surgery (minute).
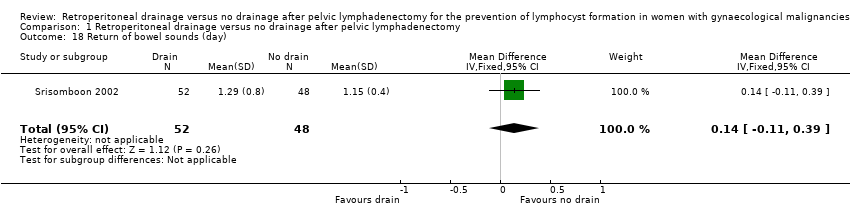
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 18 Return of bowel sounds (day).
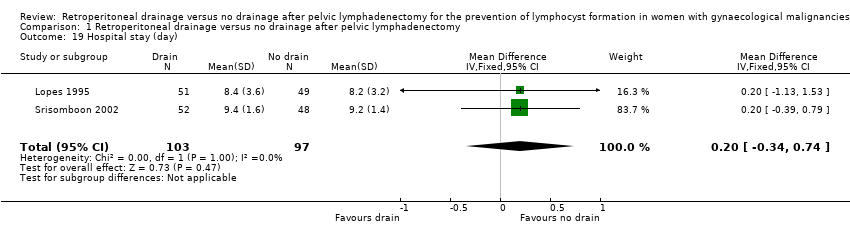
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 19 Hospital stay (day).
| Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for gynaecological malignancies | ||||||
| Patient or population: Women with gynaecological malignancies | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery | Study population | RR 0.76 | 204 | ⊕⊕⊕ | ||
| 172 per 1000 | 131 per 1000 | |||||
| Medium‐risk population | ||||||
| 161 per 1000 | 122 per 1000 | |||||
| Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery | Study population | RR 3.25 | 237 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 140 per 1000 | |||||
| Medium‐risk population | ||||||
| 36 per 1000 | 117 per 1000 | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery | Study population | RR 0.72 | 180 | ⊕⊕⊕⊕ | ||
| 112 per 1000 | 81 per 1000 | |||||
| Medium‐risk population | ||||||
| 110 per 1000 | 79 per 1000 | |||||
| Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery | Study population | RR 1.48 | 232 | ⊕⊕⊕⊕ | ||
| 171 per 1000 | 253 per 1000 | |||||
| Medium‐risk population | ||||||
| 171 per 1000 | 253 per 1000 | |||||
| Long‐term lymphocyst formation: symptomatic at 12 months after surgery | Study population | RR 7.12 | 232 | ⊕⊕⊕⊕ | ||
| 9 per 1000 | 64 per 1000 | |||||
| Medium‐risk population | ||||||
| 9 per 1000 | 64 per 1000 | |||||
| Febrile morbidity | Study population | RR 1.76 | 571 | ⊕⊕⊕⊕ | ||
| 39 per 1000 | 69 per 1000 | |||||
| Medium‐risk population | ||||||
| 39 per 1000 | 69 per 1000 | |||||
| Pelvic infection | Study population | RR 0.42 | 571 | ⊕⊕⊕ | ||
| 18 per 1000 | 8 per 1000 | |||||
| Medium‐risk population | ||||||
| 19 per 1000 | 8 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Wide confidence interval. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery Show forest plot | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.04, 13.35] |
| 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.26, 8.37] |
| 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery Show forest plot | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.30, 1.71] |
| 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.38] |
| 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.89, 2.45] |
| 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.12 [0.89, 56.97] |
| 7 Febrile morbidity Show forest plot | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.87, 3.55] |
| 8 Pelvic infection Show forest plot | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.62] |
| 9 Wound infection Show forest plot | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.18] |
| 10 Wound dehiscence Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 6.89] |
| 11 Fistula Show forest plot | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.34, 4.57] |
| 12 Bowel obstruction Show forest plot | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.12] |
| 13 Leg oedema Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.77] |
| 14 Deep venous thrombosis Show forest plot | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.38, 10.84] |
| 15 Symptomatic ascites Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.92] |
| 16 Blood transfusion Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.21] |
| 17 Duration of surgery (minute) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐15.35, 23.75] |
| 18 Return of bowel sounds (day) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.11, 0.39] |
| 19 Hospital stay (day) Show forest plot | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.34, 0.74] |

