Drenaje retroperitoneal versus ningún drenaje después de la linfadenectomía pélvica para la prevención de formación de linfoquistes en pacientes con neoplasias ginecológicas
Resumen
Antecedentes
Ésta es una versión actualizada de la revisión Cochrane original publicada en el Número 6, 2014. La linfadenectomía pélvica se asocia con complicaciones significativas que incluyen la formación de linfoquistes y morbilidades relacionadas. El drenaje retroperitoneal mediante succión se ha recomendado como un método para prevenir dichas complicaciones. Sin embargo, los resultados de estudios recientes han cuestionado esta política.
Objetivos
Evaluar los efectos del drenaje retroperitoneal versus ningún drenaje después de la linfadenectomía pélvica sobre la formación de linfoquistes y sobre las morbilidades relacionadas en pacientes con cáncer ginecológico.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, Número 3, 2017) en The Cochrane Library, en las bases de datos electrónicas MEDLINE (1946 a segunda semana de marzo 2017), Embase (1980 a semana 12, 2017) y en las listas de citas de las publicaciones relevantes. También se realizaron búsquedas en los registros de ensayos para los ensayos en curso el 20 de mayo 2017.
Criterios de selección
Ensayos controlados aleatorizados (ECA) que compararon el efecto del drenaje retroperitoneal versus ningún drenaje después de la linfadenectomía pélvica en pacientes con cáncer ginecológico. El drenaje retroperitoneal se definió como la colocación de drenajes de succión pasivos o activos en los espacios pélvicos retroperitoneales. Ningún drenaje se definió como la no colocación de drenajes de succión pasivos o activos en los espacios pélvicos retroperitoneales.
Obtención y análisis de los datos
Los estudios se evaluaron mediante criterios de calidad metodológica. Para los datos dicotómicos se calcularon los riesgos relativos (RR) y los intervalos de confianza (IC) del 95%. Los datos continuos se compararon mediante la diferencia de medias (DM) y los IC del 95%.
Resultados principales
Desde la última versión de esta revisión no se han identificado nuevos estudios para inclusión. Esta revisión incluyó cuatro estudios con 571 mujeres. Con respecto a los resultados a corto plazo (dentro de las cuatro semanas posteriores a la cirugía), el drenaje retroperitoneal se asoció con una tasa comparable de formación de linfoquistes en general cuando se consideraron juntos todos los métodos de tratamiento del peritoneo pélvico (dos estudios; 204 mujeres; RR 0,76; IC del 95%: 0,04 a 13,35; evidencia de calidad moderada). Cuando el peritoneo pélvico se dejó abierto, las tasas de formación de linfoquistes en general (un estudio; 110 mujeres; RR 2,29, IC del 95%: 1,38 a 3,79) y de formación de linfoquistes sintomáticos (dos estudios; 237 mujeres; RR 3,25, IC del 95%: 1,26 a 8,37) fueron mayores en el grupo de drenaje. A los 12 meses después de la cirugía, las tasas de formación de linfoquistes en general fueron comparables entre los grupos (un estudio; 232 mujeres; RR 1,48, IC del 95%: 0,89 a 2,45; evidencia de calidad alta). Sin embargo, hubo una tendencia hacia un mayor riesgo de formación de linfoquistes sintomáticos en el grupo con drenajes (un estudio; 232 mujeres; RR 7,12, IC del 95%: 0,89 a 56,97; evidencia de calidad baja).
Conclusiones de los autores
La colocación de sondas de drenaje retroperitoneales no tiene efectos beneficiosos sobre la prevención de la formación de linfoquistes después de la linfadenectomía pélvica en pacientes con neoplasias ginecológicas. Cuando el peritoneo pélvico se deja abierto, la colocación de una sonda de drenaje se asocia con un riesgo mayor de formación de linfoquistes sintomáticos a corto y a largo plazo. Se encontró que la calidad de la evidencia mediante los criterios GRADE fue de calidad moderada a alta para la mayoría de los resultados, excepto para la formación de linfoquistes sintomáticos a los 12 meses después de la cirugía, y el riesgo de sesgo fue incierto o bajo.
PICO
Resumen en términos sencillos
Drenajes versus ningún drenaje después de la linfadenectomía pélvica para prevenir la formación de linfoquistes en pacientes con cáncer ginecológico
Antecedentes
Ésta es una versión actualizada de la revisión Cochrane original publicada en el Número 6, 2014.
La linfadenectomía pélvica (extirpación de las glándulas linfáticas que se encuentran en la pelvis) es un componente importante del tratamiento quirúrgico de los cánceres ginecológicos para detectar si el cáncer se ha extendido. Sin embargo, puede provocar complicaciones, especialmente la formación de linfoquistes (acumulación de líquido linfático en la pelvis). Los linfoquistes pueden causar hinchazón en las piernas, bloqueo del uréter (un tubo que lleva la orina desde el riñón hasta la vejiga urinaria), dolor pélvico, formación de coágulos en las piernas y en las venas pélvicas, trastorno de la motilidad intestinal e infección. La colocación de drenajes de succión para eliminar el líquido linfático que se acumula en el área operatoria entre el peritoneo y la pared abdominal posterior se ha recomendado tradicionalmente para prevenir dichas complicaciones, pero la evidencia de su efectividad no está clara.
Pregunta de la revisión
El objetivo de esta revisión es comparar los efectos de los drenajes versus ningún drenaje para prevenir la formación de linfoquistes después de la linfadenectomía pélvica.
Principales hallazgos
Las principales búsquedas en las bases de datos se actualizaron en marzo 2017. Se identificaron cuatro estudios (571 mujeres) para inclusión. Las mujeres tenían principalmente cáncer de cuello uterino y de endometrio, y solo un estudio también incluyó mujeres con cáncer de ovario. Los hallazgos demostraron que la colocación de drenajes de succión no es efectiva para la prevención de los linfoquistes, especialmente cuando el peritoneo (revestimiento pélvico) se deja abierto. En realidad, dicha práctica aumenta el riesgo de formación de linfoquistes a corto y a largo plazo, con sus síntomas relacionados.
Calidad de la evidencia
La revisión incluye cuatro ensayos clínicos de calidad moderada a alta (riesgo de sesgo incierto o bajo) en su análisis final.
Authors' conclusions
Summary of findings
| Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for gynaecological malignancies | ||||||
| Patient or population: Women with gynaecological malignancies | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery | Study population | RR 0.76 | 204 | ⊕⊕⊕ | ||
| 172 per 1000 | 131 per 1000 | |||||
| Medium‐risk population | ||||||
| 161 per 1000 | 122 per 1000 | |||||
| Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery | Study population | RR 3.25 | 237 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 140 per 1000 | |||||
| Medium‐risk population | ||||||
| 36 per 1000 | 117 per 1000 | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery | Study population | RR 0.72 | 180 | ⊕⊕⊕⊕ | ||
| 112 per 1000 | 81 per 1000 | |||||
| Medium‐risk population | ||||||
| 110 per 1000 | 79 per 1000 | |||||
| Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery | Study population | RR 1.48 | 232 | ⊕⊕⊕⊕ | ||
| 171 per 1000 | 253 per 1000 | |||||
| Medium‐risk population | ||||||
| 171 per 1000 | 253 per 1000 | |||||
| Long‐term lymphocyst formation: symptomatic at 12 months after surgery | Study population | RR 7.12 | 232 | ⊕⊕⊕⊕ | ||
| 9 per 1000 | 64 per 1000 | |||||
| Medium‐risk population | ||||||
| 9 per 1000 | 64 per 1000 | |||||
| Febrile morbidity | Study population | RR 1.76 | 571 | ⊕⊕⊕⊕ | ||
| 39 per 1000 | 69 per 1000 | |||||
| Medium‐risk population | ||||||
| 39 per 1000 | 69 per 1000 | |||||
| Pelvic infection | Study population | RR 0.42 | 571 | ⊕⊕⊕ | ||
| 18 per 1000 | 8 per 1000 | |||||
| Medium‐risk population | ||||||
| 19 per 1000 | 8 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Wide confidence interval. | ||||||
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews, Issue 1, 2010 (Charoenkwan 2014).
Pelvic lymphadenectomy, the removal of all or most of the lymph nodes surrounding major pelvic blood vessels, is an important component of the surgical management of gynaecological malignancies, particularly those originating from the cervix, endometrium, and ovary. Knowledge of the metastatic status of the removed pelvic lymph nodes provides valuable staging and prognostic information that guides postoperative adjuvant treatment. In addition, removal of both bulky and microscopic metastatic nodes can potentially improve treatment outcome. However, the procedure is associated with postoperative complications, among which lymphocyst formation and its related morbidities are important (Benedetti‐Panici 1997; Yamamoto 2000).
A lymphocyst, defined as a collection of lymphatic fluid in the retroperitoneal space (the space between the peritoneum and the posterior abdominal wall), is well known as a specific complication of pelvic lymphadenectomy (Benedetti‐Panici 1997; Ilancheran 1988; Livingston 1980; Petru 1989). The reported incidence of lymphocyst following gynaecological cancer surgery varies from 1% to 29% (Lopes 1995). These differences can be explained by the use of various surgical techniques (methods and instruments used to remove the lymph nodes and secure lymphatic vessels; sharp dissection with suture ligation or coagulation), the difference in extent of lymphadenectomy, and the use of different diagnostic methods (ultrasonography or computerised tomography (CT) scan) (Srisomboon 2002; Yamamoto 2000). The mechanism of lymphocyst formation is unknown. However, previous radiotherapy, node metastasis, and prophylactic heparin have been considered as possible risk factors (Yamamoto 2000). Most lymphocysts occur within the first two to four weeks after surgery (Conte 1990). Although frequently asymptomatic, lymphocysts can lead to leg oedema, ureteral obstruction, pelvic pain, deep venous thrombosis, ileus (intestinal stasis), secondary infection, and fistula (abnormal passage that connects an abscess, cavity, or hollow organ to the body surface or to another hollow organ) (Franchi 2007).
Retroperitoneal drainage has been traditionally recommended as a method to prevent lymphocyst formation and associated postoperative morbidities (Symmond 1961; Symmond 1966; Van Nagell 1976). The procedure is performed by the placement of passive or active suction drains to remove lymphatic fluid or blood that accumulates in the operative fields. This practice has become a surgical dogma. However, recent studies have challenged this policy by demonstrating that there is no advantage to the routine use of retroperitoneal drainage following radical hysterectomy and pelvic lymphadenectomy (Franchi 2007; Lopes 1995; Patsner 1995; Srisomboon 2002). In fact, the drain itself, as a foreign body, could disturb the reparative and absorptive functions of the peritoneum and contribute to the formation of lymphocyst (Maitland 1970).
Our aim was to determine whether there is clear evidence to support the omission of retroperitoneal drainage following pelvic lymphadenectomy in people with gynaecological malignancies.
Objectives
To assess the effects of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy on lymphocyst formation and related morbidities in people with gynaecological cancer.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) that compared the effect of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy in women with gynaecological cancer. However, we excluded quasi‐RCTs. The trials that were included in the final analysis had clear random allocation criteria with appropriate allocation concealment. In addition, the included studies did not have significant violations of allocation procedure and exclusions after allocation.
Types of participants
Participants were women with gynaecological malignancies who had undergone pelvic lymphadenectomy with or without para‐aortic lymphadenectomy as part of their surgical treatment, regardless of lymphadenectomy approaches (transperitoneal or extraperitoneal) and surgical approach (open or laparoscopic).
Types of interventions
Main intervention
Retroperitoneal drainage, defined as placement of passive or active suction drains in pelvic retroperitoneal spaces following pelvic lymphadenectomy, regardless of drainage route (transabdominal or transvaginal), surgical management of pelvic peritoneum (left open or sutured closed), and surgical management of vaginal stump (open or closed).
Comparison intervention
No drainage, defined as no placement of passive or active suction drains in pelvic retroperitoneal spaces following pelvic lymphadenectomy, regardless of surgical management of pelvic peritoneum (left open or sutured closed) and surgical management of vaginal stump (open or closed).
Types of outcome measures
We recorded the following outcomes where information was available.
Primary outcomes
Rate of lymphocyst formation; asymptomatic and symptomatic (categorical data). The diagnosis of lymphocyst must have been made by imaging studies such as ultrasound, computerised tomography (CT), or magnetic resonance imaging (MRI). The techniques used for diagnosis were described.
Secondary outcomes
-
Rate of related postoperative morbidities: febrile morbidity, wound infection, wound dehiscence, leg oedema, deep venous thrombosis, bowel obstruction, fistula formation (categorical data).
-
Blood transfusion (categorical data).
-
Change of related serum chemistry: protein and albumin level (continuous data), albumin infusion (categorical data).
-
Time to the return of bowel sound (continuous data).
-
Duration of surgery (continuous data).
-
Postoperative hospital stay (continuous data).
Search methods for identification of studies
Electronic searches
We obtained all articles that described RCTs of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy in women with gynaecological cancer from the following sources.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2017, Issue 3) in all fields (Appendix 1).
-
The MEDLINE electronic database (1946 to March week 2, 2017) (Appendix 2).
-
The Embase electronic database (1980 to Week 12, 2017) (Appendix 3).
We performed the latest searches on 22 March 2017.
Searching other resources
-
We searched the following sources for ongoing trials: WHO International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/) and ClinicalTrials.gov (www.clinicaltrials.gov).
-
We searched electronic databases including GreyNet.org (www.greynet.org/), the Ohio College Library Center (OCLC) WorldCat dissertations and theses (www.oclc.org/support/services/firstsearch/documentation/dbdetails/details/WorldCatDissertations.en.html), and index to theses (ProQuest Dissertations & Theses: UK & Ireland) to identify the possible relevant conference abstracts and proceedings.
-
We handsearched reports of conferences from the following sources: Gynecologic Oncology (Annual Meeting of the Society of Gynecologic Oncology); International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society); Annual Meeting of the European Society of Medical Oncology (ESMO); and Biennial Meeting of the European Society of Gynaecologic Oncology (ESGO).
-
We searched the citation lists of relevant publications, systematic reviews, review articles, abstracts of scientific meetings, and included studies.
-
We conducted personal communication with experts, specialists in the field, and the study authors of relevant publications in an attempt to identify unpublished studies.
We performed the latest searches of these resources on 20 May 2017.
Data collection and analysis
Selection of studies
Both review authors (KC and CK) undertook the study selection. Both review authors screened the titles and abstracts of articles identified by the search. We discarded studies that were clearly ineligible but aimed to be overly inclusive rather than risk losing relevant studies. Next we obtained full‐text copies of the eligible articles. Both review authors independently assessed whether the studies met the inclusion criteria, resolving any disagreements by discussion. We sought further information from the study authors of papers containing insufficient information to make a decision about eligibility.
Data extraction and management
The review authors independently extracted information using a pre‐designed data extraction form. We resolved any discrepancies by discussion. For each included trial, we collected information regarding the location of the study, methods of the study, the participants (age range, eligibility criteria), nature of the interventions, and data relating to the outcomes specified above. Where possible, we sought missing data from study authors.
Assessment of risk of bias in included studies
Methodological quality
The two review authors then independently assessed the quality of all eligible studies, following the guidelines of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008) and resolving any discrepancies by discussion. We conducted the assessment by detailed description of the following standardised items.
-
Was the assigned treatment adequately concealed prior to allocation? (Appendix 4)
-
Were the treatment and control group comparable at entry?
-
Were the outcomes of participants who withdrew or were excluded after allocation described and included in an intention‐to‐treat analysis?
-
Were the participants blinded to assignment status following allocation?
-
Were the treatment providers blinded to assignment status?
-
Were the care programmes, other than the trial options, identical?
-
Were the outcome assessors blinded to assignment status?
-
Were the withdrawals or loss to follow‐up less than 10% of the study population?
We rated each item as follows:
-
clearly yes: rate A;
-
not sure: rate B (seek details from study authors);
-
clearly no: rate C.
The information regarding methodological quality is presented graphically, providing a context for discussing the reliability of the results (Figure 1; Figure 2). We examined the funnel plot for each outcome for the possibility of publication bias.
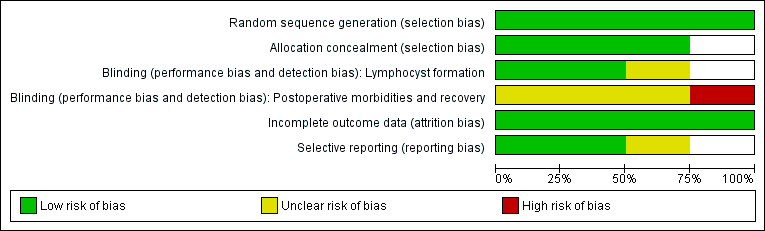
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Data synthesis
We performed statistical analysis in accordance with the guidelines for statistical analysis developed by The Cochrane Collaboration (Deeks 2008).
Subgroup analysis and investigation of heterogeneity
We examined heterogeneity (variations) between the results of different studies by inspecting the forest plot of a meta‐analysis for variation in effects. We also considered formal statistical tests, such as the statistical tests of homogeneity of 2 x 2 tables and the I2 value, in conjunction with the graphical approaches to determine between‐study differences.
For categorical data, we expressed results for each study as a risk ratio (RR) with 95% confidence interval (CI) and combined these for meta‐analysis using Review Manager 5 software (RevMan 2012).
For continuous data, we expressed results from each study as a mean difference (MD) with 95% CI and combined these for meta‐analysis using Review Manager 5 software. Meta‐analytic methods for continuous data assume that the underlying distribution of the measurements is normal. Where data were clearly skewed and results reported in the publication as median and range with non‐parametric tests of significance, we reported the results separately in the Results section of the review.
For meta‐analysis, we used the fixed‐effect or random‐effects model depending on the outcome of the tests of homogeneity.
Sensitivity analysis
We planned to perform sensitivity analyses where there was uncertainty or disagreement regarding inclusion of studies, data extraction, and missing data/dropouts.
GRADE rating and Summary of findings
Both review authors (KC and CK) rated the quality of the evidence based on the approach proposed by the GRADE Working Group (GRADE 2013). We used the GRADE checklist and GRADE Working Group quality of evidence definitions (Meader 2014). We downgraded the evidence from high quality by one level for each serious (or by two for each very serious) limitation. Definitions used for grading of evidence included:
-
High quality: Findings of the review provide a very good indication of the likely effect. The likelihood that the effect will be substantially different is low.
-
Moderate quality: Findings of the review provide a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate.
-
Low quality: Findings of the review provide some indication of the likely effect. However, the likelihood that it will be substantially different is high.
-
Very low quality: Findings of the review do not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high.
We presented the main results in a 'Summary of findings' table generated using GRADEpro software (GRADEpro). We presented the following outcomes in the 'Summary of findings' table:
-
Short‐term lymphocyst formation
-
Long‐term lymphocyst formation
-
Febrile morbidity
-
Pelvic infection
Results
Description of studies
The database searches up to March 2017 and trial registries in May 2017 identified 196 records. After removing duplicates, we screened 160 titles and abstracts records and excluded 148.
Results of the search
We considered 12 studies to be relevant and chose them for further assessment. We obtained copies of the full‐text of these potentially relevant references, and both review authors independently assessed them for eligibility.
We excluded six studies because they were not RCTs (see Characteristics of excluded studies).
We excluded two RCTs because the randomisation was not between retroperitoneal drainage and no drainage after pelvic lymphadenectomy. Franchi 1997 compared closure of pelvic and parietal peritoneum (with placement of a T‐shape suction drain through the vagina) to no peritoneal closure (but the vagina closed and two abdominal drains placed). Morice 2001 compared placement of a low‐pressure drain in the aortic area to no placement of para‐aortic drain after complete para‐aortic lymphadenectomy. However, most participants had suction drains placed in the pelvis.
We included four RCTs in the meta‐analysis (Benedetti‐Panici 1997; Franchi 2007; Lopes 1995; Srisomboon 2002) (see Figure 3 and Characteristics of included studies).
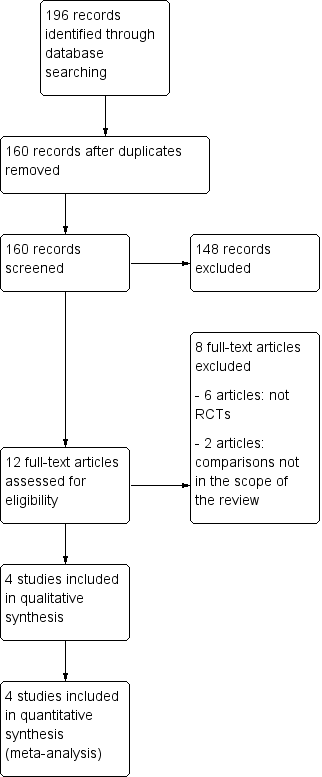
PRISMA flow diagram.
Included studies
Participants
The review included a total of 571 women. The number of women in the included studies was 100 (Lopes 1995), 137 (Benedetti‐Panici 1997), 100 (Srisomboon 2002), and 234 (Franchi 2007). The countries represented were the United Kingdom (Lopes 1995), Italy (Benedetti‐Panici 1997), and Thailand (Srisomboon 2002). Franchi 2007 is a European Organisation for Research and Treatment of Cancer‐Gynaecological Cancer Group (EORTC‐GCG) multi‐institutional trial that recruited participants from 12 European cancer centres. In three studies (Franchi 2007; Lopes 1995; Srisomboon 2002), the majority of women had early‐stage cervical carcinoma. In Benedetti‐Panici 1997, the women's diagnoses was almost equally distributed between endometrial (early), cervical (early and locally advanced), and ovarian carcinoma (all stages). Baseline characteristics of the women were comparable between the two groups in all studies.
Interventions
For women randomised to the use of drains, two suction drains were placed retroperitoneally alongside the site of node dissection and brought out through the abdominal wall in Benedetti‐Panici 1997, Lopes 1995, and Srisomboon 2002. In Franchi 2007, two passive or active suction drains were placed in the retroperitoneal fossa and inserted via the vagina or the abdominal route, according to the institution's policy. The drains were removed when the loss was less than 50 mL in 24 hours in three studies (Benedetti‐Panici 1997; Franchi 2007; Srisomboon 2002), and less than 100 mL in 24 hours in Lopes 1995.
In three studies, the pelvic peritoneum was left open in all cases (Benedetti‐Panici 1997; Franchi 2007; Lopes 1995). In Srisomboon 2002, the pelvic peritoneum was closed in the drain group and left open in the no‐drain group.
In Benedetti‐Panici 1997, Franchi 2007, and Srisomboon 2002, the vaginal cuff was primarily closed in all women. In Lopes 1995, the vaginal cuff edge was oversewn, leaving the vaginal vault open.
Outcomes
Three studies provided data on short‐term lymphocyst formation rate (Lopes 1995 (overall; asymptomatic and symptomatic together); Benedetti‐Panici 1997 (overall and symptomatic); Srisomboon 2002 (overall; asymptomatic and symptomatic together)). However, the surveillance schedule appeared to differ among studies. In Lopes 1995, an abdominal ultrasound scan was performed approximately eight weeks after the surgery to identify any asymptomatic Iymphocysts. In Srisomboon 2002, transabdominal and transvaginal ultrasounds were performed at four, eight, and 12 weeks after surgery to detect lymphocyst formation. Weekly abdominal and pelvic ultrasounds were performed on 110 of 137 women to detect lymphocyst formation in Benedetti‐Panici 1997. Lymphocysts were followed up in order to assess their clinical behaviour. When no lymphocysts were detected at the third examination, monitoring was discontinued.
The only study that provided data on rate of long‐term lymphocyst formation (both asymptomatic and symptomatic) was Franchi 2007.
Risk of bias in included studies
Allocation
Allocation concealment was clearly adequate in Srisomboon 2002, in which randomisation was performed by using sequentially numbered, sealed, opaque assignment envelopes. We considered the concealment of allocation potentially adequate in Benedetti‐Panici 1997 and Lopes 1995, although we did not obtain further details. In Lopes 1995, the random allocation was done using numbered envelopes kept in the operating theatre. However, the authors did not specifically describe whether or not the envelopes were sealed and opaque. In Benedetti‐Panici 1997, it was stated that "Randomization was centralized and computer‐based". Franchi 2007 recruited participants from 12 European cancer centres; no detailed explanation on allocation concealment method was provided.
Blinding
For the outcome of lymphocyst formation, the outcome assessors were unaware of the ongoing study in Benedetti‐Panici 1997, and unaware of the group allocation in Srisomboon 2002. The blinding of outcome assessors was not reported in Lopes 1995 and Franchi 2007.
For the rate of related postoperative morbidities the blinding of participants, treatment providers, and outcome assessors was not documented in Benedetti‐Panici 1997, Franchi 2007, and Lopes 1995. In Srisomboon 2002, the participants, treatment providers, and outcome assessors were not blinded.
Incomplete outcome data
All studies addressed incomplete data for the lymphocyst formation outcome (see the corresponding 'Risk of bias' tables under Characteristics of included studies).
Selective reporting
In Lopes 1995 and Srisomboon 2002, the outcomes prespecified in the Methods section were reported. In the other two studies, the rate of asymptomatic lymphocyst formation and the rate of asymptomatic and symptomatic lymphocyst formation at one month after surgery were not specifically reported (Benedetti‐Panici 1997; Franchi 2007).
The 'Risk of bias' graph and 'Risk of bias' summary are shown in Figure 1 and Figure 2.
Effects of interventions
For the outcomes with data available, the number of studies contributing usable data ranged from one to four. When compared to no drainage, retroperitoneal drainage after pelvic lymphadenectomy was associated with the following.
-
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation within four weeks after surgery, when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together (2 studies (Benedetti‐Panici 1997; Srisomboon 2002); 204 women; risk ratio (RR) 0.76, 95% confidence interval (CI) 0.04 to 13.35 (Analysis 1.1)). However, when the pelvic peritoneum was left open in all cases, the rate of overall lymphocyst formation within four weeks after surgery was significantly higher in the drain group (1 study (Benedetti‐Panici 1997); 110 women; RR 2.29, 95% CI 1.38 to 3.79 (Analysis 1.1)).
-
Significantly higher rate of symptomatic lymphocyst formation within four weeks after surgery when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together as well as when the pelvic peritoneum was left open in all cases (2 studies (Benedetti‐Panici 1997; Srisomboon 2002); 237 women; RR 3.25, 95% CI 1.26 to 8.37 (Analysis 1.2)).
-
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation at eight weeks after surgery, either when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together (2 studies (Lopes 1995; Srisomboon 2002); 180 women; RR 0.72, 95% CI 0.30 to 1.71 (Analysis 1.3)) or when each method of peritoneum management was considered separately (1 study (Lopes 1995) with pelvic peritoneum left open in all cases; 91 women; RR 0.89, 95% CI 0.35 to 2.26 (Analysis 1.3) and 1 study (Srisomboon 2002) with pelvic peritoneum closed in the drain group; 89 women; RR 0.19, 95% CI 0.01 to 3.79 (Analysis 1.3)).
-
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation at 12 weeks after surgery when the pelvic peritoneum was closed in the drain group (1 study (Srisomboon 2002); 83 women; RR 0.13, 95% CI 0.01 to 2.38 (Analysis 1.4)).
-
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation at 12 months after surgery (1 study (Franchi 2007); 232 women; RR 1.48, 95% CI 0.89 to 2.45 (Analysis 1.5)) and a trend toward increased risk of symptomatic lymphocyst formation at 12 months after surgery (1 study (Franchi 2007); 232 women; RR 7.12, 95% CI 0.89 to 56.97 (Analysis 1.6)) when the pelvic peritoneum was left open in all cases.
-
Comparable rate of postoperative febrile morbidity, regardless of the methods of pelvic peritoneum management (4 studies (Benedetti‐Panici 1997; Franchi 2007; Lopes 1995; Srisomboon 2002); 571 women; RR 1.76, 95% CI 0.87 to 3.55 (Analysis 1.7)).
-
Comparable rate of postoperative pelvic infection regardless of the methods of pelvic peritoneum management (4 studies (Benedetti‐Panici 1997; Franchi 2007; Lopes 1995; Srisomboon 2002); 571 women; RR 0.42, 95% CI 0.11 to 1.62 (Analysis 1.8)).
-
Comparable rate of postoperative wound infection when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together (2 studies (Franchi 2007; Srisomboon 2002); 334 women; RR 0.29, 95% CI 0.07 to 1.18 (Analysis 1.9)). However, when the pelvic peritoneum was left open in all cases, there was a significant decrease in the rate of postoperative wound infection in the drain group (1 study (Franchi 2007); 234 women; RR 0.13, 95% CI 0.02 to 0.98 (Analysis 1.9)).
-
Comparable rate of wound dehiscence (2 studies (Benedetti‐Panici 1997; Lopes 1995); 237 women; RR 0.99, 95% CI 0.14 to 6.89 (Analysis 1.10)), fistula formation (3 studies (Benedetti‐Panici 1997; Franchi 2007; Lopes 1995); 471 women; RR 1.24, 95% CI 0.34 to 4.57 (Analysis 1.11), bowel obstruction (2 studies (Franchi 2007; Srisomboon 2002); 334 women; RR 0.20, 95% CI 0.01 to 4.12 (Analysis 1.12)), leg oedema (1 study (Benedetti‐Panici 1997); 137 women; RR 2.71, 95% CI 0.75 to 9.77 (Analysis 1.13)), deep venous thrombosis (2 studies (Benedetti‐Panici 1997; Franchi 2007); 371 women; RR 2.02, 95% CI 0.38 to 10.84 (Analysis 1.14)), symptomatic ascites (1 study (Benedetti‐Panici 1997); 137 women; RR 0.68, 95% CI 0.12 to 3.92 (Analysis 1.15)), and need for blood transfusion (2 studies (Benedetti‐Panici 1997; Srisomboon 2002); 237 women; RR 0.91, 95% CI 0.68 to 1.21 (Analysis 1.16)).
-
Comparable duration of surgery (1 study (Srisomboon 2002); 100 women; mean difference (MD) 4.20 minutes, 95% CI ‐15.35 to 23.75 (Analysis 1.17)). Similarly, there was no significant difference in duration of surgery in the two studies that reported this outcome as a median (10 minutes; 190 minutes in the drain group, 180 minutes in the no‐drain group in Benedetti‐Panici 1997 and 5 minutes; 240 minutes in the drain group, 245 minutes in the no‐drain group in Franchi 2007).
-
Comparable time interval to the return of bowel sounds (1 study (Srisomboon 2002); 100 women; MD 0.14 day, 95% CI ‐0.11 to 0.39 (Analysis 1.18)). Similarly, there was no significant difference in the time interval to the return of bowel sounds in the two studies that reported this outcome as a mean only (Lopes 1995, 0.02 day; 1.04 day in the drain group, 1.02 day in the no‐drain group) and as a median (Franchi 2007, 0 day; 2 days in the drain group, 2 days in the no‐drain group).
-
Comparable postoperative decrease of total protein (‐0.1 mg%; 0.6 mg% in the drain group, 0.7 mg% in the no‐drain group) and albumin (‐0.1 mg%; 0.5 mg% in the drain group, 0.6 mg% in the no‐drain group) in the only study that reported this outcome as a median (Benedetti‐Panici 1997).
-
Comparable duration of postoperative hospital stay (2 studies (Lopes 1995; Srisomboon 2002); 200 women; MD 0.20 day, 95% CI ‐0.34 to 0.74 (Analysis 1.19)). However, postoperative hospital stay was significantly longer in the drain group in a study that reported this outcome as a median (4 days; 11 days in the drain group, 7 days in the no‐drain group) (Benedetti‐Panici 1997).
Discussion
Summary of main results
The incidence of lymphocyst formation varied among the included studies. This could be due to different surgical techniques, as well as different methods and criteria for diagnosis. The available evidence suggests that placement of retroperitoneal drains does not reduce the incidence of lymphocyst formation after pelvic lymphadenectomy in women with gynaecological cancer. In fact, if the pelvic peritoneum is not closed, the drain placement is associated with a higher incidence of symptomatic lymphocyst formation within a month and potentially so at 12 months following surgery. This could be explained by the drain itself acting as a foreign body and interfering with the reparative and absorptive function of the pelvic peritoneum. The practice of leaving the pelvic peritoneum open appears to be an effective alternative method of pelvic retroperitoneal drainage that allows lymphatic fluid to be reabsorbed by the peritoneal surface without the need to insert foreign material into the surgical site. Of note, when the pelvic peritoneum is left open, fluid produced from the pelvic lymphadenectomy area may freely circulate in the abdominal cavity. Ascites could develop as a result of an imbalance between fluid production from the retroperitoneal area and fluid absorption by the peritoneal surface. Having the tube drain in place might lower the risk of developing ascites. However, the rate of symptomatic ascites was evidently the same between women with a drain (3%) and without a drain (4%) (Benedetti‐Panici 1997).
Cost‐effectiveness is an important issue that has not been adequately addressed in studies on effects of drainage on lymphocyst formation. However, one would assume that drainage requires more materials, more personnel, and more time to spend supervising postoperative care. All of these together increase healthcare costs. In addition, women's comfort is another issue that needs special attention. It could be assumed that women without a drain would be more comfortable and more ambulant.
Leaving the vaginal vault open is another method of passive retroperitoneal drainage. In one included study (Lopes 1995), this practice was applied in combination with non‐closure of the pelvic peritoneum. That study found that women without tube drains had a higher incidence of serosanguinous fluid loss through the vagina in the first postoperative day (12% versus 4%). After the first day, the loss continued in 4% of the women without the tube drains and 2% of those with the tube drains. There are no data on the effect of leaving the vaginal vault open alone as a preventive measure for lymphocyst formation following pelvic lymphadenectomy.
Overall completeness and applicability of evidence
The number of eligible studies that examined the effects of retroperitoneal drainage following pelvic lymphadenectomy was limited. In addition, only a small number of studies contributed data for individual outcome measures. Furthermore, there were differences in the details of the surgical procedures among the included trials, for example in method of pelvic peritoneum management (open or closed), vaginal vault management (open or closed), type of drains (active or passive), route of drains (abdominal or vaginal), exact location for placement of drains, and criteria for removal of drains. One important discrepancy of surgical technique among the included studies should be noted. In Srisomboon 2002, the pelvic peritoneum was sutured closed in the drain group and left open in the no‐drain group, whereas in the other three included studies the pelvic peritoneum was left open in all women regardless of the group allocation. Closing the pelvic peritoneum in the group with drains and leaving it open in the group without drains is the comparison of two sets of surgical management strategies that consider both placement of the tube drains and management of the pelvic peritoneum together, not the drain placement alone. When this study is combined with the other studies in the final analysis, the result should therefore be interpreted with this discrepancy in mind, and bias in favour of the drain group is expected. However, the results of the final analysis appear to be in the same direction whether this study was included or excluded. This finding would confirm that placement of retroperitoneal tube drains has no benefit in preventing lymphocyst formation. This information should be applicable to a wide range of gynaecological cancer operations that include pelvic lymphadenectomy as a part of the entire procedure.
Quality of the evidence
In general, the methodological quality of the included studies was acceptable, with some limitations. The allocation concealment appeared adequate or potentially adequate in all trials. All studies addressed incomplete data on the main outcome, that is lymphocyst formation. The important limitation of the included studies was the process of blinding. Given the nature of the study intervention (retroperitoneal tube drainage), blinding of participants and care providers is difficult. On the other hand, blinding of outcome assessors is possible. However, for the diagnosis of lymphocyst formation by ultrasound, blinding of the outcome assessors was clearly documented in only two of the four included studies (Benedetti‐Panici 1997; Srisomboon 2002). Although there are objective criteria for ultrasound diagnosis of pelvic lymphocyst, the final diagnostic decision sometimes involves a subjective component. In such a case, lack of blinding could introduce bias into the results. Also, for the rate of related postoperative morbidities, the blinding of participants, treatment providers, and outcome assessors was not documented in Benedetti‐Panici 1997, Franchi 2007, and Lopes 1995. In Srisomboon 2002, the participants, treatment providers, and outcome assessors were not blinded.
The possibility of publication bias should be kept in mind. In an attempt to assess its presence, we examined the funnel plots for all main outcomes. The findings were not suggestive of publication bias. However, we did not reach a meaningful conclusion on this issue due to the limited number of included studies.
We found the quality of evidence using the GRADE approach to be moderate to high for most outcomes except for symptomatic lymphocyst formation at 12 months after surgery (see summary of findings Table for the main comparison). The quality of the evidence regarding symptomatic lymphocyst formation within 4 weeks after surgery, both asymptomatic and symptomatic lymphocyst formation at 8 weeks and 12 months after surgery, and febrile morbidity were high. We downgraded the evidence to moderate for both asymptomatic and symptomatic lymphocyst formation within 4 weeks after surgery and pelvic infection due to a wide confidence interval. Based on a wide confidence interval and small number of events in the analysis, we downgraded the evidence to low quality for symptomatic lymphocyst formation at 12 months after surgery.
Agreements and disagreements with other studies or reviews
There are a few retrospective and prospective non‐RCTs examining the effect of retroperitoneal tube drainage on the prevention of lymphocyst formation after pelvic lymphadenectomy in women with gynaecological cancer. The results and conclusions from these studies are in keeping with the findings from the present review. In Jensen 1993, the records of 115 women with early‐stage cervical cancer were retrospectively reviewed. There was no difference between the participants with drains and without drains in mean operative time, mean estimated blood loss, transfusion rate, febrile morbidity rates, incidence of pelvic cellulitis, length of postoperative ileus, and total hospital stay. However, the participants with drains had an increased rate of rehospitalisation and morbidity directly related to the presence of the drains. Patsner 1995 is a prospective non‐RCT comparing the effects of closed‐suction drainage versus no drainage after radical abdominal hysterectomy and bilateral pelvic lymphadenectomy for 120 women with stage IB cervical cancer. In this study, all operations were performed in a uniform manner by one surgeon. The vaginal cuff was closed and the pelvic peritoneum was left open in all cases. In addition, an omental J‐flap was brought into the pelvis in all women and secured to the pelvic floor. There was no increase in postoperative lymphocyst formation, pelvic infection, and fistula in the group without drains. Of note, the rate of lymphocyst formation was 6.7% in the drain group and 0% in the no‐drain group. The exact role of the omental J‐flap in the prevention of lymphocyst formation is unknown. In another prospective non‐RCT comparing the effects of closed suction pelvic drainage versus no suction drainage following pelvic/pelvic‐para‐aortic lymphadenectomy for 143 participants with various gynaecological malignancies (Bafna 2001), rates of lymphocyst formation were comparable between the study groups (7.3% in the drain group and 2.7% in the no‐drain group). The pelvic peritoneum was sutured closed in the drain group and left open in the no‐drain group.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

PRISMA flow diagram.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery.
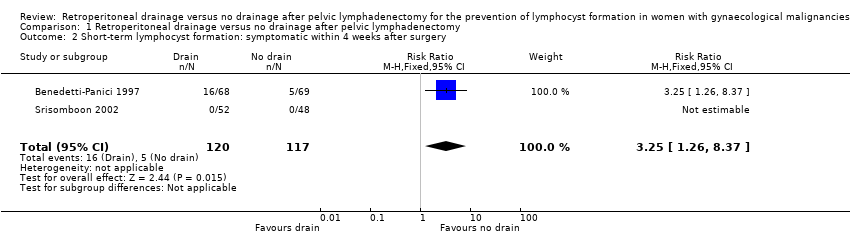
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery.
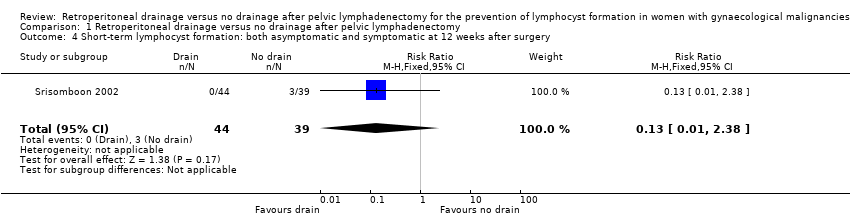
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery.
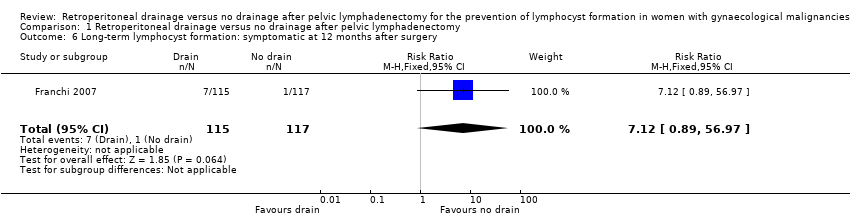
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery.
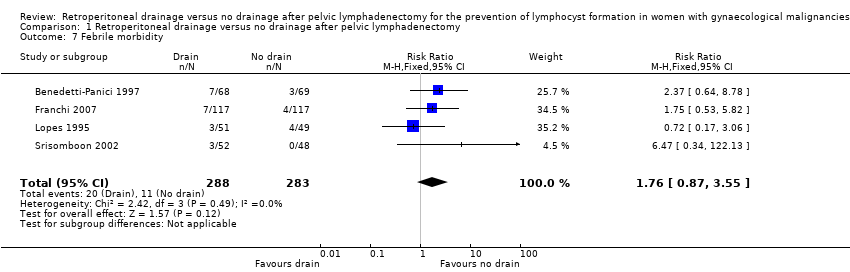
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 7 Febrile morbidity.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 8 Pelvic infection.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 9 Wound infection.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 10 Wound dehiscence.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 11 Fistula.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 12 Bowel obstruction.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 13 Leg oedema.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 14 Deep venous thrombosis.

Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 15 Symptomatic ascites.
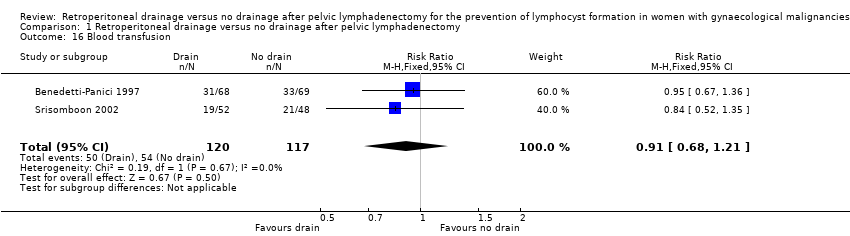
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 16 Blood transfusion.
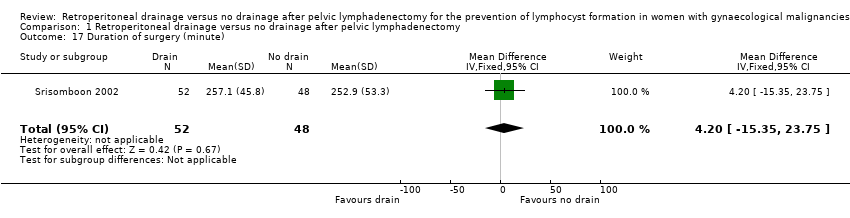
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 17 Duration of surgery (minute).
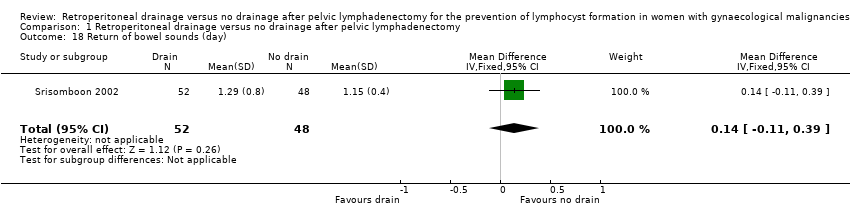
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 18 Return of bowel sounds (day).
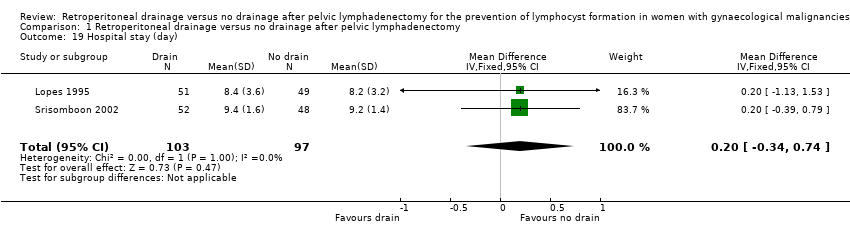
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 19 Hospital stay (day).
| Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for gynaecological malignancies | ||||||
| Patient or population: Women with gynaecological malignancies | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery | Study population | RR 0.76 | 204 | ⊕⊕⊕ | ||
| 172 per 1000 | 131 per 1000 | |||||
| Medium‐risk population | ||||||
| 161 per 1000 | 122 per 1000 | |||||
| Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery | Study population | RR 3.25 | 237 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 140 per 1000 | |||||
| Medium‐risk population | ||||||
| 36 per 1000 | 117 per 1000 | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery | Study population | RR 0.72 | 180 | ⊕⊕⊕⊕ | ||
| 112 per 1000 | 81 per 1000 | |||||
| Medium‐risk population | ||||||
| 110 per 1000 | 79 per 1000 | |||||
| Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery | Study population | RR 1.48 | 232 | ⊕⊕⊕⊕ | ||
| 171 per 1000 | 253 per 1000 | |||||
| Medium‐risk population | ||||||
| 171 per 1000 | 253 per 1000 | |||||
| Long‐term lymphocyst formation: symptomatic at 12 months after surgery | Study population | RR 7.12 | 232 | ⊕⊕⊕⊕ | ||
| 9 per 1000 | 64 per 1000 | |||||
| Medium‐risk population | ||||||
| 9 per 1000 | 64 per 1000 | |||||
| Febrile morbidity | Study population | RR 1.76 | 571 | ⊕⊕⊕⊕ | ||
| 39 per 1000 | 69 per 1000 | |||||
| Medium‐risk population | ||||||
| 39 per 1000 | 69 per 1000 | |||||
| Pelvic infection | Study population | RR 0.42 | 571 | ⊕⊕⊕ | ||
| 18 per 1000 | 8 per 1000 | |||||
| Medium‐risk population | ||||||
| 19 per 1000 | 8 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Wide confidence interval. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery Show forest plot | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.04, 13.35] |
| 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.26, 8.37] |
| 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery Show forest plot | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.30, 1.71] |
| 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.38] |
| 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.89, 2.45] |
| 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.12 [0.89, 56.97] |
| 7 Febrile morbidity Show forest plot | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.87, 3.55] |
| 8 Pelvic infection Show forest plot | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.62] |
| 9 Wound infection Show forest plot | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.18] |
| 10 Wound dehiscence Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 6.89] |
| 11 Fistula Show forest plot | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.34, 4.57] |
| 12 Bowel obstruction Show forest plot | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.12] |
| 13 Leg oedema Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.77] |
| 14 Deep venous thrombosis Show forest plot | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.38, 10.84] |
| 15 Symptomatic ascites Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.92] |
| 16 Blood transfusion Show forest plot | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.21] |
| 17 Duration of surgery (minute) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐15.35, 23.75] |
| 18 Return of bowel sounds (day) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.11, 0.39] |
| 19 Hospital stay (day) Show forest plot | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.34, 0.74] |

