| Ketoprofen 50 mg compared with placebo for acute postoperative pain |
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 50 mg Comparison: placebo |
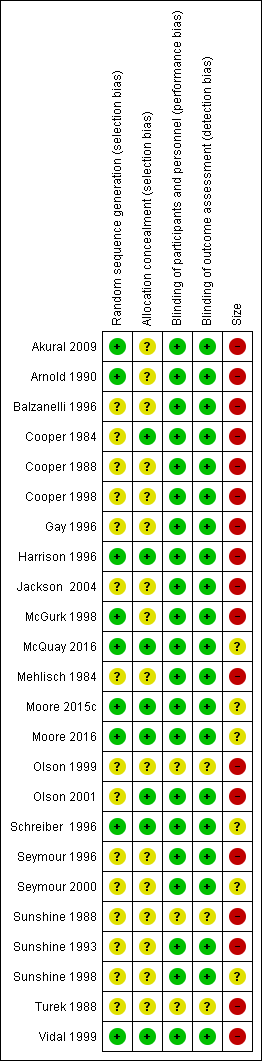
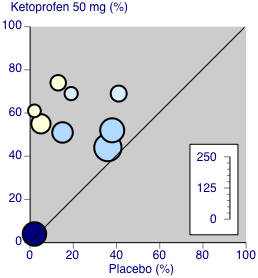
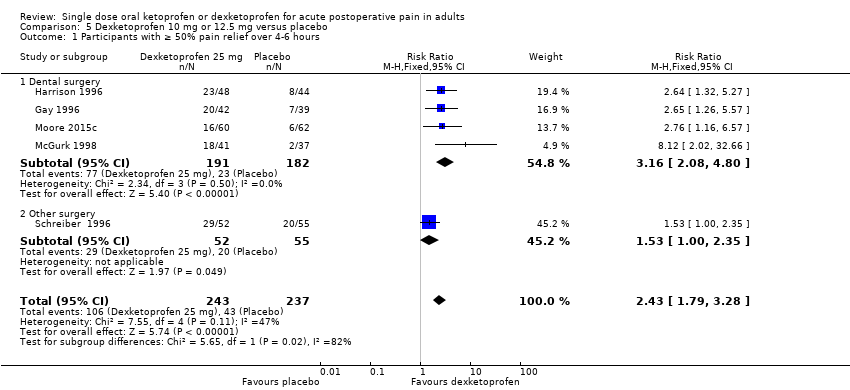
| Participants with ≥ 50% pain relief over 4‐6 hours | 570 in 1000 | 230 in 1000 | RR 2.5 (2.0 to 3.1) NNT 2.9 (2.4 to 3.7) | 8 studies 594 participants | High quality | Good quality studies, important outcome available, robust numbers. |
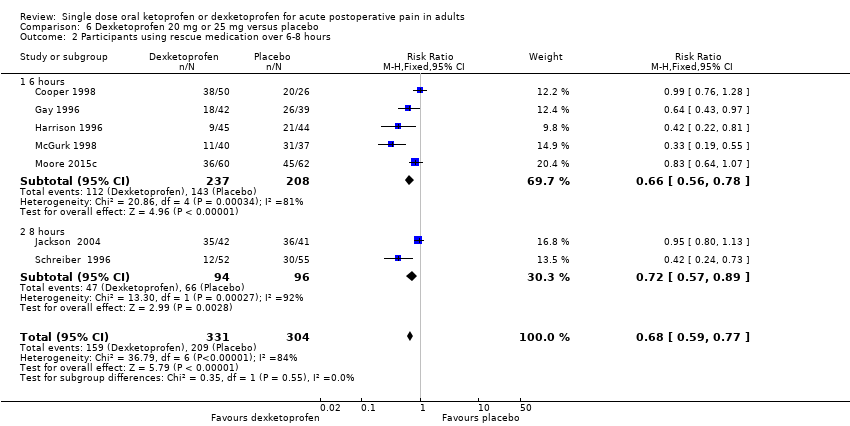
| Median (mean) time to use of rescue medication | Approximately 5 hours (3.4 hours) | Approximately 3 hours (2.5 hours) | Not estimated | 1 study 77 participants (5 studies, 342 participants) | Very low quality | Small numbers of participants. |
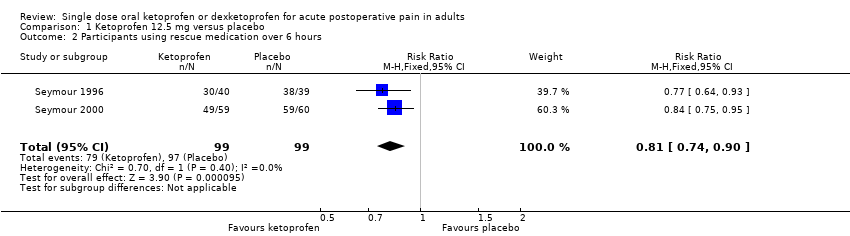
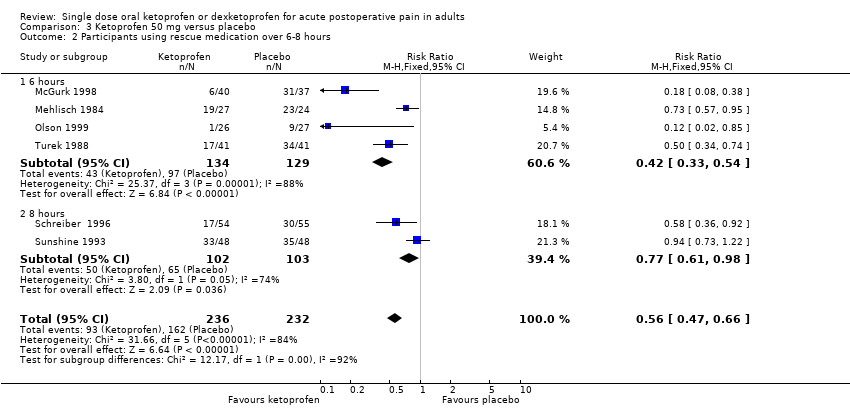
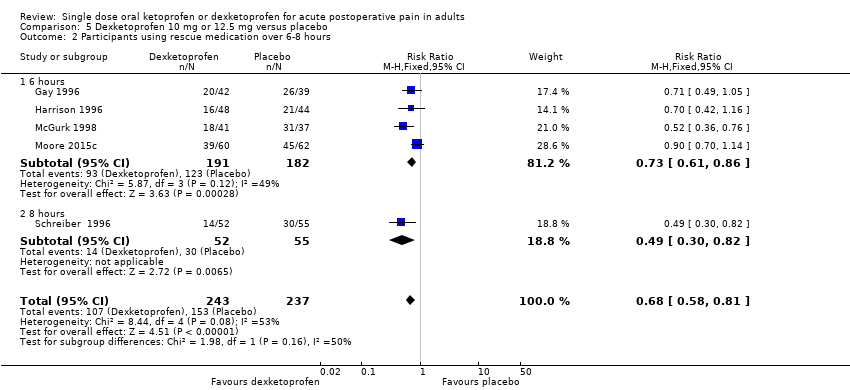
| Participants using rescue medication over 6 hours | 320 in 1000 | 750 in 1000 | RR 0.42 (0.33 to 0.52) NNTp 2.3 (1.8 to 3.1) | 4 studies 263 participants | High quality | Reasonable numbers of participants and high event rate. |
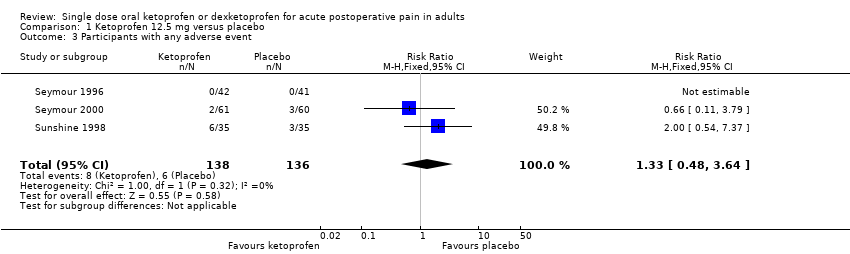
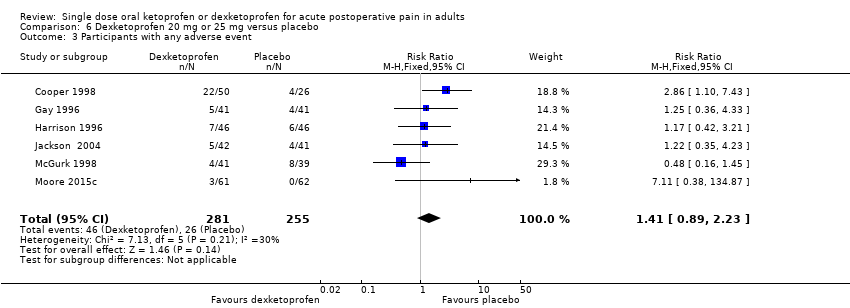
| Participants with ≥ 1 adverse event following a single dose | 180 in 1000 | 110 in 1000 | RR 1.6 (0.98 to 2.8) NNH not calculated | 5 studies 342 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 688 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. |
| We used the following descriptors for levels of evidence (EPOC 2015). -
High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially differenta is low. -
Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially differenta is moderate. -
Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially differenta is high. -
Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially differenta is very high. a Substantially different: a large enough difference that it might affect a decision. |