Dosis única oral de ketoprofeno y dexketoprofeno para el dolor postoperatorio agudo en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007355.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 25 mayo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For the original review: JB, SD, and RAM carried out searching, data extraction, and analysis, including assessment of study quality. HJM helped with analysis and acted as arbitrator. All review authors contributed to the writing of the protocol and review.
For this update: HG and SD carried out searching, data extraction, and analysis, including assessment of risk of bias and quality of the evidence. RAM acted as arbitrator; he did not participate in searching, or data extraction, analysis, assessment of risk of bias and quality of the evidence for the included studies in which he was an author. All authors contributed to the writing of the review.
Sources of support
Internal sources
-
Oxford Pain Research Funds, UK.
External sources
-
NHS Cochrane Collaboration Programme Grant Scheme, UK.
-
NIHR Biomedical Research Centre Programme, UK.
Declarations of interest
HG: none known; HG is a retired geriatrician and has treated patients with acute pain.
SD: none known.
PW: none known.
RAM is an author of three of the included trials. He has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
Acknowledgements
This review received infrastructure support from the Oxford Pain Relief Trust. Professor Henry McQuay and Jodie Barden were authors on an earlier version of this review.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS), or the Department of Health.
Menarini Group provided copies of published and unpublished studies for dexketoprofen for the earlier review; the company markets products containing both ketoprofen and dexketoprofen.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 May 25 | Single dose oral ketoprofen or dexketoprofen for acute postoperative pain in adults | Review | Helen Gaskell, Sheena Derry, Philip J Wiffen, R Andrew Moore | |
| 2009 Oct 07 | Single dose oral ketoprofen and dexketoprofen for acute postoperative pain in adults | Review | Jodie Barden, Sheena Derry, Henry J McQuay, R Andrew Moore | |
| 2009 Jul 08 | Single dose oral ketoprofen and dexketoprofen for acute postoperative pain in adults | Protocol | Jodie Barden, Sheena Derry, Henry J McQuay, R Andrew Moore | |
Differences between protocol and review
We changed the title to 'ketoprofen or dexketoprofen' to clarify that the interventions were not taken together.
This review included updated 'Risk of bias' and GRADE assessments, and 'Summary of findings' tables. We have not included sensitivity analyses by size and Oxford Quality Score.
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in four years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Pain [*drug therapy];
- Administration, Oral;
- Analgesics, Non‐Narcotic [*administration & dosage, adverse effects];
- Anti‐Inflammatory Agents, Non‐Steroidal [*administration & dosage, adverse effects];
- Dental Care;
- Ketoprofen [*administration & dosage, adverse effects, *analogs & derivatives];
- Pain, Postoperative [*drug therapy];
- Randomized Controlled Trials as Topic;
- Stereoisomerism;
- Time Factors;
Medical Subject Headings Check Words
Adult; Aged; Female; Humans; Male; Middle Aged;
PICO

Study flow diagram.
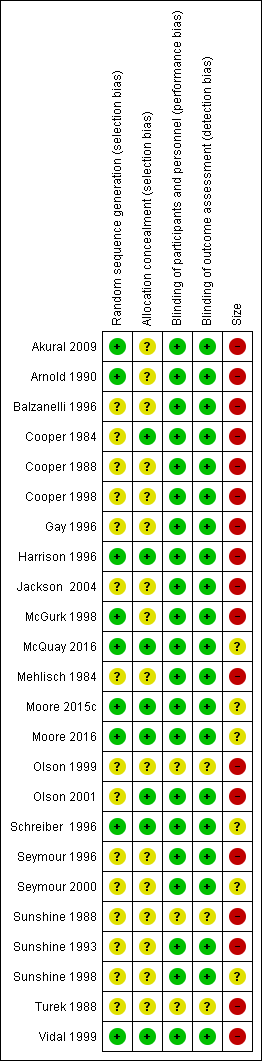
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 3 Ketoprofen 50 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over four to six hours.
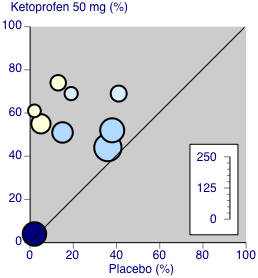
Ketoprofen 50 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.
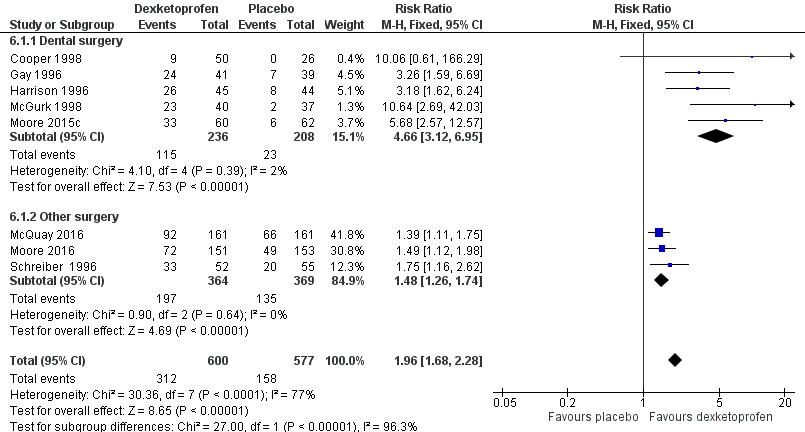
Forest plot of comparison: 6 Dexketoprofen 20 mg or 25 mg versus placebo, outcome: 6.1 Participants with at least 50% pain relief over four to six hours.

Dexketoprofen 20/25 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.

Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.
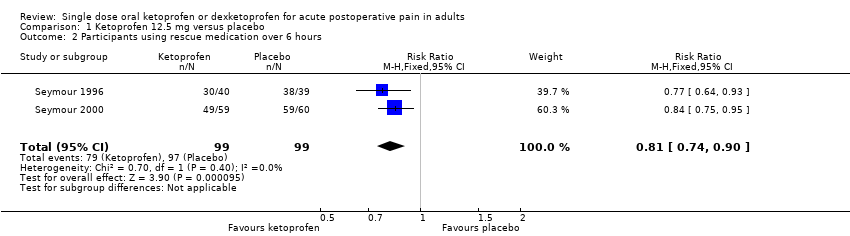
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.
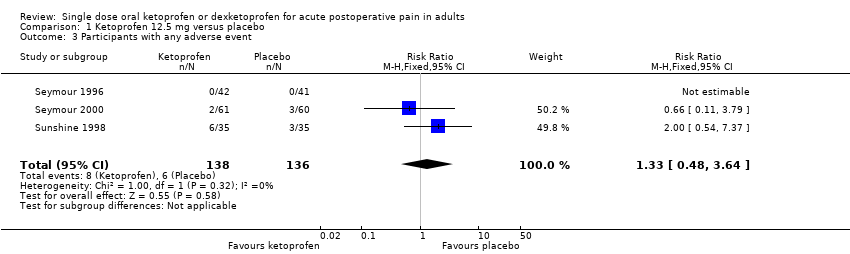
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.

Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.

Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
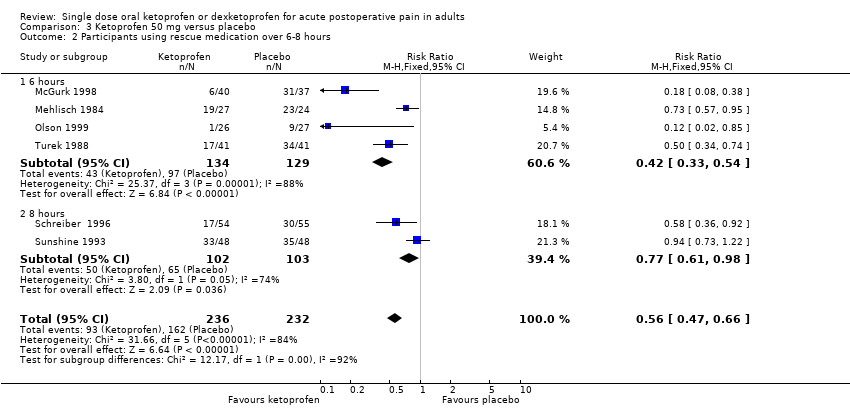
Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 3 Participants with any adverse event.
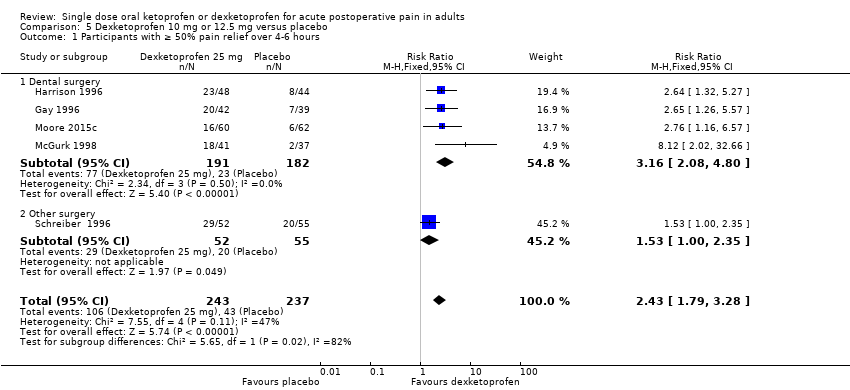
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
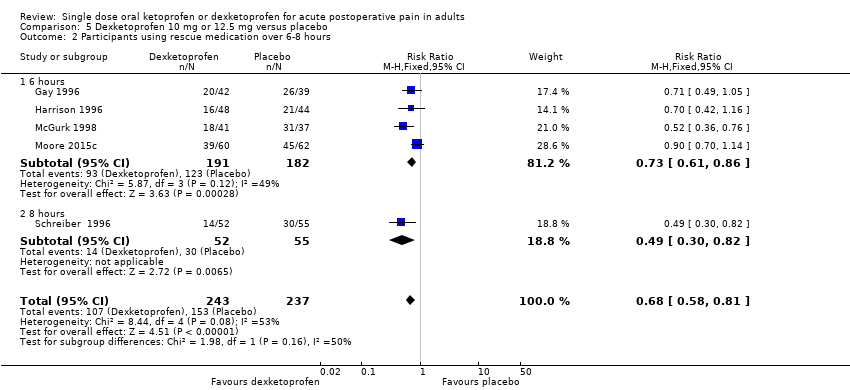
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
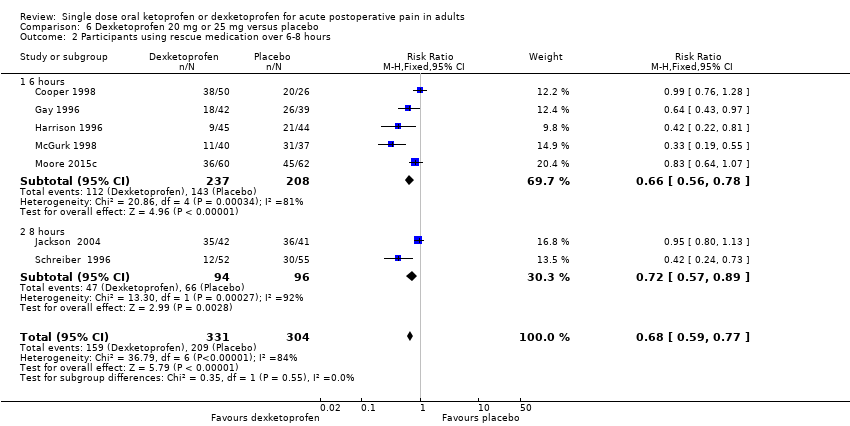
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
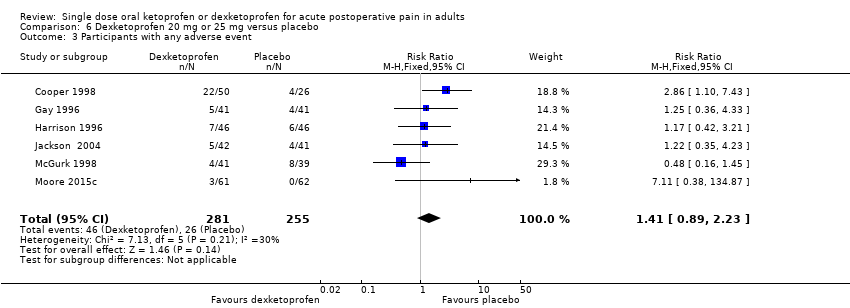
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 3 Participants with any adverse event.
| Ketoprofen 25 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 6 hours | 620 in 1000 | 120 in 1000 | RR 4.9 (3.5 to 6.9) NNT 2.0 (1.8 to 2.3) | 8 studies 535 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 5.3 hours (4.6 hours) | 1.6 hours (2.5 hours) | Not estimated | 2 studies 188 participants (5 studies 277 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 460 in 1000 | 79 in 1000 | RR 0.60 (0.52 to 0.69) NNTp 3.0 (2.4 to 4.1) | 6 studies 402 participants | Moderate | Modest numbers of participants and events. |
| Participants with ≥ 1 adverse event following a single dose | 100 in 1000 | 91 in 1000 | RR 1.2 (0.68 to 2.0) NNH not calculated | 7 studies 490 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 8 studies 535 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Ketoprofen 50 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 50 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 570 in 1000 | 230 in 1000 | RR 2.5 (2.0 to 3.1) NNT 2.9 (2.4 to 3.7) | 8 studies 594 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | Approximately 5 hours (3.4 hours) | Approximately 3 hours (2.5 hours) | Not estimated | 1 study 77 participants (5 studies, 342 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 320 in 1000 | 750 in 1000 | RR 0.42 (0.33 to 0.52) NNTp 2.3 (1.8 to 3.1) | 4 studies 263 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 180 in 1000 | 110 in 1000 | RR 1.6 (0.98 to 2.8) NNH not calculated | 5 studies 342 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 688 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Dexketoprofen 10 mg‐12.5 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 10 mg‐12.5 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 440 in 1000 | 180 in 1000 | RR 2.4 (1.8 to 3.3) NNT 3.9 (3.0 to 5.7) | 5 studies 480 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 3.6 hours (4.9 hours) | 1.4 hours (3.6 hours) | Not estimated | 1 study 122 participants (3 studies 253 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 490 in 1000 | 680 in 1000 | RR 0.73 (0.61 to 0.86) NNTp 5.3 (3.5 to 11) | 4 studies 373 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 68 in 1000 | 96 in 1000 | RR 0.70 (0.36 to 1.4) NNH not calculated | 4 studies 380 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 6 studies 574 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Dexketoprofen 20 mg or 25 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 20 mg or 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 520 in 1000 | 270 in 1000 | RR 2.0 (1.6 to 2.2) NNT 4.1 (3.3 to 5.2) | 8 studies 1177 participants | High quality | Good quality studies, important outcome available, robust numbers |
| Median (mean) time to use of rescue medication | 4.7 hours (5.2 hours) | 1.8 hours (3.6 hours) | Not estimated | 3 studies 281 participants (3 studies, 251 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 470 in 1000 | 690 in 1000 | RR 0.66 (0.56 to 0.78) NNTp 4.7 (3.3 to 8.0) | 5 studies 445 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 160 in 1000 | 100 in 1000 | RR 1.4 (0.89 to 2.2) NNH not calculated | 6 studies 536 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 1271 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for one additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [2.68, 6.63] |
| 2 Participants using rescue medication over 6 hours Show forest plot | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 3 Participants with any adverse event Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 8 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [3.48, 6.85] |
| 1.1 Dental surgery | 6 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [3.50, 7.36] |
| 1.2 Other surgery | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.77, 8.86] |
| 2 Participants using rescue medication over 6 hours Show forest plot | 6 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.52, 0.69] |
| 3 Participants with any adverse event Show forest plot | 7 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.97, 3.14] |
| 1.1 Dental surgery | 3 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.04 [4.23, 19.30] |
| 1.2 Other surgery | 5 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.40, 2.28] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 6 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.47, 0.66] |
| 2.1 6 hours | 4 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.2 8 hours | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 3 Participants with any adverse event Show forest plot | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.98, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 6 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [3.02, 6.08] |
| 1.1 Dental surgery | 4 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [4.67, 14.86] |
| 1.2 Other surgery | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 3.00] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 4 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.67] |
| 2.1 6 hours | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.38, 0.65] |
| 2.2 8 hours | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.89] |
| 3 Participants with any adverse event Show forest plot | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.79, 3.28] |
| 1.1 Dental surgery | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [2.08, 4.80] |
| 1.2 Other surgery | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.00, 2.35] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.81] |
| 2.1 6 hours | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.86] |
| 2.2 8 hours | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.82] |
| 3 Participants with any adverse event Show forest plot | 4 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.68, 2.28] |
| 1.1 Dental surgery | 5 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.12, 6.95] |
| 1.2 Other surgery | 3 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.26, 1.74] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 7 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.77] |
| 2.1 6 hours | 5 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.78] |
| 2.2 8 hours | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.89] |
| 3 Participants with any adverse event Show forest plot | 6 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.23] |

