Pentoxifilina para la hepatitis alcohólica
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: randomised, double‐blind, parallel design trial. | |
| Participants | Country: USA. | |
| Interventions | Intervention: pentoxifylline 400 mg three times a day, orally, 28 days. | |
| Outcomes | Primary outcome measure(s): 28‐day survival and progression to hepatorenal syndrome. 12/50 (24%) in pentoxifylline group died. 24/52 (46%) in control group died. Hepatorenal syndrome developed in 6/50 (21%) in pentoxifylline group and 22/52 (42%) in control group. | |
| Notes | Compliance with the intervention regimen was lower in the pentoxifylline group. In the pentoxifylline group 12 discontinued treatment, one dropped out and was not included in the analysis, seven withdrew due to adverse events, four did not adhere to the regimen and/or all the follow‐up appointments. In the control group four discontinued treatment, one due to adverse events, and three did not adhere to the regimen and/or all the follow‐up appointments. In March 2008 a letter was written to Akriviadis for clarification of sample size calculation, pre‐published protocol, and trial sponsorship. No response has so far been received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | An independent person randomly selected sealed envelopes. |
| Allocation concealment? | Low risk | Drugs were coded and distributed by hospital pharmacy; tablets were enclosed in opaque capsules. |
| Blinding? | Low risk | Placebo was packaged in identical opaque capsules; placebo had similar size and appearance to treatment. |
| Incomplete outcome data addressed? | Low risk | The number and reasons for withdrawals were given and six‐month survival data was obtained for all. |
| Free of selective reporting? | Low risk | Predefined, clinically relevant, and expected outcomes are reported. |
| Free of other bias? | Unclear risk | Intention‐to‐treat analysis was not performed; one drop‐out was not included in the analysis. Sample size calculation was not reported. |
| Methods | Trial design: randomised, double‐blind, parallel design trial. | |
| Participants | Country: France. | |
| Interventions | Intervention: pentoxifylline 400 mg three times a day, orally, duration not described. | |
| Outcomes | Primary outcome measure(s): death at 2 months. In the subgroup with severe alcoholic hepatitis of those who received pentoxifylline 14% died, and of those who received control 46% died within 2 months. Secondary outcome measure(s): death at 6 months. In the subgroup with severe alcoholic hepatitis of those who received pentoxifylline 27% died, and of those who received control 31% died within 2 months. Personal communication with the coordinating investigator Didier Lebrec tells us that from the subgroup of participants with alcoholic hepatitis, 19 participants in each group died. Meaning 19/71 died in the pentoxifylline group and 19/61 died in the control group. | |
| Notes | The numbers of participants randomised to either pentoxifylline or placebo in the subgroup with severe alcoholic hepatitis are not given. The outcome measures for these participants are reported as percentages. In March 2008 a letter was written and we telephoned Didier Lebrec where he kindly clarified the number of participants with alcoholic hepatitis in each intervention group and the number of those participants who died. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Centrally controlled, computer generated randomisation sequence. |
| Allocation concealment? | Low risk | Centrally controlled, computer generated randomisation sequence. |
| Blinding? | Low risk | Described as double‐blind and placebo used. |
| Incomplete outcome data addressed? | Unclear risk | No withdrawals or drop‐outs were reported. |
| Free of selective reporting? | Unclear risk | The trial was registered on clinical trials.gov (NCT00162552) which listed out predefined outcome measures: primary outcome measure: survival rate at 2 months. Secondary outcome measures: survival rate at 6 months; number of patient with liver transplantation; complications (bacterial infection, renal insufficiency, hepatic encephalopathy, gastrointestinal bleeding); fibrotest and acutest at 2 and 6 months; TNF alpha and IL6 plasma concentration at 2 and 6 months. Only 2 and 6 month mortality was reported in this publication. |
| Free of other bias? | Unclear risk | Sample size calculation was reported on clinicaltrilas.gov, calculated for participants with severe cirrhosis, data analysed from participants with alcoholic hepatitis was a subgroup analysis. |
| Methods | Trial design: randomised, parallel design pilot trial. | |
| Participants | Country: USA. | |
| Interventions | Intervention: pentoxifylline 1200 mg once a day, orally, 10 days. | |
| Outcomes | Primary outcome measure(s): biochemical parameters and plasma TNF levels. Renal impairment, fever and mortality were also recorded. Biochemical parameters are reported as means for each group. "Significantly less renal impairment and fever" in the pentoxifylline group, data not given. For mortality, the article states: "Thirty day mortality was higher in controls compared to treated patients (1 vs 3, p = not significant)", meaning that one participant in the pentoxifylline group died and three in the control group died. Secondary outcome measure(s): not specified. Adverse events: not reported. Period of follow‐up: 10 days after treatment follow‐up of biochemical data, 30 days after treatment follow‐up of survival data. | |
| Notes | In March 2008 a letter was written to McHutchinson for clarification of: what exactly constituted 'standard treatment'; if there were any co‐interventions; allocation sequence generation; allocation concealment; blinding; loss to follow‐up; outcome measure data; sample size calculation; pre‐published protocol; and trial sponsorship. The letter was later returned to sender. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, but the method was not described |
| Allocation concealment? | Unclear risk | No allocation concealment method was described |
| Blinding? | Unclear risk | Described as blind, but the method was not described. |
| Incomplete outcome data addressed? | Unclear risk | No withdrawals were reported. |
| Free of selective reporting? | High risk | Hepatic‐related morbidity was not reported. Renal impairment, fever, and mortality were not pre‐defined as primary outcomes. |
| Free of other bias? | Unclear risk | Intention‐to‐treat analysis was not reported. Sample size calculation not reported. Risk of multiple publication bias, as this pilot trial led to the trial of Akiviadis et al 2000. |
| Methods | Trial design: randomised, parallel design trial. | |
| Participants | Country: India. | |
| Interventions | Intervention: dose and regimen of pentoxifylline not described, duration 4 weeks. | |
| Outcomes | Primary outcome measure(s): end‐of‐study survival or hepatorenal syndrome. 4/14 in the pentoxifylline group died, and 7/16 in the control group died. Of those who died, hepatorenal syndrome occurred in 2/4 of the pentoxifylline group and 6/7 of the control group. | |
| Notes | In March 2008 a letter was written to Paladugu for clarification of: sequence generation; allocation concealment; blinding; loss to follow‐up; outcome measure data; sample size calculation; pre‐published protocol; and trial sponsorship. No response has so far been received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, but the method was not described. |
| Allocation concealment? | Unclear risk | Described as randomised, but the method was not described. |
| Blinding? | Unclear risk | Not described as blind, but described as placebo controlled. |
| Incomplete outcome data addressed? | Unclear risk | No withdrawals were reported. |
| Free of selective reporting? | Low risk | Reporting on mortality, hepatorenal syndrome and TNF levels was according to objectives and pre‐defined outcome measures. |
| Free of other bias? | Unclear risk | Sample size calculation was not reported. |
| Methods | Trial design: randomised, parallel design trial. | |
| Participants | Country: India. | |
| Interventions | Intervention: pentoxifylline 400 mg three times a day, orally, 28 days. | |
| Outcomes | Primary outcome measure(s): short‐term survival. 6/25 in the pentoxifylline group died, and 10/25 in the control group died. Of those who died, hepatorenal syndrome occurred in 5/25 of the pentoxifylline group and 6/25 of the control group. | |
| Notes | No raw data was given for the laboratory variables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, but the method was not described. |
| Allocation concealment? | Unclear risk | Described as randomised, but the method was not described. |
| Blinding? | Low risk | Described as double‐blind and placebo controlled. |
| Incomplete outcome data addressed? | Unclear risk | No withdrawals were reported. |
| Free of selective reporting? | Low risk | Outcome measures were pre‐defined and reported, ie, short‐term survival and laboratory parameters. Hepatic‐related morbidity was reported on. |
| Free of other bias? | Unclear risk | Sample size calculation was not reported. |
M = men.
W = women.
g = gram.
mg = milligram.
mm3 = millimetre cubed.
DF = discriminant factor
vs = versus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Participants had alcoholic cirrhosis, not alcoholic hepatitis. | |
| Comment on Akriviadis 2000. | |
| Participants had alcoholic cirrhosis, there was no data presented for participants with alcoholic hepatitis. | |
| Participants had non‐alcoholic steatohepatitis. | |
| Case‐controlled study. Twenty‐nine participants took pentoxifylline. | |
| Retrospective, participants not randomised. | |
| Retrospective, participants not randomised. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, double‐blind, placebo control, phase III. |
| Participants | Alcoholic hepatitis, in adults and seniors. |
| Interventions | Pentoxifylline. |
| Outcomes | Not specified. |
| Notes | Sponsor is the University of Wisconsin, Madison. Completion date is July 2007. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Adipose tissue involvement in alcohol‐induced liver inflammation in human: study of pro‐ and anti‐inflammatory cytokines and adipokines. |
| Methods | Observational, prospective study, phase III. |
| Participants | Alcoholic hepatitis or alcoholic cirrhosis in adults and seniors. |
| Interventions | Not specified. |
| Outcomes | Not specified. |
| Starting date | November 2006. |
| Contact information | http://ClincalTrials.gov/show/NCT00388323 |
| Notes | Sponsor is Assistance Publique Hôpitaux de Paris. Completion date is October 2008. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality using the fixed effect model Show forest plot | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| Analysis 1.1  Comparison 1 All‐cause mortality, pentoxifylline versus control, Outcome 1 Mortality using the fixed effect model. | ||||
| 2 Mortality using the random effects model Show forest plot | 5 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| Analysis 1.2  Comparison 1 All‐cause mortality, pentoxifylline versus control, Outcome 2 Mortality using the random effects model. | ||||
| 3 Mortality according to risk of bias Show forest plot | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| Analysis 1.3  Comparison 1 All‐cause mortality, pentoxifylline versus control, Outcome 3 Mortality according to risk of bias. | ||||
| 3.1 Low risk of bias | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.92] |
| 3.2 High risk of bias | 4 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hepatic‐related mortality using fixed‐effect model Show forest plot | 3 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.22, 0.71] |
| Analysis 2.1  Comparison 2 Hepatic‐related mortality, Outcome 1 Hepatic‐related mortality using fixed‐effect model. | ||||
| 2 Hepatic‐related mortality using the random‐effects model Show forest plot | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.85] |
| Analysis 2.2  Comparison 2 Hepatic‐related mortality, Outcome 2 Hepatic‐related mortality using the random‐effects model. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 5 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.52, 0.82] |
| Analysis 3.1  Comparison 3 Sensitivity analysis, all‐cause mortality, Outcome 1 Mortality. | ||||
| 1.1 Mortality, sensitivity analysis with all missing mortality data survived | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| 1.2 Mortality, sensitivity analysis with all missing mortality data died | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Variceal bleeding Show forest plot | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.42, 11.32] |
| Analysis 4.1  Comparison 4 Hepatic‐related morbidity, pentoxifylline versus control, Outcome 1 Variceal bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum creatinine Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐1.14, ‐0.87] |
| Analysis 5.1  Comparison 5 Biochemical parameters, pentoxifylline versus control, Outcome 1 Serum creatinine. | ||||
| 2 Serum bilirubin Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐1.55 [‐5.10, 2.00] |
| Analysis 5.2  Comparison 5 Biochemical parameters, pentoxifylline versus control, Outcome 2 Serum bilirubin. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tumour necrosis factor Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [1.59, 6.48] |
| Analysis 6.1  Comparison 6 Post‐hoc outcome measures, TNF levels, Outcome 1 Tumour necrosis factor. | ||||
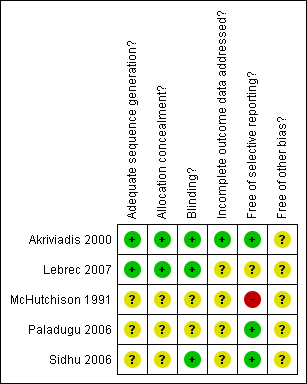
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Trial sequential analysis of the cumulative meta‐analysis of the effect of pentoxifylline on all‐cause mortality in participants with alcoholic hepatitis. The required information size of 1169 is calculated based on an a priori intervention effect of 20% (APHIS), a risk of type 1 error of 5%, and a power of 80%. The event rate in the control group is 39%, which is based on a meta‐analytic estimate of the control event rate of all the included trials. Although the cumulated z‐curve (blue curve) crosses the traditional boundary of 5% significance (horizontal red line), it does not cross the trial sequential monitoring boundary (red curve), implying that there is no firm evidence for an effect of 20% risk ratio reduction (RRR) when the cumulative meta‐analysis is adjusted for multiple testing on accumulating data.

Trial sequential analysis of the cumulative meta‐analysis of the effect of pentoxifylline on hepatic‐related mortality in participants with alcoholic hepatitis. The required information size of 1636 is calculated based on an a priori intervention effect of 20% (APHIS), a risk of type 1 error of 5% and a power of 80%. The event rate in the control group is 38%, which is based on a meta‐analytic estimate of the control event rate of all the included trials. Although the cumulated z‐curve (blue curve) crosses the traditional boundary of 5% significance (horizontal red line), it does not cross the trial sequential monitoring boundary (red curve), implying that there is no firm evidence for an effect of 20% risk ratio reduction (RRR) when the cumulative meta‐analysis is adjusted for multiple testing on accumulating data.
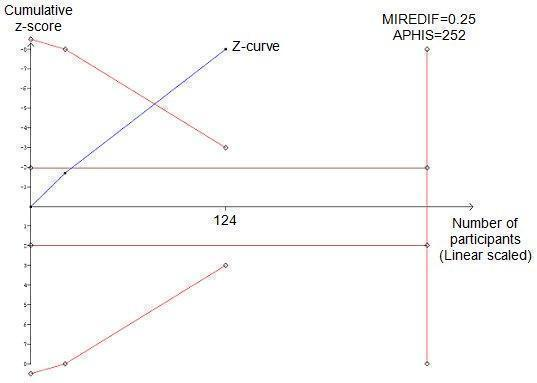
Trial sequential analysis of the cumulative meta‐analysis of the effect of pentoxifylline on serum creatinine in participants with alcoholic hepatitis. The required information size of 252 is calculated based on an intervention effect of 0.25 (mg/dl) (APHIS), a risk of type 1 error of 5% and a power of 80%. The cumulated z‐curve (blue curve) crosses the trial sequential monitoring boundary implying that there is firm evidence for a beneficial effect of 0.25 (mg/dl) decrease in serum creatinine when the cumulative meta‐analysis is adjusted for multiple testing on accumulating data.

Trial sequential analysis of the cumulative meta‐analysis of the effect of pentoxifylline on serum bilirubin in participants with alcoholic hepatitis. The trial sequential monitoring boundary is not calculated because the actual information size is less than 1% of the information size required. This is calculated based on an intervention effect of 1.00 (mg/dl) suggested by the one trial with low risk of bias.

Trial sequential analysis of the cumulative meta‐analysis of the effect of pentoxifylline on levels of TNF in participants with alcoholic hepatitis. The required information size of 318 is calculated based on an intervention effect of 4.00 pg/ml, suggested by the one trial with low risk of bias (LBHIS) (Akriviadis 2000), a risk of type 1 error of 5% and a power of 80%. The cumulated z‐curve (blue curve) does not cross the trial sequential monitoring boundary implying that there is no firm evidence for a potentially harmful effect of 4.00 pg/ml when the cumulative meta‐analysis is adjusted for multiple testing on accumulating data.

Comparison 1 All‐cause mortality, pentoxifylline versus control, Outcome 1 Mortality using the fixed effect model.
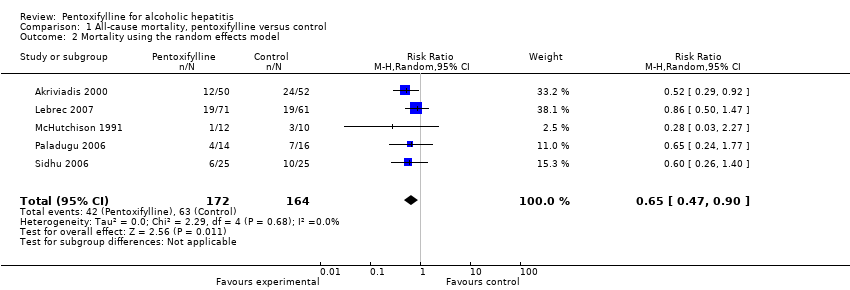
Comparison 1 All‐cause mortality, pentoxifylline versus control, Outcome 2 Mortality using the random effects model.
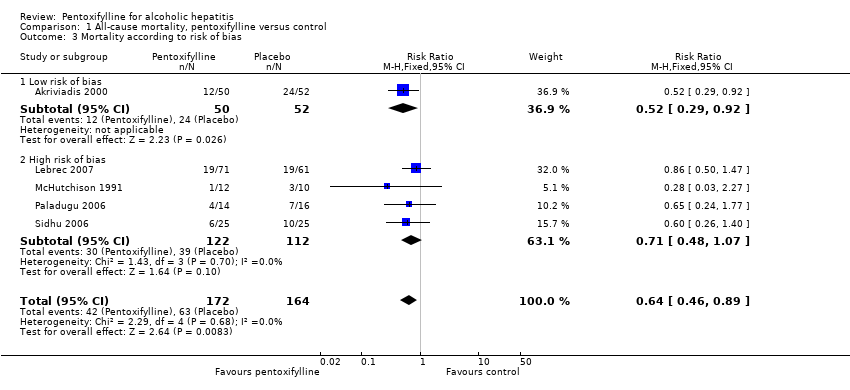
Comparison 1 All‐cause mortality, pentoxifylline versus control, Outcome 3 Mortality according to risk of bias.
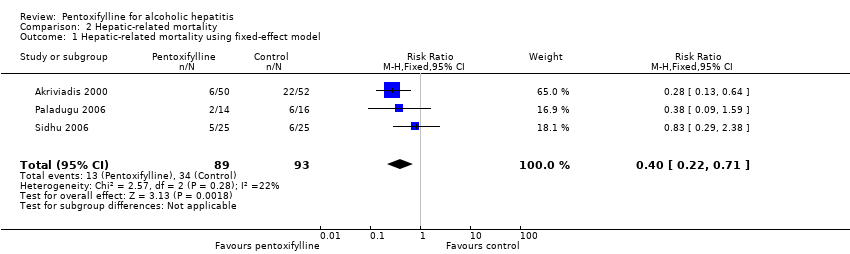
Comparison 2 Hepatic‐related mortality, Outcome 1 Hepatic‐related mortality using fixed‐effect model.

Comparison 2 Hepatic‐related mortality, Outcome 2 Hepatic‐related mortality using the random‐effects model.

Comparison 3 Sensitivity analysis, all‐cause mortality, Outcome 1 Mortality.

Comparison 4 Hepatic‐related morbidity, pentoxifylline versus control, Outcome 1 Variceal bleeding.

Comparison 5 Biochemical parameters, pentoxifylline versus control, Outcome 1 Serum creatinine.

Comparison 5 Biochemical parameters, pentoxifylline versus control, Outcome 2 Serum bilirubin.
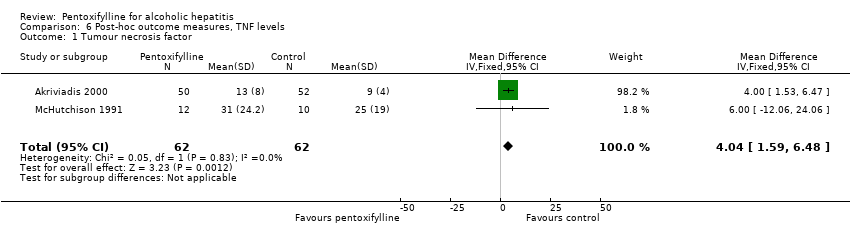
Comparison 6 Post‐hoc outcome measures, TNF levels, Outcome 1 Tumour necrosis factor.
| Outcome measure | Type of data | Pentoxifylline group | Control group | Statistical test | P value |
| Hepatic encephalopathy | Dichotomous | 9/50 (18%) | 13/52 (25%) | Fisher’s exact test | 0.133 |
| Withdrawals due to adverse events | Dichotomous | 7/50 (14%) | 1/52 (2%) | Fisher’s exact test | 0.026 |
| Outcome measure | Type of data | Pentoxifylline group | Control group | Statistical test | T value | P value |
| Blood urea nitrogen | Continuous | Mean 23 | Mean 38 | Student’s T test | 2.3426 | 0.021131 |
| Prothrombin time | Continuous | Mean 5 | Mean 5 | Student’s T test | 0 | 1 |
| Occurrence of adverse event as reported by Akriviadis et al | Pentoxifylline | Control |
| Transient diarrhoea | 4 | 2 |
| Epigastric discomfort or pain with or without vomiting | 13 | 5 |
| Severe gastrointestinal symptoms and headache | 3 | 0 |
| Diarrhoea | 1 | 0 |
| Epigastric pain | 1 | 0 |
| Severe headache | 1 | 0 |
| Generalised skin rash | 1 | 0 |
| Headache and gastrointestinal symptoms | 0 | 1 |
| Urinary tract infection | 1 | 0 |
| Spontaneous bacterial peritonitis | 3 | 4 |
| Cryptococcal septicaemia | 1 | 0 |
| Bronchopneumonia | 1 | 0 |
| Pneumonia | 0 | 1 |
| Staphylococcal bacteraemia | 0 | 1 |
| Necrotising pancreatitis | 0 | 1 |
| Intracranial bleeding | 1 | 0 |
| Vaginal bleeding | 1 | 0 |
| Posttraumatic epidural haematoma | 1 | 0 |
| Total | 33 | 15 |
| Reason for loss to follow‐up | Pentoxifylline | Control | Data collected |
| Participant dropped out | 1 | 0 | None, participant excluded from analysis. |
| Incomplete regimen and/or incomplete follow‐up appointment | 4 | 3 | No data collected due to missed appointments, but mortality at 2 and 6 months follow‐up was assessed. |
| Treatment withdrawal due to adverse events | 7 | 1 | Adverse events and mortality at 2 and 6 months follow‐up were assessed. |
| Total | 12 | 4 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality using the fixed effect model Show forest plot | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| 2 Mortality using the random effects model Show forest plot | 5 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 3 Mortality according to risk of bias Show forest plot | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| 3.1 Low risk of bias | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.92] |
| 3.2 High risk of bias | 4 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hepatic‐related mortality using fixed‐effect model Show forest plot | 3 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.22, 0.71] |
| 2 Hepatic‐related mortality using the random‐effects model Show forest plot | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 5 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.52, 0.82] |
| 1.1 Mortality, sensitivity analysis with all missing mortality data survived | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| 1.2 Mortality, sensitivity analysis with all missing mortality data died | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Variceal bleeding Show forest plot | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.42, 11.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum creatinine Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐1.14, ‐0.87] |
| 2 Serum bilirubin Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐1.55 [‐5.10, 2.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tumour necrosis factor Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [1.59, 6.48] |

