Tratamiento para el síndrome de salida torácica
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007218.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 26 noviembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
B Povlsen wrote the first draft of the 2014 review and B Povlsen and SD Povlsen reviewed the updated database searches and co‐ordinated the subsequent comments into the final review. T Hansson made valuable comments to the subsequent drafts and all participated in assessing the selected papers.
Sources of support
Internal sources
-
Department of Orthopaedics, Guy's & St Thomas Hospitals NHS Foundation Trust, UK.
External sources
-
No sources of support supplied
Declarations of interest
None of the members of the review team have conflicts of interest.
Acknowledgements
The authors are grateful for the assistance of the Cochrane Neuromuscular Disease Group. The Trials Search Co‐ordinator of the Cochrane Neuromuscular Disease Group (Angela Gunn) carried out the literature searches for the review. A Belzberg and M Dorsi contributed to the initial review, which formed the basis of this update. The Cochrane Neuromuscular Disease Group editorial base receives support from the MRC Centre for Neuromuscular Diseases.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Nov 26 | Treatment for thoracic outlet syndrome | Review | Bo Povlsen, Thomas Hansson, Sebastian D Povlsen | |
| 2010 Jan 20 | Treatment for thoracic outlet syndrome | Review | Bo Povlsen, Allan Belzberg, Thomas Hansson, Michael Dorsi | |
| 2008 Jul 16 | Treatment for thoracic outlet syndrome | Protocol | Bo Povlsen, Allan Belzberg, Thomas Hansson, Michael Dorsi | |
Differences between protocol and review
We did not search evidence‐based medicine reviews: ACP Journal Club or the Cochrane Database of Systematic Reviews (CDSR).
The review authors assessed the quality of studies using the Cochrane 'Risk of bias' tool (Higgins 2011) rather than the earlier methodological quality assessment process described in the protocol.
At this update we added some comments on dealing with bilateral cases under 'Unit of analysis issues'.
At this update, secondary outcome measures of 'change in disability' and 'change in paresthesias' were included.
Finlayson 2011 was included for review as a double‐blind randomized control trial. The protocol stipulated that we would accept the author's diagnosis of TOS if attempts had been made to stratify the patients' diagnosis of TOS into the three sub‐types. This criterion could not be applied here; however, we felt this paper should be included due to the high quality of blinding, randomization, outcome measurement, and low risk of bias.
A Belzberg and M Dorsi, who authored the protocol and original version of the review, were not involved in the update. Instead SD Povlsen, who was not involved in authoring the protocol or original version of the review, was co‐author of the update.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
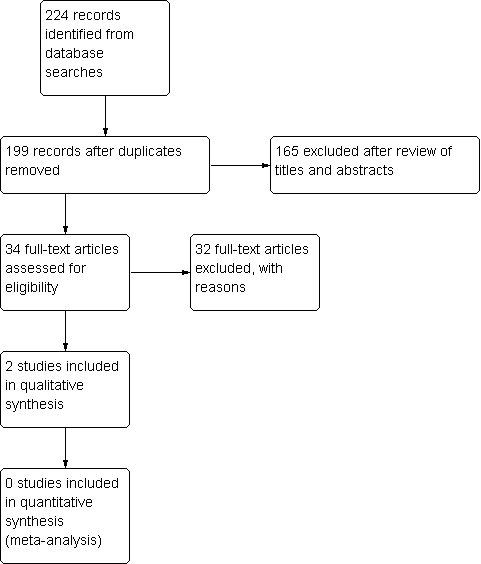
Study flow diagram.

Risk of bias summary: review authors' judgments about each 'Risk of bias' item for each included study. Red (‐) = high risk of bias, yellow (?) = unclear risk of bias, green (+) = low risk of bias.

