Tratamiento para el síndrome de salida torácica
Resumen
Antecedentes
El síndrome de salida torácica (SST) es uno de los diagnósticos más polémicos de la medicina clínica. A pesar de muchos informes de intervenciones quirúrgicas y no quirúrgicas, no existe una investigación científica rigurosa sobre este síndrome que sustente el tratamiento basado en pruebas. Ésta es la primera actualización de una revisión publicada por primera vez en 2010.
Objetivos
Evaluar los efectos beneficiosos y adversos de las intervenciones quirúrgicas y no quirúrgicas disponibles para el tratamiento del SST después de un mínimo de seis meses desde la intervención.
Métodos de búsqueda
El 23 junio 2014, se hicieron búsquedas en el registro especializado del Grupo Cochrane de Enfermedades Neuromusculares (Cochrane Neuromuscular Disease Group), CENTRAL, The Database of Abstracts of Reviews of Effects (DARE), MEDLINE, EMBASE, CINAHL Plus y AMED. También se buscó en las listas de referencias de los ensayos identificados.
Criterios de selección
Se seleccionaron los estudios aleatorios o cuasialeatorios que incluían a participantes con diagnóstico de SST de cualquier tipo (neurogénico, vascular y “dudoso”), sin limitaciones en cuanto al idioma de la publicación.
Se aceptaron estudios que examinaban cualquier intervención dirigida al tratamiento del SST.
La medida de resultado primaria fue el cambio de la calificación del dolor en una escala analógica visual validada o similar, al menos seis meses después de la intervención.
Los resultados secundarios fueron el cambio en la fuerza muscular, la discapacidad, las experiencias de parestesia (adormecimiento y sensación de cosquilleo) y los efectos adversos de las intervenciones.
Obtención y análisis de los datos
Tres autores, de forma independiente, seleccionaron los ensayos para ser incluidos y extrajeron los datos. Los autores evaluaron el riesgo de sesgo de los estudios incluidos según los métodos recomendados en el Manual Cochrane para las Revisiones Sistemáticas de Intervenciones (Cochrane Handbook for Systematic Reviews of Interventions).
Resultados principales
Las dificultades de esta revisión se deben a la ausencia de criterios diagnósticos de aceptación general del SST, y se tuvo que depender exclusivamente del diagnóstico de SST realizado por los investigadores en los estudios examinados. Se identificó un estudio que comparaba la progresión natural con una intervención activa. Se encontraron tres ensayos controlados aleatorios (ECA), aunque sólo dos de ellos tenían un seguimiento de seis meses o más, que fue el seguimiento mínimo necesario para la inclusión en la revisión. El primer ensayo que cumplió con los requisitos incluyó a 55 participantes con SST de “tipo dudoso” y comparó la resección transaxilar de la primera costilla (RTPC) con la neuroplastia supraclavicular del plexo braquial (NSPB). El ensayo presentó un alto riesgo de sesgo. La RTPC redujo más el dolor que la NSPB. No hubo efectos adversos en ningún grupo. El segundo ensayo que cumplió con estos requisitos analizó a 37 pacientes con SST de cualquier tipo y comparó el tratamiento con una inyección de toxina botulínica (BTX, por sus siglas en inglés) en los músculos escalenos con una inyección de placebo de solución salina. Este ensayo tenía un bajo riesgo de sesgo. No hubo ningún efecto significativo del tratamiento con la inyección de BTX sobre el placebo en cuanto al alivio del dolor o las mejorías en la discapacidad, aunque el mismo mejoró significativamente la parestesia a los seis meses de seguimiento. No hubo ningún evento adverso del tratamiento con BTX por sobre la inyección salina.
Conclusiones de los autores
Las dificultades de esta revisión se deben a la ausencia de criterios diagnósticos de aceptación general para el diagnóstico del SST. Hubo pruebas de muy mala calidad de que la resección transaxilar de la primera costilla disminuyó el dolor más que la neuroplastia supraclavicular, pero no hay pruebas procedentes de ensayos aleatorios de que es mejor que ningún tratamiento. Hay pruebas moderadas para sugerir que el tratamiento con inyecciones de BTX no produjo grandes mejorías sobre las inyecciones de placebo de solución salina. No hay pruebas a partir de ECA para el uso de otros tratamientos utilizados en la actualidad. Se necesita una definición acordada para el diagnóstico del SST, especialmente la forma dudosa, medidas de resultado acordadas y ensayos aleatorios de alta calidad que comparen el resultado de las intervenciones con ningún tratamiento y entre sí.
PICOs
Resumen en términos sencillos
Tratamiento para el síndrome de salida torácica
Pregunta de la revisión
Se examinaron las pruebas acerca del efecto de cualquier tratamiento para el síndrome de salida torácica (SST).
Antecedentes
El SST es uno de los diagnósticos más polémicos en medicina. El término SST representa tres síndromes relacionados: una forma en la que el plexo braquial (una colección de nervios en el cuello y axila) es comprimido; una forma en la que los vasos sanguíneos principales del tórax superior son comprimidos; y el SST dudoso o inespecífico doloroso. El paciente que presenta SST puede tener síntomas como dolor en el hombro y el cuello que puede propagarse al brazo y al frente del tórax; debilidad; cambio en las sensaciones; inflamación; y una restricción del suministro de sangre al brazo afectado. Las diversas causas del SST incluyen una costilla extra en el cuello, diferencias en la forma de los huesos de la columna vertebral, bandas de tejido anormales debajo de la piel y anomalías en la forma en que los músculos en el lado del cuello se adhieren a los huesos. El SST a menudo se asocia con lesiones anteriores.
Hay una falta de normas ampliamente aceptadas para realizar el diagnóstico del SST, de manera que con objeto de realizar esta revisión, se decidió depender del diagnóstico de SST realizado por los investigadores de los estudios examinados. El SST a menudo se diagnostica después de descartar otras causas de síntomas unilaterales de dolor en el brazo, debilidad, pérdida de sensibilidad, o las tres. La mayoría de las personas con diagnóstico de SST presenta la forma dudosa.
Se efectuaron búsquedas amplias de ensayos clínicos de los tratamientos para el SST. Se deseaba descubrir si algún tratamiento es efectivo y si los tratamientos presentan efectos perjudiciales.
Características de los estudios
A partir de la búsqueda sistemática, se identificaron dos ensayos. Un ensayo comparó la cirugía para extraer la primera costilla (resección transaxilar de la primera costilla) con cirugía en la cual el cirujano liberó los nervios de los tejidos circundantes (neuroplastia) sin extraer una costilla, en 55 pacientes con SST de tipo dudoso. Los participantes no habían respondido a los tratamientos no quirúrgicos. El seguimiento promedio fue de 37 meses. Un segundo ensayo analizó a 19 pacientes sometidos a la administración doble ciego de una inyección individual de BTX (relajante muscular) en los músculos escalenos del cuello, y a 18 pacientes del grupo de placebo que no recibieron ninguna inyección activa, con un seguimiento de seis semanas, tres meses y, críticamente con objeto de realizar esta revisión, seis meses.
Resultados y calidad de las pruebas
Hay pruebas de muy baja calidad de que la extracción de una costilla redujo más el dolor en los pacientes con SST “dudoso” en comparación con un procedimiento de neuroplastia. Se identificaron cuestiones en el diseño de estudio que podrían haber afectado el resultado del ensayo. No hubo efectos adversos en ningún grupo. No hubo ensayos de cirugía versus ningún tratamiento. El ensayo que comparó la intervención con inyección de BTX versus placebo aportó pruebas de calidad moderada de que este procedimiento no reduce significativamente las puntuaciones del dolor o la discapacidad a largo plazo, aunque no hubo ningún evento adverso asociado con el procedimiento en comparación con placebo.
Esta revisión sistemática demostró que no hay pruebas suficientes de que las intervenciones establecidas para el SST sean útiles para aliviar el dolor. Hasta que se realicen ensayos clínicos aleatorios de alta calidad que comparen las diversas intervenciones para el SST, la decisión de tratar y la elección apropiada del tratamiento tendrá que basarse en las preferencias del paciente y del profesional.
Las pruebas están actualizadas hasta junio de 2014.
Authors' conclusions
Background
Description of the condition
Thoracic outlet syndrome (TOS) is one of the most controversial diagnoses in clinical medicine. In this review, the term TOS represents three related syndromes, where the symptoms do not arise from underlying conditions, such as tumours: compression of the brachial plexus leading to confirmed neurophysiological abnormality (neurogenic TOS); compression of the subclavian artery or vein leading to objectively‐visualized vascular compression (vascular TOS); and a non‐specific or disputed type of TOS, where no objective tests can confirm either vascular or neurological abnormalities. The differential diagnosis of unilateral arm pain, weakness, or sensory loss, individually or combined, includes all of these syndromes but they are rare. The majority of people with TOS have the disputed form rather than neurogenic or vascular TOS. The objective diagnosis of (disputed) TOS is a challenge and generally accepted diagnostic criteria are lacking. Various anatomical anomalies have been offered as causes of TOS, including narrowing of the thoracic outlet by a cervical rib (cervical rib syndrome); extra bands of fascia ;or an abnormal origin or insertion of the anterior or medial scalene muscles. The person affected by TOS may experience pain affecting the shoulder and neck region and also radiating into the arm; paresis or paralysis of muscles innervated by the brachial plexus; and altered sensation. The arterial pulses in the arm may be reduced and there may be ischemia and edema (Huang 2004; Wilbourn 1999). Despite many reports on conservative and surgical intervention, complications, outcomes and success rates, rigorous scientific investigation of this syndrome and its management is lacking. This review aimed to systematically examine the evidence for the effectiveness of established interventions for the treatment of TOS.
Epidemiology of TOS
Despite the fact that the term 'thoracic outlet syndrome' was coined in 1956 (Peet 1956), there are no good estimates of its prevalence (Wilbourn 1990). Cadaver dissection has suggested that only 10% of the population have what is considered 'normal' anatomy bilaterally of the thoracic outlet (Junoven 1995). The prevalence of symptomatic TOS has been estimated to be 10 per 100,000 people (Edwards 1999).
Etiology of TOS
The etiology and mechanisms underlying TOS are complex and not well understood. Vascular compromise is estimated to account for only five per cent of all cases (Fechter 1993). Vascular TOS arises from compression in two different and distinct anatomic spaces. Arterial TOS (ATOS) results from compression of the subclavian artery as it passes through the triangle formed by the scalenus anticus and medius muscles and the first rib. Venous TOS (VTOS) results from compression of the subclavian vein as it re‐enters the chest more anteriorly, passing adjacent to the junction of the clavicle and first rib which is further reinforced by the subclavius muscle and tendon. VTOS may be further divided into four distinct presentations: (1) acute thrombosis; (2) chronic stenosis (effort thrombosis); (3) intermittent obstruction without thrombosis; and (4) complete obstruction. Ninety‐five per cent of people with TOS have only neurological symptoms. Whilst 'true neurogenic TOS' with characteristic clinical findings in the C8/T1 nerve root distribution is rare, and accounts for only about one to three per cent of all cases of TOS, 'disputed TOS' with its neurological symptoms but unconfirmed objective confirmation accounts for at least 90% of all operations for TOS in the United States (Wilbourn 1990). Factors considered influential in the development of TOS include trauma and the presence of a cervical rib (Sheth 2001).
Symptoms of TOS
Common to all types of TOS, individuals frequently report pain, which can lead to significant disability. The range of complaints reported in the literature includes pain affecting the neck, shoulder, upper extremity or hand. Weakness is another common symptom. Vascular TOS may also present with edema and cyanosis of the upper extremity with diminished pulses. True neurogenic TOS meanwhile can present with atrophy of the abductor pollicis brevis and intrinsic muscles of the hand. Disputed TOS meanwhile will still present with chronic pain of the upper extremity, but EMG and nerve conduction studies will reject diagnosis of true neurogenic TOS (Huang 2004; Wilbourn 1999).
Description of the intervention
Successful prevention and treatment of pain, muscular weakness and disability related to TOS are clinically challenging and heavily dependent on which of the three types of TOS the person is suffering from. No prospective randomized trials concerning the treatment of VTOS exist. Accepted treatments for this entity include heparinization, thrombolysis and thoracic outlet decompression, either alone or in various combinations (Illig 2010). The severity of any arterial compression guides treatment. Urschel 1998 describes the various surgeries: patients who have symptoms undergo rib resection if cervical or first rib arterial compression is responsible for poststenotic dilatation of the axillary subclavian artery. The transaxillary approach is preferred and the surgeon removes both the first and cervical ribs, without resecting the artery. Following decompression, the dilatation of the artery usually subsides. When the first or cervical rib causes compression and an aneurysm, with or without thrombus, is present, rib resection is accompanied by excision and grafting of the aneurysm. In this case the surgeon uses a combined supraclavicular and infraclavicular approach. In the most severe cases, in which the TOS compression causes thrombosis of the axillary subclavian artery or emboli in the lower part of the arm, the patient will undergo resection of the first rib with removal of the thrombus and emboli (thrombectomy and embolectomy), repair or replacement of the affected arteries, and undergo destruction of the sympathetic nerve trunk in the thoracic region, a procedure called "dorsal sympathectomy" (Urschel 1998).
While non‐operative and operative approaches have been described in the literature, no firm evidence exists for any approach in any of the three types of TOS. Non‐operative management typically involves strategies to reduce and redistribute pressure, and traction through the use of physiotherapy (Lindgren 1997) or orthoses (Nakatsuchi 1995). There are also several surgical approaches described in the literature. Surgical procedures fall into three main groups: (1) soft‐tissue procedures (scalenus release, neurolysis); (2) cervical rib excision; and (3) excision of the first thoracic rib (Sheth 2001). The outcome of treatment is said to be influenced by a number of factors such as gender, workers' compensation schemes, the position of the arm during work, and fixed joint abnormalities (Green 1991).
Why it is important to do this review
We undertook this review because of the complex nature of TOS, because of the pain and chronic morbidity that affects people with this condition, and the limited data available to guide treatment decisions. We planned to investigate each of the three types of TOS independently if evidence had been available. This is the first update of a review first published in 2010.
Objectives
To evaluate the beneficial and adverse effects of the available operative and non‐operative interventions for the treatment of TOS a minimum of six months after the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs of non‐operative and operative interventions for the treatment of TOS. We have reported evidence from high quality observational studies in the Discussion. These were prospective studies of consecutive case series with the outcomes preferably assessed by an individual who was not directly associated with delivering the intervention.
Types of participants
We included participants receiving any non‐operative or operative interventions for TOS of any etiology and type, though excluding patients with compression from malignancies. There was no restriction for age, sex, socioeconomic status, method of diagnosis, or duration of symptoms. We relied on the authors of the included papers to have appropriately diagnosed the participants.
Types of interventions
Any intervention aimed at treating TOS. These included but were not limited to the following:
-
appliances, for example orthoses and neck collar;
-
physical therapies, for example joint range of motion exercises, muscle stretching and strengthening;
-
medications, for example non‐steroidal anti‐inflammatory drugs (NSAIDs), corticosteroid injections and muscle relaxants;
-
operation, both soft‐tissue and bony procedures.
Types of outcome measures
Primary outcomes
The primary outcome was change in pain at least six months after the intervention preferably measured as change on a validated visual analogue scale (VAS) or similar.
Secondary outcomes
The secondary outcome measures were:
-
change in strength of potentially affected muscle groups at least six months after the intervention, measured with the Medical Research Council (MRC) scale which ranges from 0 = complete paralysis to 5 = normal;
-
change in disability of the affected upper extremity at least six months after the intervention, measured using the Disabilities of the Arm, Shoulder and Hand questionnaire (DASH), and physical and mental Short Form 36 Health Survey (SF‐36) or similar;
-
change in paresthesias of the affected upper extremity at least six months after the intervention using VAS or similar;
-
adverse effects of any treatment regimen.
Search methods for identification of studies
On 23 June 2014, we searched The Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2014, Issue 6 in The Cochrane Library), MEDLINE (January 1966 to June 2014), EMBASE (January 1980 to June 2014), CINAHL Plus (January 1937 to June 2014), and Allied and Complementary Medicine (AMED) (January 1985 to June 2014).
Furthermore, we performed additional searches of clinicaltrials.gov (15 July 2014) and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (15 July 2014) for ongoing trials.
Electronic searches
The detailed search strategies are in the appendices: Cochrane Neuromuscular Disease Group Specialized Register (Appendix 1), CENTRAL (Appendix 2), MEDLINE (Appendix 3), EMBASE (Appendix 4), CINAHL Plus (Appendix 5), and AMED (Appendix 6).
We would have considered studies in languages other than English, but none of the studies required translation.
For ClinicalTrials.gov and ICTRP, the search terms used were 'thoracic outlet syndrome', 'costoclavicular syndrome', 'scalenus anticus syndrome', 'superior thoracic aperture syndrome', and 'cervical rib syndrome'.
Searching other resources
We reviewed the bibliographies of the identified trials for any additional trials.
Data collection and analysis
Selection of studies
Three review authors independently and in duplicate, in a non‐blinded fashion, examined the title, keywords and abstract of reports identified from electronic searching for evidence of two criteria:
-
Is it a randomized or quasi‐randomized clinical trial?
-
Does it involve an intervention for the treatment of TOS?
If the report fulfilled these criteria or if the authors were not able to assess this from the title, keywords or abstract then the full article was obtained. There were no disagreements amongst authors regarding the inclusion or exclusion of any of the papers but any disagreement would have been resolved by discussion, to reach a consensus.
Data extraction and management
Two review authors independently extracted data from the included trial onto a data extraction form. The review authors contacted trial authors for further information when appropriate. One author entered data into the Cochrane software Review Manager 5 (RevMan 5) (RevMan 2012) and a second author checked the data entry. Any disagreement between authors in the extraction would have been resolved by a final check by a third party.
Assessment of risk of bias in included studies
For each study included, two authors independently completed a data extraction form to asses the risk of bias. We used the Cochrane 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This takes into account: secure method of randomization; concealment of allocation; blinding (including blinding of participants, blinding of investigators, blinding of outcome assessors); attrition bias; completeness of follow‐up; and other sources of bias. We obtained missing information from the authors whenever possible. Any disagreement between authors in the assessment would have been resolved by comparison of notes and further discussion of the studies until a consensus was reached.
Measures of treatment effect
We planned to analyse the three types of TOS individually. Where possible we would have calculated the mean difference (MD) and 95% confidence interval (CI) for continuous outcomes; and risk ratio and 95% CI for dichotomous outcomes.
Since we identified only two randomized trials for inclusion, we described in the Discussion some prospective trials reporting consecutive series of patients that were assessed by someone other than the person providing the intervention. Furthermore, the number assessed had to have exceeded 80% of those treated during a particular period.
Unit of analysis issues
We reported the number of bilateral cases of TOS in included studies. We stated whether randomization was applied to participants, or to arms, or noted that this information was not available.
Dealing with missing data
When the data were not available we attempted to retrieve them from the authors of the original trials, but neither main author of Sheth 2005 had supplementary data available.
Assessment of heterogeneity
To identify heterogeneity we would have examined the forest plots. If the CI of two studies had not overlapped or the I2 statistic had exceeded 50%, we would have suspected heterogeneity. Heterogeneity would have been dealt with by examining causes for heterogeneity and performing analyses taking account of these differences. In the event of unexplained heterogeneity, we would have used a random‐effects model.
Assessment of reporting biases
Not applicable.
Data synthesis
Since only two trials were included and they compared different treatment methods, no data synthesis was possible.
If more than one trial with a specific treatment or prevention approach had been identified, we would have calculated a pooled estimate of the treatment effect across the trials using RevMan. The initial analysis would have been performed with a fixed‐effect analysis.
Subgroup analysis and investigation of heterogeneity
Since only two trials were included, which investigated different interventions, subgroup analyses were not possible.
For future updates of this review, if the data are available, we will compare the effect of interventions in the following subgroups of participants:
-
presence or absence of cervical rib or elongated C7 transverse process;
-
acute (symptoms of less than six months' duration) or chronic (symptoms for six months or more); and
-
male or female.
Economic issues
We planned to consider cost and cost‐effectiveness in the Discussion, but no data were available.
Results
Description of studies
Results of the search
References found by the most recent searches were:
-
MEDLINE ‐ 116
-
EMBASE ‐ 37
-
AMED ‐ 6
-
CINAHL Plus ‐ 39
-
NMD Register ‐ 10
-
CENTRAL ‐ 12
-
DARE ‐ 4
The total number of studies was 224, but 25 of the studies overlapped, yielding a net total of 199 unique articles. Based on review of the abstracts, the authors obtained the full‐text articles for 34 studies, and reviewed these in detail. From the 34 studies we identified three RCTs. We excluded one of them because of an insufficient duration of follow‐up (Taskaynatan 2007), after we had contacted the trial authors, who were unable to provide supplementary information. We included the other two RCTs in this review. See Figure 1 for a flow diagram of the study selection process.
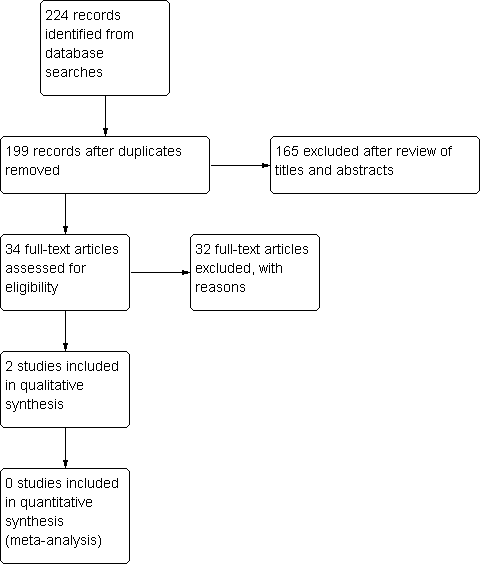
Study flow diagram.
Searches of ClinicalTrials.gov and ICTRP yielded no results of ongoing clinical trials.
Included studies
We included two trials: Sheth 2005 and Finlayson 2011.
Sheth 2005 evaluated the effects of transaxillary first rib resection (TFRR) (n = 24; bilateral procedure in two cases) versus supraclavicular neuroplasty of the brachial plexus (SNBP) (n = 25), on patient‐reported pain and numbness in 55 participants with the disputed type of TOS. Participants with anomalous elongated C7 transverse processes (cervical ribs), intrinsic weakness (characteristic of neurogenic TOS), and vascular TOS were excluded. For the two bilateral procedures, the participants, as opposed to their arms, were randomized, although no explicit information was available regarding whether the outcomes for each arm were reported separately.
Finlayson 2011 evaluated the effects of injection of botulinum toxin into the middle and anterior scalene muscles (n = 20; n = 19 analyzed) versus a placebo saline injection into the same muscles (n = 18) on patient‐reported pain, disability and paresthesias. The 37 analyzed participants were not limited by diagnosis of a specific type of TOS, but were excluded if they had previously undergone a scalenectomy. There was no information regarding occurrence of bilateral TOS and how this would have been handled within the framework of the study.
Excluded studies
We excluded 32 studies after full text review, see Characteristics of excluded studies. All but one were not randomised trials. We excluded one randomised trial for insufficient follow‐up (shorter than six months) after the trial authors of the original paper were unable to provide additional data (Taskaynatan 2007).
Risk of bias in included studies
This review was complicated by a lack of generally accepted diagnostic criteria for the diagnosis of TOS. We had to rely exclusively on the diagnosis of TOS as made by researchers in the reviewed studies. This in itself creates a high risk of bias in all the identified studies.
Sheth 2005 was at high risk of selection bias, as randomization was based on odd or even hospital number. There was no blinding. It was unclear how the VAS assessments were performed or whether they were complete, and unclear whether there may have been other sources of bias.
Finlayson 2011 was at a low risk of selection bias, with randomization carried out using a random number generator by a statistician who was not a co‐investigator. The syringes, prepared by a member of the team who was aware of the allocation sequence, were subsequently passed to the blinded injector. The allocation could in theory have been passed on to unblind the injectors, although the authors believe this not to have occurred. Participants, assessors, and those carrying out the procedure were blinded to the intervention. The distinction between self‐reported adverse events and adverse events subsequently reported via completion of a checklist is unclear, and the duration of such events is not mentioned.
The review authors' judgments about each 'Risk of bias' item for these included studies are presented in Figure 2.
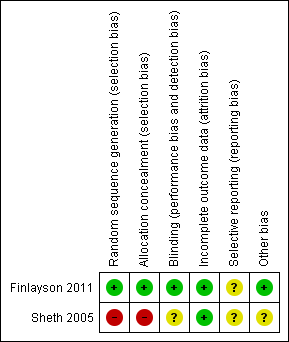
Risk of bias summary: review authors' judgments about each 'Risk of bias' item for each included study. Red (‐) = high risk of bias, yellow (?) = unclear risk of bias, green (+) = low risk of bias.
Effects of interventions
Transaxillary first rib resection (TFRR) versus supraclavicular neuroplasty of the brachial plexus (SNBP)
Change in pain at least six months after the intervention
Both interventions in Sheth 2005 resulted in significantly decreased pain and numbness after surgery at a mean follow‐up of 37 months (SEM ± 5 months). The TFRR conferred superior results to SNBP with respect to the pain rating on a zero to 100 mm range VAS scale (39 ± 7 versus 61 ± 7) with an estimated difference in the treatment effects of ‐22.0 (95% CI ‐41.9 to ‐2.1), percentage of pain relief (52 ± 8% versus 30 ± 78%), equating to an estimated difference of 22.0% (95% CI ‐0.8% to 44.8%). Pain rating on a nine‐point affective scale from "none" to "very intolerable" was 3.7 ± 0.4 with TFRR versus 5.1 ± 0.5 with SNBP, an estimated difference of ‐1.4 points (95% Cl ‐2.7 to ‐0.1).
Change in strength at least six months after the intervention
The study reports did not provide data on motor strength and no further data were available from the authors.
Change in disability at least six months after the intervention
The study reports did not provide data on change in disability and no further data were available from the authors.
Change in paresthesias at least six months after the intervention
Sheth 2005 reported change in paresthesias, but did not use VAS or similar to quantify the change, and we could not therefore include data in this review.
Adverse events
None of the participants experienced adverse effects from the interventions.
Injection of botulinum toxin (BTX) versus placebo injection of saline into the middle and anterior scalene muscles
Change in pain at least six months after the intervention
In Finlayson 2011, injection with BTX yielded no significant improvement in pain ratings on VAS over a placebo injection of saline. Whilst at baseline, median pain ratings for the treatment group was 46 mm (interquartile range 22 mm to 68 mm) and 63 mm for the placebo group (interquartile range 51 mm to 69 mm), at six months' follow‐up there was no significant difference between the two groups in terms of pain ratings using VAS, with a mean difference of ‐5.65 mm (95% CI ‐19.3 mm to 8.0 mm) in favor of less pain in the treatment arm.
Change in strength at least six months after the intervention
Change in motor strength was not reported and no further data were available from the authors.
Change in disability at least six months after the intervention
At six months' follow‐up, there was no significant difference in terms of change from baseline between the treatment and placebo groups using any of the questionnaires used, namely DASH, or SF‐36 mental and physical health surveys. Using DASH, there was a mean reduction in disability in the treatment group of 0.96 mm compared to a mean reduction of 3.19 mm in the placebo group, representing a non‐significant mean difference between groups at six months of 1.63 mm in favor of decreased disability in the placebo group (95% CI ‐5.7 mm to 9.0 mm).
Using SF‐36 physical, there was a mean increase in disability in the treatment group of 1.01 mm compared to a mean increase of 1.12 mm in the placebo group, representing a non‐significant difference between groups at six months of 0.09 mm in favor of decreased disability in the placebo group (95% CI ‐3.7 mm to 3.9 mm).
Using SF‐36 mental, there was a mean increase in disability in the treatment group of 2.89 mm compared to a mean increase of 0.01 mm in the placebo group, representing a non‐significant difference between groups at six months of 4.58 mm in favor of decreased disability in the placebo group (95% CI ‐1.4 mm to 10.6 mm).
Change in paresthesias at least six months after the intervention
At six months' follow‐up, the treatment group had a mean reduction in paresthesias from baseline (using VAS) of 7.63 mm, compared to a mean increase in paresthesias from baseline in the placebo group of 3.89 mm. The resultant calculated mean difference between groups at six months was statistically significant: ‐13.63 mm in favor of more relief in the treatment group (95% CI ‐26.3 mm to ‐1.0 mm).
Adverse events
Over the course of the six‐month follow‐up period, patients were encouraged to report any adverse events and subsequently filled in a checklist of adverse events that they believed to have occurred at some point during that period. In total, there were fewer adverse events in the treatment group (21) compared with the placebo group (40), suggesting no specific adverse effects were associated with the injection of the botulinum toxin .
Discussion
This review was complicated by a lack of generally accepted diagnostic criteria for the diagnosis of thoracic outlet syndrome (TOS). We had to rely exclusively on the diagnosis of TOS by researchers in the reviewed studies. We aimed to evaluate the effectiveness of various established interventions for TOS. An extensive search of the literature identified only two studies that met our inclusion criteria. Most studies were retrospective; the few prospective studies that we identified lacked randomization or adequate follow‐up.
Sheth 2005 is one of only two prospective randomized trials for any established intervention for TOS with a follow‐up of at least six months. Transaxillary first rib resection (TFRR) and supraclavicular neuroplasty of the brachial plexus (SNBP) are both associated with a reduction in pain postoperatively in people with the disputed type of TOS. In this group of patients TFRR provided superior results compared to SNBP for all outcome measures. A limitation of this study is that it excluded people with an elongated C7 transverse process (anomalous cervical rib) or signs and symptoms of neurogenic or vascular TOS. Thus, the diagnosis of disputed TOS was based solely on the subjective criteria set forth by the senior author. There is no report of the socioeconomic status of the participants or whether they were involved in ongoing litigation. Furthermore, the two cases of bilateral TOS, both of which appeared in the TFRR group, appear to have been recorded in the results as a single entry representing a patient instead of reporting outcomes for each limb individually. This may affect patient reporting of sensations of pain if the limbs were differentially affected by the procedure. Finally, the participants and assessors were not blinded to the specific intervention and importantly there was no control group.
Finlayson 2011 was the second prospective randomized trial that met our inclusion criteria. This study provides evidence to suggest that at six months, botulinum toxin injections into the scalene muscles offer no benefit in terms of reduction of pain and disability, in patients with diagnosis of any type of TOS, over a placebo injection of saline. However, there was a significant improvement in the experience of paresthesias in the treatment group over placebo. There were no adverse effects associated with the treatment procedure over placebo. The lack of change in pain and disability at six months versus placebo could have been explained by the effect of the drug wearing off; however, there were also no significant improvements in these outcomes at six weeks or three months. In fact, the only significant improvement reported in the paper was an improvement in paresthesias at six months only.
Whilst this study has benefits over the design in Sheth 2005, in that participants and assessors were blinded as to the intervention and that a control group was included, there remain some limitations. Firstly, the possibility of a treatment allocation bias exists, as the syringes were prepared by an investigator who was aware of the allocation sequence, which could in theory have been passed on to unblind the injectors. However, the trial authors believe this not to have occurred. Secondly, given that the mean duration of TOS symptoms in each group was long (treatment arm, six years; placebo, three years), participants could have suffered from chronic pain syndrome with central sensitization, in which case it would be expected that the treatment yielded no benefit. However, this possibility was not assessed by the authors. Thirdly, the authors state that their assessment was powered to detect a change of 20 mm on VAS from a mean baseline pain level of 40 mm. However, no baseline pain levels were set for inclusion or exclusion of participants, and there may well have been a floor effect, confounding the conclusion that the treatment had no effect. Fourthly, whilst the interscalene triangle is often considered the most common anatomical location of compression (Huang 2004), making injection into the scalene muscles seem ideal, botulinum toxin injections in themselves cannot be judged to have no effect in the treatment of TOS until other potential anatomical locations are trialled, such as the pectoralis minor and subclavius muscles. Fifthly, as people with any type of TOS could be included in this study, there remains a possibility that the treatment could have been beneficial for patients with a specific type of TOS; however, this was not examined in the study. Sixthly, although bilateral cases were reported in both the treatment and placebo groups, as with Sheth 2005 such cases appear not to have documented outcomes for each limb separately, potentially leaving the door open to patient‐reporting bias. Finally, and perhaps the most important point, causing us to downgrade the quality of evidence in this paper from 'high' to 'moderate' despite the low risk of bias and the direct study design comparing treatment arms with placebo, there appeared to be some baseline heterogeneity especially in pain scores between the treatment arm (median VAS 46 mm; interquartile range 22 to 68 mm) and the placebo group (median VAS 63 mm; interquartile range 51 to 99 mm). This baseline heterogeneity could affect the true interpretation of the results when the groups are compared at six months' follow‐up; however, the size of this effect cannot be quantified here, as this paper offered no statistical analysis of this potential baseline group heterogeneity.
Other evidence (from excluded studies)
Our search identified numerous retrospective studies and a few prospective randomized clinical trials of non‐operative interventions for TOS but none that had a follow‐up of six months for the primary outcome measure, pain.
Taskaynatan 2007 performed a randomized prospective trial to investigate the effects of cervical traction added to exercise and heat pack therapy in 40 people with TOS of non‐defined type. The participants were randomly divided into two groups. The control group received heat pack therapy and an exercise program; the experimental group received heat pack therapy, an exercise program, and cervical traction. The final outcome was assessed three weeks after the intervention. Outcome measures included the response to provocative manoeuvres and a Likert Scale rating of improvement in pain and numbness. Both interventions produced improvement in some of the provocative maneuvers and pain in most patients (75% control group versus 90% experimental group, P > 0.05). The difference in numbness scores between the groups was statistically significant in favor of adding cervical traction (80% versus 20%, P < 0.001). Although this study was a randomized controlled trial, it was excluded from our review because it did not meet the criteria for follow‐up of at least six months. The authors did not describe the method used for sequence generation or allocation concealment. In addition, neither the participants nor the investigators were blinded to the interventions. Thus, the risk of selection and assessment bias was high.
Lindgren 1997 published a prospective descriptive study of 119 people with possible TOS who were treated with a non‐operative inpatient rehabilitation program and instructions for home exercises to restore the normal function of their cervical spine and upper thoracic aperture. Patient satisfaction with the intervention at the end of the mean 11.4 (range 4‐24) days' inpatient period was 88%. The authors reported following the patients for a mean of 24.6 months, but did not provide standardized data at the long‐term follow‐up timepoint. Further, 30 of the 119 participants included in the study were found to have pathology other than TOS accounting for their symptoms. There was no assessment of compliance with the home exercises. The lack of comparison groups, blinding, standardization of patient diagnosis, and use of validated outcome measures introduced additional risk of bias.
Gülbahar 2005 reported a prospective series of 34 people with a subtype of disputed TOS, known as droopy shoulder syndrome, who were prescribed postural correction and shoulder girdle strengthening exercises. Compliance and symptom outcome were assessed at a mean (SD) follow‐up of 13.7 (5.0) months, and the patients were divided into two groups—regular or irregular―with regard to their adherence to exercise programs. Patients that completed the exercise program had significantly better results in pain on a VAS scale, satisfaction with the treatment, and radiographic assessment. Pretreatment equivalence was not established between the two groups and there was no randomization, therefore the risk of selection bias was high.
Jordan 2000 conducted a prospective single‐blind trial of people with TOS of probable neurogenic type who received intrascalene injections of either botulinum toxin, or lidocaine and steroids. One month after injection, 14 of 22 participants (64%) in the botulinum group reported greater than 50% reduction in symptoms compared to 4 of 22 participants in the lidocaine and steroid group. There was no information available regarding the method used to allocate the participants to a specific group, nor any information about the characteristics of the participants in each group. Thus, there was a high risk of selection bias.
There are numerous retrospective case series supporting the various established surgical interventions for TOS including scalenectomy, scalenotomy, division of fibrous bands, first rib resection, cervical rib resection or a combination of two or more of these procedures from either a supraclavicular or transaxillary approach. However, these retrospective studies lack randomization, blinding, and standardized outcome assessment and therefore have a high risk of selection, allocation, and assessment bias. There are a few prospective series of consecutive patients that underwent surgical intervention for TOS.
Martens 1980 reported on a consecutive series of 67 patients with various types of TOS who had undergone surgical intervention after failing non‐operative therapy. The patients were contacted by telephone or letter and their long‐term outcomes were categorized as excellent, satisfactory or poor. Surgical approaches included supraclavicular, posterior thoracoplasty, and transaxillary. Satisfactory results were reported for 75% of posterior thoracoplasty, 64% of supraclavicular, and 100% of transaxillary approaches. The paper did not report the statistical analysis used to compare the outcomes between the surgical groups. There was no attempt to randomize patients to the various surgical interventions, blind the patients or assessors, or attempt to account for unbalanced attrition rates across the surgical groups and therefore the risk of selection and assessment bias was high.
Sällström 1983 reported on a consecutive series of 63 patients with TOS who underwent transaxillary first rib resection. Three had venous thrombosis and the others were in no specific defined subgroup. The patients were evaluated at regular intervals after surgery with a final evaluation at a mean of 2.5 years. Eighty‐one per cent of patients reported at least marked improvement of symptoms. However, the lack of comparison groups, blinding, and validated outcome measures introduce significant risk of assessment bias.
Balci 2003 prospectively studied 47 people with TOS. The authors subdivided the patients into four TOS subtypes: neurogenic upper plexus, neurogenic lower plexus, arterial, and venous. Nineteen patients had an anomalous cervical rib. Forty‐nine surgical procedures were performed, including first rib resection (n = 28), cervical rib resection (n =10), first and cervical rib resection (n = 9), and thrombectomy (n = 2). Follow‐up, consisting of clinic visit, phone conversation, or mailed questionnaire, was conducted at one and two months postoperatively and with a long‐term follow‐up at a mean of 4.6 years. At long‐term follow‐up, 75% of upper plexus and 50% of lower plexus patients remained asymptomatic. There was no difference in success when the various surgical groups were compared. The overall morbidity rate was 17% and included incisional pain, pneumothorax, intercostobrachial neuralgia, wound infection, and wound hematoma. The patients were not randomized to undergo the various surgical interventions, and the outcome measurement was not standardized, therefore the risk of selection and assessment bias was high.
Landry 2001 reported a prospective observational cohort study of people with disputed TOS who were evaluated by an independent medical examiner over an eight year period. The authors performed the initial examination, but were not involved with any interventions. At a mean follow‐up of 4.2 years, the study participants completed a standardized telephone interview or a mailed questionnaire. Of the 79 survey respondents, 15 had undergone surgical intervention. Most patients reported improved symptoms and were able to return to work. Surgical intervention did result in additional relief of symptoms compared to non‐operative therapy. The lack of randomization, high attrition rate (42%), and lack of patient allocation conferred a high risk of selection and assessment bias.
Bhattacharya 2003 reported an observational study of a consecutive series of 60 people who had undergone supraclavicular neurolysis or transaxillary first rib resection for TOS of various types. Study participants were identified from a prospective patient database and evaluated using a standardized questionnaire that was mailed or completed via telephone. The median follow‐up was 43 months (range 4 to 102 months). At least fair improvement of symptoms was reported in 90% of the cases. There was no difference in outcome with regards to type of TOS or type of surgical intervention. There was no attempt to randomize patients to various surgical interventions, and the assessors were not blind to which intervention had been performed, therefore the risk of selection and assessment bias was high.
Potential biases in the review process
We believe that there is a high likelihood that all studies of randomized controlled trials for the treatment of TOS as stipulated by our inclusion and exclusion criteria have been identified, given that only a few studies appeared, and that these few studies re‐appeared across several databases. As a result, we believe the potential bias in the review process with regards to study selection to be low. However, there are areas in which the protocol gave insufficient guidance, requiring ad hoc decisions to be made. For example, in updates of this review we included additional secondary outcomes of disability and paresthesias that were not mentioned in the protocol. We took the scales for measurement from the papers in which these outcomes were reported. Furthermore, the protocol provided insufficient guidance for the method of reporting the occurrence of adverse events. These too were included in the review as reported by the individual study, which in the case of Finlayson 2011 did not include duration of events, only the number of occurrences. As the first review that we know of to analyze the field of randomized controlled trials for the treatment of TOS, this review represents the first evidence of its kind.

Risk of bias summary: review authors' judgments about each 'Risk of bias' item for each included study. Red (‐) = high risk of bias, yellow (?) = unclear risk of bias, green (+) = low risk of bias.

