Intervenciones conservadoras para el tratamiento de la fractura del tercio medio de la clavícula en adolescentes y adultos
Appendices
Appendix 1. Search strategies (Janurary 2014 to January 2016)
Cochrane Central Register of Studies (CRS Online)
#1 MESH DESCRIPTOR clavicle EXPLODE ALL TREES (66)
#2 (clavic* or midclavic* or collarbone):TI,AB,KY (225)
#3 #1 OR #2 (225)
#4 MESH DESCRIPTOR Fracture Healing (333)
#5 MESH DESCRIPTOR Fracture Fixation EXPLODE ALL TREES (1004)
#6 MESH DESCRIPTOR Fractures, Bone EXPLODE ALL TREES (3120)
#7 fracture*:TI,AB,KY (9958)
#8 #4 OR #5 OR #6 OR #7 (9974)
#9 #3 AND #8 (102)
#10 31/01/2014 TO 31/01/2016:DL (181817)
#11 #9 AND #10 (50)
MEDLINE (OvidSP)
1 Clavicle/ (4763)
2 (clavic* or midclavic* or collarbone).tw. (8153)
3 1 or 2 (9807)
4 Fracture Healing/ (10158)
5 exp Fracture Fixation/ (49809)
6 exp Fractures, Bone/ (149256)
7 fracture*.tw. (191373)
8 4 or 5 or 6 or 7 (237311)
9 3 and 8 (2997)
10 Randomized controlled trial.pt. (422103)
11 Controlled clinical trial.pt. (92600)
12 randomized.ab. (344099)
13 placebo.ab. (171790)
14 Drug therapy.fs. (1876199)
15 randomly.ab. (247903)
16 trial.ab. (359106)
17 groups.ab. (1540591)
18 or/10‐17 (3746261)
19 exp Animals/ not Humans/ (4176097)
20 18 not 19 (3223958)
21 9 and 20 (331)
Embase (Ovid Online)
1 Clavicle/ (5030)
2 (clavic* or midclavic* or collarbone).tw. (9473)
3 or/1‐2 (10957)
4 exp Fracture Healing/ or exp Fracture Treatment/ or exp Fracture/ (246703)
5 fracture*.tw. (219328)
6 or/4‐5 (306053)
7 and/3,6 (3289)
8 Randomized Controlled Trial/ (388340)
9 Controlled Clinical Study/ (391120)
10 random*.ti,ab. (1029619)
11 Randomization/ (68588)
12 Intermethod Comparison/ (203346)
13 placebo.ti,ab. (223165)
14 (compare or compared or comparison).ti. (387195)
15 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (1315917)
16 (open adj label).ti,ab. (47583)
17 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (171854)
18 Double Blind Procedure/ (124856)
19 parallel group*1.ti,ab. (17424)
20 (crossover or cross over).ti,ab. (75833)
21 ((assign* or match or matched or allocation) adj5 (alternate or group*1 or intervention*1 or patient*1 or subject*1 or participant*1)).ti,ab. (221526)
22 (assigned or allocated).ti,ab. (263126)
23 (controlled adj7 (study or design or trial)).ti,ab. (228125)
24 (volunteer or volunteers).ti,ab. (187553)
25 Human Experiment/ (345374)
26 trial.ti. (189895)
27 or/8‐26 (3415088)
28 (exp Animal/ or Animal.hw. or Nonhuman/) not (exp Human/ or Human Cell/ or (human or humans).ti.) (5397275)
29 27 not 28 (2962221)
30 7 and 29 (461)
31 (2014* or 2015* or 2016*).em,dd. (3508884)
32 30 and 31 (136)
LILACS (BIREME)
Mh Clavicle OR Tw clavic$ OR Tw midclavic$ OR Tw collarbone [Words] and Mh Fracture healing OR Mh Fracture fixation OR Mh Fractures OR Tw fracture$ [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh Randomized controlled trials OR Mh Random allocation OR Mh Double‐blind method OR Mh Single‐blind method) AND NOT (Ct animals AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh Research design) AND NOT (Ct animals AND NOT (Ct human and Ct animals)) OR (Ct comparative study OR Ex E05.337$ OR Mh Follow‐up studies OR Mh Prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animals AND NOT (Ct human and Ct animals))) [Words] (20)
Orthopaedic Proceedings (The Bone and Joint Journal)
Title: clavic* or midclavic* or collarbone
Abstract or title: random*
Orthopaedic Proceedings = 14
ISRCTN Registry
fracture AND (clavicle OR clavicular OR midclavicle OR midclavicular) = 9
WHO International Clinical Trials Registry Platform
clavic* AND fracture* OR midclavic* AND fracture* OR mid‐clavic* AND fracture* = 40
ClinicalTrials.gov
fracture AND (clavicle OR clavicular OR midclavicle OR midclavicular) = 31
Appendix 2. Previous search strategies 2008 to 2014
The Cochrane Library (Wiley Online Library)
#1 MeSH descriptor: [Clavicle] this term only (75)
#2 (clavic* or midclavic* or collarbone):ti,ab,kw (159)
#3 (#1 or #2) (159)
#4 MeSH descriptor: [Fracture Healing] this term only (364)
#5 MeSH descriptor: [Fracture Fixation] explode all trees (1124)
#6 MeSH descriptor: [Fractures, Bone] explode all trees (3803)
#7 (fracture*):ti,ab,kw (8448)
#8 (#4 or #5 or #6 or #7) (8464)
#9 (#3 and #8) in Trials (54)
MEDLINE (PubMed)
(((Clavicle [mh] OR clavic* [tw] OR collarbone [tw]) AND (Fracture Healing [mh] OR Fracture Fixation [mh] OR Fractures, Bone [mh] OR fracture* [tw]) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT humans [mh])))) AND 2008:2014 [edat] (89)
Embase (Ovid Online)
1 Clavicle/ (4726)
2 (clavic$ or midclavic$ or collarbone).tw. (9091)
3 or/1‐2 (10567)
4 exp Fracture Healing/ or exp Fracture Treatment/ or exp Fracture/ (232404)
5 fracture$.tw. (209206)
6 or/4‐5 (291855)
7 and/3,6 (3094)
8 Clinical trial/ (896067)
9 Randomized controlled trial/ (367792)
10 Randomization/ (64730)
11 Single blind procedure/ (18891)
12 Double blind procedure/ (122402)
13 Crossover procedure/ (39662)
14 Placebo/ (245995)
15 Randomi?ed controlled trial$.tw. (99640)
16 Rct.tw. (13563)
17 Random allocation.tw. (1368)
18 Randomly allocated.tw. (20584)
19 Allocated randomly.tw. (1974)
20 (allocated adj2 random).tw. (823)
21 Single blind$.tw. (14643)
22 Double blind$.tw. (150221)
23 ((treble or triple) adj blind$).tw. (377)
24 Placebo$.tw. (206316)
25 Prospective study/ (261999)
26 or/8‐25 (1429455)
27 Case study/ (23741)
28 Case report.tw. (270245)
29 Abstract report/ or Letter/ (920220)
30 or/27‐29 (1208582)
31 26 not 30 (1391203)
32 limit 31 to human (1265124)
33 and/7,32 (181)
34 (2008$ or 2009$ or 2010$ or 2011$ or 2012$ or 2013$ or 2014$).em. (7323623)
35 33 and 34 (108)
LILACS (BIREME)
Mh Clavicle OR Tw clavic$ OR Tw midclavic$ OR Tw collarbone [Words] and Mh Fracture healing OR Mh Fracture fixation OR Mh Fractures OR Tw fracture$ [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh Randomized controlled trials OR Mh Random allocation OR Mh Double‐blind method OR Mh Single‐blind method) AND NOT (Ct animals AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh Research design) AND NOT (Ct animals AND NOT (Ct human and Ct animals)) OR (Ct comparative study OR Ex E05.337$ OR Mh Follow‐up studies OR Mh Prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animals AND NOT (Ct human and Ct animals))) [Words] (18)
Orthopaedic Proceedings (The Bone and Joint Journal)
Title: clavic* or midclavic* or collarbone
Abstract or title: random*
Orthopaedic Proceedings = 13
Appendix 3. Results of the searches in previous versions of the review
We updated the search from December 2008 to January 2014. We screened a total of 282 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (0), the Cochrane Central Register of Controlled Trials (54), MEDLINE (89), Embase (108), LILACS (18) and Orthopaedic Proceedings (13). We did not identify any potentially eligible studies from trial registers or other sources.
The search update resulted in the identification of one potentially eligible study, for which we obtained a full report. We excluded this study (Bajuri 2011). We also excluded one study that was classified as ongoing in the last version of this review, after contact with the primary study author (Roberti 2008). A flow diagram summarising the study selection process is shown in Figure 1.
Overall, there are now a total of three included studies, four excluded studies, no ongoing trials and no studies awaiting classification.
The search of five main electronic databases for completed research yielded 159 references (Figure 1). JB and ML screened the titles and abstracts for references and firstly excluded 152 citations. Among these, 115 were duplicated citations or not relevant, 31 were excluded because they did not meet the inclusion criteria for participants or interventions, and six were not randomised or quasi‐randomised trials. The remaining seven potentially relevant studies were evaluated from full trial reports. Three trials were included (Andersen 1987a; Hoofwijk 1988; Lubbert 2008). A translation of one report (Jensen 1985) showed it that was a report of Andersen 1987a. One full report and two abstracts, one of which reported interim results (Hoofwijk 1986), were available for Hoofwijk 1988. Finally, one study (Thompson 2005) was excluded. Two trials were translated to English (see Acknowledgements). Of the two further trials identified from Trial Registers, one was excluded (Talbot 2008) and one is ongoing (Roberti 2008a).

Study flow diagram

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 1 Shoulder function.
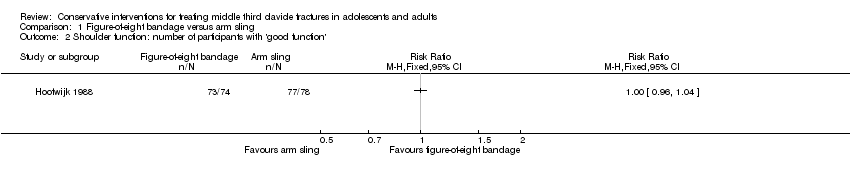
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 2 Shoulder function: number of participants with 'good function'.
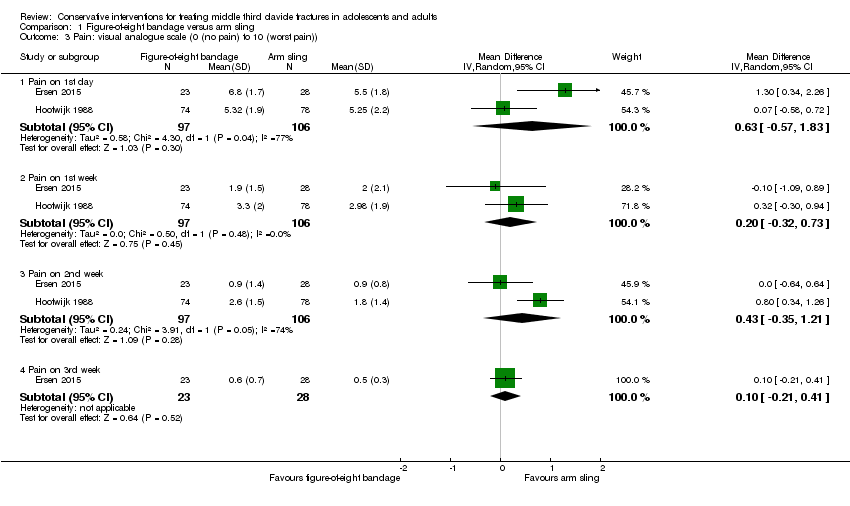
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 4 Pain: duration of painkiller consumption (days).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 5 Clinical healing: time to clinical fracture consolidation (weeks).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 6 Adverse event.

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 7 Time to return to previous activities (weeks).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 8 Patient dissatisfaction with course of treatment.

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)).

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 2 Pain: number of painkillers (tablets/28 days).

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 3 Treatment failure.
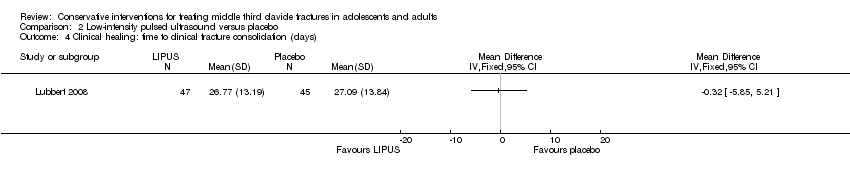
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 4 Clinical healing: time to clinical fracture consolidation (days).
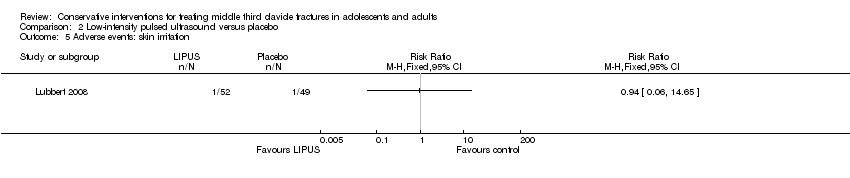
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 5 Adverse events: skin irritation.

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 6 Time to return to previous activities (days).
| Figure‐of‐eight bandage compared with arm sling for treating fractures of the middle third of the clavicle | ||||||
| Patient or population: patients (mainly young male adults) with fractures of the middle third of the clavicle Settings: hospital (initially) Intervention: figure‐of‐eight bandage Comparison: arm sling | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Arm sling | Figure‐of‐eight bandage | |||||
| Shoulder function Constant score (0 to 100 points: higher = better) Follow‐up: 6 to 12 months | Mean (SD) population Constant score 89 (7)¹ | Mean function in the figure‐of‐eight bandage groups was 0.75 points lower (3.72 lower to 2.39 higher) | MD ‐0.75 points (‐3.72 to 2.39) | 51 | ⊕⊝⊝⊝ | The 95% CI does not include a clinically important difference.³ Shoulder function was measured using non‐validated measures in two other trials (61 and 152 participants). Both trials found evidence of similar shoulder function in the two groups |
| Pain (early) Visual Analogue Scale ‐ VAS (0 (no pain) to 10 (worst pain)) Follow‐up: 2 weeks | Mean pain in the arm sling groups ranged from | Mean pain in the figure‐of‐eight bandage groups was 0.43 points higher (0.35 lower to 1.21 higher) | MD 0.43 points (‐0.35 to 1.21) | 203 (2 studies) | ⊕⊝⊝⊝ | The 95% CI do not include a clinically important difference. A third trial (data for 61 participants) provided very low quality evidence based on a non‐validated scoring system of more pain and discomfort during the course of treatment in the figure‐of‐eight group |
| Treatment failure (Number of participants who have undergone or are being considered for a surgical intervention) | See comment | See comment | Not estimable | ‐ | See comment | Poorly reported outcome. One trial (152 participants) reported that one participant in the arm sling group had successful surgery for a secondary plexus nerve palsy |
| Clinical healing ‐ time to clinical fracture consolidation (weeks) | Mean clinical healing in the arm sling group was 3.6 weeks | Mean clinical healing in the figure‐of‐eight bandage group was 0.20 weeks longer (0.11 week shorter to 0.51 week longer) | MD 0.20 weeks (‐0.11 to 0.51) | 148 | ⊕⊝⊝⊝ | In addition, there were four non‐unions in the figure‐of‐eight group; none were problematic |
| Adverse events ‐ total participants with adverse events | See comment | See comment | Not estimable | ‐ | See comment | The very low evidence quality data for individual adverse outcomes (poor cosmetic appearance; change in allocated treatment due to pain and discomfort, worsened fracture position on healing; shortening > 15 mm; non‐symptomatic non‐union and permanent pain) did not confirm a difference between the two groups⁵ |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial |
| Return to previous activities ‐ Resumption of school/work (weeks) | Mean time to return to previous activities ranged across control groups from | Mean time to return to previous activities (weeks) ‐ resumption of school/work in the intervention groups was 0.12 weeks lower (0.69 lower to 0.45 higher) | MD ‐0.12 weeks (‐0.69 to 0.45) | 176 (2 studies) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ These are based on the Constant score in healthy people as reported in Yian 2005. ² We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of blinding. We downgraded the evidence one further level for imprecision given the wide confidence interval and that the available data were from only one trial. ³ For the purposes of this review, the minimally clinical important difference was considered to be 10 points for the Constant score (Kukkonen 2013). ⁴ We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence one further level for inconsistency given the considerable heterogeneity between the findings of the two groups (I² = 74%). ⁵ Data for individual adverse outcomes (poor cosmetic appearance; change in allocated treatment due to pain and discomfort, worsened fracture position on healing; shortening > 15 mm; non‐symptomatic non‐union and permanent pain) confirmed a difference between the two groups. ⁶ We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence one further level for imprecision given the low numbers of participants contributing data to this outcome. | ||||||
| Low‐intensity pulsed ultrasound compared with placebo for treating fractures of the middle third of the clavicle | ||||||
| Patient or population: patients with fractures of the middle third of the clavicle Settings: hospital Intervention: low‐intensity pulsed ultrasound Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Low‐intensity pulsed ultrasound | |||||
| Shoulder function | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the trial |
| Pain Visual analogue scale ‐ VAS (0 (no pain) to 10 (worst pain)) Follow‐up: in the 28‐day treatment period | Mean pain in the control group was | Mean pain in the intervention group was 0.04 points lower (0.61 lower to 0.53 higher) | MD ‐0.04 (95% CI ‐0.61 to 0.53) | 101 (1 study) | ⊕⊕⊕⊝ | |
| Treatment failure ‐ Number who had surgical procedure | See comment | See comment | RR 1.13 (0.37 to 3.47) | 101 (1 study) | ⊕⊕⊕⊝ | Only one trial assessed this comparison |
| Clinical healing ‐ Time to clinical fracture consolidation (days) | Mean clinical healing in the control group was 27.09 days | Mean clinical healing: time to clinical/radiographic fracture consolidation (days) in the intervention group was 0.32 days lower (5.85 lower to 5.21 higher) | MD ‐0.32 weeks (‐5.85 to 5.21) | 101 (1 study) | ⊕⊕⊕⊝ | |
| Adverse events ‐ total of adverse events (Skin irritation) Follow‐up: during the intervention | See comment | See comment | RR 0.94 (0.06 to 14.65) | 101 (1 study) | ⊕⊕⊕⊝ | Only one trial assessed this comparison |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the trial |
| Return to previous activities ‐ Resumption of work (days) | Mean time to return to previous activities in the control group was | Mean time to return to previous activities (days) ‐ resumption of work in the intervention group was 1.95 weeks higher (2.18 lower to 6.08 higher) | MD 1.95 days (‐2.18 to 6.08) | 101 (1 study) | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ We downgraded the evidence one level for imprecision given the wide confidence interval and that the available data were from only one trial. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Shoulder function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Constant score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 ASES score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Shoulder function: number of participants with 'good function' Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Pain on 1st day | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.57, 1.83] |
| 3.2 Pain on 1st week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.32, 0.73] |
| 3.3 Pain on 2nd week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.35, 1.21] |
| 3.4 Pain on 3rd week | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.21, 0.41] |
| 4 Pain: duration of painkiller consumption (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Clinical healing: time to clinical fracture consolidation (weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse event Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Poor cosmetic appearance post fracture healing | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.55, 3.16] |
| 6.2 Change in allocated treatment due to pain and discomfort | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.35, 25.83] |
| 6.3 Worsened fracture position on healing | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.44] |
| 6.4 Shortening > 15 mm | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.35, 2.90] |
| 6.5 Non‐union | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 6.6 Permanent pain at mean 10 months | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 7 Time to return to previous activities (weeks) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Resumption of school/work | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.69, 0.45] |
| 7.2 Resumption of sports activities | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.48, 0.28] |
| 8 Patient dissatisfaction with course of treatment Show forest plot | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.03, 7.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain: number of painkillers (tablets/28 days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Treatment failure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Number who had surgical procedure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Clinical healing: time to clinical fracture consolidation (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events: skin irritation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Time to return to previous activities (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Resumption of household activities | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Resumption of professional work | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Resumption of sport | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

