Duloxetine for treating painful neuropathy or chronic pain
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group, randomised, double‐blind, placebo‐controlled trial in fibromyalgia | |
| Participants | 207 men or women over 18 years who fulfilled American College of Rheumatology (ACR) criteria for fibromyalgia, and scoring 4 or more on the pain intensity item of the Fibromyalgia Impact Questionnaire (FIQ) | |
| Interventions | Duloxetine 60 mg twice daily versus placebo for 12 weeks with a 20 day titration phase. Follow‐up at 12 weeks | |
| Outcomes | FIQ pain score, SF‐36 brief pain inventory | |
| Notes | Greater use of antidepressants in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Assignment to treatment groups was determined by a computer‐generated random sequence. |
| Allocation concealment? | Low risk | Used an interactive voice response system. Treatment was double‐blind for 12 weeks. The number of placebo capsules was similarly adjusted to maintain the blinding. |
| Blinding? | Low risk | Double‐blind for all assessments in therapy phase, single‐blind in run‐in phase |
| Incomplete outcome data addressed? | High risk | 46/104 (44%) in duloxetine and 37/103 (36%) in placebo group discontinued treatment but all dropouts accounted for and last observation carried forward |
| Free of selective reporting? | Unclear risk | As above in incomplete outcome data |
| Free of other bias? | Low risk | Antidepressants used more in the placebo group but this would bias against the treatment arm |
| Methods | Parallel group, randomised, double‐blind, placebo‐controlled trial of duloxetine in women with fibromyalgia | |
| Participants | Women only, >18 years of age who met criteria for primary fibromyalgia as defined by the American College of Rheumatology, and had a score of >4 on the average pain severity item of the Brief Pain Inventory (BPI) at randomisation 354 participants | |
| Interventions | Duloxetine 60 mg daily, duloxetine 60 mg twice daily and placebo | |
| Outcomes | Brief pain inventory (average pain severity), SF‐36, BPI interference scale | |
| Notes | Company sponsored and run trial. Fibromyalgia Impact Questionnaire abandoned in favour of BPI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Women who met entry criteria following the screening phase were randomly assigned to one of three treatment groups: duloxetine 60 mg daily, duloxetine 60 mg twice daily (forced titration from 60 mg daily for 3 days to 60 mg twice daily), or placebo, with randomisation in a 1:1:1 ratio. Random assignment of the patients to treatment groups was applied within two stratified groups, those with and those without current major depressive disorder |
| Allocation concealment? | Unclear risk | Probably low risk of bias as previous trial used an adequate method |
| Blinding? | Low risk | Double‐blind |
| Incomplete outcome data addressed? | High risk | High proportion of dropouts: 138 (39%) patients withdrew during the 12‐week therapy phase, 41 (35%) from the duloxetine 60 mg QD group, 45 (39%) from the duloxetine 60 mg BID group, and 52 (43%) from the placebo group (P = 0.407). Matched across groups but high rate of loss. 'Partial intention to treat analysis'. All randomised patients with a baseline and at least one post‐baseline visit with efficacy data were included in the efficacy analyses, while all randomised patients were included in the safety analyses. |
| Free of selective reporting? | Unclear risk | See incomplete outcome data above |
| Free of other bias? | Low risk | |
| Methods | Parallel group, randomised, double‐blind, placebo‐controlled trial of duloxetine in patients with painful diabetic neuropathy | |
| Participants | Participants, at least 18 years of age, had daily pain due to polyneuropathy caused by type 1 or type 2 diabetes mellitus, which was present for a minimum of 6 months. This pain had to have begun in the feet with relatively symmetrical onset. The diagnosis was confirmed by a score of at least 3 on the Michigan Neuropathy Screening Instrument (MNSI). Participants were required to have a minimum score of 4 on the 24‐hour Average Pain Score rated on an 11‐point (0‐10) Likert scale. 457 participants | |
| Interventions | Duloxetine 20 mg daily, 60 mg daily or 60 mg twice daily versus placebo | |
| Outcomes | 24‐hour average pain score, SF‐36, patient global impression of change, night pain | |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomly assigned in a 1:1:1:1 ratio by a computer generated random sequence. |
| Allocation concealment? | Low risk | Patient numbers were assigned consecutively at each study site. The interactive voice response system was used to assign blister cards containing the study drug to each patient confirmed through interactive voice response system entry of a confirmation number on the card. |
| Blinding? | Low risk | Double‐blind |
| Incomplete outcome data addressed? | High risk | All analyses were undertaken as an intention‐to‐treat analysis. All patients were analysed in the safety analysis and all patients with at least one post entry data point were analysed in an intention to treat analysis. Dropout rate was 25% with significantly more in the higher dose treatment groups. |
| Free of selective reporting? | Unclear risk | See above |
| Free of other bias? | Low risk | Company sponsored and run trial |
| Methods | Parallel group, randomised, double‐blind, placebo‐controlled trial in participants with diabetic peripheral neuropathic pain | |
| Participants | Participants > 18 years, with pain due to bilateral peripheral neuropathy caused by type 1 or type 2 diabetes mellitus. The pain had to begin in the feet with relatively symmetrical onset and be present for at least 6 months. Participants had to have a mean score of > 4 when assessed for 24‐hour average pain severity on the Michigan Neuropathy Screening Instrument (MNSI)11‐point Likert scale (from the patient diary prior to randomisation), and stable glycaemic control. Concomitant pain medications excluded 348 participants | |
| Interventions | Duloxetine 60 mg daily or duloxetine 60 mg twice daily versus placebo | |
| Outcomes | 24‐hour average pain severity, patent global impression of clinical change, pain at rest, Brief Pain Inventory (BPI) severity, Clinical Global Impression of Pain (CGI) severity, SF‐McGill pain questionnaire, BPI interference scale | |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation performed at visit 3 in a 1:1:1 ratio. Assignment to treatment groups was determined by a computer‐generated random sequence using an interactive voice response system. |
| Allocation concealment? | Low risk | Participants received either of (or a combination of, depending on their randomly assigned treatment) the following: 30 mg capsules of duloxetine hydrochloride or placebo capsules identical to duloxetine capsules. Participants randomly assigned to each treatment group were instructed to take two capsules (by mouth) every morning and every evening. |
| Blinding? | Low risk | Double‐blind |
| Incomplete outcome data addressed? | Low risk | Dropouts were 52/340 (15%). Analysis was by intention‐to‐treat. |
| Free of selective reporting? | Low risk | See above |
| Free of other bias? | Low risk | |
| Methods | Parallel group, randomised, double‐blind, placebo controlled trial in fibromyalgia | |
| Participants | Female and male outpatients > 18 years of age who met criteria for fibromyalgia as defined by the American College of Rheumatology. Participants were required to have a score > 4 on the average pain severity item (in the past 24 hours) of the Brief Pain Inventory (BPI‐modified short form) at screening and at baseline. Patients with or without current major depressive disorder were included and evaluated for the presence of psychiatric disorders using the Mini International Neuropsychiatric Interview (MINI). Prior to randomisation, participants were required to discontinue any medications that might interfere with the evaluation of pain improvement, including analgesics (with the exception of up to 325 mg/day of aspirin for cardiac prophylaxis and paracetamol up to 2 g/day for pain), antidepressants, anticonvulsants, or other medications taken for fibromyalgia or pain. 520 participants | |
| Interventions | Duloxetine 20 mg daily, 60 mg daily or 60 mg twice daily versus placebo | |
| Outcomes | BPI average pain severity score, SF 36, patient global impression of clinical change | |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Assignment to treatment groups was determined by a computer‐generated random sequence and each stratum (depressed and non‐depressed) was randomly assigned within sites to achieve a relative balance across treatments. |
| Allocation concealment? | Unclear risk | Unclear although other trials from the same group have been adequate |
| Blinding? | Low risk | Double‐blind |
| Incomplete outcome data addressed? | High risk | 35 to 40% dropout at the 3 month interim analysis phase and up to 46% dropout for the 6 month phase. 'Intention‐to‐treat unless otherwise specified'. Safety analyses in all patients and others with data for at least 1 measure |
| Free of selective reporting? | Unclear risk | See above |
| Free of other bias? | Low risk | |
| Methods | Parallel group, randomised, placebo‐controlled trial of duloxetine in diabetic peripheral neuropathic pain | |
| Participants | Male or female > 18 years and > 6 months diabetic peripheral neuropathic pain secondary to type 1 or 2 diabetes (distal and symmetrical). At randomisation score > 3 on Michigan Neuropathy Screening Instrument and average > 4 on 24 hour pain scale. Stable glucose control and HBA1c <12. Multiple exclusions including other pain medications except paracetamol and aspirin 334 participants | |
| Interventions | Duloxetine 60 mg daily, 60 mg twice daily or placebo | |
| Outcomes | 24 hour average pain score (Likert 11‐point), SF‐36, BPI interference, patient reported global clinical impression of change, night pain, clinical global impression ‐ pain severity, clinical global impression of change | |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed at the site level in that randomisation codes were assigned to sites in blocks, but there was no further stratification. Participants were randomly assigned to treatment in a 1:1:1 ratio. Assignment to a treatment group was determined by a computer‐generated random sequence using an interactive voice response system (IVRS). |
| Allocation concealment? | Low risk | The IVRS was used to assign blister cards containing study drug to each patient |
| Blinding? | Low risk | Double‐blind |
| Incomplete outcome data addressed? | High risk | Drop outs were 29/114 (25%) in duloxetine 60 mg daily, 34/112 (30%) in duloxetine 60 mg twice daily and 23/108 (21.3%) in the placebo group. |
| Free of selective reporting? | Unclear risk | An intent‐to‐treat principle was used in the analyses of all efficacy variables. For each efficacy variable, the analysis included all randomised patients with a baseline and at least one non‐missing observation after baseline. |
| Free of other bias? | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Analysis performed at only seven weeks of treatment. Excluded as predefined analysis at 8 weeks | |
| Trial of duloxetine in depression. Pain scales as secondary outcome measures only. It was not clear what sort of pain the participants had (for example musculoskeletal, neuropathic, headache) and the levels of pain at baseline were low compared to the included trials. | |
| Not a randomised controlled study but a report of three trials included in this review | |
| Not a double‐blind trial | |
| Open label study with dosage control only | |
| Summary report of 3 studies included in this review | |
| Not double‐blind ‐ extension of Goldstein 2005 | |
| Open study, not blinded |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A global, multicenter, randomised, double‐blind, placebo controlled study comparing the safety and efficacy of ABT‐894, duloxetine and placebo in subjects with diabetic neuropathic pain |
| Methods | Randomised, double‐blind, placebo control, single group assignment, safety/efficacy study. Phase II |
| Participants | Male and female 18 to 75 Inclusion criteria:
|
| Interventions | Drug: ABT‐894, 1 mg, 2 mg, 4 mg twice daily |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | July 2007 to October 2008 |
| Contact information | Wolfram Nothaft, MD Abbott |
| Notes | Completed |
| Trial name or title | A phase 2a, randomised, double‐blind, placebo‐ and active‐controlled, parallel‐group, multicenter study to assess the safety and efficacy of ADL5859 100 mg BID in subjects with neuropathic pain associated with diabetic peripheral neuropathy |
| Methods | Phase II randomised, double blind, parallel assignment, safety/efficacy study |
| Participants | Male and female subjects between 18 and 75 years of age. Diabetes mellitus (type I or II) that is documented to be under stable glycaemic control over a period of at least 3 months, as indicated by a HbgAIc of ≤ 12% and a stable dose of insulin or oral diabetic medication for 90 days prior to starting study medication. Evidence of symmetrical, bilateral pain in the lower extremities due to diabetic peripheral neuropathy (DPN). Presence of daily pain due to DPN for at least 3 months. Score ≥ 3 on the physical examination portion of the Michigan Neuropathy Screening Instrument (MNSI). Average weekly pain score of ≥ 4 on the numeric pain rating scale (NPRS) for symmetrical neuropathic pain in the feet and legs |
| Interventions | Drug: ADL5859 |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2007. Completed August 2008 |
| Contact information | Bruce Berger Adolor Corporation Exton Office |
| Notes |
| Trial name or title | Pilot study of use of duloxetine for the treatment of phantom limb pain |
| Methods | Non‐randomised, open label, uncontrolled, single group assignment, safety/efficacy study |
| Participants | Male or female 18 to 75 years
|
| Interventions | Duloxetine |
| Outcomes | Primary outcome measure
Secondary outcome measures:
|
| Starting date | January to June 2007 |
| Contact information | Asif Chaudhry, MD 713‐798‐7999 ext 96363 Ken Woods 713‐791‐1414 ext 2247 |
| Notes | Completed |
| Trial name or title | A comparison of strategies for switching patients from amitriptyline to duloxetine for the management of diabetic peripheral neuropathic pain |
| Methods | Phase IV |
| Participants | Male or female 18 years or over. Diagnosed with diabetic peripheral neuropathic pain, taking the same dose of amitriptyline once daily at bedtime for at least four weeks. Stable glycaemic control |
| Interventions | Duloxetine switch from amitriptyline |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 2005 completed 2007 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 |
| Notes | Completed but not published |
| Trial name or title | Duloxetine versus placebo in the treatment of patients with diabetic peripheral neuropathic pain in China |
| Methods | Phase 3 randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), placebo control, parallel assignment, safety/efficacy study |
| Participants | Male or female, 18 to 75, pain due to bilateral peripheral neuropathy caused by type I or type II diabetes with the pain beginning in the feet and present for at least 6 months. Score of 4 or greater on the Brief Pain Inventory on the 24‐hour average pain item |
| Interventions | Duloxetine 60 mg daily for 12 weeks |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | December 2006 to February 2008 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 Mon‐Fri 9 AM ‐ 5 PM Eastern time UTC/GMT ‐ 5 hours, EST |
| Notes | Closed and completed |
| Trial name or title | Maintenance of effect of duloxetine 60 mg once daily in patients with diabetic peripheral neuropathic pain |
| Methods | Phase IV open label, uncontrolled, single group assignment, safety/efficacy study |
| Participants | Male or female 18 years or older. Have pain due to bilateral peripheral neuropathy caused by type I or type II diabetes with the pain beginning in the feet and present for at least 6 months. Score of 4 or greater on the Brief Pain Inventory (BPI) on the 24‐hour average pain item |
| Interventions | All subjects receive duloxetine 30 mg daily, orally for 1 week followed by duloxetine 60 mg daily, orally for 7 weeks, then maintenance at 60 mg daily, orally for responders to 6 months and rescue at 120 mg daily, orally for non‐responders to 6 months |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | April 2006 to October 2007 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 Mon‐Fri 9 AM ‐ 5 PM Eastern time UTC/GMT ‐ 5 hours, EST |
| Notes | Completed but publication status unclear |
| Trial name or title | A 1‐year safety study of duloxetine in patients with fibromyalgia syndrome |
| Methods | Phase III randomised, double‐blind, dose comparison, parallel assignment, safety study |
| Participants | Male or female 18 years or older who meet criteria for primary fibromyalgia syndrome as defined by the American College of Rheumatologists |
| Interventions | Duloxetine ? dose |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | July 2005 completed March 2007 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY(1‐877‐285‐4559) OR 1‐317‐615‐4559 |
| Notes | Completed publication status unclear |
| Trial name or title | A superiority study of LY248686 versus placebo in the treatment of patients With diabetic peripheral neuropathic pain |
| Methods | Randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), placebo control, parallel assignment, safety/efficacy study. Phase III |
| Participants | Male or female outpatients aged 20 years or older but less than 80 years at the time of consent.
|
| Interventions | Duloxetine 40 mg or 60 mg orally daily versus placebo |
| Outcomes | Primary outcome measures: Reduction in average pain severity as measured by an 11‐point Likert scale (Time Frame: 12 weeks)
|
| Starting date | November 2007 to August 2009 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY(1‐877‐285‐4559) OR 1‐317‐615‐4559 Mon‐Fri 9 AM‐5 PM Eastern time (UTC/GMT‐5 hours,EST) |
| Notes | Recruiting |
| Trial name or title | An open‐label, randomised comparison of duloxetine, pregabalin, and the combination of duloxetine and gabapentin among patients with inadequate response to gabapentin for the management of diabetic peripheral neuropathic pain |
| Methods | Randomised, open label, uncontrolled, parallel assignment, safety/efficacy study |
| Participants | Male or female 18 years or older, diagnosed with diabetic neuropathic pain. Patient has an average daily pain score greater than or equal to 4 on an 11‐point Likert scale, and patient or provider feel that a change from the current gabapentin therapy for pain management is warranted. Patient is currently treated with gabapentin greater than or equal to 900 mg/d, has been prescribed the current dose for at least 4 weeks, and has been at least 80% compliant with dosing, according to patient report. Patient must agree not to change dose of gabapentin between visits 1 and 2. Stable glycaemic control |
| Interventions | Duloxetine, gabapentin, pregabalin |
| Outcomes | Primary outcome measure Mean change from baseline, weekly mean of daily 24 hour average pain score, pregabalin to duloxetine
|
| Starting date | September 2006 ‐ October 2009 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY(1‐877‐285‐4559) OR 1‐317‐615‐4559 Mon‐Fri 9 AM‐5 PM Eastern time (UTC/GMT‐5 hours,EST) |
| Notes | Recruiting |
| Trial name or title | Flexible dosed duloxetine versus placebo in the treatment of fibromyalgia |
| Methods | Phase IV randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), placebo control, parallel assignment, safety/efficacy study |
| Participants | Male or female patients.
|
| Interventions | Duloxetine 60 to 120 mg daily for 24 weeks |
| Outcomes | Primary outcome measure
Secondary outcome measures:
|
| Starting date | June 2008 to February 2010 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY(1‐877‐285‐4559) OR 1‐317‐615‐4559 Mon‐Fri 9 AM‐5 PM Eastern time (UTC/GMT‐5 hours,EST) |
| Notes | Recruiting |
| Trial name or title | A superiority study of LY248686 versus placebo in the treatment of patients with diabetic peripheral neuropathic pain ‐ extension phase |
| Methods | Phase III randomised, open label, dose comparison, parallel assignment, safety/efficacy study in Japan |
| Participants | Male or female 20 to 80 years Outpatients who have completed the 13‐week treatment in the preceding study (Protocol No.0715N0831). Patients with latest HbA1c ≤ 9.0% before Visit 7 |
| Interventions | Duloxetine 40 mg to 60 mg daily for 1 year |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | March 2008 to February 2010 |
| Contact information | Eli Lilly and Company 1‐877‐CTLILLY(1‐877‐285‐4559) OR 1‐317‐615‐4559 Mon‐Fri 9 AM‐5 PM Eastern time (UTC/GMT‐5 hours,EST) |
| Notes | Recruiting |
| Trial name or title | Pilot, open, randomised clinical trial to assess the efficacy of duloxetine in the treatment of fibromyalgy in patients with infection by HIV 1+ |
| Methods | Phase III randomised, open label, active control, parallel assignment, safety/efficacy study |
| Participants | Male or female
|
| Interventions | Duloxetine 60 mg daily |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | January 2007 to December 2009 |
| Contact information | Negredo Eugenia, MD,PhD Lluita contra la Sida Foundation Germans Trias i Pujol Hospital Badalona, Barcelona, Spain, 08916 |
| Notes | Active not recruiting |
| Trial name or title | A double‐blind, randomised, parallel groups investigation into the effects of pregabalin, duloxetine and amitriptyline on aspects of pain, sleep, and next day performance in patients suffering from diabetic peripheral neuropathy |
| Methods | Phase II and III randomised, double‐blind, active control, parallel assignment |
| Participants | Male or female
|
| Interventions | Duloxetine, amitriptyline and pregabalin |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | February 2007 to December 2008 |
| Contact information | Professor AN Nicholson 01483 683719 |
| Notes | Recruiting |
| Trial name or title | Three way interaction between gabapentin, duloxetine, and donepezil in patients with diabetic neuropathy |
| Methods | Randomised, double‐blind (subject, investigator, outcomes assessor), parallel assignment |
| Participants | Male or female. Diagnosis of diabetic neuropathy. Age 18 to 80 |
| Interventions | Group 1: Donepezil 5 mg once per day for 12 weeks. Gabapentin will be added to all groups at week 9. Group 2: Will receive duloxetine 30 mg twice a day for 12 weeks. Gabapentin will be added to all groups at week 9. Group 3: Will receive a combination of donepezil 2.5 mg and duloxetine 30mg for 12 weeks. Gabapentin will be added to all groups at week 9. Group 4: Will receive placebo pills. Gabapentin will be added to all groups at week 9. |
| Outcomes | Primary outcome measures:
|
| Starting date | February 2008 to July 2010 |
| Contact information | Regina Curry, RN, CCRC 336‐716‐4294 Wake Forest University Baptist Medical Center Winston‐Salem, North Carolina, United States, 27157 |
| Notes | Recruiting |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with >50% improvement of pain at 12 weeks or less Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 1 Number of patients with >50% improvement of pain at 12 weeks or less. | ||||
| 1.1 Duloxetine 20 mg daily | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.98, 2.09] |
| 1.2 Duloxetine 60 mg daily | 3 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.34, 2.03] |
| 1.3 Duloxetine 120 mg daily | 3 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.35, 2.04] |
| 1.4 All doses | 3 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.35, 1.97] |
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less. | ||||
| 2.1 Duloxetine 20 mg daily | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.05, 0.15] |
| 2.2 Duloxetine 60 mg daily | 3 | 618 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.37, ‐0.71] |
| 2.3 Duloxetine 120 mg daily | 3 | 612 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.49, ‐0.83] |
| 3 Number of patients with >30% improvement in pain at 12 weeks or less Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 3 Number of patients with >30% improvement in pain at 12 weeks or less. | ||||
| 3.1 Duloxetine 60 mg daily | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.27, 1.83] |
| 3.2 Duloxetine 120 mg daily | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.30, 1.86] |
| 3.3 All doses | 2 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.30, 1.82] |
| 4 Mean improvement in SF‐36 Physical subscore at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 4 Mean improvement in SF‐36 Physical subscore at 12 weeks or less. | ||||
| 4.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.42, 1.88] |
| 4.2 Duloxetine 60 mg daily | 2 | 410 | Mean Difference (IV, Random, 95% CI) | 2.51 [1.00, 4.01] |
| 4.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Random, 95% CI) | 2.80 [1.04, 4.55] |
| 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less Show forest plot | 2 | 1019 | Mean Difference (IV, Fixed, 95% CI) | 1.67 [0.71, 2.64] |
| Analysis 1.5  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less. | ||||
| 5.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.98, 3.20] |
| 5.2 Duloxetine 60 mg daily | 2 | 410 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.12, 2.96] |
| 5.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.69, 3.77] |
| 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less. | ||||
| 6.1 Duloxetine 20 mg daily | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐2.37, 8.17] |
| 6.2 Duloxetine 60 mg daily | 2 | 421 | Mean Difference (IV, Fixed, 95% CI) | 5.58 [1.74, 9.42] |
| 6.3 Duloxetine 120 mg daily | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 8.19 [4.33, 12.05] |
| 7 Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 7 Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less. | ||||
| 7.1 Duloxetine 20 mg daily | 1 | 219 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.56, 0.10] |
| 7.2 Duloxetine 60 mg daily | 3 | 660 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.78, ‐0.41] |
| 7.3 Duloxetine 120 mg daily | 3 | 655 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.80, ‐0.44] |
| 8 Mean improvement in BPI severity ‐ average pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 8 Mean improvement in BPI severity ‐ average pain at 12 weeks or less. | ||||
| 8.1 Duloxetine 60 mg daily | 2 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.38, ‐0.57] |
| 8.2 Duloxetine 120 mg daily | 2 | 428 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.91, ‐0.41] |
| 9 Mean improvement in pain at rest (night pain) at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 9 Mean improvement in pain at rest (night pain) at 12 weeks or less. | ||||
| 9.1 Duloxetine 20 mg daily | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.90, 0.34] |
| 9.2 Duloxetine 60 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.27, ‐0.57] |
| 9.3 Duloxetine 120 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.45, ‐0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with = or >50% improvement of pain at = or <12 weeks Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 1 Number of patients with = or >50% improvement of pain at = or <12 weeks. | ||||
| 1.1 Duloxetine 20 mg | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.91, 2.14] |
| 1.2 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.20, 2.06] |
| 1.3 Duloxetine 120 mg daily | 3 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.36, 2.19] |
| 1.4 All doses | 3 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.37, 2.13] |
| 2 Number of patients with = or >50% improvement of pain at >12 weeks Show forest plot | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.25, 2.11] |
| Analysis 2.2  Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 2 Number of patients with = or >50% improvement of pain at >12 weeks. | ||||
| 2.1 Duloxetine 60 mg daily | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.10, 2.27] |
| 2.2 Duloxetine 120 mg daily | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.15, 2.45] |
| 3 Number of patients with = or >30% improvement of pain at = or <12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 3 Number of patients with = or >30% improvement of pain at = or <12 weeks. | ||||
| 3.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.79] |
| 3.2 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.85] |
| 3.3 Duloxetine 120 mg daily | 2 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.86] |
| 3.4 All doses | 2 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.25, 1.80] |
| 4 Mean improvement in the SF36 mental component summary subscore Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 4 Mean improvement in the SF36 mental component summary subscore. | ||||
| 4.1 Duloxetine 20 mg | 1 | 223 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐2.37, 3.99] |
| 4.2 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Random, 95% CI) | 3.31 [0.59, 6.02] |
| 4.3 Duloxetine 120 mg daily | 3 | 691 | Mean Difference (IV, Random, 95% CI) | 3.09 [1.47, 4.70] |
| 5 Mean improvement in the SF36 physical component summary subscore Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 5 Mean improvement in the SF36 physical component summary subscore. | ||||
| 5.1 Duloxetine 20 mg | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐1.92, 3.54] |
| 5.2 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐0.33, 2.89] |
| 5.3 Duloxetine 120 mg daily | 3 | 691 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.50, 3.10] |
| 6 Mean improvement in the SF36 Bodily Pain subscore Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 6 Mean improvement in the SF36 Bodily Pain subscore. | ||||
| 6.1 Duloxetine 60 mg daily | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 8.2 [3.20, 13.20] |
| 6.2 Duloxetine 120 mg daily | 2 | 403 | Mean Difference (IV, Fixed, 95% CI) | 8.42 [4.80, 12.03] |
| 7 Mean improvement in the patient reported global impression of change at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 7 Mean improvement in the patient reported global impression of change at 12 weeks or less. | ||||
| 7.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.96, ‐0.12] |
| 7.2 Duloxetine 60 mg daily | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.73, ‐0.18] |
| 7.3 Duloxetine 120 mg daily | 2 | 513 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.81, ‐0.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients with any adverse event Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 1 Proportion of patients with any adverse event. | ||||
| 1.1 Duloxetine 60 mg daily | 4 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.12, 1.30] |
| 1.2 Duloxetine 120 mg daily | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.09, 1.30] |
| 2 Nausea Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 2 Nausea. | ||||
| 2.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 3.00] |
| 2.2 Duloxetine 60 mg daily | 3 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.33, 3.83] |
| 2.3 Duloxetine 120 mg daily | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.00, 4.45] |
| 3 Somnolence Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 3 Somnolence. | ||||
| 3.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.43] |
| 3.2 Duloxetine 60mg daily | 3 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.62, 4.74] |
| 3.3 Duloxetine 120 mg daily | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.61 [2.74, 7.74] |
| 4 Dry mouth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 4 Dry mouth. | ||||
| 4.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.47] |
| 4.2 Duloxetine 60 mg daily | 2 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.12, 3.77] |
| 4.3 Duloxetine 120 mg daily | 2 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [1.93, 6.04] |
| 5 Dizziness Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 5 Dizziness. | ||||
| 5.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.33] |
| 5.2 Duloxetine 60 mg daily | 3 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.15, 3.03] |
| 5.3 Duloxetine 120 mg daily | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.55, 3.97] |
| 6 Adverse event leading to cessation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 6 Adverse event leading to cessation. | ||||
| 6.1 Duloxetine 20 mg daily | 2 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.78, 2.39] |
| 6.2 Duloxetine 60 mg daily | 5 | 1215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.20, 2.32] |
| 6.3 Duloxetine 120 mg daily | 6 | 1414 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.74, 3.05] |
| 6.4 All doses | 6 | 2220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.48, 2.52] |
| 7 Serious adverse event Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 7 Serious adverse event. | ||||
| 7.1 Duloxetine 20 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Duloxetine 60 mg daily | 5 | 1219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.32, 1.33] |
| 7.3 Duloxetine 120 mg daily | 5 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.35] |
| 7.4 All doses | 5 | 1856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.25] |

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Duloxetine versus placebo in the treatment of painful neuropathy: Number of patients with >50% improvement of pain at <12 weeks.

Duloxetine versus placebo in the treatment of pain: Mean improvement in pain at 12 weeks.

Duloxetine versus placebo in the treatment of pain: Number of patients with >30% improvement in pain at <12 weeks.
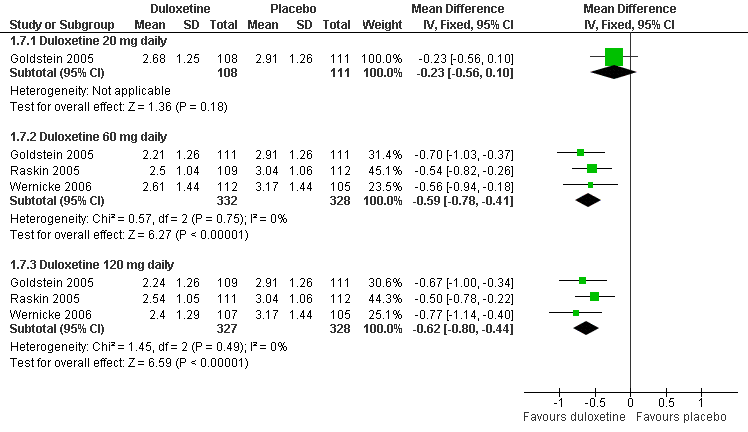
Duloxetine versus placebo in the treatment of pain: Patient reported global impression of change.

Duloxetine versus placebo in the treatment of pain: BPI severity ‐ average pain.

Duloxetine versus placebo in the treatment of fibromyalgia: >30% improvement <12 weeks.

Duloxetine versus placebo in the treatment of fibromyalgia: SF36 Bodily Pain.

Adverse events leading to cessation of treatment.

Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 1 Number of patients with >50% improvement of pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 3 Number of patients with >30% improvement in pain at 12 weeks or less.
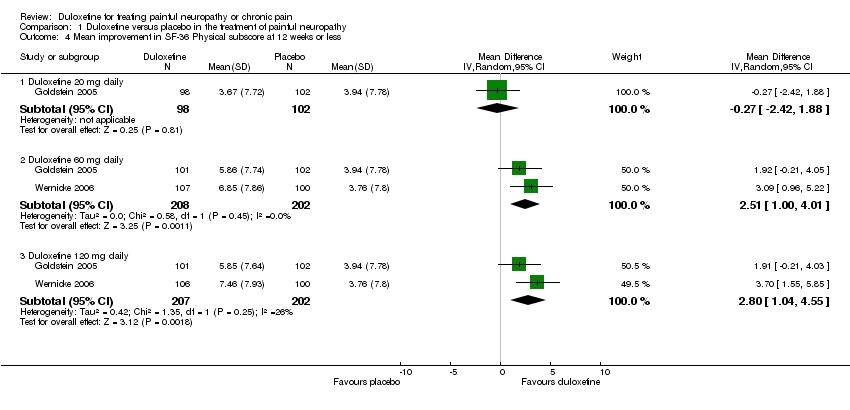
Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 4 Mean improvement in SF‐36 Physical subscore at 12 weeks or less.
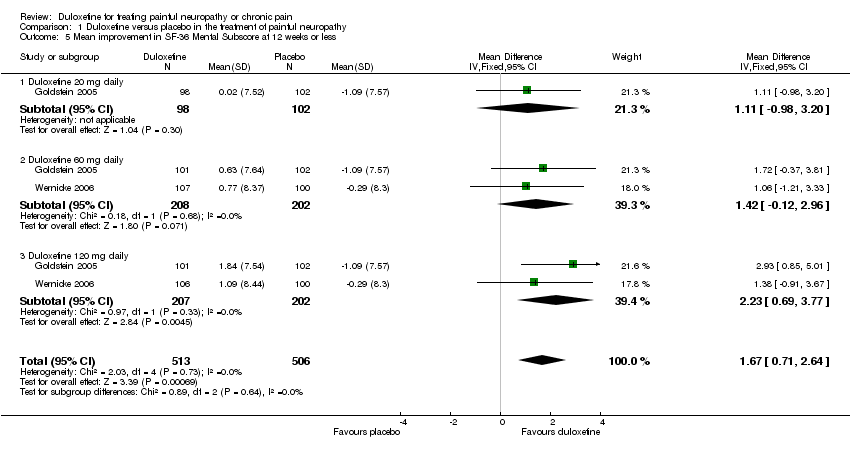
Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 7 Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less.
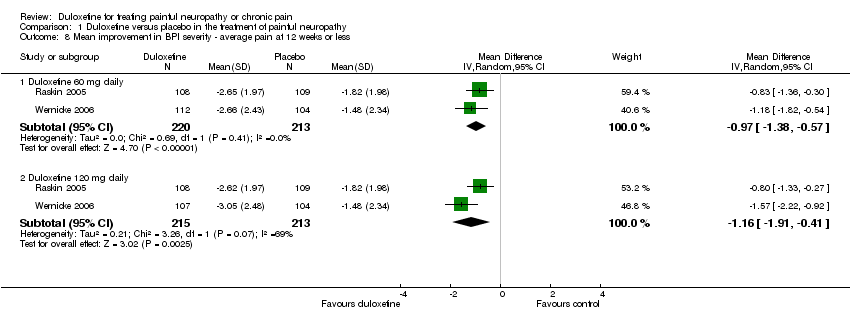
Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 8 Mean improvement in BPI severity ‐ average pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful neuropathy, Outcome 9 Mean improvement in pain at rest (night pain) at 12 weeks or less.

Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 1 Number of patients with = or >50% improvement of pain at = or <12 weeks.
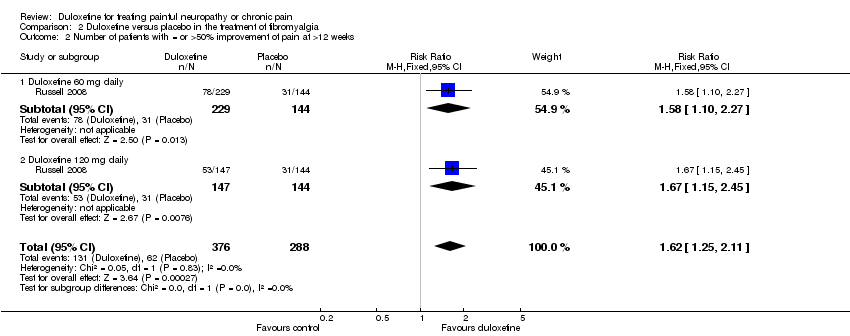
Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 2 Number of patients with = or >50% improvement of pain at >12 weeks.

Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 3 Number of patients with = or >30% improvement of pain at = or <12 weeks.
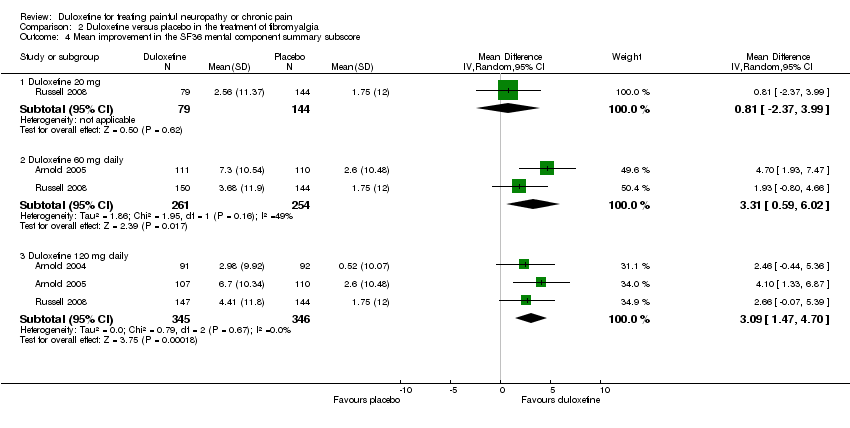
Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 4 Mean improvement in the SF36 mental component summary subscore.

Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 5 Mean improvement in the SF36 physical component summary subscore.
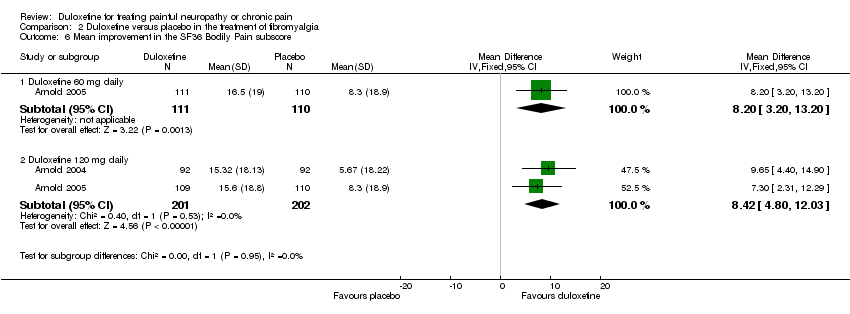
Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 6 Mean improvement in the SF36 Bodily Pain subscore.

Comparison 2 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 7 Mean improvement in the patient reported global impression of change at 12 weeks or less.

Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 1 Proportion of patients with any adverse event.

Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 2 Nausea.
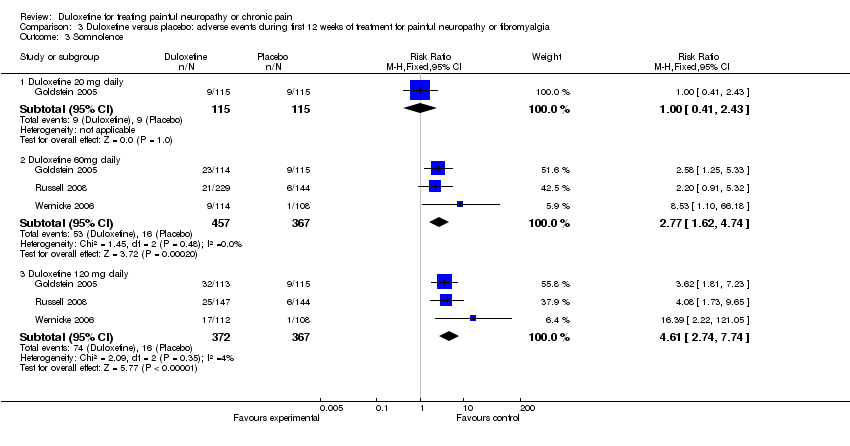
Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 3 Somnolence.

Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 4 Dry mouth.

Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 5 Dizziness.
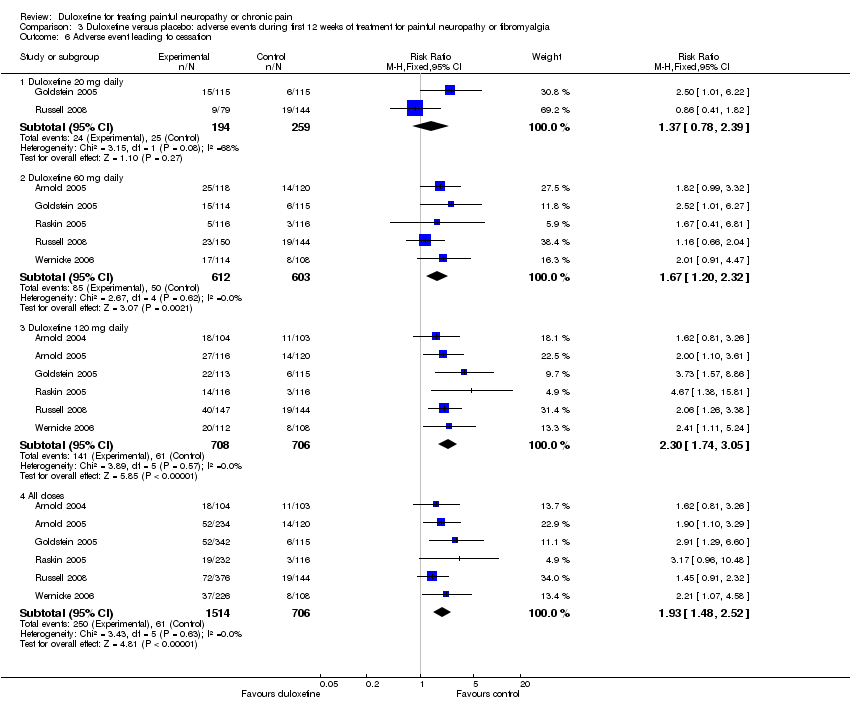
Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 6 Adverse event leading to cessation.

Comparison 3 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 7 Serious adverse event.
| Duloxetine for treating painful neuropathy or chronic pain | ||||||
| Patient or population: patients with treating painful neuropathy or chronic pain Settings: Intervention: Duloxetine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Duloxetine | |||||
| Greater than 50% improvement of diabetic peripheral neuropathic pain‐ Duloxetine 60 mg daily | 288 per 10002 | 475 per 1000 | RR 1.65 | 655 | ⊕⊕⊕⊝ | Number needed to treat (NNT) for 50% or more improvement in pain with duloxetine 60 mg daily = 6 (CI 5 to 10)4 |
| Improvement in diabetic peripheral neuropathic pain ‐ Duloxetine 60 mg daily | The mean improvement in diabetic peripheral neuropathic pain ‐ duloxetine 60 mg daily in the control groups was | The mean Improvement in diabetic peripheral neuropathic pain ‐ Duloxetine 60 mg daily in the intervention groups was | 618 | ⊕⊕⊕⊝ | ||
| Greater than 30% improvement in diabetic peripheral neuropathic pain ‐ Duloxetine 60 mg daily | 429 per 1000 | 656 per 1000 | RR 1.53 | 442 | ⊕⊕⊕⊝ | NNT for 30% or more improvement in pain with duloxetine 60 mg daily = 5 (CI 3 to 8) |
| Greater than 50% improvement of fibromyalgia pain ‐ Duloxetine 60 mg daily | 233 per 1000 | 366 per 1000 | RR 1.57 | 528 | ⊕⊕⊕⊝ | NNT for 50% or more improvement in fibromyalgia pain with duloxetine 60 mg daily = 8 (CI 5 to 17) |
| Adverse event leading to cessation ‐ Duloxetine 60 mg daily | 83 per 1000 | 139 per 1000 | RR 1.67 | 1215 | ⊕⊕⊕⊝ | 'Number needed to harm' (NNH) for cessation of duloxetine treatment at duloxetine 60 mg daily = 17 (CI 12 to 50) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidance | ||||||
| 1 Titled 24 hour pain score or 24 hour pain severity 2 Assumed risks generated from placebo population 3 Three trials only all company sponsored and performed but all trials pre‐registered on clinical trials.gov have been published. No publication bias detected. 4 CI = confidence interval 5 Risk of bias relevant in 2 of 3 studies. Quality of evidence graded moderate because of high dropout and company sponsorship of all studies. 6 Titled 24 hour pain score or 24 hour pain severity 7 Risk of bias relevant in 2 of 3 studies. Quality of evidence graded moderate because of high dropout and company sponsorship of all studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with >50% improvement of pain at 12 weeks or less Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 20 mg daily | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.98, 2.09] |
| 1.2 Duloxetine 60 mg daily | 3 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.34, 2.03] |
| 1.3 Duloxetine 120 mg daily | 3 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.35, 2.04] |
| 1.4 All doses | 3 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.35, 1.97] |
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 20 mg daily | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.05, 0.15] |
| 2.2 Duloxetine 60 mg daily | 3 | 618 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.37, ‐0.71] |
| 2.3 Duloxetine 120 mg daily | 3 | 612 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.49, ‐0.83] |
| 3 Number of patients with >30% improvement in pain at 12 weeks or less Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 60 mg daily | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.27, 1.83] |
| 3.2 Duloxetine 120 mg daily | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.30, 1.86] |
| 3.3 All doses | 2 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.30, 1.82] |
| 4 Mean improvement in SF‐36 Physical subscore at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.42, 1.88] |
| 4.2 Duloxetine 60 mg daily | 2 | 410 | Mean Difference (IV, Random, 95% CI) | 2.51 [1.00, 4.01] |
| 4.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Random, 95% CI) | 2.80 [1.04, 4.55] |
| 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less Show forest plot | 2 | 1019 | Mean Difference (IV, Fixed, 95% CI) | 1.67 [0.71, 2.64] |
| 5.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.98, 3.20] |
| 5.2 Duloxetine 60 mg daily | 2 | 410 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.12, 2.96] |
| 5.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.69, 3.77] |
| 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐2.37, 8.17] |
| 6.2 Duloxetine 60 mg daily | 2 | 421 | Mean Difference (IV, Fixed, 95% CI) | 5.58 [1.74, 9.42] |
| 6.3 Duloxetine 120 mg daily | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 8.19 [4.33, 12.05] |
| 7 Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 20 mg daily | 1 | 219 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.56, 0.10] |
| 7.2 Duloxetine 60 mg daily | 3 | 660 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.78, ‐0.41] |
| 7.3 Duloxetine 120 mg daily | 3 | 655 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.80, ‐0.44] |
| 8 Mean improvement in BPI severity ‐ average pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Duloxetine 60 mg daily | 2 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.38, ‐0.57] |
| 8.2 Duloxetine 120 mg daily | 2 | 428 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.91, ‐0.41] |
| 9 Mean improvement in pain at rest (night pain) at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Duloxetine 20 mg daily | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.90, 0.34] |
| 9.2 Duloxetine 60 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.27, ‐0.57] |
| 9.3 Duloxetine 120 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.45, ‐0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with = or >50% improvement of pain at = or <12 weeks Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 20 mg | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.91, 2.14] |
| 1.2 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.20, 2.06] |
| 1.3 Duloxetine 120 mg daily | 3 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.36, 2.19] |
| 1.4 All doses | 3 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.37, 2.13] |
| 2 Number of patients with = or >50% improvement of pain at >12 weeks Show forest plot | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.25, 2.11] |
| 2.1 Duloxetine 60 mg daily | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.10, 2.27] |
| 2.2 Duloxetine 120 mg daily | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.15, 2.45] |
| 3 Number of patients with = or >30% improvement of pain at = or <12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.79] |
| 3.2 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.85] |
| 3.3 Duloxetine 120 mg daily | 2 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.86] |
| 3.4 All doses | 2 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.25, 1.80] |
| 4 Mean improvement in the SF36 mental component summary subscore Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 20 mg | 1 | 223 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐2.37, 3.99] |
| 4.2 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Random, 95% CI) | 3.31 [0.59, 6.02] |
| 4.3 Duloxetine 120 mg daily | 3 | 691 | Mean Difference (IV, Random, 95% CI) | 3.09 [1.47, 4.70] |
| 5 Mean improvement in the SF36 physical component summary subscore Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐1.92, 3.54] |
| 5.2 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐0.33, 2.89] |
| 5.3 Duloxetine 120 mg daily | 3 | 691 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.50, 3.10] |
| 6 Mean improvement in the SF36 Bodily Pain subscore Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 60 mg daily | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 8.2 [3.20, 13.20] |
| 6.2 Duloxetine 120 mg daily | 2 | 403 | Mean Difference (IV, Fixed, 95% CI) | 8.42 [4.80, 12.03] |
| 7 Mean improvement in the patient reported global impression of change at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.96, ‐0.12] |
| 7.2 Duloxetine 60 mg daily | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.73, ‐0.18] |
| 7.3 Duloxetine 120 mg daily | 2 | 513 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.81, ‐0.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients with any adverse event Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 60 mg daily | 4 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.12, 1.30] |
| 1.2 Duloxetine 120 mg daily | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.09, 1.30] |
| 2 Nausea Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 3.00] |
| 2.2 Duloxetine 60 mg daily | 3 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.33, 3.83] |
| 2.3 Duloxetine 120 mg daily | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.00, 4.45] |
| 3 Somnolence Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.43] |
| 3.2 Duloxetine 60mg daily | 3 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.62, 4.74] |
| 3.3 Duloxetine 120 mg daily | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.61 [2.74, 7.74] |
| 4 Dry mouth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.47] |
| 4.2 Duloxetine 60 mg daily | 2 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.12, 3.77] |
| 4.3 Duloxetine 120 mg daily | 2 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [1.93, 6.04] |
| 5 Dizziness Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.33] |
| 5.2 Duloxetine 60 mg daily | 3 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.15, 3.03] |
| 5.3 Duloxetine 120 mg daily | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.55, 3.97] |
| 6 Adverse event leading to cessation Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 2 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.78, 2.39] |
| 6.2 Duloxetine 60 mg daily | 5 | 1215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.20, 2.32] |
| 6.3 Duloxetine 120 mg daily | 6 | 1414 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.74, 3.05] |
| 6.4 All doses | 6 | 2220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.48, 2.52] |
| 7 Serious adverse event Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 20 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Duloxetine 60 mg daily | 5 | 1219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.32, 1.33] |
| 7.3 Duloxetine 120 mg daily | 5 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.35] |
| 7.4 All doses | 5 | 1856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.25] |

