Anestésicos locales y anestesia regional versus analgesia convencional para la prevención del dolor posoperatorio persistente en adultos y niños
Resumen
Antecedentes
La anestesia regional puede reducir la tasa de dolor posoperatorio persistente (DPP), un trastorno frecuente y debilitante. Esta revisión fue publicada originalmente en 2012 y se actualizó en 2017.
Objetivos
Comparar los anestésicos locales y la anestesia regional versus analgesia convencional para la prevención del DPP más allá de tres meses en adultos y niños sometidos a la cirugía electiva.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL, MEDLINE y Embase hasta diciembre 2016, sin restricciones de idioma. Se utilizó una combinación de búsqueda de texto libre y una búsqueda de vocabulario controlado. Se limitaron los resultados a los ensayos controlados aleatorios (ECA). Se actualizó esta búsqueda en diciembre 2017, pero estos resultados todavía no se han incorporado a la revisión. Se realizó una búsqueda manual en las listas de referencias de los estudios incluidos, artículos de revisión y resúmenes de congresos. Se realizaron búsquedas en el registro de revisiones sistemáticas PROSPERO para obtener revisiones sistemáticas relacionadas.
Criterios de selección
Se incluyeron ECA que comparaban la anestesia local o regional versus analgesia convencional con un resultado del dolor más allá de tres meses después de la cirugía electiva, no ortopédica.
Obtención y análisis de los datos
Al menos dos autores de la revisión evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos y los eventos adversos. Se estableció contacto con los autores de los estudios para obtener información adicional. Los resultados se presentan como odds ratios agrupados (OR) con intervalos de confianza (IC) del 95%, basados en modelos de efectos aleatorios (método de la varianza inversa). Los estudios se analizaron por separado por intervención quirúrgica, aunque se agruparon los resultados informados en diferentes intervalos de seguimiento. Se compararon los resultados con los modelos de Bayesian y los clásicos (frecuentistas). Se investigó la heterogeneidad. Se evaluó la calidad de la evidencia con GRADE.
Resultados principales
En esta revisión actualizada, se identificaron 40 nuevos ECA y siete estudios en curso. En total, se incluyeron 63 ECA en la revisión, aunque sólo fue posible sintetizar los datos sobre la anestesia regional para la prevención del DPP más allá de tres meses después de la intervención quirúrgica a partir de 41 estudios, con un total de 3143 participantes en el análisis inclusivo.
La síntesis de evidencia de siete ECA estuvo a favor de la anestesia epidural para la toracotomía, lo cual sugiere que las probabilidades de presentar DPP tres a 18 meses después de una epidural para la toracotomía fueron de 0,52 en comparación con ninguna epidural (OR 0,52; IC del 95%: 0,32 a 0,84; 499 participantes, evidencia de calidad moderada). De igual manera, la síntesis de la evidencia de 18 ECA estuvo a favor de la anestesia regional para la prevención del dolor persistente tres a 12 meses después de la intervención quirúrgica por cáncer de mama con un OR de 0,43 (IC del 95%: 0,28 a 0,68; 1297 participantes, evidencia de baja calidad). El agrupamiento de los datos a los tres a ocho meses después de la intervención quirúrgica a partir de cuatro ECA estuvo a favor de la anestesia regional después de la cesárea con un OR de 0,46; (IC del 95%: 0,28 a 0,78; 551 participantes, evidencia de calidad moderada). La síntesis de evidencia de tres ECA que investigaron la infusión continua con un anestésico local para la prevención del DPP tres a 55 meses después de la cosecha para el injerto óseo de la cresta ilíaca (ICBG, por sus siglas en inglés) fue no concluyente (OR 0,20; IC del 95%: 0,04 a 1,09; 123 participantes, evidencia de baja calidad). Sin embargo, la síntesis de evidencia de dos ECA también estuvo a favor de la infusión de anestésicos locales intravenosos para la prevención del DPP tres a seis meses después de la intervención quirúrgica por cáncer de mama con un OR de 0,24 (IC del 95%: 0,08 a 0,69; 97 participantes, evidencia de calidad moderada).
No se sintetizó la evidencia para los subgrupos quirúrgicos de amputación del miembro, reparación de la hernia, cirugía cardíaca y laparotomía. No fue posible agrupar la evidencia sobre los efectos adversos debido a que los estudios incluidos no los examinaron de forma sistemática, y los informaron de manera dispersa. La heterogeneidad clínica, la deserción, y los datos de resultado dispersos obstaculizaron la síntesis de la evidencia. El alto riesgo de sesgo causado por los datos faltantes y la falta de cegamiento a través de algunos estudios incluidos redujeron la confianza en los hallazgos. Por lo tanto, los resultados deben ser interpretados con cautela.
Conclusiones de los autores
Se establece la conclusión de que hay evidencia de calidad moderada de que la anestesia regional puede reducir el riesgo de desarrollo de DPP tres a 18 meses después de la toracotomía y tres a 12 meses después de la cesárea. Hay evidencia de baja calidad de que la anestesia regional puede reducir el riesgo de desarrollar DPP tres a 12 meses después de la intervención quirúrgica por cáncer de mama. Hay evidencia de calidad moderada de que la infusión intravenosa de anestésicos locales puede reducir el riesgo de desarrollar DPP tres a seis meses después de la intervención quirúrgica por cáncer de mama.
Las conclusiones son debilitadas considerablemente por el tamaño y el número pequeño de estudios, por el sesgo de realización, el sesgo nulo, la deserción y los datos faltantes. Se necesitan estudios más grandes y de alta calidad, que incluyan niños. Se advierte que la presente síntesis de la evidencia, (excepto para la cirugía de mama) se basa exclusivamente en unos pocos estudios pequeños. Debe señalarse que no es posible extender las conclusiones a otras intervenciones quirúrgicas o técnicas de anestesia regional, por ejemplo, no es posible concluir que el bloqueo paravertebral reduce el riesgo de DPP después de la toracotomía. Hay siete estudios en curso y 12 estudios en espera de clasificación que pueden cambiar las conclusiones de la revisión actual una vez que se publiquen e incorporen.
PICO
Resumen en términos sencillos
Anestesia local y regional en el momento de la intervención quirúrgica para prevenir el dolor persistente a más largo plazo después de la cirugía
Pregunta de la revisión
Se planificó determinar si la administración de anestésicos locales (medicación adormecedora) en el momento de la intervención quirúrgica reduce el riesgo de dolor que persiste durante tres meses y más después de la intervención quirúrgica. La comparación se realizó con analgésicos solos, como los opiáceos y los fármacos antiinflamatorios no esteroideos.
Antecedentes
El dolor que persiste mucho tiempo después de la cirugía se llama el dolor posoperatorio persistente (DPP), y no es poco común. El daño tisular y la lesión nerviosa pueden cambiar las vías de dolor y la sensibilidad al dolor por lo cual el dolor persiste durante meses. El paciente también puede sentir el dolor más intensamente o con un estímulo que normalmente no se percibe como dolor. Estos cambios pueden ser permanentes. La aplicación de anestésicos locales cerca de los nervios, los manojos de nervios o las raíces nerviosas en el sistema nervioso central, del mismo modo que una epidural, puede interrumpir la conducción de los impulsos de dolor del sitio quirúrgico al sistema nervioso central. El tratamiento efectivo del dolor agudo puede prevenir el DPP. La infiltración en la herida utiliza un tubo especialmente diseñado con orificios múltiples que se coloca dentro de la herida para aplicar el anestésico local.
Características de los estudios
La evidencia está actualizada hasta diciembre 2016. Se encontraron 63 ensayos controlados aleatorios (ECA) con participantes sometidos a cirugía abierta de tórax, corazón, mama, abdominal, vascular, ginecológica y otras, pero no a cirugía ortopédica. Los ECA son estudios en los que los pacientes son asignados al azar a uno u otro de los diferentes tratamientos estudiados. Los estudios incluyeron solo adultos, y se realizaron principalmente en Europa y América del Norte y algunos en China, Egipto y Brasil. Los tipos de intervención quirúrgica incluyeron cirugía con una tasa alta de eventos de dolor persistente después de la intervención quirúrgica, como la cirugía de mama, la amputación del miembro y la abertura el tórax y la intervención quirúrgica con un riesgo menor pero con números altos de procedimientos, como la cesárea.
Fue posible agrupar los resultados de 41 ECA que incluían a un total de 3143 participantes para el análisis inclusivo. El seguimiento se realizó para 1331 participantes a los tres meses, 1443 participantes a los seis meses, 326 participantes a los 12 meses y 43 participantes a los 20 meses o más después de la intervención quirúrgica. Los ECA no informaron las complicaciones quirúrgicas ni anestésicas de forma consistente y hubo poca información disponible sobre las mismas. Los estudios fueron financiados principalmente por las instituciones que realizaron los estudios.
Resultados clave
La anestesia regional redujo el número de pacientes que presentaron dolor persistente después de ser sometidos a la cirugía no ortopédica. Para la cirugía abierta de tórax, la administración de una epidural redujo a la mitad las probabilidades de que el paciente presente dolor posoperatorio persistente a los tres a 18 meses después de la intervención quirúrgica (siete ECA, 499 participantes, evidencia de calidad moderada). Siete pacientes necesitaron ser tratados de esa forma para que uno logre un beneficio.
Para la prevención del dolor persistente tres a 12 meses después de la intervención quirúrgica por cáncer de mama, siete pacientes necesitaron anestesia regional para que uno logre un beneficio (18 ECA, 1297 participantes, evidencia de baja calidad). Se observó que la infusión de un anestésico local en una vena redujo el riesgo de dolor persistente tres a seis meses después de la cirugía de mama (2 ECA, 97 participantes, evidencia de calidad moderada), y tres pacientes necesitaron ser tratados para que uno logre un beneficio. La anestesia regional redujo en más de la mitad las probabilidades de que una mujer presente dolor persistente después de la cesárea (cuatro ECA, 551 participantes, evidencia de calidad moderada). El número de pacientes tratadas para que una logre un beneficio fue de 19.
La infusión continua de un anestésico local en el sitio de donde se obtuvo tejido óseo del hueso de la cadera no redujo claramente el número de pacientes con dolor persistente a los tres a 55 meses (tres ECA, 123 participantes, evidencia de baja calidad).
No fue posible sintetizar la evidencia sobre la amputación del miembro, la reparación de la hernia, la cirugía cardíaca o abdominal debido a las diferencias en cómo se proporcionó el tratamiento o cómo se informaron los resultados.
Calidad de la evidencia
Se encontró evidencia consistente que apoya la administración de anestesia regional en adultos para prevenir el dolor persistente después de varios tipos de cirugía. Sin embargo, se observaron variaciones en los tamaños del efecto, y en diferentes momentos después de la intervención quirúrgica. En algunos estudios no fue posible realizar el cegamiento al tratamiento recibido y los resultados son afectados por el número pequeño de estudios y participantes y las pérdidas durante el seguimiento de los participantes con el transcurso del tiempo. La evidencia era por lo tanto de calidad baja o moderada.
Authors' conclusions
Summary of findings
| Should thoracic epidural anaesthesia or conventional pain control be used to prevent persistent pain after open thoracotomy | ||||||
| Patient or population: people undergoing open thoracotomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional pain control | Thoracic epidural anaesthesia | |||||
| Persistent pain 3 to 18 months after thoracotomy (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 18 months after surgery.) | Study population | OR 0.52 (0.32 to 0.84) | 499 | ⊕⊕⊕⊝ | All studies investigated persistent pain after open thoracotomy. The results cannot be extended to video‐assisted thoracotomy or other (minimally invasive) surgeries of the chest. The five of the seven included studies using thoracic epidural anaesthesia showed the strongest effect. The results cannot be extended to other interventions like paravertebral blocks. Conventional pain control with opioids and NSAID was the comparator. Event rates of persistent pain after thoracotomy were reported between 25% to 65% Regional anaesthesia may prevent persistent (chronic) pain after open thoracotomy in one out of seven people treated, thoracic epidural anaesthesia in one out of five people treated. | |
| 525 per 1000 | 332 per 1000 | |||||
| Low | ||||||
| 250 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 500 per 1000 | 310 per 1000 | |||||
| Adverse effects of epidural anaesthesia ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of epidural anaesthesia were not systematically reported and due to their low frequency are better investigated in patient registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1While outcome observers' blinding was described, study participants were not blinded; this is acceptable because participant and provider blinding is difficult in regional anaesthesia. | ||||||
| Should regional anaesthesia or conventional pain control be used to prevent persistent pain following breast cancer surgery | ||||||
| Patient or population: women with breast cancer undergoing elective surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional pain control | Paravertebral block | |||||
| Persistent pain 3 to 12 months after breast cancer surgery (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 12 months after surgery.) | Study population | OR 0.43 (0.28 to 0.68) | 1297 | ⊕⊕⊝⊝ | Conventional pain control with opioids and NSAID was the comparator. Event rates of persistent pain after breast cancer were reported around 30%. Pooling all studies, regional anaesthesia may prevent persistent pain after breast surgery in one out of every seven women. Limiting the analysis to paravertebral block, the number of women needed to treat for one person to benefit was 11. | |
| 427 per 1000 | 239 per 1000 | |||||
| Low | ||||||
| 200 per 1000 | 95 per 1000 | |||||
| High | ||||||
| 600 per 1000 | 387 per 1000 | |||||
| Adverse effects of paravertebral block for breast cancer surgery | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of regional anaesthesia after breast surgery were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded quality of evidence by one level because conclusions may be considerably weakened by performance bias, shortcomings in allocation concealment, considerable attrition and incomplete outcome data. | ||||||
| Should local or regional anaesthesia be used for the prevention of chronic pain after caesarean section | ||||||
| Patient or population: women after caesarean section Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Local or regional anaesthesia | |||||
| Persistent pain 3 to 8 months after caesarean section (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 8 months after surgery.) | Study population | OR 0.46 | 551 participants | ⊕⊕⊕⊝ | Event rates of persistent pain after caesarean section are reported around 10%. The number of women needed to be treated for one woman to benefit from regional anaesthesia after caesarean section was 19. | |
| 179 per 1000 | 91 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 100 per 1000 | 49 per 1000 | |||||
| Adverse effects of local or regional anaesthesia ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of local or regional anaesthesia after caesarean section were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The results are based on only four, mostly smaller studies. Meta‐analysis results based on small numbers tend to overestimate the effects. | ||||||
| Should continuous donor site local anaesthetic infusion or conventional pain control be used for the prevention of persistent postoperative pain after iliac crest bone graft harvesting | ||||||
| Patient or population: people after iliac crest bone graft harvesting Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Continous donor site local anaesthetic infusion | |||||
| Persistent pain 3 to 55 months after iliac crest bone graft harvesting (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 55 months after surgery) | Low | OR 0.20 | 123 | ⊕⊕⊝⊝ | We accepted study author classification of the presence of persistent postoperative pain. Some assessed only pain vs no pain, others pain and dysaesthesia vs none. Event rates of persistent pain after iliac crest bone graft harvesting were reported between 20% to 40% and was assumed to be around 30%. | |
| 200 per 1000 | 48 per 1000 | |||||
| Moderate | ||||||
| 400 per 1000 | 118 per 1000 | |||||
| High | ||||||
| 600 per 1000 | 231 per 1000 | |||||
| Adverse effects of continuous local anaesthetic infusion ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of regional anaesthesia after iliac crest bone graft harvesting were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The results are based on only three small studies. Meta‐analysis results based on small numbers tend to overestimate the effects. Including an additional RCT with continuous outcomes in a Bayesian evidence synthesis further strengthens the evidence favouring the intervention (Blumenthal 2005). | ||||||
| Should continuous intravenous local anaesthetic infusion or conventional pain control be used for the prevention of persistent pain after breast cancer surgery | ||||||
| Patient or population: women with breast cancer undergoing elective surgery Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Continous intravenous local anaesthetic infusion | |||||
| Persistent pain 3 to 6 months after breast cancer surgery (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 6 months after surgery.) | Study population | OR 0.24 | 97 | ⊕⊕⊕⊝ | Event rates of persistent pain after breast cancer surgery ranged in this population between 20% to 40%. One in three women benefited on average from continuous intravenous infusion of local anaesthetics after breast cancer surgery. | |
| 370 per 1000 | 123 per 1000 | |||||
| Low | ||||||
| 200 per 1000 | 57 per 1000 | |||||
| High | ||||||
| 600 per 1000 | 265 per 1000 | |||||
| Adverse effects of continuous local anaesthetic infusion ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of intravenous infusion of local anaesthetics after breast cancer surgery were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded quality of evidence by one level because conclusions may be considerably weakened by the small number of studies included. These two studies are however consistent and of high methodological quality. Still, meta‐analysis results based on small numbers tend to overestimate the effects. | ||||||
Background
Description of the condition
Pain arising from a surgical intervention and persisting beyond three months is termed persistent postoperative pain (PPP) (Kehlet 2006). PPP continues to be frequent and is sometimes severe, but often neglected (Bayman 2014; Gewandter 2015; Kehlet 2006; Perkins 2000). The risk of developing PPP varies from 5% after minor surgery to 50% for phantom limb pain or postmastectomy pain syndrome (Jung 2003; Perkins 2000). Young age, the surgical procedure and perioperative pain predict PPP, while genetic risk factors remain unknown (Lewis 2015; Montes 2015). PPP may be only mild or it may be severely disabling (Kehlet 2006). Even the relatively low risk (about 10%) of developing PPP after caesarean section is a major concern due to the frequency of caesarean sections (Sng 2009). Most clinical studies focus on acute postoperative pain, and few address the preventive effects of regional anaesthesia on PPP (MacRae 2001; MacRae 2008). Recent reviews deplored the poor quality of available studies and documented the high event rate after a variety of surgical interventions, from hernia repair to breast surgery (MacRae 2001; MacRae 2008). Our current review focuses on the ability of local anaesthetics or regional anaesthesia to reduce the risk of PPP.
Pain pathways, and hence pain perception, can be modulated, sensitized and permanently altered (Woolf 2000). Persistent pain, postoperative hyperalgesia and allodynia (Kehlet 2006), after surgery are the consequence of neuronal plasticity, that is permanent synaptic neuronal changes in the peripheral and central nervous system in response to tissue trauma and nerve injury; where hyperalgesia refers to pain felt more intensely and allodynia describes a painful sensation after a stimulus that normally is not perceived as pain (Wilder‐Smith 2006).
Description of the intervention
Before or after surgery, local anaesthetics may be applied locally to interrupt the conduction of pain impulses from the site of injury to the central nervous system. If local anaesthetics are applied locally at the site of surgery this is called local anaesthesia. If local aesthetics are applied close to nerves, but at a distance from the surgical site, this is called regional anaesthesia. Sometimes, local aesthetics are also applied intravenously. All three modes of administration of local aesthetics may prevent the central sensitization described in the Description of the condition. Epidural and spinal anaesthesia act at the nerve roots while nerve blocks, plexus anaesthesia and wound infiltration inhibit peripheral nerves. By blocking sympathetic nerves, local anaesthetics may also have desirable effects on bowel motility or unwanted effects on blood pressure. Systemically (for example intravenously) administered local anaesthetics might also exert beneficial effects including preventing PPP, hyperalgesia and allodynia (Duarte 2005; Herroeder 2007; Lavand'homme 2005; Strichartz 2008; Vigneault 2011). As in our previous review, in this update we also focused on the pre‐emptive (Kissin 1996), use of local anaesthetics with or without opioids or other adjuvants intravenously or in regional anaesthesia.
The local and regional anaesthesia techniques described above can be used as an alternative or in addition to conventional pain control. Opioids like morphine, non‐steroidal anti‐inflammatory drugs (NSAIDs) such as ibuprofen, and other analgesics like paracetamol (acetaminophen in the USA) are the most frequently used conventional pain killers. They are administered systemically and, therefore, often cause systemic side effects that limit their use, like the nausea and constipation caused by opioids or kidney damage as a result of use of NSAIDs. We have provided an explanation of regional anaesthesia and conventional analgesia in Appendix 1.
How the intervention might work
We hypothesize that preventing pain transmission using local or regional anaesthesia during or soon after surgery, or both, reduces the risk of PPP (Atchabahian 2015b; Woolf 1993). Local anaesthetics applied close to the nerves will block pain perception and prevent the central sensitization in the spinal cord that leads to hyperalgesia and PPP (Kehlet 2006) (see: Description of the condition). However, systemic toxicity of local anaesthetics is well described (Brown 1995), either as a side effect after absorption or when given intravenously (Herroeder 2007; Strichartz 2008). Anti‐hyperalgesic effects of systemic lidocaine persist days beyond drug delivery and cannot be explained by sodium channel blockage. The actual mechanism remains elusive (Strichartz 2008).
Our review focused on preventive analgesia. We defined preventive analgesia as antinociception with local anaesthetics or regional anaesthesia to reduce the risk of PPP regardless of the timing of the intervention in relation to surgery (Kissin 2000). We did not study if local anaesthetics or regional anaesthesia were more effective if applied before, during or after surgery (Bong 2005; Lavand'homme 2011).
Why it is important to do this review
PPP is frequent and difficult to treat (Kehlet 2006). Hence prevention of PPP is paramount (Gewandter 2015). We are interested in investigating whether local anaesthetics or regional anaesthesia prevent PPP several months after surgery. Clinical trials report conflicting results. For example, epidural anaesthesia may reduce the risk of PPP after thoracotomy (Ju 2008; Lu 2008; Senturk 2002), but these effects have not been consistently reproduced (Ochroch 2006). Our previous review and evidence synthesis (Andreae 2012), favoured regional anaesthesia for PPP after breast cancer surgery and thoracotomy; but these inferences were based on a few small studies and plagued by unit‐of‐analysis issues. Also we found that pertinent studies reported repeated outcomes at different and disparate follow‐up intervals (Andreae 2012). We did not find enough studies to allow us to make inferences for other surgical subgroups. No other meta‐analysis is presently available on the effect of local or regional anaesthesia on PPP six to 12 months after surgery. A systematic review by Ong focused mostly on immediate postoperative pain control and the timing of regional anaesthesia (Ong 2005); some have questioned his results and methods (Møiniche 2002). Existing narrative reviews of regional anaesthesia for PPP have not attempted evidence synthesis (MacRae 2001; MacRae 2008). Terkawi 2015a sought to synthesize the evidence on paravertebral block for the prevention of PPP, but found the outcome reporting of available randomized controlled trials (RCTs) disparate and hence evidence synthesis difficult.
Objectives
To compare local anaesthetics and regional anaesthesia versus conventional analgesia for the prevention of PPP beyond three months in adults and children undergoing elective surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with a randomized, controlled design. We also included single‐blinded studies because regional anaesthesia causes numbness of the affected body part and, therefore, neither participant nor anaesthesia provider can be reliably blinded to the intervention. However, blinding of the outcome observer was a prerequisite for inclusion in this review.
Types of participants
We included studies in adults and children undergoing elective surgical procedures, encompassing general, thoracic, abdominal, vascular, gynaecological and other surgery. This included the main groups of surgery with a high event rate of persistent pain after surgery, such as breast surgery, limb amputation and thoracotomy, but also groups with a lower baseline risk but high surgical volume, such as caesarean section.
We excluded studies in participants undergoing orthopaedic procedures as they are covered by another Cochrane Review (Atchabahian 2015a).
Types of interventions
We included studies comparing local anaesthetics or regional anaesthesia versus conventional pain control (Appendix 1).
Interventions
We included studies comparing local anaesthetics and regional anaesthesia versus conventional pain control.
We defined local anaesthetics as any pharmacological agents acting on the sodium channel to block nerve conduction (Movassaghian 2013; Rodriguez‐Navarro 2011).
The inclusion criteria for the intervention groups were as follows.
Studies administering local anaesthetics or regional anaesthesia, including:
-
studies that employed local anaesthetics or regional anaesthesia for any length of time during the perioperative period;
-
studies that employed local anaesthetics by any route (Appendix 1);
-
studies that may also have employed adjuvants or opioids, either locally or systemically, in any one group.
The exclusion criteria for the intervention groups were:
-
studies that only compared different regional anaesthesia techniques or varying dose regimens of local anaesthetics during the same perioperative time span;
-
studies using local anaesthetics for other than anaesthetic or analgesic purposes (for example as anti‐arrhythmics).
The inclusion criteria for the comparator groups were:
-
studies that used conventional postoperative pain control (Appendix 1).
Types of outcome measures
We studied primary and secondary outcomes as follows.
Primary outcomes
Our primary outcome was persistent postoperative pain (PPP) at three or more months after surgery.
We defined PPP as new pain, (which did not exist before the operation), but lasting beyond three months after surgery. We defined our primary outcome of interest as a dichotomous contrast, namely the presence versus absence of pain elicited at that clinical encounter. We accepted the dichotomous pain outcomes as reported in the studies, mostly contrasting pain versus no pain, even though definitions varied at times. Use of pain medication is by some assessed as a dichotomous outcome (no pain medication versus pain medication) or as an ordinal outcome (no pain medication versus non‐opioid pain medication versus opioid pain medication) (Lavand'homme 2005). Some primary study authors define the presence or absence of pain in their study as pain exceeding a given threshold on a continuous pain scale, analogous to responder analysis. We accepted the thresholds used by the study authors, though they sometimes employed different scales or instruments. This responder analysis (Andreae 2015c; Dworkin 2009a), also employed during our previous version of this review (Andreae 2015), counts the number of people with an outcome above a defined threshold. Responder analysis informed our approach to missing data imputation (Andreae 2013b), as detailed below (Dealing with missing data). We discussed responder analysis and the heterogeneity of outcome reporting in greater detail in (Overall completeness and applicability of evidence). Studies elicited the presence of pain at different follow‐up intervals beyond our cut‐off of three months and we discuss the two approaches we took (inclusive versus classical analyses) to address this heterogeneity in Data synthesis.
We also assessed differences in scores based on validated pain scales, such as the visual analogue scale (VAS); the verbal rating score; or the McGill pain questionnaire (Dworkin 2009b).
Secondary outcomes
Our secondary outcomes were as follows.
-
Allodynia and hyperalgesia
-
Use of pain medication
-
Adverse effects of techniques and agents used
Acceptable continuous measures for allodynia or hyperalgesia may, for example, be the area of punctuate allodynia or hyperalgesia measured with von Frey hair (Lavand'homme 2005).
For adverse events we accepted any definition by the authors of the primary studies, who in the previous version of this review (Andreae 2012), sparsely reported on adverse events and most anecdotally or in narrative form. We discuss in Overall completeness and applicability of evidence, that registries are better suited to assess adverse events after regional anaesthesia given their rare occurrences.
Search methods for identification of studies
We performed an electronic search of common databases and handsearched reference lists of relevant studies and conference abstracts.
Electronic searches
In December 2016 we searched for studies on local anaesthetics or regional analgesia for the prevention of PPP in the Evidence‐Based Medicine Reviews (EBMR) via OVID‐Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12), Ovid MEDLINE (1946 to December 2016), and Ovid Embase (1980 to December 2016).
We performed an additional search in December 2017 and added the results to Studies awaiting classification to be incorporated into the next update of this review.
We limited the results in MEDLINE using the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). As there is, as yet, no Cochrane Highly Sensitivity Search Strategy for Embase, we limited the results in Embase using a filter we found at the University of Alberta library, based on a trial done in MEDLINE (Glanville 2006; University of Alberta Library Guide 2014).
We combined a free text search with a controlled vocabulary search, covering from the inception of the database to the present.
We searched for studies using local or regional anaesthesia for painful postsurgical conditions with an outcome follow‐up of weeks or months. Our MEDLINE, Embase and CENTRAL search terms are reproduced in the appendices (see:Appendix 2; Appendix 3; Appendix 4).
We did not impose a language restriction.
Searching other resources
We conducted a handsearch of the reference lists of included studies, review articles and other identified relevant studies for additional citations, and in the conference abstracts of the International Anesthesia Research Society (IARS) and the European Society of Regional Anaesthesia (ESRA) for 2005 through to 2007.
Because the yield of the handsearch was very low, we did not update this search in 2015.
We followed links for related articles in Pubmed Central. We searched the PROSPERO systematic review registry (Booth 2012), for related systematic reviews, which might list relevant studies.
Data collection and analysis
We present a diagram illustrating the process of the searches and selection and we followed the recommendations of the QUORUM and PRISMA statements (Moher 1999; Moher 2010; Figure 1).
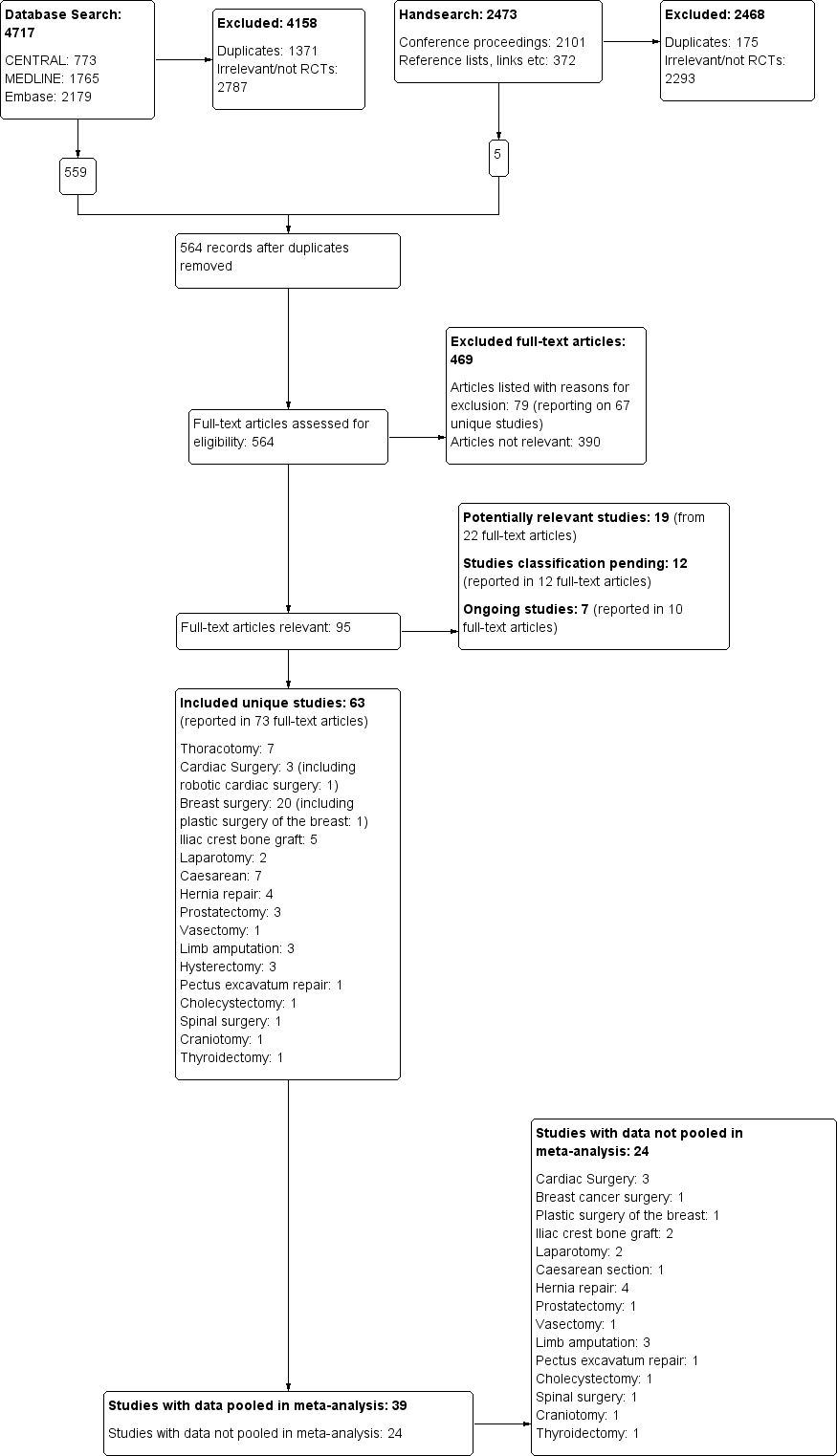
The study flow diagram documents the search and selection process. We included 63 studies. We were able to pool data from 39 of the 63 included studies in our inclusive analysis; data from 24 studies were not available or otherwise could not be pooled (Appendix 11).
Selection of studies
We completed screening and data extraction using DistillerSR, a web‐based systematic review software.
The review authors (EJW, MSC, JLL, JYC, DAA and MHA) screened the citations and abstracts of all publications obtained by the search strategies. To avoid location bias, all articles detected by our search, (but not available via online subscription of our institutions) were requested through interlibrary loans. For studies that appeared to be eligible RCTs, we obtained and inspected the full articles to assess their relevance based on the preplanned criteria for inclusion. We noted the reasons for study exclusion and inserted them into the Characteristics of excluded studies table.
Data extraction and management
We developed a standard data collection form within DistillerSR based on a template provided by Cochrane Anaesthesia, Critical and Emergency Care (ACE) for the first version of this review (Andreae 2012). We recorded details of study design, participant characteristics, interventions and outcome measures. We performed a pilot run and revised our data sheet accordingly, published as an appendix in our previous review (Andreae 2012). For this review update, at least two review authors independently collected and extracted data (EJW, JLL, MSC, JYC, MHA and DAA), using the DistillerSR software, based on the previously used data extraction form (Andreae 2012). EJW, JLL, MSC, MHA and DAA checked and entered the data into Review Manager 5 (RevMan 5) (RevMan 2014), computer software.
We extracted the following primary outcome data on pain: any patient‐reported chronic pain outcome (dichotomous, continuous or multidimensional instrument) at three months or beyond after surgery.
Where dichotomous data on persistent postoperative pain were not reported, we attempted to obtain these from the study authors. If unavailable, we used continuous pain assessment and outcome measures (for example the VAS or the Numerical Rating Scale (NRS)) or complex instruments to evaluate chronic pain (for example the Brief Pain Inventory (BPI)).
We extracted the following secondary outcomes, where provided: allodynia and hyperalgesia, use of pain medication.
We also extracted the following data: exclusion criteria; comorbidity; regional anaesthesia technique and local anaesthetic used; quality assurance of the intervention; quality of pain control; assessment of hyperalgesia and allodynia; use of adjuvants; and surgery performed. We extracted data on adverse effects and attrition.
Assessment of risk of bias in included studies
For each report, at least two of the review authors (EJW, MSC, JLL, JYC, MHA and DAA) independently evaluated each report meeting the inclusion criteria. We contacted study authors for missing information regarding their methods. We graded study quality in a 'Risk of bias' table on the basis of a checklist of design components. This comprised randomization, concealed allocation, observer blinding, and intention‐to‐treat analysis. We extracted information on conflicts of interest and funding (see: Characteristics of included studies). We achieved consensus by informal discussion. We judged risk of bias to be unclear, high or low (Higgins 2011a).
In regional anaesthesia interventions, blinding of participants and anaesthesia providers can be difficult and hence this criterion received less weight in the evaluation of performance bias, but not with regard to detection bias. We listed excluded studies with detailed reasons (see: Characteristics of excluded studies).
If the randomization and allocation process was open to substantial bias, for example pseudo‐randomization, we did not include the study data in the data analysis.
Null bias
In response to the first version of this review (Andreae 2013b), clinicians expressed concern about null bias. Null bias might cause studies to underestimate the benefit of regional anaesthesia for the prevention of persistent pain after surgery, if the regional anaesthesia interventions were not effectively delivered (Higgins 2011a; Woods 1995). Indeed, a number of included studies reported no improved pain control in the immediate postoperative period in the experimental (regional anaesthesia) group, as evidenced by inconsequential differences in pain scores between groups perioperatively, or similar requirements of rescue analgesic medications between groups in the immediate postoperative period (Barkhuysen 2010; Baudry 2008; Bollag 2012; Can 2013; Choi 2016; Fassoulaki 2000; Ibarra 2011; Ju 2008; Karmakar 2014; Katz 1996; Lam 2015; Lee 2013; Liu 2015; Loane 2012; McKeen 2014; Micha 2012; Purwar 2015; Singh 2013; Smaldone 2010; Terkawi 2015b; Vrooman 2015; Xu 2017; Zhou 2016). Two review authors therefore extracted information on null bias for each included study and documented their judgement with supporting evidence (see: Characteristics of included studies).
Exploring the influence of attrition and follow‐up interval on effect size.
We explored the possible influence of attrition and follow‐up duration on effect size. We plotted attrition (in percent of participants lost at follow‐up from participants randomized) versus effect size (log odds ratio) for the major groups of studies investigating regional anaesthesia for the prevention of persistent postoperative pain, where we had most studies with repeated measurements. We connected repeated sequential effect measures at consecutive follow‐up visits within one study. We wanted to test the hypothesis that increasing attrition and outcome reporting at later follow‐ups leads to bias in the effect size estimation. If we found evidence to refute our null hypothesis of no association, then pooling studies reporting outcomes at different follow‐up intervals or with differential attrition might lead to biased pooled estimates and we would avoid this mode of analysis.
Measures of treatment effect
As the summary statistic for our dichotomous primary outcome, we chose the odds ratio (OR) (Bland 2000). We reported the OR with 95% confidence intervals (CI). We calculated the number needed to treat for an additional beneficial outcome (NNTB) for the surgical subgroups, for example, for thoracotomy and breast cancer surgery, but not for the overall effect across all types of surgery (Cook 1995). We used the open source statistical software package R (R 2015), to compute the NNTB and its 95% CI according to the Cochrane Handbook for Systematic Reviews of Interventions chapter 12.5.4.3 Computing absolute risk reduction or NNTB from an OR (Schünemann 2011a), as documented in Appendix 5.
Risk ratios (RR) and ORs are equally accepted measures of treatment effect (Deeks 2011). The planned integration of dichotomous outcomes with continuous outcomes implied the use of ORs (see: Data synthesis). After this integration turned out to be of marginal importance for our analysis, we decided to stick to our protocol to eliminate any reasonable doubt about a postanalysis decision that might inappropriately influence our results (Andreae 2008).
For the continuous pain scales we calculated the mean difference between groups when all studies in a given subgroup used the same scale, and standardized mean differences (SMD) between groups when studies being compared used different scales.
Unit of analysis issues
Some studies have the surgical site (e.g. left or right hernia) as unit of analysis as opposed to the study participant (Bell 2001; Kurmann 2015), which could, in theory, confound results as absorbed lidocaine from the treated site could exert effects on the non‐treated site if they were randomized to discordant interventions (Strichartz 2008).
For our inclusive evidence synthesis (Analysis 1.1; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11), we pooled studies reporting outcomes at variable follow‐up intervals. When one study reported the results at several subsequent follow‐up intervals, we used only the latest outcome reported, because the most sustained effect would be most interesting clinically.
Dealing with missing data
We checked with the study authors for any missing information and reported data inconsistencies in the Characteristics of included studies. We specified in the tables if we were unable to obtain data.
Assessment of heterogeneity
We grouped studies in subgroups based on surgical interventions. Depending on the surgery, PPP has a different natural history (MacRae 2008). We feel these differences argue against pooling or comparing studies across surgical disciplines (Deeks 2011). We investigated study heterogeneity at the subgroup level using a Chi2 test and calculation of the I2 statistic (Higgins 2002). We followed the thresholds suggested in the Cochrane Handbook for Systematic Reviews of Interventions for the interpretation of I2 statistic (Deeks 2011).
Assessment of reporting biases
We contacted study authors to request missing data. We countered time lag bias by repeating our search just prior to submission of our work.
We considered an examination of publication bias using graphical and statistical tests (e.g. funnel plot, Egger's test (Sterne 2011)).
Data synthesis
In anticipation of diversity in reporting (Andreae 2012), in this update including additional studies with earlier and later follow‐up intervals at three months and beyond 12 months, we planned to pool studies reporting outcomes at different intervals after surgery and to build one coherent hierarchical Bayesian model (Andreae 2017a; Carter 2015), described in detail elsewhere (Andreae 2015). We thereby followed Ioannidis 2008, who explicitly proposed Bayesian methods to synthesize heterogeneous studies to overcome disparity in study design and reporting. In addition we performed a classical (frequentist) stratified evidence synthesis by surgical subgroup and follow‐up interval as in our initial publication (Andreae 2012). Frequentist inference, throughout this review, refers to the classical statistical approaches of significance and hypothesis testing proposed by Fisher and Neyman–Pearson, respectively, in contrast to the Bayesian statistical paradigm of updating a prior probability with new data (Andreae 2015c; Andreae 2018; Gelman 2014).
Inclusive model
For the inclusive evidence synthesis, we did not pool the data across different surgical disciplines. Instead, we grouped studies in broad surgical categories (e.g. thoracotomy, limb amputation, breast cancer surgery, etc.) based on the different natural history of PPP after each surgery. Where we had sufficient studies for a surgical procedure, that is, the inclusive analysis in breast surgery (Analysis 1.3), we organized the studies according to the regional anaesthesia intervention employed.
Pooling across different follow‐up intervals
We pooled studies reporting results at different follow‐up intervals to get a single stable estimate of the effect in a given surgical subgroup. Stratifying both by follow‐up and surgical subgroup would have led to very few studies at each follow‐up for each subgroup and hence unstable and variable pooled effect estimates. We counted each study only once, using the last follow‐up, if results were reported at more than one, and ordered them in the forest plots according to the duration of follow‐up. For example, in Analysis 1.3 synthesizing the dichotomous outcome persistent postoperative pain after breast cancer surgery, we pooled studies reporting this outcome at three, six and 12 months.
The underlying assumption is that follow‐up duration and attrition do not alter the effect estimate and we tested this hypothesis as described under Assessment of risk of bias in included studies and Incomplete outcome data (attrition bias), (Levene 2015). We describe how we dealt with unit of analysis issues in studies reporting outcomes at several follow‐up intervals under (Unit of analysis issues), and for the Bayesian model below.
Stratified analysis
We compared the results of our inclusive model with a classical (frequentist) stratified analysis where we only pooled studies with similar follow‐ups in each surgical subgroup. This predictably would lead to smaller bins and hence to more variability in the estimate, including possibly contradicting results when pooling the same studies, but repeatedly at subsequent intervals. If follow‐up varied only by weeks to one month, we considered follow‐up intervals to be the same, for example data at 24 weeks or at five months with data at six months.
For both stratified and inclusive analysis, we used the inverse‐variance approach, adjusting study weights based on the extent of variation, or heterogeneity, among the varying intervention effects (Deeks 2011). Confidence intervals for the average intervention effect would be wider with the more conservative random‐effects model; this would account for any potential between‐study heterogeneity and result in a more cautious estimate of any treatment effect (DerSimonian 1986).
We pooled treatment effects following the random‐effects meta‐analysis using the Cochrane statistical software RevMan 2014, as detailed in Chapter 8.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Following the process of GRADE assessment (GRADE Working Group 2004), we generated 'Summary of findings' tables as detailed in Chapter 11.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b) using the computer software GRADEpro GDT 2015.
Bayesian model
Anticipating that some studies would report only dichotomous outcomes while other studies would report only continuous outcomes (Andreae 2012), we had planned to pool the results in one comprehensive Bayesian hierarchical model (Andreae 2017a; Ioannidis 2008).
We started with a Bayesian hierarchical model for the surgical subgroup of iliac crest bone graft harvesting (ICBG). Where dichotomous aggregate data were not available, we estimated the dichotomous data from the continuous data presented for Blumenthal 2005 (Andreae 2013b). We then pooled the data in a Bayesian model (Andreae 2013b), implemented in the statistical software OpenBugs (Lunn 2009), with the model code presented in Appendix 6.
Bayesian statistics and our all‐inclusive Bayesian hierarchical model are described elsewhere in greater detail (Andreae 2015; Andreae 2017b; Carter 2015; Gelman 2014), but essentially we first obtained study‐level estimates for studies reporting outcomes at several subsequent follow‐up intervals by pooling these in a random‐effects model. Then we pooled these study‐level pooled effect estimates with the study‐level data of studies reporting only at one specific follow‐up interval by subgroups according to surgical intervention, as described above for the classical (frequentist) model. Finally we pooled the group‐level effect estimates to obtain an overall effect estimate. We used weak priors for effect estimates. We pooled the estimates of the within‐study variance between subsequent follow‐ups across all studies, assuming that the variability of effect estimates within a study would not depend on the surgical intervention but rather on the outcome measurement, which would be similar across all studies. We pooled the within‐group variance across studies and as a sensitivity analysis estimated between‐study variance for each group. We chose our prior for the variance of the overall effect estimate, the between‐group variability to force it to represent our prior belief that effects in one group will be almost independent of effects in another surgical group, reflecting the identical approach executed in both the classical and the inclusive analysis, described above. We used one study, identified during the initial search and selection, but subsequently excluded as non‐randomized (Brull 1992), to inform our Bayesian priors for the hierarchical Bayesian model of the subgroup of ICBG. We compared results based on this informative prior with results based on a weak uninformative prior (Andreae 2013b; Andreae 2015; Gelman 2014). In this we considered the argument by Shrier, that observational studies did not differ in their effects of interventions (Shrier 2007).
Model estimation, implementation and convergence testing
We used Marcov Chain Monte Carlo (MCMC) methods implemented in OpenBugs (Carter 2015; Lunn 2009) for our ICBG Bayesian model and the Hamiltonian Monte Carlo (HMC) algorithm implemented in the probabilistic programming language Stan (RStan 2.5), to fit our all‐inclusive model. We assessed convergence looking at trace plots of our simulations. We explored the multidimensional autocorrelation of parameters using shinyStan, our purpose‐built software, to visualize objects created in the Stan language (ShinyStan 1.0). We investigated tree depth and other HMC‐specific convergence parameters (Gelman 2014). We used the Gelman‐Rubin statistic Ř to assess convergence of all parameters (Gelman 2014). Even though convergence was satisfactory, we ran the final model with four chains, and 100,000 iterations in OpenBugs (Lunn 2009), and 5000 iterations, (including 2500 warm‐up iterations) in (RStan 2.5).
Pooling groups with different timing of regional anaesthesia interventions or varying use of adjuvants in regards to the surgical intervention
For studies with several groups using local or regional anaesthesia, albeit with varying use of adjuvants or different timing of the intervention with regards to the surgical procedure, or both, we pooled all groups employing local or regional anaesthesia and compared them against the comparator. If the first group received a regional anaesthesia intervention before incision (preoperative or pre‐emptive) and the second group received it after incision (postoperative or preventive), we pooled the (first and second) groups employing local anaesthetics against the (third) control groups not employing any local anaesthetics (that is using only conventional pain control instead). Similarly, if there were multiple study groups using (different) regional anaesthesia, one with and one without an adjuvant analgesic, we pooled the results from both groups and compared them to the control group using conventional analgesic methods.
Subgroup analysis and investigation of heterogeneity
Where there were enough studies in one group, we calculated the I2 statistic (Higgins 2002). We followed the thresholds suggested in the Cochrane Handbook for Systematic Reviews of Interventions for the interpretation of the I2 statistic (Deeks 2011).
We investigated studies employing adjuvant therapy, using different regional anaesthesia modalities, and studies providing continuous postoperative regional anaesthesia as a subgroup.
Sensitivity analysis
We tested the sensitivity of our results to our model assumptions and calculated the effect estimates for our pooled subgroups (e.g. breast cancer surgery and thoracotomy) for the random‐effects model versus the fixed‐effect model. For the Bayesian model, we tested the influence of different priors on the pooled estimate (Gelman 2014), comparing the use of a non‐RCT (Brull 1992), to inform our Bayesian priors versus the use of a weak, non‐informative prior for our Bayesian hierarchical model, for the subgroup of Illiac crest bone graft harvesting only, as reported in greater detail elsewhere (Andreae 2013b).
'Summary of findings' table and GRADE
We used the GRADE approach to assess the quality of the evidence (Langendam 2013). We imported import data from RevMan 2014, using GRADEpro GDT 2015, to create 'Summary of findings' tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5). These tables summarize the magnitude of the effects of the interventions examined, the total sum of all available data and their consistency, weighing them against the internal and external validity of the studies, or lack thereof. We assessed the overall quality of evidence for each outcome. We downgraded the evidence from 'high quality' by one level for serious (or by two levels for very serious) study limitations (risk of bias, e.g. performance bias, shortcomings in allocation concealment, considerable attrition and incomplete outcome data) serious inconsistency, heterogeneity or imprecision of effect estimates. We reported the effect of local or regional anaesthesia on the prevention of PPP at three months or beyond by surgical subgroups after thoracotomy (summary of findings Table for the main comparison), breast cancer surgery (summary of findings Table 2; summary of findings Table 5), caesarean section (summary of findings Table 3), and ICBG (summary of findings Table 4).
Results
Description of studies
Results of the search
The searches for this updated review were undertaken in September 2014 to January 2015, again in April 2015, and for a final time in December 2016. We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12), Ovid MEDLINE (1946 to April 2016), and Ovid Embase (1980 to April 2016).
For the original review, the searches were undertaken in February and March 2008 and rerun between February and August 2010 and again between April and May 2012 (Andreae 2012).
The search and selection process is illustrated in a flow diagram (Figure 1).
Electronic search
The electronic search yielded a total of 4717 references matching the predefined search parameters: 773 in CENTRAL, 1765 in MEDLINE, 2179 in Embase; among them were 1371 duplicates. The review authors (EJW, JLL, MSC, JC, MHA and DAA) screened these and excluded 2787 references as irrelevant or not RCTs. We added 11 study reports from an updated search in December 2017 to Studies awaiting classification.
Handsearch
We did not repeat the handsearch for this update. For the first version of this review (Andreae 2012), in our handsearch of the conference proceedings, we looked at 2101 references. We found 372 references in the reference lists of included studies or review articles, or by following links in PubMed and Google to other relevant studies. This resulted in a total of 2473 references; 175 were duplicates and 2293 were excluded as irrelevant or not RCTs.
Unpublished data
We identified one unpublished study, which was included in the meta‐analysis (Katz 1996).
Selection process
Three review authors (EJW, JLL, MHA) obtained full‐text copies of 564 articles for further assessment (see: Figure 1). Six review authors (EJW, JLL, MSC, JC, MHA and DAA) selected 63 studies for inclusion in this review (see: Characteristics of included studies). We found seven ongoing studies for assessment upon completion (ISRCTN46621916; Liew 2011; Michael 2014; NCT00418457; NCT01626755; NCT02002663; Theodoraki 2016) (see Characteristics of ongoing studies).
Data extraction
Seven study reports were only available as a conference abstracts. For three of these, we could not identify any follow‐up report and obtained no additional data (Katsuly‐Liapis1996; Okur 2016; Smaldone 2010). We were able to resolve all disagreements with regard to data extraction, study inclusion and quality assessment by informal discussion. Data extraction and quality assessment for the remaining four studies was resolved with help from the respective study authors (Besic 2014; Choi 2016; Micha 2012; Tecirli 2014).
Incomplete and raw data
In spite of contacting study authors, we were unable to obtain appropriate or adequate data for five studies (Burney 2004; Chiu 2008; Di‐Gennaro 2013; McKeen 2014; Pinzur 1996).
Included studies
We identified 63 RCTs studying regional anaesthesia or local anaesthetics for the prevention of PPP in this updated review (see: Characteristics of included studies), 40 of these were newly included in this update. For ease of orientation, Appendix 7 summarizes the surgical operations, type of anaesthesia, timing of intervention, adjuvant therapy and outcomes of the pooled studies. Four included studies reported their results in several published manuscripts (Kairaluoma 2006; Katz 1996; Katz 2004; Singh 2007). When two manuscripts were published by the same authors and reported the same participant numbers, we judged them to be reporting on just one and the same study; we used this data set only once (Kairaluoma 2006; Katz 1996; Katz 2004; Singh 2007). We reviewed studies reported in English and several other languages, including Danish (Bach 1988), French (Baudry 2008; Mounir 2010), German (Weihrauch 2005), Japanese (Hirakawa 1996), Mandarin (Lu 2008), and Spanish (Ibarra 2011).
Descriptive characteristics of participants
We pooled the data of 3143 study participants in our inclusive analysis (Appendix 8), with 499 participants after thoracotomy, 116 participants after cardiac surgery, 1297 participants after breast cancer surgery, 661 participants after caesarean section, 123 participants after ICBG, 150 participants after prostatectomy, 297 participants after hysterectomy, with outcomes ranging from 3 to 48 months after surgery.
We pooled the data organized by surgery type with outcomes at 3, 6, 12, 20, or 48 months. A breakdown of the number of participants by surgery and time point is provided in Appendix 8. One study on participants undergoing pectus excavatum repair took place in children and adolescents older than 10 years, but was the only study of its surgery type and we did not, therefore, include it in the meta‐analysis (Weber 2007). Only adults (> 18 years) could be included in the meta‐analysis; the youngest population had a mean age in the experimental group of 25 years plus or minus a standard deviation of five years (Blumenthal 2005).
Patient characteristics
Reflecting the diversity of surgical interventions, the participants' age, sex and comorbidities varied widely and were sparsely reported. Breast surgery and caesarean section studies included only female participants. Studies on limb amputation included predominantly male participants.
Types of surgery
We listed the surgical interventions investigated in the pooled studies (thoracotomy, breast cancer surgery, hysterectomy, ICBG, caesarean section, prostatectomy) in Appendix 7. We grouped studies in broad categories (thoracotomy, cardiac surgery, breast surgery, caesarean section, laparotomy, and prostatectomy) with similar characteristics. We reported breast surgery (Albi‐Feldzer 2013; Baudry 2008; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Grigoras 2012; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lee 2013; Micha 2012; Strazisar 2012; Strazisar 2014; Tecirli 2014; Terkawi 2015b) including cosmetic breast surgery (Bell 2001), in the same subgroup, but performed a sensitivity analysis excluding plastic surgery.
Characteristics of regional anaesthesia interventions
Regional anaesthesia modalities and timing of perioperative blockade
We summarized the use of regional techniques in (Appendix 7).
Epidural anaesthesia was used in majority of the thoracotomy studies (Can 2013; Comez 2015; Ju 2008; Lu 2008; Senturk 2002). Exceptions included one study using intercostal nerve block (Katz 1996), and one employing wound irrigation (Liu 2015). Wound irrigation and instillation were used in three of the studies on ICBG (Blumenthal 2005; Gundes 2000; Singh 2007), while local infiltration techniques were used in the others (Barkhuysen 2010; O'Neill 2014). For laparotomy surgery, both studies employed epidural anaesthesia (Katz 2004; Lavand'homme 2005), whereas in hysterectomy both studies employed spinal anaesthesia (Sprung 2006; Wodlin 2011). Within the other surgical subgroups, studies investigated different regional anaesthetic techniques: for breast surgery, mostly paravertebral block (Gacio 2016; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013), with and without some local infiltration (Albi‐Feldzer 2013), some used intravenous local anaesthesia (Grigoras 2012; Terkawi 2015b), others used only local infiltration (Baudry 2008; Besic 2014; Strazisar 2012; Strazisar 2014); for caesarean section, mostly transverse abdominal plain block (Bollag 2012; Loane 2012; McKeen 2014; Singh 2013), and peritoneal instillation (Shahin 2010); for hernia repair, mainly local/wound infiltration.
The experimental arms in two studies on breast cancer surgery used intravenous lidocaine (Grigoras 2012; Terkawi 2015b). Dermal patches, Bier block, ultra long‐acting or slow‐release local anaesthetic compounds were not studied.
In thoracotomy, all studies used continuous regional anaesthesia in the perioperative period. In the breast cancer surgery subgroup, only those with topical (Fassoulaki 2000; Fassoulaki 2005), or intravenous administration (Grigoras 2012; Terkawi 2015b), of local anaesthesia used continuous perioperative regional anaesthesia. Caesarean section studies employed mostly single‐shot interventions with the exception of two studies that used continuous wound irrigation perioperatively (Lavand'homme 2007; O'Neill 2012). In ICBG, three of the studies used continuous postoperative wound irrigation (Blumenthal 2005; O'Neill 2014; Singh 2007). In the remaining surgical subgroups, there were only a handful of studies utilizing continuous application of regional anaesthetics (Brown 2004; Chiu 2008; Gupta 2006; Lavand'homme 2005; Pinzur 1996; Vrooman 2015).
Two studies tested the hypothesis that blocking ischaemic limb pain prior to amputation prevents the central sensitization that might otherwise lead to persistent pain afterwards (Karanikolas 2006; Katsuly‐Liapis1996). The latter comparison was not planned in our protocol and hence these data were not presented.
Primary outcomes
As a prerequisite for inclusion, studies had to employ an instrument to subjectively measure patient discomfort (Appendix 7). The study authors primarily used a dichotomous outcome, that is presence or absence of (phantom) pain. They also used several continuous pain scales (verbal rating scale (VRS), visual analogue scale (VAS), numeric rating scale (NRS), bodily pain sub‐component of the Short Form Health Survey (SF‐36)). Nine studies did not record pain as a dichotomous outcome but only used continuous pain scales (Blumenthal 2005; Chiu 2008; Gupta 2006; McKeen 2014; O'Neill 2014; Singh 2013; Sprung 2006; Vrooman 2015; Wodlin 2011). One did record pain as a dichotomous outcome but did not report it in the manuscript, and provided the review authors with the data via email (Kurmann 2015). Nine studies (Brown 2004; Burney 2004; Gupta 2006; Karanikolas 2006; Karmakar 2014; Katz 2004; ; McKeen 2014; Sprung 2006; Wodlin 2011), reported continuous complex outcome instruments, like the McGill questionnaire (Dworkin 2009b), or the Short Form Health Survey (SF‐36) (Ware 1992), which are recommended in consensus statements for the assessment of chronic pain (Gewandter 2015; Turk 2006).
Duration of follow‐up
A minimum of three months' follow‐up was required for inclusion. Most studies focused on, and most patient data were collected at three or six months' follow‐up (Appendix 7).
Secondary outcomes
Allodynia and hyperalgesia and other outcome measures
Nine studies investigated allodynia and hyperalgesia (Bell 2001; Blumenthal 2005; Bollag 2012; Grigoras 2012; Gundes 2000; Ju 2008; Kurmann 2015; Lavand'homme 2005; Lavand'homme 2007). The heterogeneity of surgical interventions precluded any evidence synthesis. Fifteen studies used other (additional) outcome measures, like McGill questionnaire (Dworkin 2009b), Short Form Health Survey (SF‐36) (Ware 1992), Mental Health Inventory 18 (Beusterien 1996), Pain Disability Index (Tait 1990), or "interference with life" (Bollag 2012; Brown 2004; Burney 2004; Gupta 2006; Karanikolas 2006; Katz 2004; Lavand'homme 2005; McKeen 2014; Pinzur 1996; Sprung 2006; Wodlin 2011).
Reporting of adverse effects
Most reporting on long‐term adverse effects was sparse, sporadic and anecdotal, rather than prospective and systematic. Two RCTs investigated the risk of women in labour developing backache after epidural anaesthesia during labour as primary outcome (Howell 2001; Loughnan 2002), but did not meet the inclusion criteria of the main analysis.
Risk factors and pre‐existing pain
The included studies did not elicit or compare the known risk factors for the development of PPP between the experimental and control groups. We are therefore unable to comment on to what degree a difference between the groups may have introduced bias (Fassoulaki 2008). As people who present for thoracotomy and breast cancer are usually pain free, pre‐existing pain is unlikely to be a confounder for these pooled subgroups (Gottschalk 2006). This may be very different for people undergoing limb amputation; they may have suffered from prolonged and excruciating ischaemic pain prior to surgery.
Excluded studies
We excluded 79 studies, a summary of which can be found in the Characteristics of excluded studies table. No study was excluded exclusively for lack of observer blinding. We excluded three studies for pseudo‐randomization (Bach 1988; da Costa 2011; Nikolajsen 1997). One study (da Costa 2011), also failed other inclusion criteria.
Studies awaiting classification
As reported on 22 January 2009, SS Reuben was accused of publishing fraudulent data. Up to 22 papers have been, or will be, retracted by the journals in which they have been published, as detailed in the retraction notice in Anesthesia and Analgesia, 20 February 2009 (Shafer 2009). It appears that Reuben 2006 is not among the list of retracted manuscripts, however we have placed it in the classification pending section on the advice of Cochrane Anaesthesia, Critical and Emergency Care.
Further, 11 studies from an updated search in December 2017 are currently awaiting classification (see Characteristics of studies awaiting classification).
Ongoing studies
There are seven ongoing studies (ISRCTN46621916; Liew 2011; Michael 2014; NCT00418457; NCT01626755; NCT02002663; Theodoraki 2016). These seven studies will be assessed when they have been completed. A summary of the studies can be found in the Characteristics of ongoing studies table.
Risk of bias in included studies
The risk of bias is detailed in the risk of bias tables (Characteristics of included studies), the risk of bias graph (Figure 2), and is summarized in the methodological quality summary (Figure 3).
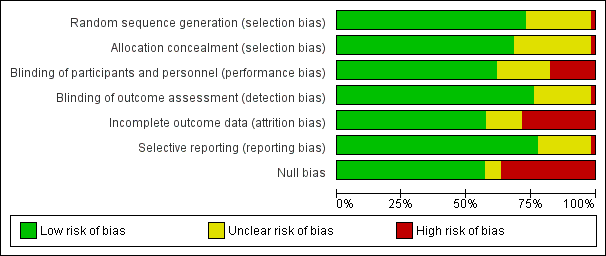
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
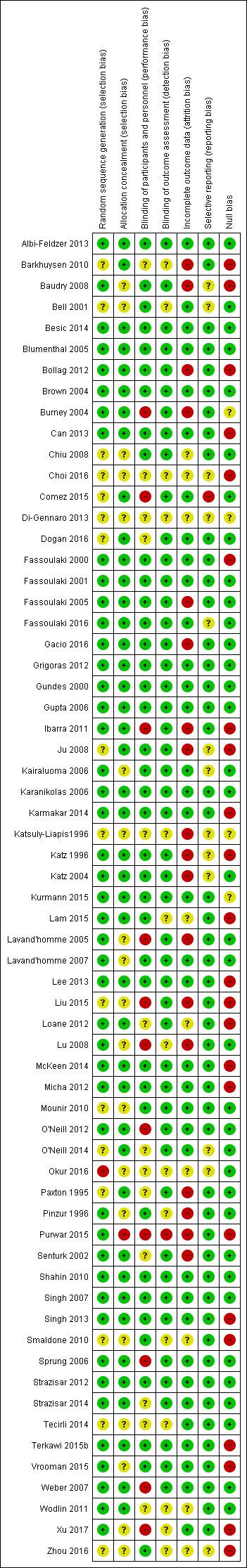
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Allocation
Sequence generation
Twelve studies did not detail the process of sequence generation (Bell 2001; Chiu 2008; Choi 2016; Comez 2015; Dogan 2016; Gacio 2016; Ju 2008; Katsuly‐Liapis1996; Liu 2015; Mounir 2010; Paxton 1995; Zhou 2016). Study authors' responses provided additional unpublished information for some studies (Can 2013; Fassoulaki 2000; Fassoulaki 2001; Gacio 2016; Gundes 2000; Ibarra 2011; Lavand'homme 2007; Purwar 2015; Senturk 2002). We excluded three studies for pseudo‐randomization (Bach 1988; da Costa 2011; Nikolajsen 1997) (Appendix 9). A general finding was that the most recently published articles overall provided much more detail on this process in their study manuscripts.
Concealment of allocation
The majority of studies utilized adequate concealment of allocation, using sealed, opaque envelopes opened just prior to the regional anaesthesia intervention. Allocation concealment was not detailed in 16 studies (Baudry 2008; Bell 2001; Chiu 2008; Choi 2016; Kairaluoma 2006; Katsuly‐Liapis1996; Lavand'homme 2005; Lavand'homme 2007; Liu 2015; Lu 2008; Mounir 2010; Okur 2016; Pinzur 1996; Vrooman 2015; Xu 2017; Zhou 2016).
Blinding
We did not exclude any studies for detection bias, and only outcome assessment blinding was a prerequisite for inclusion. Some study authors reported difficulties in keeping the participants and providers blinded due to the need to adjust dosing or preoperative pain control prior to limb amputation (Nikolajsen 1997), or the obvious immediate clinical effects of regional anaesthesia, that is numbness of the affected body part (Lavand'homme 2005; Senturk 2002). Most participants will note the obvious effects of regional anaesthesia, like motor weakness and sensory loss, and guess their allocation. This made effective blinding of participants and practitioners almost impossible. In other cases, different methods of anaesthesia between the groups led to awareness of group allocation by participants and physicians conducting the study, such as one group with spinal anaesthesia versus another with spinal‐epidural anaesthesia (O'Neill 2012), or thoracic epidural anaesthesia in the intervention arm versus patient‐controlled analgesia (PCA) in the control arm (Weber 2007).
Many study authors detailed (in the publication or via further communications) efforts to blind study participants, physicians and caregivers well as outcome assessors (Albi‐Feldzer 2013; Baudry 2008; Blumenthal 2005; Bollag 2012; Brown 2004; Can 2013; Chiu 2008; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Fassoulaki 2016; Gacio 2016; Grigoras 2012; Gundes 2000; Gupta 2006; Ju 2008; Kairaluoma 2006; Karanikolas 2006; Karmakar 2014; Katz 1996; Katz 2004; Kurmann 2015; Lavand'homme 2007; McKeen 2014; Mounir 2010; Shahin 2010; Singh 2007; Terkawi 2015b; Vrooman 2015). Some reported double blinding but did not provide details (Bell 2001; Comez 2015; Paxton 1995; Pinzur 1996). Six studies described outcome assessor blinding, without detail on personnel or participant blinding (Burney 2004; Dogan 2016; Ibarra 2011; Lam 2015; Lavand'homme 2005; O'Neill 2012), but nine other studies neither described nor confirmed it (Bell 2001; Choi 2016; Katsuly‐Liapis1996; Liu 2015; Lu 2008; Okur 2016; Wodlin 2011; Xu 2017; Zhou 2016).
Obviously, performance bias may weaken the conclusions of our review. The placebo effect may be particularly strong for pain outcomes and remains unknown for long‐term outcomes. Our conclusions are considerably weakened by shortcomings in allocation concealment, considerable attrition and incomplete outcome data. Six studies employed adjuvants (Bollag 2012; Brown 2004; Fassoulaki 2005; Gacio 2016; Lavand'homme 2005; Sprung 2006), only in the experimental group, potentially introducing bias, but this did not affect the results for the breast cancer surgery subgroup and was not pertinent for the thoracotomy subgroup.
Incomplete outcome data
There was a trend toward more adequate addressing of incomplete outcome data in more recent studies (Albi‐Feldzer 2013; Bell 2001; Blumenthal 2005; Brown 2004; Can 2013; Comez 2015;Dogan 2016; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2016; Gacio 2016; Grigoras 2012; Gundes 2000; Gupta 2006; Kairaluoma 2006; Karanikolas 2006; Karmakar 2014; Kurmann 2015; Lavand'homme 2007; McKeen 2014; Mounir 2010; O'Neill 2012; Okur 2016; Purwar 2015; Shahin 2010; Singh 2007; Sprung 2006; Terkawi 2015b; Vrooman 2015; Weber 2007; Xu 2017). compared to those that are older (Katsuly‐Liapis1996; Katz 1996; Katz 2004; Lavand'homme 2005; Paxton 1995; Senturk 2002). Study authors reported high attrition rates, due to loss to follow‐up as well as the high mortality of the participant groups studied. This potentially introduces bias. One study excluded randomized participants that the surgeon deemed inoperable but did not consider an intention‐to‐treat analysis (Senturk 2002). Only seven studies performed a formal intention‐to‐treat analysis (Albi‐Feldzer 2013; Kairaluoma 2006; Karmakar 2014; Kurmann 2015; Singh 2007; Sprung 2006; Terkawi 2015b). In four studies, there was no attrition at all (Comez 2015; Grigoras 2012; ; ; Weber 2007; Xu 2017).
In our graphical exploration of the influence of attrition and follow‐up interval on effect size shown in an attrition effect size graph (Figure 4), we did not find any association. In other words, we found no evidence to reject the null hypothesis that attrition and follow‐up intervals have no influence on effect size estimation.
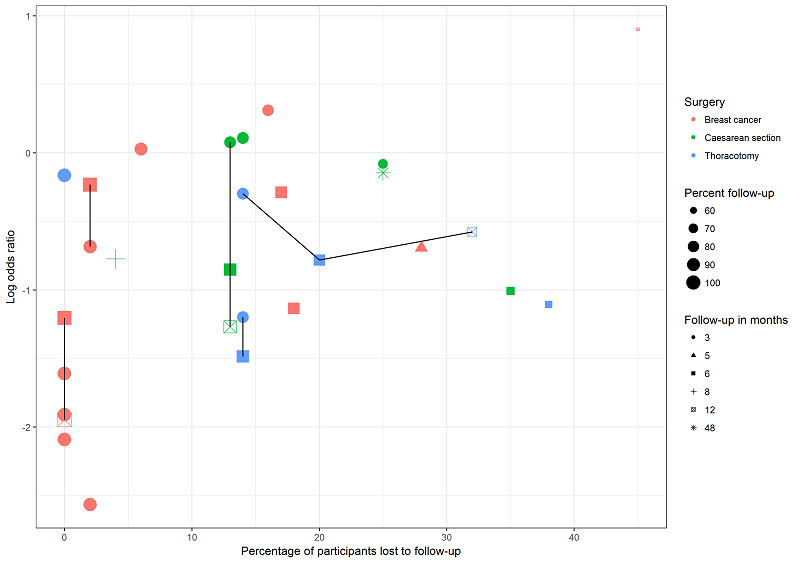
This graph plots attrition versus effect size (log odds ratio) for studies investigating regional anaesthesia for the prevention of persistent pain after thoracotomy (blue), breast surgery (pink) and caesarean section (green). Symbol size decreases with attrition. Repeated follow‐ups within one study are linked with a black line. We are unable to discern any association between attrition, follow‐up time and effect measure; this lends support to our decision to pool studies reporting outcomes at different follow‐up intervals and with different attrition.
Selective reporting
We contacted the authors of 37 included studies during this update, and 23 in the original systematic review for clarification of study methodology or to obtain further unpublished data. We found no contact information for the authors of three studies (Choi 2016; Katsuly‐Liapis1996; Zhou 2016).
Selective reporting was a concern regarding adverse effects. Several studies reported adverse effects as 'none', but did not detail, if patients were asked about any side effects and if so which. This may reflect reporting bias (Albi‐Feldzer 2013; Karmakar 2014; Pinzur 1996). Where reported, information on adverse effects in the included studies was mostly anecdotal and not reported separately by group (Can 2013; Kairaluoma 2006; Katz 2004; Lavand'homme 2007; Paxton 1995; Singh 2007; Weber 2007). The studies made very general statements about the side effects, such as, "no clinical signs or symptoms of local anaesthetic toxicity were noted in any patient" (Gundes 2000), and "Only one patient (in the placebo group) developed lymphedema, while no post‐surgery infection or other complications were reported" (Terkawi 2015b).
Undue sponsor influence (conflict of interest)
The source of funding and conflict of interest statements for many studies were either addressed in the manuscript or clarified in correspondence with study authors, with the exception of eleven studies for which no information was available (Baudry 2008; Brown 2004; Chiu 2008; Choi 2016; Gupta 2006; Ibarra 2011; Kairaluoma 2006; Katsuly‐Liapis1996; Lam 2015; Lu 2008; Paxton 1995). The studies were mostly supported by funds from the department or the institution. For those studies that described support by outside funding, we did not find any undue influence by the sponsors.
Null bias
The occurrence of ‘null bias’ is due to interventions being insufficiently well delivered (Higgins 2011a; Woods 1995). A number of included studies report insufficient pain control in the immediate postoperative period, as evidenced by inconsequential differences in pain scores between groups perioperatively, or similar requirements of rescue analgesic medications between groups in the immediate postoperative period (Barkhuysen 2010; Baudry 2008; Bollag 2012; Can 2013; Choi 2016; Fassoulaki 2000; Ibarra 2011; Ju 2008; Karmakar 2014; Katz 1996; Lam 2015; Lee 2013; Liu 2015; Loane 2012; McKeen 2014; Micha 2012; Purwar 2015; Singh 2013; Smaldone 2010; Terkawi 2015b; Vrooman 2015; Xu 2017; Zhou 2016). These studies are at high risk of null bias as the intervention was possibly not applied correctly or at high enough dosages for a true treatment effect in the immediate postoperative period. This likely blunted the treatment effect at three or more months postoperatively, because poor pain control in the postoperative period is probably an important driver of persistent pain after surgery (Lewis 2015; Gottschalk 2006).
Other potential sources of bias
Reporting bias
The small numbers of studies found in each subgroup precluded a formal study of publication bias by graphical analysis or the test proposed by Egger 1997 in most subgroups. At least 10 studies should be included in the meta‐analysis to make a funnel plot or an Egger test useful because with fewer studies the power of the tests is insufficient to distinguish chance from real asymmetry (Sterne 2011). We present a funnel plot for the breast surgery subgroup (Figure 5), which is inconclusive, especially considering that it is based on only 11 studies and includes several repeated observations for some among them. We acknowledge some degree of publication bias. Some studies, which failed to demonstrate substantial benefit beyond three months, could not be included because published aggregate data were insufficient for inclusion. In some studies we could not get the individual participant data (Blumenthal 2005; Burney 2004; Chiu 2008; McKeen 2014; Pinzur 1996), even though this did not affect any inferences we made.
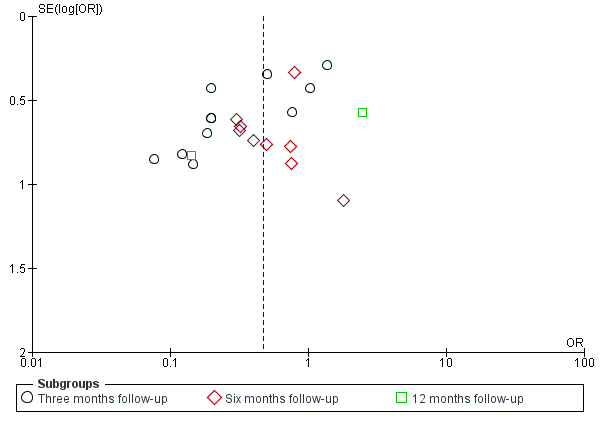
The funnel plot for breast surgery including all outcomes at any follow‐up interval for all breast surgery studies is inconclusive for publication bias.
In spite of considerable efforts outcome data were not available for some studies, as detailed also in the table Characteristics of included studies, this potentially introduced bias in our review and may reflect underlying publication bias.
Assessment of pre‐existing pain and risk factors for persistent postsurgical pain
There are risk factors for the development of PPP (Kehlet 2006). The severe ischaemic pain prior to limb amputation may be a predictor for PPP after amputation (Karanikolas 2006). Most studies did not assess risk factors or baseline pain. An exception to this were studies reporting continuous outcomes via the Short Form Health Survey (SF‐36), in which some studies report baseline values for comparison (Brown 2004; Gupta 2006; Karmakar 2014; Sprung 2006; Wodlin 2011).
Effects of interventions
See: Summary of findings for the main comparison Thoracic epidural anaesthesia versus conventional pain control to prevent persistent pain after open thoracotomy; Summary of findings 2 Regional anaesthesia compared to conventional pain control for breast cancer surgery; Summary of findings 3 Local or regional anaesthesia for the prevention of chronic pain after caesarean section; Summary of findings 4 Continous donor site local anaesthetic infusion for the prevention of persistent postoperative pain after iliac crest bone graft harvesting; Summary of findings 5 Continous intravenous local anaesthetic infusion for the prevention of persistent pain after breast cancer surgery
Regional anaesthesia for the prevention of persistent postoperative pain three or more months after surgery
We report an inclusive evidence synthesis (Data synthesis/inclusive model), whereby we synthesize outcomes observed at different follow‐up intervals (Analysis 1.1; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Figure 6). We used only the latest available follow‐up time point for each study included in the analysis (Data synthesis/inclusive model). We compared our results with the classical (frequentist) evidence synthesis stratified by follow‐up interval as in the previous versions of this review (Andreae 2012) (Analysis 2.1; Analysis 2.3; Analysis 2.4;Analysis 2.5Analysis 2.6; Analysis 2.7; Analysis 2.8). A census of included participants grouped according to surgery is in Appendix 8. We presented the data in 'Summary of findings' tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5), for persistent pain after thoracotomy, breast cancer surgery, caesarean section subgroups, intravenous local anaesthetic infusion and for local infiltration to reduce the risk of persistent pain at the donor site after iliac crest bone graft harvesting.
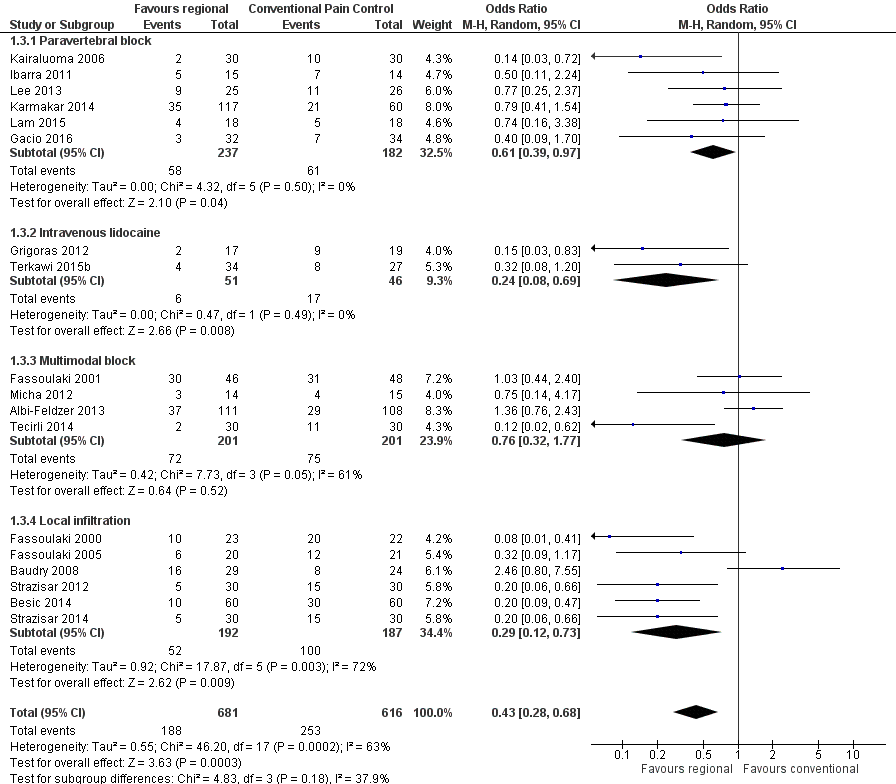
Forest plot of comparison 1. Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), outcome 1.3, PPP three to 12 months after breast cancer surgery
1. Thoracotomy
In an inclusive analysis summarized in (summary of findings Table for the main comparison), including the latest possible time point for each study for an overall estimate of effect, we found an overall benefit to regional anaesthesia for preventing persistent post‐thoracotomy pain (Analysis 1.1). This analysis included a total 499 participants from seven studies (Can 2013; Comez 2015; Ju 2008; Katz 1996; Liu 2015; Lu 2008; Senturk 2002) and found an overall effect clearly favouring regional anaesthesia, with an OR of 0.52, (95% CI 0.32 to 0.84, P = 0.008). The I² statistic, (measuring between‐study heterogeneity), was 14%, indicating little statistical heterogeneity between the studies pooled. Limiting the analysis only to those five studies (Can 2013; Comez 2015; Ju 2008; Lu 2008; Senturk 2002) that had employed epidural anaesthesia favoured regional anaesthesia even more (OR 0.41, 95% CI 0.25 to 0.67), without changing the inferences.
Including 499 participants in seven studies, the NNTB for the subgroup thoracotomy is 7 with a 95% CI 4 to 23, for an assumed corresponding risk of 0.5. High risk of bias from missing data across a number of included studies reduced our confidence in the findings. However, the risk of detection bias was low in the included studies on PPP after thoracotomy. Cryotherapy can arguably cause neuropathy (Ju 2008; Mustola 2011), and is clinically different from conventional pain therapy. Liu 2015, used continuous wound infiltration instead of the epidural analgesia employed in all the other included studies. To perform a sensitivity analysis, we excluded Ju 2008 or Liu 2015, or both; while this reduced I2, the statistical heterogeneity observed, the exclusions did not alter the inferences. In other words, the resulting change in confidence intervals are not clinically relevant.
Stratified analysis
We compared this with a classical (frequentist) analysis and pooled five studies on regional anaesthesia for the prevention of PPP after thoracotomy in 428 participants with dichotomous outcomes at three months after thoracotomy (Analysis 2.1). This resulted in an OR 0.70 (95% CI 0.40 to 1.20) favouring regional anaesthesia, but the results are imprecise leaving doubts as to their clinical relevance (Can 2013; Comez 2015; Ju 2008; Liu 2015; Lu 2008). Excluding Liu 2015, the only study employing wound infiltration instead of epidural analgesia, resulted in similar inferences (OR 0.60, 95% CI 0.35 to 1.02, P = 0.06). We pooled these same four studies (Can 2013; Comez 2015; Ju 2008; Lu 2008) plus one more (Senturk 2002), with dichotomous pain outcomes at six months after thoracotomy including data from 370 participants (Analysis 2.1). This resulted in OR 0.39 (95% CI 0.24 to 0.63), strongly favouring regional anaesthesia (P = 0.0001). Only one study, Ju 2008, an insufficient number for meta‐analysis, reported outcomes at 12 months in 77 participants, but results were inconclusive with an OR of 0.56 (95% CI 0.23 to 1.39). Similarly, only one small study (Katz 1996) reported outcomes at 20 months in 23 participants, showing no benefit for the intervention with an OR 1.17 (95% CI 0.22 to 6.08).
2. Cardiac surgery
We did not conduct any meta‐analysis of the three studies in cardiac surgery (Chiu 2008; Dogan 2016; Vrooman 2015), due to very high statistical heterogeneity (I2 = 83%), possibly due to different regional anaesthesia modalities employed. Chiu 2008 employed a continuous wound infusion, parasternal blocks were utilized in Dogan 2016, while Vrooman 2015 used lidocaine patches.
3. Breast cancer surgery
In our inclusive analysis of overall effect (Analysis 1.3; summary of findings Table 2; Figure 6), we included 18 studies (Albi‐Feldzer 2013; Baudry 2008; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Gacio 2016; Grigoras 2012; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013; Micha 2012; Strazisar 2012; Strazisar 2014; Tecirli 2014; Terkawi 2015b) and 1297 participants, which resulted in an overall treatment effect (OR 0.43, 95% CI 0.28 to 0.68) suggesting a clear benefit of regional anaesthesia (P = 0.0003). The inferences were not affected whether or not we included the study on plastic surgery of the breast (Bell 2001), or the study investigating intravenous infusions of local anaesthetics (Terkawi 2015b). (As an aside, Bell 2001 randomized participants to receive local anaesthetic infiltration of one breast, while the other side was infiltrated with placebo. Absorbed systemic lidocaine might have attenuated the development of PPP on the untreated side, leading to a diminished signal). We observed substantial heterogeneity among included studies (I2 = 63%). Limiting the studies to those six studies (participants = 419) that investigated paravertebral block as the intervention (Gacio 2016; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013), still favoured regional anaesthesia (OR 0.61, 95% CI 0.39 to 0.97; NNTB 11), and reduced the statistical heterogeneity to zero (I2 = 0%). Including 1297 participants in 18 studies, the NNTB for the subgroup breast cancer surgery is 7 with a 95% CI 6 to 13, for an assumed corresponding risk of 0.3.
This review was not planned as a comparison of different regional anaesthesia modalities and it is problematic to make inference by a crude subgroup stratification as in (Analysis 1.3). We will plan an a priori‐designed network analysis and meta‐regression for our next review update (Andreae 2015c; Andreae 2018; Thompson 2002).
Stratifed analysis
We compared this inclusive analysis with the stratified classical (frequentist) analyses by follow‐up interval; we pooled 11 studies on regional anaesthesia for breast surgery with dichotomous pain outcomes at three months postoperatively (Albi‐Feldzer 2013; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Grigoras 2012; Karmakar 2014; Lee 2013; Strazisar 2012; Strazisar 2014; Tecirli 2014), including a total of 966 participants (Analysis 2.3). Their evidence synthesis (OR 0.34, 95% CI 0.19 to 0.61) favoured regional anaesthesia (P = 0.0003). However, an I² statistic of 72% suggested considerable statistical heterogeneity.
Similarly, we pooled nine studies on regional anaesthesia for breast surgery with dichotomous pain outcomes at six months postoperatively (Bell 2001; Fassoulaki 2005; Gacio 2016; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Micha 2012; Terkawi 2015b), including a total of 515 participants (Analysis 2.3). The result strongly favoured regional anaesthesia (OR 0.56, 95% CI 0.37 to 0.84; P = 0.005; I2 = 0%). For a more conservative estimate, we had included the only one of the seven studies that investigated plastic surgery of the breast (Bell 2001), which has a different pathologic mechanism of persistent pain after breast cancer surgery, and the study investigating intravenous infusion of local anaesthetics (Terkawi 2015b); however, the inferences were the same with or without inclusion of these studies (OR 0.56, 95% CI 0.37 to 0.87). The results at six months are much less heterogeneous (I² statistic = 0%).
Finally, we present the pooled results of two studies on regional anaesthesia with dichotomous pain outcomes at 12 months after breast cancer surgery (Baudry 2008; Kairaluoma 2006), including 113 participants in total (Analysis 2.3), but caution that these studies are highly heterogeneous, both statistically (I² statistic = 88%), and clinically, as one utilized local infiltration (Baudry 2008), and the other paravertebral block (Kairaluoma 2006). In Baudry 2008, the experimental treatment failed to reduce the severity of immediate postoperative pain and the results at 12 months did not favour regional anaesthesia, with an OR of 2.46, and wide confidence interval which crosses the midline (95% CI 0.80 to 7.55). Kairaluoma 2006, with improved immediate pain control in the experimental group, however, did strongly favour the experimental intervention, with an OR of 0.14 (95% CI 0.03 to 0.72).
4. Caesarean section
In an inclusive analysis (Analysis 1.4), evaluating the overall effect across all time points, we included four studies (Bollag 2012; Lavand'homme 2007; Loane 2012; Shahin 2010), totaling 551 participants, but excluding O'Neill 2012, which had zero events in both arms (Deeks 2011). The results strongly favoured the use of regional anaesthesia for the prevention of PPP after caesarean section, with an OR of 0.46 (95% CI 0.28 to 0.78, P = 0.004) and little heterogeneity at both the study and subgroup level (I2 = 0% for both); the NNTB for caesarean section is 19 with a 95% CI (14 to 49) for an assumed corresponding risk of 0.1 (summary of findings Table 3).
We performed an inclusive analysis (Analysis 1.5) evaluating two studies reporting continuous outcomes on 110 participants but it was inconclusive (pooled SMD 0.14 (95% CI ‐0.34 to 0.61) (McKeen 2014; Singh 2013). Neither study demonstrated a clear improvement in immediate postoperative pain control or a reduction of the risk of persistent postoperative pain.
Stratified analysis
We again compared the results of our inclusive analysis with a conservative stratified analysis, where we pooled two studies after caesarean section (Pfannenstiel incision), including 137 participants with dichotomous pain outcomes at three months postoperatively (Bollag 2012; Loane 2012) but excluding O'Neill 2012, which had zero events in both arms (Deeks 2011) (Analysis 2.4). Evidence synthesis resulted in an OR of 1.09 (95% CI 0.39 to 3.07), suggesting no benefit of regional anaesthesia. Both of these studies (Bollag 2012; Loane 2012), used transversus abdominis plane blocks, with single‐shot interventions suggesting relative clinical homogeneity, which is complemented by the lack of statistical heterogeneity (I² statistic = 0%) in this analysis. We did not pool one study in this analysis (O'Neill 2012), as there were no events in either arm (making the OR undeterminable). The Cochrane Handbook for Systematic Reviews of Interventions suggests the standard practice in this instance is to exclude these studies from a meta‐analysis (Deeks 2011).
We pooled three studies after caesarean section (Pfannenstiel incision), including 492 participants (Bollag 2012; Lavand'homme 2007; Shahin 2010), with dichotomous pain outcomes at six months postoperatively (Analysis 2.4). Their analysis resulted in an OR of 0.44 (95% CI 0.26 to 0.74), clearly favouring regional anaesthesia at this follow‐up interval. Bollag 2012 administered transversus abdominus plane block, Lavand'homme 2007 used continuous postoperative wound irrigation, and Shahin 2010, peritoneal instillation, both as single‐shot interventions. The interventions were clinically heterogeneous, and one must be cautious when interpreting this evidence synthesis. However, all three studies individually favoured regional anaesthesia.
We decided not to include two studies in our analysis above (Bamigboye 2013; Kindberg 2009), because they studied chronic pelvic pain (Bamigboye 2013) and dyspareunia (Kindberg 2009) as their outcomes after postpartum surgical repair. These conditions are materially different from persistent postoperative pain, our primary outcome. The pre‐existing pain in Bamigboye 2013 and the nonelective traumatic nature of the surgical intervention in Kindberg 2009 led the authors ultimately to exclude the studies from the review. However, a sensitivity analysis including those two studies did not alter the inferences.
5. Iliac crest bone graft
We performed the inclusive analysis (Analysis 1.6; summary of findings Table 4) to synthesize the effect of local anaesthesia on PPP after iliac crest bone grafting across all available time points, and included three studies with a total of 123 participants (Barkhuysen 2010; Gundes 2000; Singh 2007). This analysis could not include Blumenthal 2005, which reported only continuous outcomes. The overall OR for the effect was 0.20 (95% CI 0.04 to 1.09, P = 0.06), with an I2 statistic demonstrating moderate heterogeneity, but was inconclusive.
We were able to include one additional study (Blumenthal 2005), in a Bayesian analysis (Appendix 6). We could not include one study reporting no pain outcome (O'Neill 2014). We described the approach separately (Andreae 2013b). We pooled four RCTs with 159 participants with continuous (Blumenthal 2005), or dichotomous (Barkhuysen 2010; Gundes 2000; Singh 2007), pain outcomes at 3, 6 and 12 months after iliac crest bone graft harvesting in our Bayesian evidence synthesis. Results favoured continuous infusion of the donor site with local anaesthetic after iliac crest bone graft harvesting with an OR 0.1, (95% Bayesian credible intervals (BCI 95%) 0.01 to 0.59); NNTB 3 (BCI 95% 2 to 10). Clinical inferences were unaffected by the minor changes in effect estimates (OR 0.12, BCI 95% 0.02 to 0.63; NNTB 3, BCI 95% 2 to 10), whether we included a fifth non‐randomized observational study (Brull 1992), as proposed in Andreae 2013b, or not.
Stratifed analysis
No classical (frequentist) analysis was possible for the effects of local anaesthesia on PPP following iliac crest bone graft, as there were only three studies that met our inclusion criteria, one with available data at three months (Gundes 2000), one with data at 12 months (Barkhuysen 2010), and one other study with available data at 55 months postoperatively (Singh 2007). Two additional studies in the iliac crest bone graft surgical subcategory met the inclusion criteria, but reported only continuous pain data (Blumenthal 2005) or no pain outcome (O'Neill 2014). The study at three months (Gundes 2000), included a total of 45 participants and found that perioperative wound instillation of bupivacaine decreased postoperative pain, with an OR of 0.14 (95% CI 0.02 to 0.86). At almost four years postoperatively, one study with 20 participants (Singh 2007) also found that wound irrigation with local anaesthetic reduced chronic pain after iliac crest bone graft, with an OR 0.03 (95% CI 0.00 to 0.68). However, local infiltration of bupivacaine showed no clear reduction in persistent postoperative pain in another study at 12 months (Barkhuysen 2010).
6. Limb amputation
We did not pool two studies investigating the effect of epidural anaesthesia on chronic pain (phantom limb pain) after limb amputation at six months (Karanikolas 2006; Katsuly‐Liapis1996). PPP may be different from phantom limb pain and timing of nociception may be much more important for the latter (Karanikolas 2006). Pooling groups of participants receiving epidural analgesia during different pre‐, intra‐ and postoperative intervals may be seen as arbitrary and controversial. We did not pool these studies in Analysis 1.7 and Analysis 2.5 for these reasons. We excluded two studies on pre‐amputation epidural analgesia (Bach 1988; Nikolajsen 1997) for pseudo‐randomization, as discussed in Appendix 9.
7. Laparotomy
We did not pool data from two studies with data at six months on 189 laparotomy participants (Analysis 1.8; Analysis 2.6), because the I² statistical estimate of 82% and 90%, respectively suggested excessive statistical heterogeneity.
The study on epidural anaesthesia for laparotomy for major gynaecological surgery (Katz 2004), provided insufficient evidence to reject the null hypothesis of no effect with an OR of 0.81 (95% CI 0.35 to 1.88) at six months, while the study on thoracic epidural anaesthesia for colonic resection (xiphopubic incision) (Lavand'homme 2005), favoured regional anaesthesia with an OR of 0.04 (95% CI 0.01 to 0.22) at six months and OR of 0.08 (95% CI 0.01 to 0.45) at 12 months. We can only hypothesize that the more effective pain control in Lavand'homme 2005, compared to the no‐improved‐pain‐control in the immediate postoperative period in the experimental group in Katz 2004 might explain the heterogeneity. Alternatively, differences in surgical specialties may explain this heterogeneity.
8. Hernia repair
We did not pool data for our inclusive analysis (Analysis 1.9), including only the six‐month time point for Mounir 2010 and the 12‐month time point for Kurmann 2015, not synthesizing the data on hernia repairs, because statistical heterogeneity at both the study and subgroup level was deemed excessive with an I2 statistic of 93%.
Stratified analysis
We did not pool two studies after inguinal hernia repair, including 389 hernias (Kurmann 2015; Mounir 2010), with outcome data at three months postoperatively (Analysis 2.7). An I2 statistic of 93% suggested marked heterogeneity; one study used participants while the other used hernias as unit of analysis. Both studies employed infiltration locally or into the wound, with a single shot post‐incision. However, Mounir 2010 used spinal anaesthesia, whereas Kurmann 2015 employed either spinal or general anaesthesia, at the request of the participant. The OR for Mounir 2010, using spinal anaesthesia with wound infiltration was 0.01 (95% CI 0.00 to 0.15) at three months and 0.01 (95% CI 0.00 to 0.09) at six months. In contrast, the OR of 2.61 (95% CI 0.80 to 8.48) at three months for (Kurmann 2015), favoured conventional postoperative analgesia over local infiltration. Notably, Kurmann 2015 could not show a clear and precise improvement in pain in the immediate postoperative period, while pain was improved immediately postoperatively in Mounir 2010.
9. Prostatectomy
We pooled two studies after prostatectomy that utilized regional anaesthesia with pain outcomes at three months postoperatively (Brown 2004; Gupta 2006), including a total of 150 participants. While only one of the two studies on prostatectomy collected dichotomous outcomes (Brown 2004), both reported continuous outcome data, in the form of the Short Form Health Survey (SF‐36) (Analysis 1.10). The pooled standard mean difference was inconclusive with a SMD of 0.06 (95% CI ‐0.26 to 0.38) not suggesting any benefit of regional anaesthesia (P = 0.71). Both studies reported outcomes at the same time point, (three months after surgery), thus approach and results are the same using the inclusive or the classical analysis.
10. Hysterectomy
We performed an inclusive analysis on the effect of the intervention on PPP in hysterectomy, pooling 297 participants from three studies (Purwar 2015; Sprung 2006; Wodlin 2011) performed across the above named time points (Analysis 1.11). Each study recorded the pain data as the bodily pain subcomponent of the Short Form Health Survey (SF‐36) questionnaire, which is a continuous outcome, and thus we used the mean difference as the outcome measure. The results remained inconclusive, with an overall mean difference of 1.70 (95% CI ‐1.06 to 4.46), with little heterogeneity (I2 statistic across both study and subgroup level = 0%). We performed classical analysis (Analysis 2.8) for the effects of regional or local anaesthesia on PPP after hysterectomy at three months (Purwar 2015; Sprung 2006). There were 135 participants included in the analysis, which yielded a mean difference of 1.90 (95% CI ‐1.23 to 5.02), which is inconclusive.
11. Additional comparisons
We performed an additional analysis of the effect of intravenous local anaesthesia on persistent pain after breast surgery (summary of findings Table 5); breast cancer surgery was the only surgical subgroup which has been studied thus far (Analysis 1.3.2). Two studies, one with outcomes at three months (Grigoras 2012), and one with outcomes at six months (Terkawi 2015b), and a total of 97 participants, were included in this evidence synthesis, demonstrating a meaningful benefit of the use of intravenous local anaesthetics in preventing persistent postsurgical pain in breast surgery (OR 0.24, 95% CI 0.08 to 0.69, P = 0.008).
One study on the use of regional anaesthesia for the prevention of pain after repair of pectus excavatum in children and young adults met the inclusion criteria for our review, but we were unable to include it in the primary analysis as it was the only study of its surgical subgroup (Weber 2007). Due to the rare incidence of pain in this study, the effect of epidural anaesthesia on PPP was inconclusive at both three months (OR 0.32, 95% CI 0.01 to 8.26) and six months (inestimable due to 0 events) postoperatively. We also report on a single study (Paxton 1995), that favoured local injection of bupivacaine to the vas deferens for pain after vasectomy, with an OR 0.02 (95% CI 0.00 to 0.33). Finally, we report on one study performed on plastic surgery of the breast (Bell 2001), excluded from the rest of the breast surgery subgroup as the nature of plastic surgery and the population studied are likely quite different. The results of this small study (Bell 2001), did not show a benefit to local infiltration of the wound in this subgroup at six months, with an OR 1.80 (95% CI 0.21 to 15.41).
12. Extended perioperative nociception
When we excluded single‐shot interventions to test if continuous prolonged antinociception was more effective in reducing the risk of persistent pain after surgery, the results were unchanged because either the same or too few studies were left for meta‐analysis in each surgical subgroup.
13. Anaesthesia modality
While we explored the influence of anaesthesia modality on risk reduction afforded by regional anaesthesia in sensitivity analysis, the small number of studies precluded a formal subgroup analysis of anaesthesia technique. Only epidural anaesthesia was used for thoracotomy, limb amputation and laparotomy. For other surgical interventions, studies investigated a variety of regional anaesthesia techniques (Appendix 7), with the marked diversity especially in breast surgery possibly explaining the observed heterogeneity of effect.
14. Adjuvant therapy
We examined studies employing adjuvant therapy. Because they investigated surgeries of different body parts (Fassoulaki 2005; Lavand'homme 2005), we did not pool the data (Data synthesis). A separate Cochrane Review on pharmacological interventions to prevent PPP has recently been completed (Chaparro 2013).
15. Adverse effects and long‐term sequelae after regional anaesthesia
Reporting of adverse effects and long‐term sequelae after regional anaesthesia (e.g. permanent nerve damage) was mostly anecdotal; they were not our primary or secondary outcomes and we report them only for completeness. Three studies systematically compared adverse effects between the experimental and the control groups, but these studies and the collected data sets were too heterogeneous for meta‐analysis. Details are listed in Appendix 10.
Sensitvity analysis of model assumptions
We had decided a priori to use the random‐effects model for evidence synthesis regardless of the observed I² statistic, because we anticipated clinically relevant heterogeneity and felt that the absence of observed proof for heterogeneity would be no proof for homogeneity.
Discussion
Summary of main results
Of the 63 studies identified, we pooled the data from 41 studies, enrolling a total of 3143 participants in our inclusive analysis. Follow‐up was for 1331 participants at three months, 1443 participants at six months, 326 participants at 12 months, and 43 participants at 20 or more months after surgery (Appendix 8), favouring regional anaesthesia for the prevention of persistent pain after surgery after thoracotomy, breast cancer surgery, caesarean section and iliac crest bone graft harvesting as detailed below.
Inclusive analysis
Our inclusive evidence synthesis (Data synthesis/inclusive analysis), is presented in five summary of findings tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5).
Thoracotomy
Including 499 participants in seven studies (Can 2013; Comez 2015; Ju 2008; Katz 1996; Liu 2015; Lu 2008; Senturk 2002), with outcomes between 3 and 18 months, results favoured regional anaesthesia for thoracotomy with an OR of OR of 0.52 (0.32 to 0.84), leading to a NNTB of 7, 95% CI (4 to 23) (Analysis 1.1) (summary of findings Table for the main comparison).
Cardiac surgery
We did not conduct any meta‐analysis of the three studies in cardiac surgery (Chiu 2008; Dogan 2016; Vrooman 2015), due to a very high statistical heterogeneity (I2 = 83%), possibly due to different regional anaesthesia modalities employed (Chiu 2008 employed a continuous wound infusion, parasternal blocks were utilized in Dogan 2016, while Vrooman 2015 used lidocaine patches).
Breast cancer surgery
For breast cancer surgery, based on 1297 participants in 18 studies (Albi‐Feldzer 2013; Baudry 2008; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Gacio 2016; Grigoras 2012; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013; Micha 2012; Strazisar 2012; Strazisar 2014; Tecirli 2014; Terkawi 2015b), we estimated the NNTB for breast cancer surgery as 7 with a 95% CI of 6 to 13 (summary of findings Table 2), calculated from (OR 0.43, 95% CI 0.28 to 0.68) (Analysis 1.3; Figure 6).
Caesarean section
For caesarean section (Analysis 1.4), evaluating the overall effect across all time points, we included four studies (Bollag 2012; Lavand'homme 2007; Loane 2012; Shahin 2010), totaling 551 participants. The results strongly favoured the use of regional anaesthesia for the prevention of PPP after caesarean section with an OR of 0.46 (95% CI 0.28 to 0.78). The NNTB for caesarean section is 19 with a 95% CI (14 to 49) with an assumed corresponding risk of 0.1 (summary of findings Table 3).
Illiac crest bone graft harvesting
Bayesian evidence synthesis (Data synthesis/Bayesian Evidence Synthesis), of data from 159 participants enrolled in four RCTs (Barkhuysen 2010; Blumenthal 2005; Gundes 2000; Singh 2007), favoured continuous infusion of the donor site with local anaesthetic for the reduction of PPP risk after iliac crest bone graft harvesting with an OR 0.1 (BCI 95% 0.01 to 0.59); NNTB 3 (BCI 95% 2 to 10) (Andreae 2013b), but our frequentist analysis (Analysis 1.6), (unable to include the study Blumenthal 2005, reporting only continuous outcomes) was inconclusive, pooling data from three studies (Barkhuysen 2010; Gundes 2000; Singh 2007), including a total of 123 participants (OR 0.20, 95% CI 0.04 to 1.09) (summary of findings Table 4).
Other surgical subgroups, interventions, continuous pain outcomes and results in children
We did not pool the studies investigating local or regional anaesthesia after limb amputation (Karanikolas 2006; Katsuly‐Liapis1996), laparotomy (Katz 2004; Lavand'homme 2005), or hernia repair (Kurmann 2015; Mounir 2010), as the sparse study data were clinically and statistically too heterogeneous (Analysis 1.7; Analysis 1.8; Analysis 1.9).
The inclusive analysis of two studies (Brown 2004; Gupta 2006), reporting continuous outcomes for prostatectomy were inconclusive, with a SMD of 0.06 (95% CI ‐0.26 to 0.38) (Analysis 1.10), as were those pooling three studies (Purwar 2015; Sprung 2006; Wodlin 2011), for hysterectomy, with a MD of 1.70, 95% CI (‐1.06 to 4.46) (Analysis 1.11). A subgroup comparison pooling two studies (Grigoras 2012; Terkawi 2015b), with 97 participants showed a statistically meaningful benefit of intravenous local anaesthetics in reducing the risk of persistent postsurgical pain after breast surgery with an OR of 0.24 (95% CI 0.08 to 0.69) (Analysis 1.3.2), and a NNTB 4 95% CI (3 to 11) (summary of findings Table 5).
We included only one RCT in children and adolescents undergoing pectus excavatum repair; this study was inconclusive (Weber 2007). A single study favoured local injection of bupivacaine to the vas deferens for pain after vasectomy, with an OR 0.02 (95% CI 0.00 to 0.33) (Paxton 1995). The results of one small study on local infiltration of the breast for plastic surgery did not show a benefit to local infiltration of the wound in this subgroup at six months, with an OR 1.80 (95% CI 0.21 to 15.41) (Bell 2001).
Classical stratified analysis
Classical (frequentist) evidence synthesis pooling studies separately at different follow‐up intervals within the same surgical subgroup led to sometimes disparate, contradictory results. For thoracotomy, evidence synthesis at three months of data from five studies (Can 2013; Comez 2015; Ju 2008; Liu 2015; Lu 2008), with a total of 428 participants favoured epidural anaesthesia with an OR 0.70 but failed to reach statistical significance with a 95% CI from 0.40 to 1.20 (Analysis 2.1); in contrast at six months, data from five studies (Can 2013; Comez 2015; Ju 2008; Lu 2008; Senturk 2002), (including four studies with outcomes at three months), with 370 participants favoured epidural anaesthesia for the prevention of persistent postoperative pain at six months (OR 0.39, 95% CI 0.24 to 0.63) (Analysis 2.1). At all time points, the four studies completed at different institutions and in several countries (China and Turkey) were remarkably homogeneous in their estimates of effect measure (I2 statistic = 0% and 19%).
Likewise, in the breast cancer surgery subgroup, statistical and clinical heterogeneity was notable for the outcomes observed at three months after surgery, but much less for outcomes observed six months after surgery, with both comparisons clearly favouring regional anaesthesia. In the most conservative analysis limiting our analysis only to the two studies (Ibarra 2011; Kairaluoma 2006), using paravertebral block for breast cancer surgery at six months, evidence synthesis favoured the intervention (OR 0.37, 95% CI 0.14 to 0.94; NNTB 5; analysis shown in the previous version of this review (Andreae 2012)). Also, for example after caesarean section (Analysis 2.4), pooled effect estimates including data from 492 participants in three studies (Bollag 2012; Lavand'homme 2007; Shahin 2010), with outcomes at six months showed a strong and statistically meaningful effect (OR 0.44, 95% CI 0.26 to 0.74). However, pooling data from three studies reporting outcomes at three months after caesarean section did not favour regional anaesthesia (OR 1.09, 95% CI 0.39 to 3.07). The same studies showed different results at different follow‐up intervals (Bollag 2012; Loane 2012; O'Neill 2012).
This emphasizes the utility and need for an inclusive analysis (Data synthesis/inclusive analysis) and for more advanced (Bayesian) modelling in evidence synthesis (Andreae 2013b), reflecting the hierarchical, nested structure of interventions and outcome reporting:
-
at the very least, results in some subgroups can inform estimates of between‐study heterogeneity in other subgroups and
-
taking into account the correlation of effects observed at subsequent follow‐up intervals can lead to better estimation of the credible intervals of the pooled effect estimates.
Clinical and statistical heterogeneity of effects
While there is consistent evidence favouring regional anaesthesia for the prevention of persistent pain after surgery across different but not all surgical subgroups, regardless of which approach we chose for the analysis, we observed important statistical heterogeneity, possibly explained by 'null bias', clinical heterogeneity or differences in follow‐up intervals or attrition (Incomplete outcome data (attrition bias) between studies and diversity in the follow‐up intervals used for many of the other subgroups (Appendix 7). We failed to completely explain the observed disparity in the effect estimates for outcomes reported at different follow‐up intervals: for example after caesarean section (Analysis 2.4). The same was true to a lesser extent after thoracotomy (Analysis 2.1), where the pooled effect estimate confidence interval touched the midline at three months but not at six months in our classical (frequentist) analysis.
As in our first review (Andreae 2012), we noted a pattern at the study level, in that if pain control was not improved in the immediate postoperative period, persistent postoperative pain was less likely to be improved at three, six or twelve months (e.g. Baudry 2008; Can 2013; Ju 2008; Karmakar 2014; Kurmann 2015; Loane 2012). This may be an example of ‘null bias’ due to interventions being insufficiently well delivered (Higgins 2011a; Woods 1995). On the other hand, 'null bias' may simply reflect the clinical reality that providers with different training and skill levels provide regional anaesthesia of variable quality.
On one hand, especially in the breast surgery subgroup, (as illustrated by Figure 6, ordered by regional anaesthesia modality), local infiltration consistently failed to reduce the risk of persistent postoperative pain (Baudry 2008; Bell 2001), and as mentioned often failed to have an effect in the immediate postoperative period. At first sight, this seems to contradict the finding that intravenous administration of lidocaine did reduce the risk of persistent postoperative pain in two studies (Grigoras 2012; Terkawi 2015b), and evidence synthesis of their data favoured intravenous lidocaine over control (OR 0.24, 95% CI 0.08 to 0.69; participants = 97; I2 = 0%). We had planned to include studies that administered local anaesthetics systemically in our initial protocol (Andreae 2008), because we felt there is a physiological rationale for effect several months later (Strichartz 2008). We hypothesize that the lack of effect observed in the infiltration study (Bell 2001), is the result of systemic absorption of local anaesthetics, which would attenuate the effect in the untreated breast and diminish the effect difference observed between the breast infiltrated versus non‐infiltrated.
Surgical and anaesthetic complications were too sparsely and inconsistently reported for any conclusions to be drawn from the data included in this review. It is probable that large observational studies would be more suited to accurately estimating these risks, particularly the rare but serious risk of persistent long‐term neurological injuries after regional anaesthesia (Brull 2007; Schnabel 2010).
Overall completeness and applicability of evidence
Participants
Most included studies were performed in university settings. Other than this limitation, the inclusion and exclusion criteria did not limit the applicability of the results to people in the community. We deplore the dearth of paediatric studies (Weber 2007). On a cautionary note, there is still insufficient evidence to extrapolate the effect of one regional anaesthesia technique to another. For example, with our data on epidural anaesthesia for thoracotomy and on paravertebral block for breast cancer surgery, we cannot conclude that paravertebral blocks prevent PPP after thoracotomy.
Interventions
When we limited our evidence synthesis to almost identical regional techniques for very similar surgical interventions (epidural anaesthesia for thoracotomy or paravertebral blocks for breast cancer surgery) (data shown in the previous version of this review (Andreae 2012)), heterogeneity of effect measures was clearly reduced (Figure 6). Some may take the stance that pooling studies using different techniques, different adjuvants, even different local anaesthetic agents is never appropriate. A sceptical reader may consider different regional anaesthesia techniques or different surgical interventions clinically too diverse to justify pooling in a meta‐analysis (Deeks 2011). Others may argue that such evidence synthesis is warranted (and this type of clinical heterogeneity is immaterial) and that effective pain control in the immediate postoperative period would be a better criterion to include or exclude studies. We were not comfortable to base our decision to pool or not solely on the observed statistical heterogeneity, not least because lack of evidence for heterogeneity obviously constitutes no proof for homogeneity. Results of our evidence synthesis were indifferent to choosing a classical or more inclusive approach and suggested that regional anaesthesia reduces persistent postoperative pain after breast surgery, thoracotomy, caesarean section and iliac crest bone graft harvesting.
Comparator
Our review compared local and regional anaesthesia to conventional pain control (Appendix 1). Only one study (Lavand'homme 2005) compared the effects of the localized (for example wound infiltration) versus the systemic (for example intravenous) administration of local anaesthetics on PPP (Strichartz 2008). There is insufficient evidence to support or refute the notion that systemically administered local anaesthetics are equally effective in reducing the risk of persistent pain after surgery (Lavand'homme 2005; Strichartz 2008; Vigneault 2011), but there is evidence that intravenous local anaesthetics are also effective in reducing the risk of persistent pain after (breast cancer) surgery (Analysis 1.3).
Outcomes and follow‐up intervals
Outcomes were reported at three, six and 12 months, and beyond. We compared our inclusive analysis (which pooled studies reporting outcomes at different intervals) with a classical approach (only pooling outcomes reported at similar follow‐up intervals) (Data synthesis); we also built a novel Bayesian hierarchical model, which first pooled outcomes observed at subsequent intervals in the same study to a study‐level pooled estimated, which we then used to inform the group‐level estimate. The inclusive analysis and the Bayesian approach gave more consistent and coherent results than the classical stratified evidence synthesis, (grouping studies strictly by time to follow‐up), reminding us that meta‐analysis results are contingent on modelling choices in any approach (Deeks 2011).
Dichotomous outcomes were reported by most studies. While neither optimal nor comprehensive, dichotomous outcomes are meaningful and easy to understand for people, physicians, payers, politicians and the public alike; in other words, the media, congress aides and insurance administrators will find it easier to comprehend the benefit of regional anaesthesia when outcomes are expressed simply as a 'pain versus no pain' alternative. Many continuous outcome measures of chronic pain represent not just similar scales measuring the same outcome, but rather, different dimensions of the human pain experience that hence cannot be pooled easily by meta‐analysis. We acknowledge that the dichotomous outcomes used in our review fall short of a comprehensive assessment of the full impact of PPP on peoples' quality of life (Turk 2006).
The summary statistics extracted from the included studies did not provide the detail required to differentiate between mild and severe disabling PPP six months after surgery (Gewandter 2015). Mild versus severely disabling PPP may make an important difference (Kehlet 2006) for the individual. However, persistent pain after thoracotomy can decrease function even at low levels of pain (Gottschalk 2006). Considering the impact of even minor pain on quality of life (Gottschalk 2006; MacRae 2008), we feel that the prevention of minor PPP after thoracotomy or breast cancer surgery is clinically meaningful; this is even more so after minor or benign elective interventions like caesarean section, vasectomy, lumpectomy or iliac bone graft harvesting. Similar to responder analysis, the state of the art for the evaluation of interventions for chronic pain (Dworkin 2009a), our dichotomous effect measure is also appropriate to investigate if regional anaesthesia reduces the risk of PPP.
To judge the clinical meaningfulness of regional anaesthesia we must weigh its risks and costs against short‐term benefits, such as enhanced recovery and improved immediate pain control (Dworkin 2009a; Gottschalk 2006), plus the reduced risk for persistent postsurgical pain suggested by our evidence synthesis. Long‐term sequelae secondary to regional anaesthesia are better studied in registries, then in RCTS and meta‐analysis (Jeng 2010). The risk of regional anaesthesia is deemed very low (Brown 1995; Jeng 2010; Neal 2008; Schnabel 2010). An overall assessment of the clinical usefulness of regional anaesthesia should probably be reserved for a Cochrane Review overview.
Quality of the evidence
The 'Risk of bias' graph gives an overview of risk of bias in the included studies (Figure 2), detailed in the methodological quality summary (Figure 3). We noted several important limitations in the quality of the evidence. The nature of the interventions made participant blinding effectively impossible. Hence, performance bias may weaken the conclusions of our review. The placebo effect may be particularly strong for pain outcomes and remains unknown for long‐term outcomes. Several studies employed adjuvants only in the experimental group, potentially introducing bias, although this did not affect the pooled results for the breast cancer surgery subgroup and was not pertinent for the thoracotomy subgroup. Our conclusions are considerably weakened by high risk of bias due to incomplete outcome data, high risk of selection bias due to lack of allocation concealment and high risk of performance bias due to incomplete participant blinding across a number of the included studies (Hewitt 2005).
Influence of attrition and follow‐up interval on effect size
The included studies investigating long‐term outcomes after regional anaesthesia tended to vary in the follow‐up intervals at which they collected and reported outcomes (Appendix 7). By pooling studies with disparate outcome reporting, we greatly increased our power, because more studies and more data are available for inferences. However, this could lead to bias, if the (estimation of the) effect of the intervention were associated with the duration of follow‐up or with attrition; the attrition is likely to increase with the duration of the follow‐up period. Several reasons for a biased estimate are conceivable.
-
PPP might slowly subside with time; this would lead to lower estimates of the prevalence of PPP at later follow‐up visits, which would bias the estimates of the effects of regional anaesthesia on PPP towards the null, because both the treatment and the control group prevalence would be diminished.
-
Attrition might have a similar effect of biasing the effect estimates towards the null, simply by decreasing the sample size of available observed outcomes.
-
Attrition might however bias the effect estimates in unforeseeable ways, if loss to follow‐up were associated with the outcomes, the intervention, or other predictors of effect or risk factors for poor outcome (PPP). Indeed, it is very well conceivable that people with persistent pain are more likely to be retained in a study; people with chronic painful symptoms are more likely to continue to follow‐up and see their physician than those who have no complaints and hence no reason to attend subsequent visits. This increased probability to keep people with pain in the study (and to loose people who no longer have persistent pain), could lead to a (spurious) increase in the observed prevalence of persistent pain in the control or the treatment group and hence to false estimates of effect, even when the intervention is not (as) effective.
To refute this concern, we explored the association of attrition and follow‐up duration with effect size estimation graphically in an attrition effect size plot; we are unaware of any description of a similar graphical test, especially in the context of meta‐analysis. The resultant graph (Figure 4; Levene 2015), does not suggest any correlation of effect size estimation with follow‐up or attrition to our best judgement. Effect sizes at later follow‐up visits sometimes lead to lower and sometimes to higher estimates of effect. Loss to follow‐up leads to higher effect size estimates in some and to lower estimates in other studies, without any apparent trend. This absence of evidence to reject this null hypothesis (no association between attrition and effect), while there is no proof of lack of association, reassures us regarding our decision to pool studies with disparate follow‐up intervals or attrition (Data synthesis/inclusive analysis; Effects of interventions/inclusive analysis).
We compared our inclusive analysis with the approach taken in the previous version of this review, a classical meta‐analysis stratified by follow‐up interval (Andreae 2012); the classical approach produced contradictory results with strong evidence at one follow‐up interval and inconclusive results at another; sometimes the same studies showed conflicting results at subsequent follow‐up intervals. We feel that this is likely the result of the generally small study size leading to variability in effect estimates and therefore a priori planned to pool the studies across follow‐up periods to obtain more robust and consistent results (Data synthesis/inclusive analysis). As discussed above, we found no evidence in our graphical exploration that attrition and follow‐up duration bias effect estimates (Figure 4; Levene 2015). We also compared our analysis with a Bayesian hierarchical model and described the results elsewhere in more detail (Andreae 2015). While we obtained similar inferences in our Bayesian model, we found the estimates of the credible intervals for the OR to change substantially with our modelling choices. Classical meta‐analysis may underestimate the between‐study variability for small numbers of studies, making estimates for the confidence intervals less reliable when they rely only on a small number of studies (Cornell 2014; Song 2012).
The statistical and clinical heterogeneity in some subgroups, the dependence of the estimates of effects on model choices or the duration of follow‐up, high risk of bias from incomplete outcome data and lack of participant blinding across a number of included studies may lead sceptical readers to question the strength of the evidence favouring regional anaesthesia for the prevention of persistent pain after surgery; the variability of results is in part explained by the small size of the included studies, which some consider a risk of bias in its own right (Moore 2013).
Potential biases in the review process
Reporting and selection bias
Not all outcome data were available for inclusion (Figure 1; Results of the search; Assessment of reporting biases; Appendix 11). This potentially introduced bias in our review and may reflect publication bias. A formal analysis of publication bias by using a funnel plot or the test proposed by Egger 1997 was precluded by the small numbers of studies found in most subgroups and their similar sizes. Even though we feel that the funnel plot for breast surgery (Figure 5) is inconclusive for publication bias, we acknowledge the possibility of underlying publication bias, as we were clearly unable to include data of all identified studies as detailed in Other potential sources of bias.
Predefining subgroups based on surgical interventions, pooling studies across subsequent follow‐up intervals and identification of studies with high risk of null bias effectively reduced unexplained effect size variability, but failed to explain all statistical heterogeneity. Our results were robust in different models used in the analysis, but are undeniably contingent on model assumptions. For several subgroups study design and reporting disparity were deemed clinically too heterogeneous for classical evidence synthesis.
Additionally, though we attempted to conduct a comprehensive search, the 12 studies currently awaiting classification may be a source of potential bias.
Agreements and disagreements with other studies or reviews
Two previous narrative reviews were rather sceptical as to the potential of regional anaesthesia for the prevention of PPP (Kehlet 2006; MacRae 2008), but did not quote all the evidence analysed in this review. We are only aware of one new attempt to synthesize the evidence on regional anaesthesia for the prevention of chronic pain after surgery (Terkawi 2015a). He investigated the prevention of persistent postoperative pain after breast cancer surgery but only pooled studies employing paravertebral block. The three available RCTs reported outcomes at disparate endpoints. His evidence synthesis was inconclusive, favouring the intervention at some but not all follow‐up intervals studied (Terkawi 2015a). Several (major) studies are underway on regional anaesthesia for PPP (ISRCTN46621916; Liew 2011; Michael 2014; NCT01626755), plus one study where this is likely to be an important, albeit not the primary outcome (NCT00418457).
The effects of intravenous lidocaine several months after surgery are remarkable and match findings from another excluded study in spine surgery (Farag 2013). Another Cochrane Review on pharmacotherapy to prevent PPP in adults was published before the second included study (Terkawi 2015b) became available and hence did attempt an evidence synthesis for this outcome (Analysis 1.3.2) (Chaparro 2013).
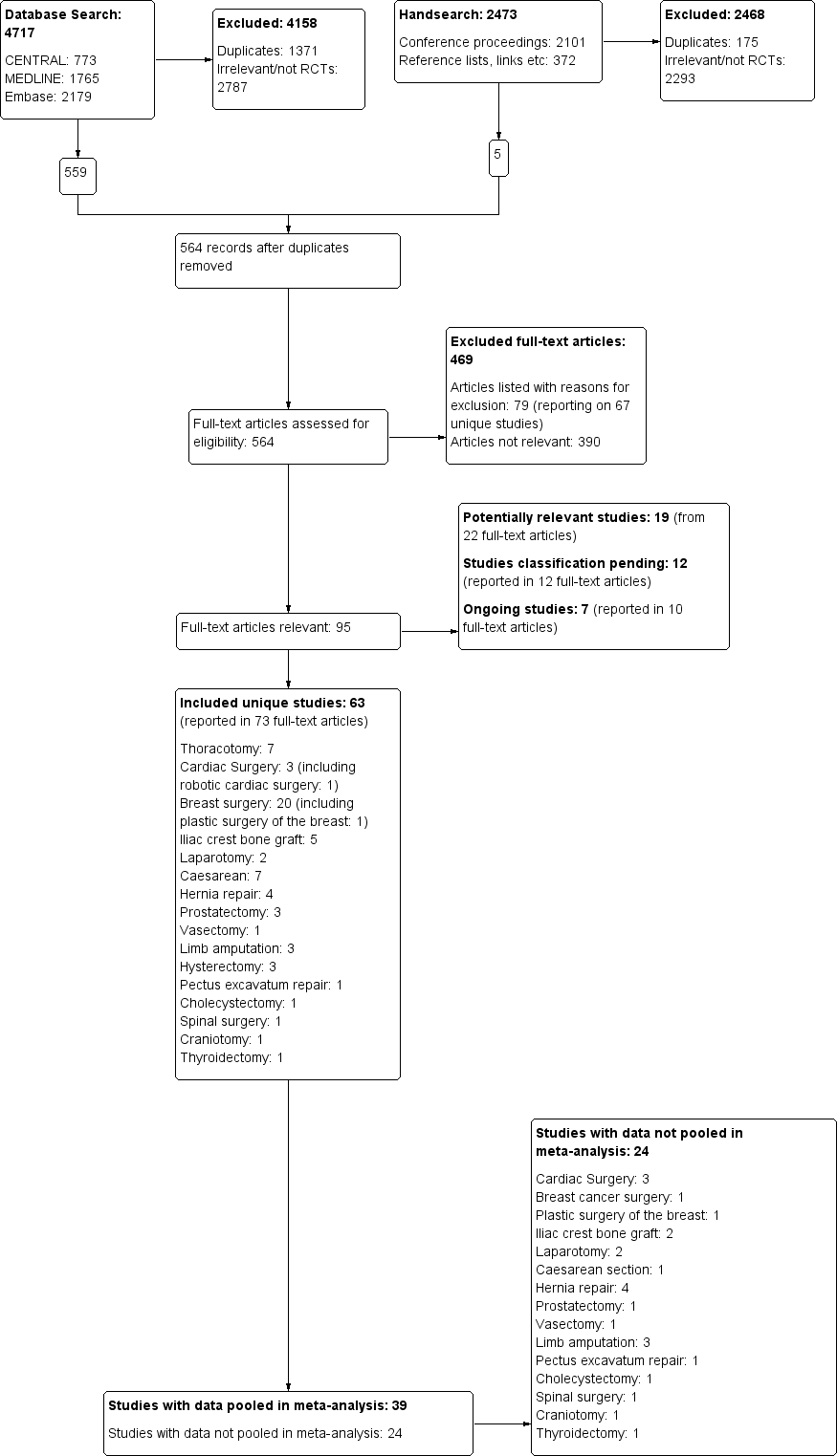
The study flow diagram documents the search and selection process. We included 63 studies. We were able to pool data from 39 of the 63 included studies in our inclusive analysis; data from 24 studies were not available or otherwise could not be pooled (Appendix 11).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Methodological quality summary: review authors' judgements about each methodological quality item for each included study
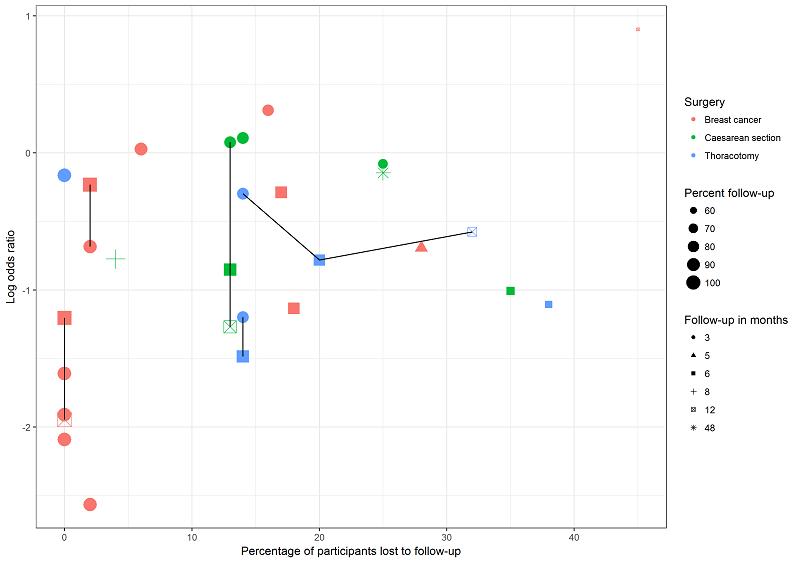
This graph plots attrition versus effect size (log odds ratio) for studies investigating regional anaesthesia for the prevention of persistent pain after thoracotomy (blue), breast surgery (pink) and caesarean section (green). Symbol size decreases with attrition. Repeated follow‐ups within one study are linked with a black line. We are unable to discern any association between attrition, follow‐up time and effect measure; this lends support to our decision to pool studies reporting outcomes at different follow‐up intervals and with different attrition.

The funnel plot for breast surgery including all outcomes at any follow‐up interval for all breast surgery studies is inconclusive for publication bias.

Forest plot of comparison 1. Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), outcome 1.3, PPP three to 12 months after breast cancer surgery

Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 1 PPP three to 18 months after thoracotomy.
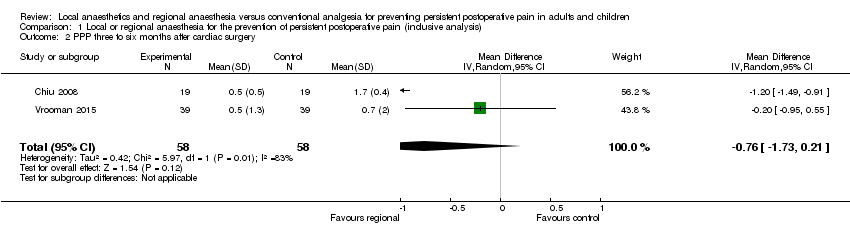
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 2 PPP three to six months after cardiac surgery.
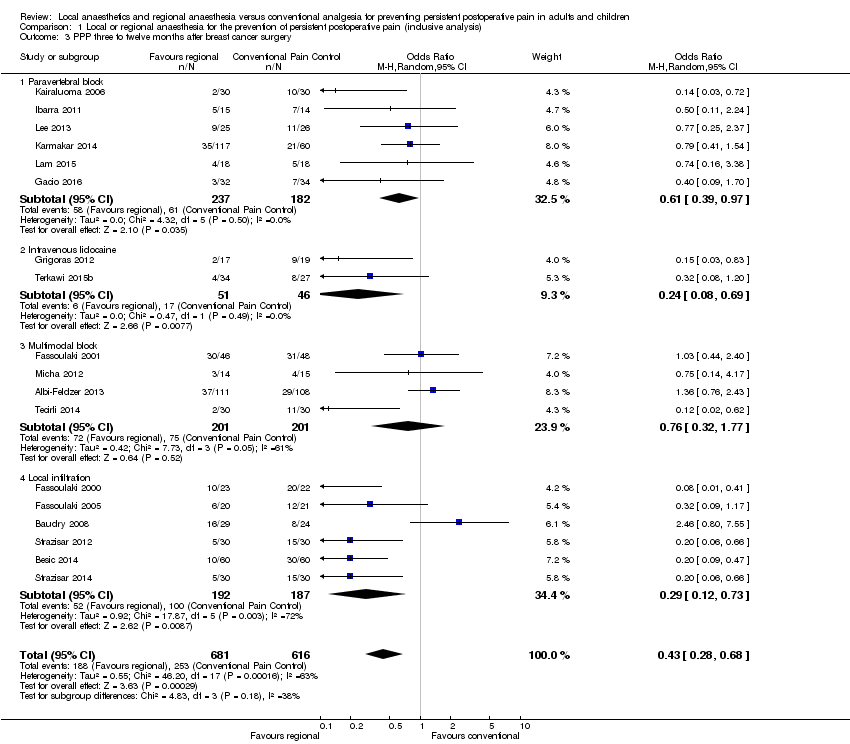
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 3 PPP three to twelve months after breast cancer surgery.

Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 4 PPP three to eight months after caesarean section.

Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 5 Pain score three to six months after caesarean section.

Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 6 PPP three to 55 months after Iliac crest bone graft.

Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 7 PPP six to 12 months after amputation.
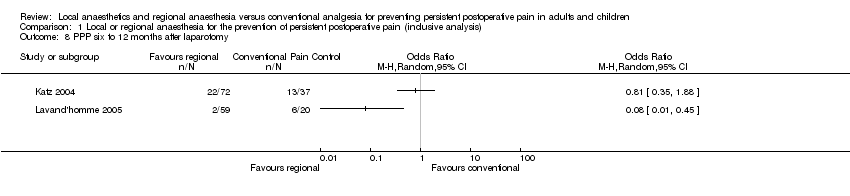
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 8 PPP six to 12 months after laparotomy.

Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 9 PPP three to 12 months after hernia repair.
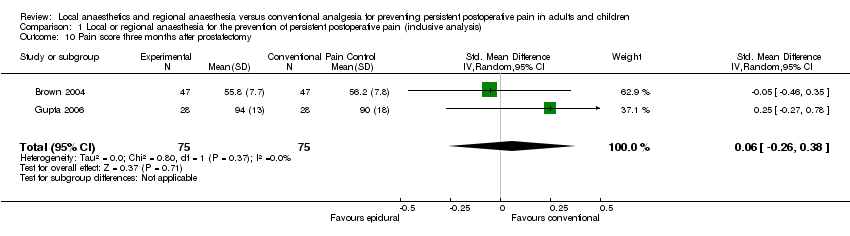
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 10 Pain score three months after prostatectomy.

Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 11 SF‐36 bodily pain score at three to six months after hysterectomy.

Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 1 PPP after thoracotomy.

Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 2 PPP after cardiac surgery.

Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 3 PPP after breast cancer surgery.

Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 4 PPP after caesarean section.

Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 5 PPP after amputation.
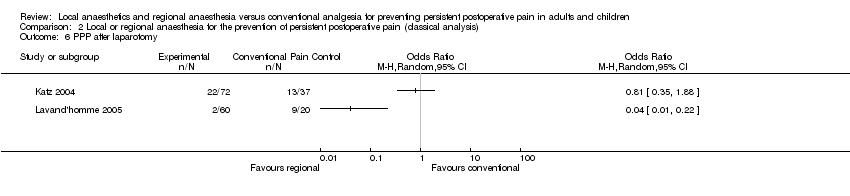
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 6 PPP after laparotomy.
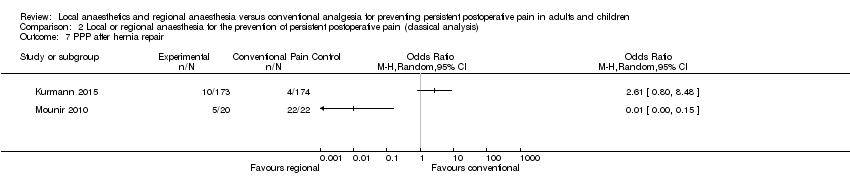
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 7 PPP after hernia repair.

Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 8 PPP after hysterectomy.
| Should thoracic epidural anaesthesia or conventional pain control be used to prevent persistent pain after open thoracotomy | ||||||
| Patient or population: people undergoing open thoracotomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional pain control | Thoracic epidural anaesthesia | |||||
| Persistent pain 3 to 18 months after thoracotomy (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 18 months after surgery.) | Study population | OR 0.52 (0.32 to 0.84) | 499 | ⊕⊕⊕⊝ | All studies investigated persistent pain after open thoracotomy. The results cannot be extended to video‐assisted thoracotomy or other (minimally invasive) surgeries of the chest. The five of the seven included studies using thoracic epidural anaesthesia showed the strongest effect. The results cannot be extended to other interventions like paravertebral blocks. Conventional pain control with opioids and NSAID was the comparator. Event rates of persistent pain after thoracotomy were reported between 25% to 65% Regional anaesthesia may prevent persistent (chronic) pain after open thoracotomy in one out of seven people treated, thoracic epidural anaesthesia in one out of five people treated. | |
| 525 per 1000 | 332 per 1000 | |||||
| Low | ||||||
| 250 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 500 per 1000 | 310 per 1000 | |||||
| Adverse effects of epidural anaesthesia ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of epidural anaesthesia were not systematically reported and due to their low frequency are better investigated in patient registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1While outcome observers' blinding was described, study participants were not blinded; this is acceptable because participant and provider blinding is difficult in regional anaesthesia. | ||||||
| Should regional anaesthesia or conventional pain control be used to prevent persistent pain following breast cancer surgery | ||||||
| Patient or population: women with breast cancer undergoing elective surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional pain control | Paravertebral block | |||||
| Persistent pain 3 to 12 months after breast cancer surgery (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 12 months after surgery.) | Study population | OR 0.43 (0.28 to 0.68) | 1297 | ⊕⊕⊝⊝ | Conventional pain control with opioids and NSAID was the comparator. Event rates of persistent pain after breast cancer were reported around 30%. Pooling all studies, regional anaesthesia may prevent persistent pain after breast surgery in one out of every seven women. Limiting the analysis to paravertebral block, the number of women needed to treat for one person to benefit was 11. | |
| 427 per 1000 | 239 per 1000 | |||||
| Low | ||||||
| 200 per 1000 | 95 per 1000 | |||||
| High | ||||||
| 600 per 1000 | 387 per 1000 | |||||
| Adverse effects of paravertebral block for breast cancer surgery | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of regional anaesthesia after breast surgery were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded quality of evidence by one level because conclusions may be considerably weakened by performance bias, shortcomings in allocation concealment, considerable attrition and incomplete outcome data. | ||||||
| Should local or regional anaesthesia be used for the prevention of chronic pain after caesarean section | ||||||
| Patient or population: women after caesarean section Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Local or regional anaesthesia | |||||
| Persistent pain 3 to 8 months after caesarean section (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 8 months after surgery.) | Study population | OR 0.46 | 551 participants | ⊕⊕⊕⊝ | Event rates of persistent pain after caesarean section are reported around 10%. The number of women needed to be treated for one woman to benefit from regional anaesthesia after caesarean section was 19. | |
| 179 per 1000 | 91 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 100 per 1000 | 49 per 1000 | |||||
| Adverse effects of local or regional anaesthesia ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of local or regional anaesthesia after caesarean section were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The results are based on only four, mostly smaller studies. Meta‐analysis results based on small numbers tend to overestimate the effects. | ||||||
| Should continuous donor site local anaesthetic infusion or conventional pain control be used for the prevention of persistent postoperative pain after iliac crest bone graft harvesting | ||||||
| Patient or population: people after iliac crest bone graft harvesting Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Continous donor site local anaesthetic infusion | |||||
| Persistent pain 3 to 55 months after iliac crest bone graft harvesting (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 55 months after surgery) | Low | OR 0.20 | 123 | ⊕⊕⊝⊝ | We accepted study author classification of the presence of persistent postoperative pain. Some assessed only pain vs no pain, others pain and dysaesthesia vs none. Event rates of persistent pain after iliac crest bone graft harvesting were reported between 20% to 40% and was assumed to be around 30%. | |
| 200 per 1000 | 48 per 1000 | |||||
| Moderate | ||||||
| 400 per 1000 | 118 per 1000 | |||||
| High | ||||||
| 600 per 1000 | 231 per 1000 | |||||
| Adverse effects of continuous local anaesthetic infusion ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of regional anaesthesia after iliac crest bone graft harvesting were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The results are based on only three small studies. Meta‐analysis results based on small numbers tend to overestimate the effects. Including an additional RCT with continuous outcomes in a Bayesian evidence synthesis further strengthens the evidence favouring the intervention (Blumenthal 2005). | ||||||
| Should continuous intravenous local anaesthetic infusion or conventional pain control be used for the prevention of persistent pain after breast cancer surgery | ||||||
| Patient or population: women with breast cancer undergoing elective surgery Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Continous intravenous local anaesthetic infusion | |||||
| Persistent pain 3 to 6 months after breast cancer surgery (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 6 months after surgery.) | Study population | OR 0.24 | 97 | ⊕⊕⊕⊝ | Event rates of persistent pain after breast cancer surgery ranged in this population between 20% to 40%. One in three women benefited on average from continuous intravenous infusion of local anaesthetics after breast cancer surgery. | |
| 370 per 1000 | 123 per 1000 | |||||
| Low | ||||||
| 200 per 1000 | 57 per 1000 | |||||
| High | ||||||
| 600 per 1000 | 265 per 1000 | |||||
| Adverse effects of continuous local anaesthetic infusion ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of intravenous infusion of local anaesthetics after breast cancer surgery were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded quality of evidence by one level because conclusions may be considerably weakened by the small number of studies included. These two studies are however consistent and of high methodological quality. Still, meta‐analysis results based on small numbers tend to overestimate the effects. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PPP three to 18 months after thoracotomy Show forest plot | 7 | 499 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.32, 0.84] |
| 2 PPP three to six months after cardiac surgery Show forest plot | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.73, 0.21] |
| 3 PPP three to twelve months after breast cancer surgery Show forest plot | 18 | 1297 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.68] |
| 3.1 Paravertebral block | 6 | 419 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.97] |
| 3.2 Intravenous lidocaine | 2 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.08, 0.69] |
| 3.3 Multimodal block | 4 | 402 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.77] |
| 3.4 Local infiltration | 6 | 379 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.73] |
| 4 PPP three to eight months after caesarean section Show forest plot | 4 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.28, 0.78] |
| 5 Pain score three to six months after caesarean section Show forest plot | 2 | 110 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.34, 0.61] |
| 6 PPP three to 55 months after Iliac crest bone graft Show forest plot | 3 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.09] |
| 7 PPP six to 12 months after amputation Show forest plot | 2 | 108 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.33] |
| 8 PPP six to 12 months after laparotomy Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 PPP three to 12 months after hernia repair Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Pain score three months after prostatectomy Show forest plot | 2 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.26, 0.38] |
| 11 SF‐36 bodily pain score at three to six months after hysterectomy Show forest plot | 3 | 297 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐1.06, 4.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PPP after thoracotomy Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Three months follow‐up | 5 | 428 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.20] |
| 1.2 Six months follow‐up | 5 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.63] |
| 2 PPP after cardiac surgery Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Three months follow‐up | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.74, 0.20] |
| 3 PPP after breast cancer surgery Show forest plot | 19 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Three months follow‐up | 11 | 966 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.61] |
| 3.2 Six months follow‐up | 9 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 3.3 12 months follow‐up | 2 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.04, 10.47] |
| 4 PPP after caesarean section Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Three months follow‐up | 2 | 137 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.07] |
| 4.2 Six months follow‐up | 3 | 492 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.26, 0.74] |
| 5 PPP after amputation Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 PPP after laparotomy Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 PPP after hernia repair Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 PPP after hysterectomy Show forest plot | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐1.23, 5.02] |
| 8.1 Three months follow‐up | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐1.23, 5.02] |

