Dacriocistorrinostomía endonasal versus externa para la obstrucción del conducto nasolagrimal
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007097.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: Cochrane Eyes and Vision
2011 version
-
Designing the review: DA
-
Co‐ordinating the review: DA
-
Data collection for the review
-
Designing electronic search strategies: Cochrane Eyes and Vision Group Trials Search Co‐ordinator
-
Undertaking manual searches: DA
-
Screening search results: DA, LD
-
Organising retrieval of papers: DA, LD
-
Screening retrieved papers against inclusion criteria: DA, LD
-
Appraising quality of papers: DA, LD
-
Extracting data from papers: DA, LD
-
Writing to authors of papers for additional information: DA
-
Providing additional data about papers: DA
-
Obtaining and screening data on unpublished studies: DA
-
-
Data management for the review:
-
Entering data into RevMan: DA, LD
-
Analysis of data: DA, LD
-
-
Interpretation of data
-
Providing a methodological perspective: DA, CJM
-
Providing a clinical perspective: DA, CJM
-
Providing a policy perspective: DA, CJM
-
Providing a consumer perspective: DA, CJM
-
-
Writing the review: DA, LD, CJM
-
Providing general advice on the review: CJM
2017 update
LJ and DA screened the search results, extracted data, assessed studies for bias and wrote to trial investigators for additional information. CJM provided general advice on the review. LJ wrote the text for the update of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital National Health Service (NHS) Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
Lona Jawaheer: none known
Caroline MacEwen: none known
Deepa Anijeet: none known
Acknowledgements
Cochrane Eyes and Vision created and executed the search strategies. We thank Catey Bunce and Daniel Morris for their comments, and Iris Gordon for her assistance with searches and obtaining articles. We also thank Anupa Shah and Jennifer Evans for author support, and Gianni Virgili for his help with statistical analysis.
We are also grateful to Hsin‐wen Wu for translating Chinese reports of trials.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 24 | Endonasal versus external dacryocystorhinostomy for nasolacrimal duct obstruction | Review | Lona Jawaheer, Caroline J MacEwen, Deepa Anijeet | |
| 2011 Jan 19 | Endonasal versus external dacryocystorhinostomy for nasolacrimal duct obstruction | Review | Deepa Anijeet, Lynne Dolan, Caroline J MacEwen | |
| 2008 Apr 23 | Endonasal versus external dacryocystorhinostomy for nasolacrimal duct obstruction | Protocol | Deepa Anijeet, Lynne Dolan, Caroline J MacEwen | |
Differences between protocol and review
We prepared a 'Summary of findings' table and a GRADE assessment which was an amendment to the protocol (Anijeet 2008).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
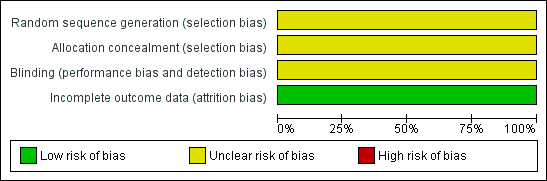
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Endonasal versus external DCR, Outcome 1 Anatomic success.

Comparison 1 Endonasal versus external DCR, Outcome 2 Subjective success.

Comparison 1 Endonasal versus external DCR, Outcome 3 Intraoperative bleeding.
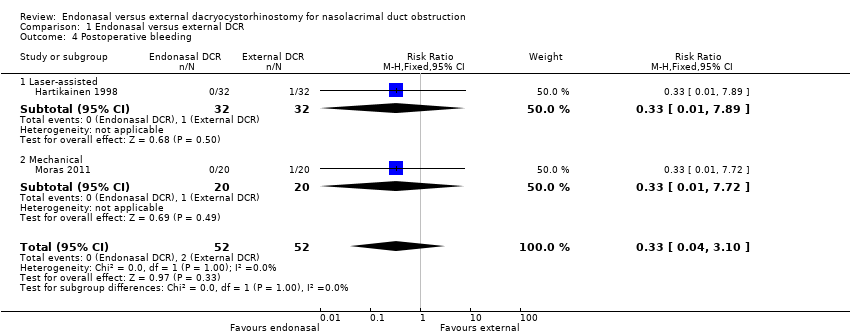
Comparison 1 Endonasal versus external DCR, Outcome 4 Postoperative bleeding.

Comparison 1 Endonasal versus external DCR, Outcome 5 Wound infection/gaping.
| Endonasal dacryocystorhinostomy (DCR) compared with external DCR for nasolacrimal duct obstruction | ||||||
| Patient or population: People with nasolacrimal duct obstruction Settings: Hospital Intervention: Endonasal DCR Comparison: External DCR | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Certainty of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| External DCR | Endonasal DCR | |||||
| Anatomic success (i.e. patent lacrimal passage after a period of at least six months after operation) | 900 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ | |||
| 621 per 1000 (468 to 828) | RR 0.69 (0.52 to 0.92) | 64 (1) | ||||
| Mechanical endonasal DCR | ||||||
| 900 per 1000 (729 to 1000) | RR 1.00 (0.81 to 1.23) | 40 | ||||
| Subjective success (i.e. resolution of symptoms of watering following surgery) | 840 per 1000 | Laser‐assisted endonasal DCR5 | ⊕⊕⊝⊝ | |||
| 588 per 1000 (428 to 815) | RR 0.70 (0.51 to 0.97) | 64 (1) | ||||
| Intraoperative bleeding | 170 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ | No cases of intraoperative bleeding reported in trial of laser‐assisted endonasal DCR | ||
| Not estimable | Not estimable | 64 (1) | ||||
| Mechanical endonasal DCR | ||||||
| 170 per 1000 (85 to 337) | RR 1.00 (0.50 to 1.98) | 40 (1) | ||||
| Postoperative bleeding | 40 per 1000 | 13 per 1000 (2 to 124) | RR 0.33 (0.04 to 3.10) | 104 (2) | ⊕⊝⊝⊝ | |
| Wound infection/gaping | 40 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ | No cases of wound infection/gaping reported in trial of laser‐assisted endonasal DCR | ||
| Not estimable | Not estimable | 64 (1) | ||||
| Mechanical endonasal DCR | ||||||
| 8 per 1000 (0 to 157) | RR 0.20 (0.01 to 3.92) | 40 (1) | ||||
| CI: confidence interval; DCR: dacryocystorhinostomy; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The assumed control risk was estimated from the control group in the included studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Anatomic success Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Laser‐assisted | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.52, 0.92] |
| 1.2 Mechanical | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 2 Subjective success Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Intraoperative bleeding Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Laser‐assisted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Mechanical | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Postoperative bleeding Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.10] |
| 4.1 Laser‐assisted | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.89] |
| 4.2 Mechanical | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 5 Wound infection/gaping Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Laser‐assisted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mechanical | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

