Irradiación parcial de la mama para el cáncer de mama precoz
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007077.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 julio 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer de mama
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
ML, BH and DF wrote the protocol.
BH extracted data, created 'Risk of bias' tables and 'Characteristics of included studies' tables, analyzed the data, wrote the results section and discussion, and responded to editorial and peer review (in consultation with ML).
ML checked the analyses, 'Risk of bias' tables, collaborated with writing the results, discussion and conclusion sections.
AS checked the extracted data and ran the search strategy.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Princess Alexandra Cancer Collaborative Group, Australia.
Declarations of interest
MH: none known.
BH: none known.
DF: none known.
AS: none known.
Acknowledgements
We thank the Princess Alexandra Hospital Cancer Collaborative Group.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Aug 30 | Partial breast irradiation versus whole breast radiotherapy for early breast cancer | Review | Brigid E Hickey, Margot Lehman | |
| 2016 Jul 18 | Partial breast irradiation for early breast cancer | Review | Brigid E Hickey, Margot Lehman, Daniel P Francis, Adrienne M See | |
| 2014 Jun 18 | Partial breast irradiation for early breast cancer | Review | Margot Lehman, Brigid E Hickey, Daniel P Francis, Adrienne M See | |
| 2008 Apr 23 | Partial breast irradiation for early breast cancer | Protocol | Margot Lehman, Brigid E Hickey, Daniel P Francis | |
Differences between protocol and review
We reported time‐to‐event data where possible for cancer‐related outcomes. We reported local relapse‐free survival (LR‐FS) rather than local relapse (LR), distant metastasis‐free survival (DM‐FS) rather than distant metastases (DM), we reported loco‐regional relapse‐free survival (L‐R R‐FS) rather than loco‐regional control (LRC) as a secondary endpoint. We added the words "elsewhere primary" to the name of the endpoint "new primary in ipsilateral breast" because this term is used in the relevant literature, in order to add clarity for the reader.
We initially indicated that we would convert doses to their biological equivalent (BED), but have in fact used equivalent dose in 2 Gy fractions (EQD2 ). This allows numerical addition of separate components of a treatment and is more readily understood by clinical radiation oncologists because it results in numbers which can be directly related to clinical experience.
We added APBI as well as PBI: modern RT techniques that reduce the treated volume allow the use of high dose per fraction to the smaller treated volume. The ongoing studies tend to use APBI, which reflects modern RT practice, making the review results more applicable.
We added blinding to assessment of risk of bias, because the lack of blinding for the primary outcome of cosmesis would be a significant cause of bias. We searched an additional database (i.e. EMBASE.com) and trial registry (WHO ICTRP) to our search strategy, and also handsearched other resources. This ensured that our searches were as comprehensive as possible, and complied with Cochrane search requirements.
We included studies which included women with ductal carcinoma in situ (RAPID) for reporting of toxicity endpoints. ELIOT used regional nodal irradiation for those women with more or more involved nodes (5% of the cohort), we excluded ELIOT from the analysis of L‐RR‐FS.
We pooled the studies in a quantitative meta‐analysis, but excluded the older studies, which used surgical, RT and systemic management practices which do not reflect current practice. We had planned sensitivity analysis based on excluding studies which used outmoded RT and surgical techniques, but as we decided to exclude them from our analysis, we did not do so. These studies were included in the previous iteration of this review, but were removed for the 2015 update.
We corrected the list and table of excluded studies so we are now compliant with MECIR guidelines, so that this list only includes studies that might reasonably be expected to be included, but which we deemed ineligible.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Breast [radiation effects];
- Breast Neoplasms [pathology, *radiotherapy, surgery];
- Combined Modality Therapy [methods];
- Disease‐Free Survival;
- Mastectomy, Segmental;
- Neoplasm Recurrence, Local [prevention & control];
- Organ Sparing Treatments [*methods];
- Radiation Dose Hypofractionation;
- Radiotherapy [adverse effects, methods];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Female; Humans;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.1 Local recurrence‐free survival.
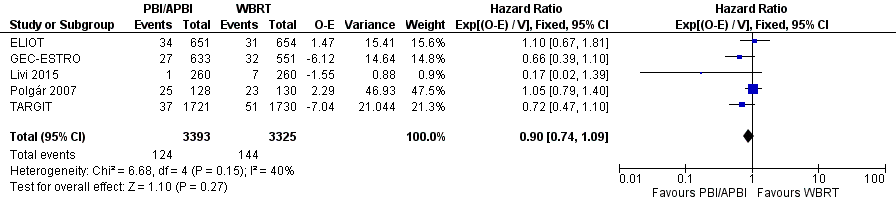
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.3 Overall survival.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.8 Cause‐specific survival.
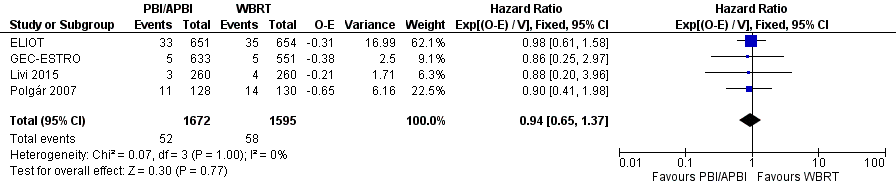
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.9 Distant metastasis‐free survival.
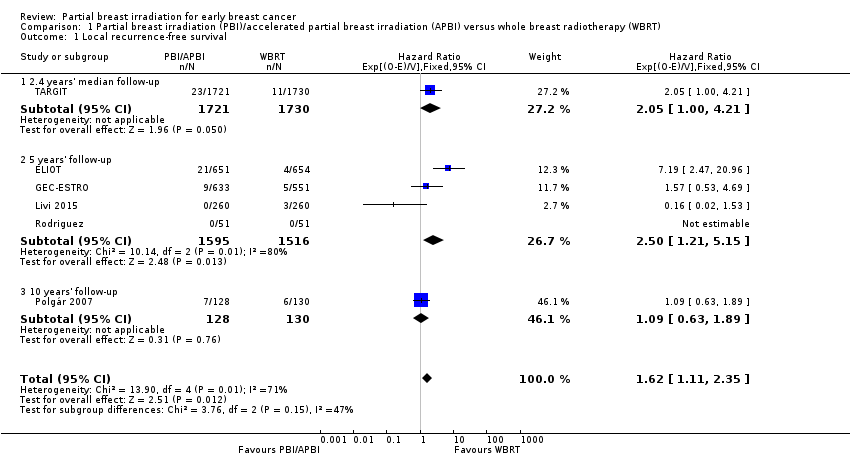
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 1 Local recurrence‐free survival.
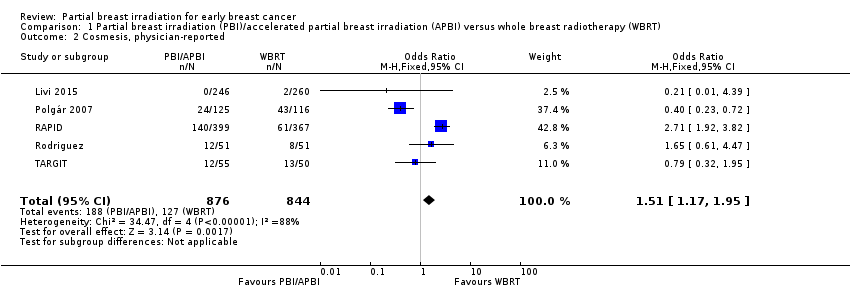
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 2 Cosmesis, physician‐reported.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 3 Overall survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 4 Acute radiotherapy (RT) skin toxicity.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 5 Late RT skin toxicity.
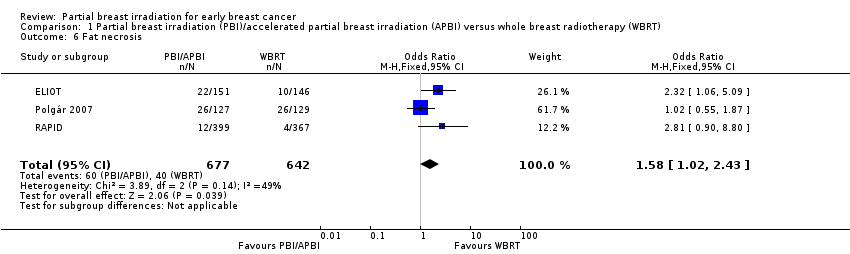
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 6 Fat necrosis.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 7 'Elsewhere primary'.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 8 Cause‐specific survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 9 Distant metastasis‐free survival.
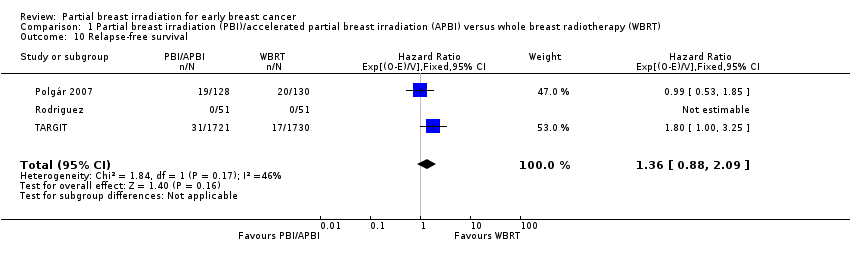
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 10 Relapse‐free survival.
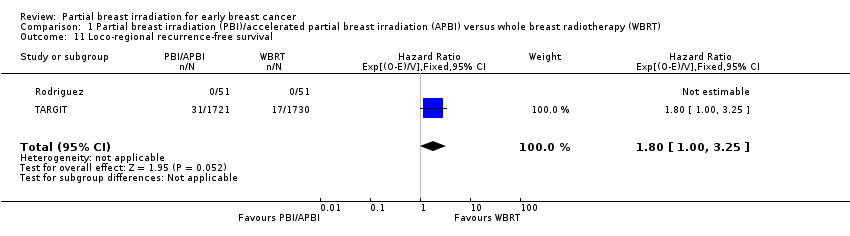
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 11 Loco‐regional recurrence‐free survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 12 Mastectomy.
| PBI/APBI for women with early breast cancer | ||||||
| Patient or population: women with early breast cancer Setting: radiotherapy centres Intervention: PBI/APBI Comparison: whole breast radiotherapy (WBRT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with WBRT | Risk with PBI/APBI | |||||
| Local recurrence‐free survival at 5 years | Study population | HR 1.62 | 6820 | ⊕⊕⊝⊝ | ‐ | |
| 10 per 10001 | 16 per 1000 | |||||
| Cosmesis assessed with 4‐point scale Follow‐up: range 29‐122 months | Study population | OR 1.51 | 1720 | ⊕⊕⊝⊝ | Cosmesis was assessed using a 4‐point scale. We reported those women with poor/fair cosmesis at final review | |
| 150 per 1000 | 218 per 1000 | |||||
| Late radiotherapy toxicity (subcutaneous fibrosis) Follow‐up: median 36 months | Study population | OR 6.58 | 766 | ⊕⊕⊕⊝ | Assessed using National Cancer Institute 3‐point scale, events were defined as: Grade II or higher toxicity Physician assessors, at 3 years' follow‐up | |
| 22 per 1000 | 128 per 1000 | |||||
| Cause‐specific survival at 5 years | Study population | HR 1.08 | 6718 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 10002 | 22 per 1000 | |||||
| Distant metastasis‐free survival at 5 years | Study population | HR 0.94 | 3267 | ⊕⊕⊕⊝ | ‐ | |
| 33 per 10002 | 31 per 1000 | |||||
| Mastectomy rate Follow‐up: range 29‐122 months | Study population | OR 1.20 | 4817 | ⊕⊕⊝⊝ | Mastectomy rate reflected both local recurrence and adverse cosmetic outcome | |
| 15 per 1000 | 18 per 1000 | |||||
| Mortality | Study population | HR 0.90 | 6718 | ⊕⊕⊕⊕ | Survival advantage from radiotherapy for breast cancer is not apparent before 15 years' follow‐up (EBCTCG 2011) | |
| 51 per 10002 | 46 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The baseline risk for the control group was calculated at the 5‐year time point from 5 studies. | ||||||
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
| Score | Definition |
| Excellent | Perfect symmetry, no visible distortion or skin changes and no visible catheter entry/exit sequelae |
| Good | Slight skin distortion, retraction or oedema, any visible telangiectasia, any visible catheter entry/exit scar or mild hyperpigmentation |
| Fair | Moderate distortion of the nipple or breast symmetry, moderate hyperpigmentation, or prominent skin retraction, oedema or telangiectasia |
| Poor | Marked distortion, oedema, fibrosis or severe hyperpigmentation |
| RTOG CTC | Grade I | Grade II | Grade III | Grade IV |
| Description | Follicular, faint or dull erythema / epilation / dry desquamation / decreased sweating | Tender or bright erythema, patchy moist desquamation / moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | Ulceration, haemorrhage, necrosis |
| RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria. | ||||
| Grade | Findings |
| 0 | No fat necrosis |
| 1 | Asymptomatic fat necrosis (only radiological or |
| 2 | Symptomatic fat necrosis not requiring medication |
| 3 | Symptomatic fat necrosis requiring medication |
| 4 | Symptomatic fat necrosis requiring surgical |
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Induration (subcutaneous fibrosis) | Increased density on palpation | Moderate increase in density, not interfering with ADL; marked increase in density and firmness on palpation with or without minimal retraction | Dysfunction interfering with ADL; very marked density, retraction or fixation | ‐ |
| Telangiectasia | Few | Moderate | Many and confluent | ‐ |
| Pain | Pain mild, not interfering with function | Moderate pain; pain or analgesics interfering with function, but not with ADL | Severe pain; pain or analgesics interfering with ADL | Disability |
| ADL: activities of daily living; NCI CTC: National Cancer Institute Common Toxicity Criteria. | ||||
| Trial | PBI/APBI dose | Fraction size (Gy) | EQD2 PBI/APBI | Control dose | Fraction size (Gy) | EQD2 Control |
| 20 Gy at surface of the applicator (attenuated to 5‐7 Gy at 1 cm) (APBI) | 80 at cavity surface 12.8 at 1 cm | 80 Gy at cavity surface 12.8 Gy at 1 cm | 40‐56 Gy/20‐28 fractions ± 10‐16 Gy boost | 2 | 40‐56 Gy ± 10‐16 Gy | |
| 30 Gy/5 daily fractions EBRT IMRT. 100% of the PTV was covered by 95% of the prescribed dose | 6 | 75 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost | 2 | 50 + 10 = 60 Gy | |
| 38.5 Gy/10 fractions bd (with 6 hour gap) Dose‐evaluation volume (that part of PTV within the breast) received 95‐107% of prescription dose | 3.85 | 74.1 Gy | 50 Gy/25 fractions or 42.5 Gy/16 fractions ± boost (10 Gy/4‐5 fractions) based on criteria such as young age or close margins, pre‐specified by centre | 2 or 2.65 | 50 or 47.1 Gy | |
| 37.5 Gy/10 fractions bd (with 6 hour gap) (APBI). PTV covered by ≥ 95% of prescribed dose, with < 105% hot spot | 3.75 | 71.22 Gy | 48 Gy/24 fractions ± 10 Gy/5 fractions boost | 2 | 48 ± 10 = 48‐58 Gy | |
| 7 × 5.2 Gy HDR (APBI) or 50 Gy/25 fractions (PBI). | 5.2 or 2 | 53.6 Gy or 50 Gy | 50 Gy/25 fractions (3D‐CRT was not used) | 2 | 50 Gy | |
| 30.3 Gy/7 fractions or 32 Gy/8 fractions HDR twice daily or 50 Gy at 0.6‐0.8 Gy/hour pulses (1 pulse per hour, 24 hours per day) PDR | 7‐8 | 41.64‐42.67 Gy | 50.0‐50.4 Gy to a reference point + 10 Gy/5 fractions boost. Electron dose was prescribed to the point of maximum dose on the beam axis (Dmax), ensuring the 85% isodose encompassed the tumour bed | 1.8‐2.0 | 48.72‐50 + 10 = 58.72‐60 Gy | |
| 21 Gy/1 fraction at 90% using 6‐9 MeV | 21 | 131.2 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost (using electrons) | 2.0 | 50 + 10 Gy | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; bd: twice daily; CT: computer tomography; EBRT: external beam radiotherapy; EQD2: equivalent dose in 2 Gy fractions; Gy: Gray; HDR: high‐dose‐rate; IMRT: intensity‐modulated radiotherapy; MeV: mega electron volt; PBI: partial breast irradiation; PDR: pulsed‐dose‐rate; PTV: planning target volume. | ||||||
| Trial | RT technique |
| Interstitial brachytherapy (88/128) EBRT using photons (40/128) | |
| intra‐operative electrons | |
| EBRT (IMRT) | |
| intra‐operative kV RT | |
| EBRT | |
| EBRT (3D‐CRT) | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; EBRT: external beam radiotherapy; IMRT: intensity‐modulated radiotherapy; RT: radiotherapy. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local recurrence‐free survival Show forest plot | 6 | 6820 | Hazard Ratio (95% CI) | 1.62 [1.11, 2.35] |
| 1.1 2.4 years' median follow‐up | 1 | 3451 | Hazard Ratio (95% CI) | 2.05 [1.00, 4.21] |
| 1.2 5 years' follow‐up | 4 | 3111 | Hazard Ratio (95% CI) | 2.50 [1.21, 5.15] |
| 1.3 10 years' follow‐up | 1 | 258 | Hazard Ratio (95% CI) | 1.09 [0.63, 1.89] |
| 2 Cosmesis, physician‐reported Show forest plot | 5 | 1720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| 3 Overall survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 0.90 [0.74, 1.09] |
| 4 Acute radiotherapy (RT) skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.09] |
| 5 Late RT skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.39] |
| 6 Fat necrosis Show forest plot | 3 | 1319 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.02, 2.43] |
| 7 'Elsewhere primary' Show forest plot | 3 | 3009 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.51, 10.41] |
| 8 Cause‐specific survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 1.08 [0.73, 1.58] |
| 9 Distant metastasis‐free survival Show forest plot | 4 | 3267 | Hazard Ratio (95% CI) | 0.94 [0.65, 1.37] |
| 10 Relapse‐free survival Show forest plot | 3 | 3811 | Hazard Ratio (95% CI) | 1.36 [0.88, 2.09] |
| 11 Loco‐regional recurrence‐free survival Show forest plot | 2 | 3553 | Hazard Ratio (95% CI) | 1.80 [1.00, 3.25] |
| 12 Mastectomy Show forest plot | 3 | 4817 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.77, 1.87] |

