Intervenciones basadas en el análisis funcional para el comportamiento desafiante en la demencia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Random assignment to intervention or control condition. The intervention was delivered through a group workshop followed by 16 in‐home treatment sessions over 12 months. The paper reports only 6 months follow‐up. | |
| Participants | 70 white and 48 African American primary caregivers (PCG) of individuals with dementia. Care recipients (CR) were required to score < 24 on MMSE, exhibit one limitation in ADLs or IADLs and display 3 problem behaviours as identified by the PCG. CR mean MMSE score was 14.53 for white participants and 10.98 for African American participants, with a mean age of 78.83. | |
| Interventions | Caregiver Skill Training Intervention based on a manual Minimal Support Condition (control) Primary aim of intervention: CR problem behaviour, CG appraisal, social support, activity, well‐being (e.g. depression & anxiety) and desire to institutionalise CR. (See Table 2) | |
| Outcomes | Revised Memory and Behaviour Problem Checklist (RMBPC) RMBPC Appraisal Leisure Time Satisfaction Measure The Center for Epidemiologic studies‐Depression Scale (CES‐D) State‐Trait Personality Inventory Desire to Institutionalise (see Table 3) | |
| Notes | Country of origin: America | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | 'Staff were not blinded to group assignment; however. intervention and assessment were never conducted by same individual'. |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Methods | Cluster randomised controlled trial. Study duration 8 months. | |
| Participants | 289 residents from 15 residential homes, of similar management structure, standards and size. Residents had to show need‐driven behaviours, which made it difficult for staff to provide them with quality care. Residents mean age was 85 years. | |
| Interventions | Caregiver training and support intervention in either: Person Centered Care (PCC) or Dementia Care Mapping (DCM) Control (Usual Care) Primary aim of the intervention: To decrease need driven dementia compromised behaviours, improve resident quality of life and reduce the use of psychotropic drugs, restraints, rates of accidents and injuries. (See Table 2) | |
| Outcomes | Cohen Mansfield Agitation Inventory (CMAI) Neuropsychiatric Inventory (NPI) Quality of life in late stage dementia (QUALID) Quality interactions schedule (QUIS) (See Table 3) | |
| Notes | Country of origin: Sydney, Australia For the purpose of this review the DCM condition was compared with usual care. Interventionist visited sites for 6 hours per day over 2 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomised at site level, using an SAS system'. |
| Allocation concealment (selection bias) | Low risk | Allocation performed by study statistician unaware of sites' identities, using a balanced incomplete‐block design, remaining sites used a complete block design. |
| Blinding of participants and personnel (performance bias) | Low risk | Used a protocol and manual. There is no report of checking treatment fidelity or adherence to the manual. Membership to the intervention or control group was masked to outcome assessors; however, it is not reported if participants and other staff members were blind to allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | 'Research assistants were trained in measurement and remained masked to group intervention by means of a signed agreement with staff and managers not to mention the intervention information' |
| Incomplete outcome data (attrition bias) | Low risk | 26 died and 4 transferred after randomisation. A further 21 died and 2 transferred after the intervention. |
| Selective reporting (reporting bias) | Low risk | Only reported total NPI score, not sub scale scores for frequency and severity. |
| Other bias | Unclear risk | Not other sources of bias identified. |
| Methods | Randomised clinical trial. Study duration 18 months. | |
| Participants | 295 care recipients (CR) with Alzheimer’s disease (AD) or other dementia syndrome, with MMSE < 24, and their family caregiver (CG), who provided a minimum of 6 months care, with four hours direct contact per day. CR mean MMSE score was 12.6, CR mean age was not reported. CGs had a mean age of 64.4, 225 were female and 70 male. | |
| Interventions | Caregiver skill (CSB) Intervention Information and Support Orientated Group Intervention (ISO) (Comparison Condition) Primary aim of intervention: Reducing emotional distress in CG & improving CG management of behaviour problems. (See Table 2) | |
| Outcomes | The Center for Epidemiologic studies‐Depression Scale (CES‐D) for CG Behaviour Management Skill Revised (BMS‐R) The Revised Memory and Problem Behaviour Checklist (RMPBC) Time to Institutionalisation (see Table 3) | |
| Notes | Country of Origin: Chicago, USA 12 weekly sessions, 5 group sessions, 7 individualised telephone contact sessions, 2 group booster sessions (6 and 12 months after enrolment) and as needed telephone contact during 12 month period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Participants were randomly assigned to treatment condition' |
| Allocation concealment (selection bias) | Low risk | Statistician generated randomised sequence of binary codes (1 or 2) for each block of 10 to 20 participants. Sequence position determined by an alphabetically ordered list of participant names within each block. Coin toss to determine group 1 or 2 as intervention or control. |
| Blinding of participants and personnel (performance bias) | Low risk | Participant assignment list and identification number exclusive to project director. Trained interviewers blind to assignment. Treatment protocol for intervention. To assure fidelity, each staff member received 40 hours training and followed a detailed manual of prescribed material for each session. Supervised implementation, corrective feedback and group sessions taped and reviewed. Intervention staff remained blind to baseline and follow‐up assessment data. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessments conducted over the telephone. Assessment of key outcomes by reviewers blind to treatment condition. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reported (23 participants terminated early, reasons included: transportation/schedule difficulties (30%), health status (22%), nursing home placement or death (13%) and other reasons/not interested (26%)). |
| Selective reporting (reporting bias) | Low risk | Only coefficients reported, however, full data set supplied by author. |
| Other bias | Low risk | No crossover or carryover effects reported. |
| Methods | Cluster randomised controlled trial with blinded assessment of outcome. Study duration: 12 months. | |
| Participants | 346 residents from 12 residential homes. The mean age of residents was 82 years. The majority of residents had a clinical dementia rating of severe. | |
| Interventions | Training and Support Intervention for nursing home staff. Control (treatment as usual) Primary aim of intervention: To reduce the proportion of residents with dementia who are prescribe neuroleptics. CG training in behavioural management techniques and person centred care, positive care planning, awareness of environmental design, ABC models, development of individualised interventions, active listening and communication and reminiscence techniques. (See Table 2) | |
| Outcomes | Cohen Mansfield Agitation Inventory (CMAI) Daily drug dosage of residents (See Table 3) | |
| Notes | Country of origin: London, Newcastle and Oxford, UK. The intervention was delivered over two days a week for 10 months by a psychologist, occupational therapist or nurse. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned' |
| Allocation concealment (selection bias) | Low risk | 'Statistician randomly assigned homes to intervention or control, stratified by region and baseline neuroleptic use. Allocations were computer generated using stratified block randomisation (fixed block size of two) with strata version 8' |
| Blinding of participants and personnel (performance bias) | Unclear risk | Statistician blinded to identification of homes. Follow‐up assessments completed by blinded research assistants. Intervention described as 'the package', however, it is not reported whether there was a manual or assessments of adherence. |
| Blinding of outcome assessment (detection bias) | Low risk | Follow‐up assessments completed by a research assistant who was not employed during the intervention period. However, the paper reports that 'because the package was designed to influence the whole care approach of staff, it is likely that the research assistant would have been able to detect which homes had received the intervention'. |
| Incomplete outcome data (attrition bias) | Low risk | All reported, some reasons reported as unknown (105 participants died, 4 moved home, 14 unknown reason). |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | No other risks identified. |
| Methods | Randomised controlled trial. Study duration: 12 months. | |
| Participants | The participants were 255 persons with Alzheimer’s disease or related disorder and their family caregiver, of which 190 were available at follow‐up. CR were to have a MMSE of < 24. Care recipients had a mean age of 80.85, with an average MMSE score of 12.05. CGs had to be at least 21 years of age, providing care for 4 hours per day for 6 months. CGs were predominantly African American with a mean age of 60.45. | |
| Interventions | Home Environmental Skill‐Building program (ESP) for family CG Control (usual care) Primary aim of intervention: CG well‐being (e.g. Mastery, skill enhancement), Burden & Distress & CR functioning (behaviour & ADL/IADL) delivered by interventionists who received 25 hours of training (See Table 2) | |
| Outcomes | Revised Memory and Behaviour Problem Checklist (RMBPC) Caregiver Burden (RMBPC) Caregiving Mastery Index Task Management Strategy Index (See Table 3) | |
| Notes | Country of origin: Philadelphia, USA 5 in home contacts, one telephone contact, Active treatment phase for the first 6 months, maintenance phase for the subsequent 6 months which consisted of 1 home contact and 3 brief telephone sessions. 12 month follow‐up data are reported in Gitlin 2005 but data reported for CR behaviour for the primary outcome measure not equivalent to the data reported in Gitlin 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised trial with comparison group. Study duration: 6 months. | |
| Participants | 272 caregivers (CG) and people with dementia (CR) with a mean age: 82.1 years, of which 220 were available at follow‐up. CR MMSE score of < 24. Caregivers had to be at least 21 years of age, English speaking and planning to live in the area for 6 months, not actively seeking a nursing home placement, managing problem behaviours and reporting upset. | |
| Interventions | Caregiver skills training in managing problem behaviours ‐ the Advanced Caregiver Training (ACT) No treatment control group Primary aim of intervention: CG confidence in managing problem behaviours and associated upset. CG, well‐being (e.g. skill enhancement, management skills, communication, perceived change and perceived benefits), burden and mood. (See Table 2) | |
| Outcomes | Incidence and frequency of problem behaviours, measured by: Agitated Behaviours in Dementia Scale ‐16 items, Revised Memory and Behaviour Problem Checklist (RMBPC) ‐ 3 items, and other behaviours ‐ families could specify other behaviours which were not listed. Caregiver upset was measured by averaging caregiver responses over all occurring behaviours, with higher scores indicating greater upset. Caregiver depression measured by CES‐D Caregiver burden measured by Zarit Burden Interview (ZBI) Caregiver change (managing care challenges, affect and somatic) ‐ Perceived Change Index (PCI) Task Managment Strategy Index Communication Index Perceived Benefits (See Table 3) | |
| Notes | Country of origin: Philadelphia, USA Occupational therapists and nurse delivered intervention.16 week active phase of 9 occupational therapy sessions and two nursing sessions (one home, one telephone) and a maintenance phase (16‐24 weeks) of three brief OT telephone contacts to reinforce strategy use. Help caregivers identify antecedents and consequences or potential modifiable triggers of the target problem behaviour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two group randomised trial. |
| Allocation concealment (selection bias) | Low risk | Stratified according to relationship (spouse vs non spouse) and randomised within each of two strata using permuted blocks. Study statistician developed a blocking number which was unknown to others. Randomisation lists and two sets of randomisation forms were prepared using opaque envelopes. The Project director randomised each participant within 48 hours of baseline interview. Project director performed randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | 10 licensed OTs and 1 nurse had 35 hours training. Treatment fidelity monitored and maintained through twice monthly meetings involving case presentations. Audiotaped 10% of home sessions for review and feedback. Documentation of contacts was kept in order to review delivery adherence. Interviewers were masked to treatment assignment. |
| Blinding of outcome assessment (detection bias) | Low risk | Interviewers masked to participants assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete outcome data addressed but specific reasons for dropout not noted, only reported as % lost to follow‐up or missed. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | None reported or determined. |
| Methods | Randomised controlled trial. Study duration: 6 weeks. | |
| Participants | 80 caregivers (CG) with a mean age of 64.4 years, providing a weekly minimum of 4 hours care to 80 care recipients (CR) with a confirmed diagnosis of Alzheimer’s disease (mean age: 77) in the mild to moderate severity range, with at least one neuropsychiatric symptom. Caregivers were mostly spouses, female and Caucasian. | |
| Interventions | Caregiver group based training intervention (Project CARE) Psychoeducational control group using similar structure to the intervention group. Primary aim: CG distress associated with CR behaviour, CG burden and CR behaviour problems. (See Table 2) | |
| Outcomes | Neuropsychiatric inventory (NPI) ‐ Severity & Distress Zarit Burden Interview (ZBI) (See Table 3) | |
| Notes | Country of origin: Boston, USA. Caregiver based multi‐component behavioural group intervention, delivered over 5‐weekly 90 minute sessions with 15 minutes individual time. The intervention was delivered in a group format (5 ‐10 members). The intervention was based on the principles of behaviour therapy and activation and designed to teach behavioural techniques for managing care recipients neuropsychiatric symptoms in the home environment. Caregivers were taught ABC behavioural analysis. The control group had a similar structure to the intervention, but consisted of only general information on aging and Alzheimer’s disease, home safety, support and techniques for improved communication. The total study duration was 6 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'We then assigned participants by block randomisation' |
| Allocation concealment (selection bias) | Low risk | 'We assigned participants by block randomisation to one of the two conditions'. Unclear as to who performed the randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Therapists had 16‐20 hours training in intervention protocols. To monitor treatment fidelity, PI consulted with therapists on a regular basis to review group sessions and assess group progress. Not all caregivers adhered to the intervention (did not submit homework). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Reported in the discussion 'It was also not possible to blind all interviewers to the caregivers treatment condition at the post‐intervention assessment' |
| Incomplete outcome data (attrition bias) | Low risk | Specific reasons for withdrawal not reported; however, the number withdrawn is recorded. (11 caregivers did not complete) |
| Selective reporting (reporting bias) | Unclear risk | NPI frequency not reported, only severity. |
| Other bias | Unclear risk | Generalisabilty to the general population difficult due to low numbers of ethnically and racially diverse individuals. |
| Methods | Randomised controlled trial. Study duration 10 weeks. | |
| Participants | 62 care recipients (CR) with a diagnosis of dementia and their co‐resident carer. Care recipients with dementia were required to be rated by their carer as mildly aggressive. Care recipient mean age was 75.95 years, with an average MMSE score of 13.3. Caregivers (CG) mean age was 68.45 and were predominantly female. | |
| Interventions | Caregiver Behaviour Management Training Programme Control group Primary aim of intervention: CR behaviour & severity and CG burden. (See Table 2) | |
| Outcomes | Rating Scale for Aggressive Behaviour in the Elderly (RAGE) Behavioural Pathology in Alzheimer's Disease Rating Scale (BEHAVE‐AD) Zarit Burden Interview (ZBI) (See Table 3) | |
| Notes | Country of origin: Kent, UK. 4 sessions over 8 weeks, providing education, ABC analysis and behavioural interventions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly allocated patients and their carers to intervention or control group' |
| Allocation concealment (selection bias) | Low risk | Randomisation was concealed from the second author. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The paper does not report the use of a manual or checking adherence to the manual. The intervention and control were conducted by the first author, only the second author was blinded to allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Second author blind to treatment allocation conducted assessments. |
| Incomplete outcome data (attrition bias) | Low risk | All reasons and number of withdrawals noted. Three patients dropped out of the trial shortly after their initial assessment: two were admitted to hospital and the third was admitted to residential care. |
| Selective reporting (reporting bias) | Low risk | All results reported. |
| Other bias | Unclear risk | Author conducted the intervention. |
| Methods | Randomised controlled trial, Pilot study. Study duration: 12 weeks. | |
| Participants | 48 patients with dementia and their family caregiver (CG). Care recipients (CR) had to be aged 65 or over and score 50 or above on the CMAI. CRs were predominantly female with a mean age of 75.8 years. Twenty had a CDR rating of mild, 17 moderate, 10 severe and 1 very severe, with an average MMSE score of 13.1. CGs were predominantly female, with a mean age of 55.6. | |
| Interventions | A home‐based Caregiver Training Programme Control (written materials only) Primary aim of intervention: To improve CG self efficacy and decrease CR problem behaviours. (See Table 2) | |
| Outcomes | Chinese version of Cohen Mansfield Agitation Inventory (CMAI) (CR Frequency of problem behaviours & CG Self efficacy). | |
| Notes | Country of origin: Northern Taiwan. 2 week in home training programme, plus telephone consultations every two weeks.The control group received educational materials and social telephone follow‐ups every two weeks. At the third week and third month assessments were conducted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned to intervention or control group' |
| Allocation concealment (selection bias) | Unclear risk | Randomised by patient registration number, odd registration numbers to intervention, even to control. The paper does not report who performed randomisation, |
| Blinding of participants and personnel (performance bias) | Low risk | 'Although caregivers knew they were in a study, they did not know whether they were in the experimental or control group'. A manual was developed by the research team as a guide for the training program. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Investigator ran the intervention, unclear as to level of blinding and who conducted assessments. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition reported (11 participants were lost to follow‐up because either the caregiver was unwilling to continue, the patient was hospitalised or the address was changed). |
| Selective reporting (reporting bias) | Low risk | All results reported. |
| Other bias | Unclear risk | None determined. |
| Methods | Randomised trial with 2 treatment and 1 control arm (for the purpose of this review the PSP condition was compared with the control condition). Study duration: 5 months. | |
| Participants | 31 family caregivers (CG) of a relative with dementia. CG had a mean age of 61.1 years and were predominantly female. Care recipients (CR) had a mean age of 80.4. | |
| Interventions | Caregiver Cognitive Behavioural Intervention (PCC) Caregiver Problem‐Solving Skills Training Intervention (PSP) Control group For the purpose of this review PSP was compared to the Control group. Primary aim of intervention: Modifying CR behavioural problems, CG stress associated with problem behaviours, CG depression and CG dysfunctional thoughts. (See Table 2) | |
| Outcomes | Memory and Behaviour Check List (MBCL) ‐ Frequency & Reaction Perception of Social Support (PSQ) Caregiver depression measured by CES‐D Perceived Stress Scale (PSS) CG dysfunctional thoughts on care (CPD) (See Table 3) | |
| Notes | Country of origin: Madrid, Spain. The paper is reported in Spanish. Our translation of this study led us to believe it was suitable for inclusion in the review as causes of behaviour were identified and hypothesis and strategies formed to alleviate the targeted behaviour. The intervention was delivered by two psychologists in one 2‐hour session a week for 8 weeks, totalling 16 hours. A post‐intervention assessment was taken after the 8 weeks, and 3 months following the end of the intervention. The total study duration was 5 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | Unsure who performed the randomisation procedure and how it was conducted. |
| Blinding of participants and personnel (performance bias) | Low risk | 'Interventionists carried out assessments, however, were unaware of membership at the time'. Due to difficulty translating the paper we were unable to establish whether the intervention has a manual or whether adherence checks were executed to ensure full delivery of the intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unable to determine due to difficulty translating the paper |
| Incomplete outcome data (attrition bias) | Low risk | Data reported. |
| Selective reporting (reporting bias) | Unclear risk | No risks identified, however, due to difficulty in translation of the paper, we have graded this as unclear. |
| Other bias | Low risk | None idenitified. |
| Methods | Randomised trial ‐ Behaviour Advisory Service compared with Usual Care. Study duration: 9 days. | |
| Participants | 71 patients with dementia and behavioural disturbance judged to be problematic with a mean age: 82.5. | |
| Interventions | Staff Training Hospital Behaviour Advisory Service Usual Care Primary aim of intervention: Modify level of patient agitation over time, appropriateness of psychotropics, length of stay, discharge destination, falls, restraint use and CG satisfaction with care provided. (See Table 2) | |
| Outcomes | Pittsburgh Agitation Scale (PAS) Medication Appropriateness Index (MAI) Discharge destination Falls Restraint use CG satisfaction with care Length of stay (See Table 3) | |
| Notes | Country or origin: South Australia Patients assessed within 24 hours of randomisation. Nurse formulated management plan with respect to non‐pharmacological strategies to help manage patients problematic behaviours, discussed the plan with ward nursing staff and provided ongoing support and education for nursing staff. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pragmatic randomised controlled trial. 'Patients were randomised' (not by ward or hospital only by patient). |
| Allocation concealment (selection bias) | Low risk | Randomisation by hospital pharmacy department using sequential sealed opaque envelopes by external person using stratified blocks. Computer generated random numbers, allocation via external person. |
| Blinding of participants and personnel (performance bias) | Unclear risk | It is not reported if a manual was used or whether checks were completed to ensure accurate delivery of the intervention. The level of blinding of participants and personnel is not reported. Adherence was not formally measured 'it is possible that, although the EPN was offering advice and providing frequent follow‐up visits to reinforce their suggestions, the ward nursing staff were not carrying out the strategies suggested'. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unsure as to the level of blinding of the EPN. Ward nurses conducted assessments unsure as to level of blinding. 'Treatment and control patients were both nursed on the same wards so it is possible that nursing staff may have picked up on useful strategies and applied them to the control group'. |
| Incomplete outcome data (attrition bias) | Low risk | All reported (4 died, 67 discharged). |
| Selective reporting (reporting bias) | Unclear risk | Only follow‐up data for the PAS is reported (in the abstract). |
| Other bias | Unclear risk | No other potential sources of bias identified. |
| Methods | Randomised controlled trial. Study duration:18 months | |
| Participants | 113 care recipients (CR) and their family caregiver (CG). CR had a mean age of 77.2 years; CG had a mean age of 63.2 years and were predominantly female. | |
| Interventions | Community Mental Health Nurses Training Intervention (CMHN) Control (usual practice) Primay aim of intervention: Training CMHNs in systematic psychosocial interventions (PSI) to help family caregivers manage behavioural changes in their relative with dementia. (See Table 2) | |
| Outcomes | The General Health Questionnaire (GHQ) The adapted‐Gilleard Problem Checklist (PC) The Hospital Anxiety and Depression Scale (HADS) The Global Deterioration Scale (GDS) (See Table 3) | |
| Notes | Country of origin: Hull, UK. 4 consecutive weekly in home visits following which CMHN exercised clinical judgment about future contact and attended in service clinical supervision with a Clinical Psychologist (Esme Moniz‐Cook) and senior nurse for the duration of the 18 month study, 2 hours, once a week for the first 6 months and once a fortnight for the following 6 months. Individual sessions were held once a month for the final 5 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Dyads (i.e. CR and CG) were randomly allocated to either condition' |
| Allocation concealment (selection bias) | Unclear risk | Randomisation procedure not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Level of blinding of participants and personnel not reported. A protocol was in place. Only two CMHNs adhered to the 4 consecutive family treatment sessions. Despite protocol‐led recommendations no relaxation or anxiety management occurred. Only two CMHNs sustained clinical supervision, noted as 'poor adherence' in the text. |
| Blinding of outcome assessment (detection bias) | Low risk | Researchers conducted baseline measures. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for withdrawal were reported (1 neighbour disengaged, 18 caregivers disengaged, 3 carers relocated, 3 spouse deceased care provided by a child). |
| Selective reporting (reporting bias) | Low risk | All outcome results at each time period reported. |
| Other bias | Low risk | Authors supervised CMHNs. |
| Methods | Randomised controlled trial. Study duration: 6 months. | |
| Participants | 105 subjects, 12 nursing and residential homes. Residents had a mean age of 83.1. Staff selected 10 residents in each home whose behavioural problems made them difficult to care for. | |
| Interventions | Staff training and Education Intervention including psychosocial management of resident's behavioural problems. Control Primary aim of intervention: To assess quality of care, resident depression and organic symptoms and resident behavioural characteristics. (See Table 2) | |
| Outcomes | Crichton Royal Behavioural Rating Scale (CRBRS) Automatic Geriatric Examination for Assisted Taxonomy (AGECAT) (depression & organic symptoms) (See Table 3) | |
| Notes | Country of origin: Manchester, UK Seven 1 hour seminars plus individual visits from a member of the hospital outreach team. An experienced psychiatric nurse then visited each residential home every week to provide support to individual staff in development of care planning skills. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Residential homes were randomised to the control or intervention group' |
| Allocation concealment (selection bias) | Low risk | 10 residential homes and 2 nursing homes were paired according to size and accreditation status. Computer generated random numbers used independently of the researchers to assign one of each pair of homes to intervention or control. Ten residents in each home were selected by staff independently of the researchers. |
| Blinding of participants and personnel (performance bias) | Low risk | Residents were unaware of carer allocation. However, 'Staff that received the training were aware of the intervention'. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The paper does not report who conducted the outcome assessments and whether they were blind to allocation. |
| Incomplete outcome data (attrition bias) | Low risk | All reported (11 died, 2 transferred and 3 withdrew consent). |
| Selective reporting (reporting bias) | Low risk | All results reported. |
| Other bias | Unclear risk | Staff who received training were aware of intervention and may have had expectations about the effects of the programme. |
| Methods | Randomised placebo controlled clinical trial. Study duration: 12 months. | |
| Participants | 149 care recipients (CR) with Alzheimer’s disease and their caregiver (CG). CRs had a mean age of 74.8 years, whilst CGs had a mean age of 65.6 and were predominantly the CR's spouses. | |
| Interventions | Caregiver Behaviour Management Techniques Intervention Haloperidol Trazodone Placebo Primary aim of intervention: To decrease CR agitated behaviours (See Table 2) | |
| Outcomes | Clinical Impression of Change (ADCS) The Consortium to Establish a Registry for Alzheimers Disease (CERAD) Behavioural Rating Scale for Dementia (BRSD) Revised Memory and Behaviour Problem Checklist (RMBPC) Cohen Mansfield Agitation Inventory (CMAI) Physical Self Maintenance (PSM) Agitated Behaviour Inventory for Dementia (ABID) Cognitive Function (MMSE) (See Table 3) | |
| Notes | Country of origin: America BMT intervention delivered over 8 weekly and 3 biweekly sessions providing information about AD, strategies for decreasing agitated behaviours, assignments and videotape training program, conducted by a therapist with masters degree. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Subjects were randomly allocated' |
| Allocation concealment (selection bias) | Low risk | 'Subjects were allocated to four study arms. Ten sites had patients randomised to medications or placebo. Eleven sites had patients randomised to medications, placebo or BMT. Treatments were assigned in randomised blocks of nine or 12'. Randomisation was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | The intervention had a protocol. Ongoing training, inter‐rater reliability checks and quality control were performed to ensure standardisation. 'To insure interviewers remained blind to treatment assignment, caregivers did not discuss any aspects of treatment with the interviewer '. |
| Blinding of outcome assessment (detection bias) | Low risk | Paper reports that in no instance was blinding compromised. Assessments conducted by blind interviewers. |
| Incomplete outcome data (attrition bias) | Low risk | The paper reports number of patients who withdrew and reasons and adverse effects (57 discontinued, major reasons for dropout included increased agitation in the trazodone arm (59%), unacceptable adverse effects in the haloperidol arm (43%), and caregiver difficulties or increased agitation in the BMT arm (35%)). |
| Selective reporting (reporting bias) | Low risk | The paper reports only total frequency score for the RMBPC but not reaction. The paper reports post‐treatment data only. |
| Other bias | Low risk | Clinicians had a treatment protocol but allowed discretion in strategies to employ and when; therefore, intervention not wholly standardised. |
| Methods | Randomised controlled trial. Study duration: 24 months. | |
| Participants | 153 community dwelling care recipients (CR) meeting criteria for Alzheimer’s disease and their caregiver (CG). CRs had a mean age of 78 years, with an average MMSE score of 16.8 and were predominantly male. CGs had a mean age of 70, and were predominantly female. | |
| Interventions | Caregiver training in behavioural management techniques with home‐based exercise program ‐ Reducing Disability in Alzheimers disease (RDAD) Control (routine medical care) Primary aim of intervention: CG management of CR problem behaviours and decreasing CR frailty and behavioural impairment. (See Table 2) | |
| Outcomes | Physical Health and Function (SF36) Affective Status ‐ Hamilton Depression Rating Scale (HDRS) & Cornell Scale for Depression in Dementia CR Physical Health & Function Revised Memory and Behaviour Problem Checklist (RMBPC) (See Table 3) | |
| Notes | Country of origin: Washington, USA. RDAD: In own home, 12 x 1 hour sessions, 2 per week for the first 3 weeks, followed by 1 for the next 4 weeks and biweekly sessions over the following 4 weeks. Followed by 3 sessions over the next 3 months conducted by health professionals experienced in dementia care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patient caregiver dyads were randomly assigned to exercise plus behavioural management techniques or routine medical care' |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was obtained from a computer program that blocked groups of 8 patients. Dyads were randomised after baseline assessment by research coordinators. |
| Blinding of participants and personnel (performance bias) | Low risk | A manual was used. Treatment adherence maintained and monitored through weekly supervision. Treatment sessions were videotaped and reviewed by independent reviewers. Unsure as to the level of blinding of other personnel and participants other than outcome assessment interviewers. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessments conducted by blind interviewers. |
| Incomplete outcome data (attrition bias) | Low risk | All reported (43 institutionalised, 2 declined to continue, 2 caregivers were ill, 2 moved, 5 caregivers declined to continue, 9 patients died and 1 caregiver died). |
| Selective reporting (reporting bias) | Unclear risk | Behavioural data not reported. |
| Other bias | Unclear risk | Training by authors |
| Methods | Randomised controlled trial. Study duration: 6 months. | |
| Participants | 95 care recipients (CR) with Alzheimer’s disease and family caregivers (CG). CR mean age: 79.95 with an average MMSE score of 14.0. CR were required to have three or more agitated or depressed behaviour problems. CG ages ranged from 22 to 91 years. CR were predominantly female. | |
| Interventions | Community Consultants Training program (STAR‐ Caregivers) Control (routine medical care) Primary aim of intervention: To train community consultants to teach CGs a systematic behavioural approach for reducing mood and behaviour problems of their CR. (See Table 2) | |
| Outcomes | Center for Epidemiologic Studies Depression Scale (CES‐D) for CG Hamilton Depression Rating Scale (HDRS) for CG Perceived Stress Scale (PSS) Caregiver Sleep Questionnaire Screen for Caregiver Burden (SCB) Short Sense of Competence Questionnaire (SSCQ) Neuropsychiatric Inventory (NPI) Revised Memory and Behaviour Problem Checklist (RMBPC) The quality of Life in Alzheimers disease (QOL‐AD) (See Table 3) | |
| Notes | Country of origin: Washington, USA. Counsultant training consisted of an initial 2 hour orientation with supervising gero‐psychologist. A standardised treatment manual that included instructions to consultants was disseminated and discussed. Consultants met the CGs in their home over 8 weekly sessions followed by four monthly phone calls. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned caregivers and care recipients to the intervention or control' |
| Allocation concealment (selection bias) | Unclear risk | The randomisation procedure is not reported; therefore, whether adequate allocation concealment was achieved is unclear. |
| Blinding of participants and personnel (performance bias) | Low risk | Follow‐up was conducted by interviewers blind to treatment assignment. Unsure as to the level of blinding of participants. A manual was used and adherence to the manual was monitored through audio taping treatment sessions and weekly supervision. (Consultants also had to successfully complete a pilot case). |
| Blinding of outcome assessment (detection bias) | Low risk | Interviewers blind to treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Reported withdrawal, however, some specific reasons are not recorded (3 care recipients hospitalised, 9 caregivers declined due to non‐specific reasons) |
| Selective reporting (reporting bias) | Low risk | The paper does not report RMBPC data for frequency at 6 months. |
| Other bias | Unclear risk | Ratings of consultant adherence not done by independent raters. |
| Methods | Randomised controlled trial. Study duration: 2 months. | |
| Participants | 31 residents and 25 staff from four assisted living residences. Residents were predominantly female, had a mean age of 85.8 years and a MMSE mean score of 16.0. The mean age of staff was 37.4 years. | |
| Interventions | Staff Training in Assisted Living Residences (STAR) based on a manual. Control ‐ usual onsite training Primary aim of intervention: Dementia specific training program to teach direct care staff to improve care and reduce problems in residents with dementia. (See Table 2) | |
| Outcomes | Geriatric Depression Scale (GDP) Clinical Anxiety Scale (CAS) Revised Memory and Behaviour Problem Checklist (RMBPC) Agitated Behaviours in Dementia (ABID) Neuropsychiatric Inventory (NPI) Short Sence of Competence Questionnaire (SSCQ) (See Table 3) | |
| Notes | Country of origin: Seattle, Washington, USA. STAR is conducted over 2 months, through 2 half day group workshops and four individualised sessions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Four assisted living residences were randomly assigned to intervention or control' |
| Allocation concealment (selection bias) | Unclear risk | Randomisation procedure not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | A training manual and protocol were used. Opportunities to discuss site specific issues that might hinder implementation or sustainability were provided. Unclear as to the level of blinding of participants and staff other than outcome assessors. |
| Blinding of outcome assessment (detection bias) | Low risk | Interviewers blind to treatment condition conducted pre‐training and post‐training assessments |
| Incomplete outcome data (attrition bias) | Low risk | No attrition to report. |
| Selective reporting (reporting bias) | Unclear risk | No RMBPC frequency data reported. Doesent state the number of participants in each group, this information had to be sought by authors. |
| Other bias | Low risk | Training by authors. |
| Methods | Randomised controlled trial | |
| Participants | 76 Care recipients with data available at 12 month follow‐up. | |
| Interventions | ||
| Outcomes | Agitated Behaviours in Dementia (ABID) (SeeTable 3) | |
| Notes | Reports the maintenance effects of Teri 2000 paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised placebo controlled clinical trial. |
| Allocation concealment (selection bias) | Low risk | 'Subjects were allocated to four study arms. Ten sites had patients randomised to medications or placebo. Eleven sites had patients randomised to medications, placebo or BMT. Treatments were assigned in randomised blocks of nine or 12'. |
| Blinding of participants and personnel (performance bias) | Low risk | 'To insure Interviewers remained blind to treatment assignment, caregivers did not discuss any aspects of treatment with the interviewer'. Clinicians had a treatment protocol. |
| Blinding of outcome assessment (detection bias) | Low risk | Paper reports that in no instance was blinding compromised. |
| Incomplete outcome data (attrition bias) | Unclear risk | Does not state dropout, however, this is reported in the previous paper. |
| Selective reporting (reporting bias) | Unclear risk | The paper only reports ABID data, however, other outcome measures were used. |
| Other bias | Low risk | No other forms of bias noted. |
| Methods | Randomised controlled trial ‐ wait list control. Study duration: 24 months. | |
| Participants | 184 dementia care recipients (CR) living in the community and their primary caregivers (CG). CR mean age was 75.72 with an average MMSE score of 14.42. Mean age of CG was 62.02. 119 completed treatment. | |
| Interventions | Caregiver Support Group Intervention (SG) Caregiver Individual Family Counselling Intervention (IFC) Wait list Control Group Primary aim of intervention: To test the effectiveness of a stress‐management approach in reducing CG stress and burden. CG changes in reports of stress, improvement in management of the CR's problem behaviours, CG increased use of social support and CG perception of treatment benefits. (See Table 2) | |
| Outcomes | Brief Symptom Inventory (BSI) Burden Interview (BI) Memory and Behaviour Problems Checklist (MBPC) Caregiver Change Interview Social Support Caregiver adequacy of support (See Table 3) | |
| Notes | Country of origin: USA Only one experimental condition offered at a time at each site (2 sites). Subjects at one site randomly assigned to either IFC or wait list, other site randomly assigned to SG or wait list. For the purposes of this review SG was compared with wait list control. The interventions were delivered over 8 sessions (8 weeks). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned to either IFC, SG or wait list control" |
| Allocation concealment (selection bias) | Unclear risk | Crossover. 1st year of study one site received intervention and then assigned to a wait list. In the 2nd year, this was reversed. Does not state actual procedure of how sites assigned, e.g. blocks |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants and staff not reported. The first author monitored sessions using audiotapes and supervision sessions to ensure that therapists implemented the treatment approach. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The paper does not report who conducted the assessments. |
| Incomplete outcome data (attrition bias) | Low risk | Dropout numbers reported, but specific reasons not reported |
| Selective reporting (reporting bias) | Low risk | One year outcome data not reported, only post‐intervention |
| Other bias | High risk | Crossover trial |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Physical activity and environmental intervention to improve sleep and agitation. | |
| Assessment of CANE measurement scale. No behavioural outcomes. | |
| Pharmacological intervention only. | |
| This paper is a systematic review. | |
| Not an RCT, the paper reports a case series using problem solving for the management of depression and agitation in long term care. | |
| Review paper of multi‐sensory therapy in psychiatric care. | |
| Snoezelen or Reminiscence therapy intervention. | |
| Snoezelen or Reminiscence intervention | |
| Snoezelen & Activity intervention | |
| Snoezelen intervention | |
| Case series. | |
| Occupational Therapy intervention | |
| Period of BPST not randomised, only pharmacotherapy part of trial randomised (information supplied by Author) | |
| Worksite based Internet multi‐media program. No behavioural component, predominantly focused on carer stress and coping | |
| Enhancing Quality of life through proving education and skills | |
| This paper reports on the maintenance effects of the CRONOS project. | |
| Not an RCT, naturalistic controlled trial with repeated measures | |
| Case Series | |
| Instructional intervention for Caregivers on bathing and specific activities. | |
| Communication improvement intervention using memory books, no behavioural analysis | |
| This paper reports the effects of the Reach study 2 year outcomes. No behavioural outcomes. | |
| Behavioural intervention, however from intervention description there is no evidence of functional analysis. | |
| Identity specific intervention regarding retention of self identity and the impact of role based treatment | |
| This was not a fully randomised controlled trial, due to only some of the care homes being randomly assigned | |
| Recreational activities intervention | |
| Therapies were standardised not individually tailored, intervention involved the use of verbal prompts for eating behaviour | |
| Not an RCT, participants were referred into the study | |
| Education and coaching intervention to provide ideas of interventions to reduce and avoid BPSD but did not involve analysis of behaviour | |
| Support & education Intervention to predominantly reduce caregiver burden | |
| Case Series | |
| Psycho‐education and caregiver health intervention | |
| Reports on a subgroup only from previous randomised controlled trial, full RUSH trial is included in the review | |
| Interactive voice response intervention.Behavioural management advice provided over the phone. | |
| Emotion oriented care intervention training staff to use an emotion oriented approach | |
| Cognitive behavioural intervention to reduce depression in family caregivers | |
| Education only intervention involving two day training on problem behaviours. | |
| Cross sectional study in care homes to investigate the relationship between apathy and quality of life. | |
| Home Environmental intervention proving occupational therapy to improve the environment. | |
| Paper reports the maintenance effects of included study Gitlin 2003, however the results have not been reported in the same format and therefore this data could not be included in the review. | |
| This paper reports on the design and method of projectACT3, however the results for this study are not yet published. | |
| Physical activity intervention, not functional analysis | |
| Occupational therapy based intervention | |
| Occupational therapy based intervention. | |
| Occupational therapy based intervention. | |
| Initial elucidation of unmet need or cause not by trained professional as this study is distance based. All contact with a trained professional is via the telephone | |
| Case Series | |
| Psychodeuctional and coaching group intervention, providing role training to help caregivers assume a more clinical belief set about care giving | |
| Reports only on the development and testing of the Savvy Caregiver Program. | |
| Psychoeducational intervention to deal with caregiver distress using activity, OT and music | |
| Psycho‐educational group program for caregivers to look at caregiver appraisal of stress and problem solving | |
| Primary outcome data not reported in continuous format (reported as dichotomous) therefore it could not be included in the meta‐analysis. | |
| Observational study. | |
| No behavioural outcome e.g. NPI or CMAI. Behaviour rated through 'hassle' scale only. Specific bathing intervention. | |
| Psychosocial intervention to involve families in care to evoke positive responses from residents when provided with items of interest | |
| Psychoeducational intervention. Randomisation unclear | |
| Therapeutic recreational activities intervention. The paper is a review with a report of a small pilot crossover experimental design | |
| Recreational Activities intervention, not functional analysis. | |
| Specific agitation study, not an RCT, cross sectional design with repeated measures | |
| Memory and coping program specifically for improving cognition not behaviour. | |
| Cognitive behavioural therapy intervention | |
| Therapeutic activities intervention, to promote comfort, QOL and dignity. | |
| Serial Trial Intervention, needs assessed but not in terms of what functions behaviours served or what where the antecedents and causes. | |
| Dementia care mapping intervention | |
| Pharmacological intervention. | |
| Activities based intervention. | |
| Review paper. | |
| Stimulation intervention | |
| Pleasant events intervention, brainstorming and activity programming | |
| Cross sectional study to discover factors associated with the use of anti‐psychotics | |
| Cross sectional study to investigate the relationship between self destructive behaviours and nursing home environments | |
| Review/discussion paper of exit seeking wandering behaviour intervention strategies. | |
| Increasing sensitivity to non verbal signals to improve psychological well‐being of caregiver. | |
| Cognitive behavioural therapy intervention, involving role play and problem solving. | |
| Activity based intervention to improve sleep/wake patterns. | |
| Specific observational wandering intervention to assess the use of mirror. | |
| Intervention to improve communication between carer and resident by observing interactions. | |
| Specific Sleep intervention, did analyse behaviour however excluded due to targeting only night time behaviour. | |
| Sleep Education intervention to deal with nighttime insomnia only. | |
| Way‐finding intervention | |
| No behavioural outcomes. | |
| Support and education intervention where caregivers dictated sessions. | |
| Counselling and support intervention with management of behaviours however from the description of the intervention it was not apparent functional analysis was utilised. | |
| Case Series | |
| Case Series, not a randomised controlled trial | |
| Systematic review of pharmacological therapies for sleep problems in later life | |
| Reports 6 month data from an NIH‐funded study, decision making educational intervention. | |
| Reality orientation intervention | |
| Systematic Literature Review paper looking at the efficacy of psychosocial approaches | |
| Randomised controlled trial lasted only 3 days where subjects acted as own controls (early group controls for late group). | |
| Psychoeducational intervention only. | |
| Sleep improvement intervention involving increasing daytime physical activity, bright light exposure and social interactions | |
| Observational study. | |
| Kit based activity intervention to reduce apathy and improve quality of life. | |
| Cognitive intervention to test the efficacy of telemedicine vs face to face treatment | |
| Case series regarding managing anxiety in people with dementia. | |
| Qualitative study. | |
| Reality orientation. Not a randomised controlled trial. | |
| Discussion paper. | |
| Social Activity Intervention. | |
| Only secondary outcomes reported. No extractable data for Primary outcomes. | |
| Psychoeducational and communication facilitation intervention. | |
| Physical Activity intervention to improve ADL's & physical performance | |
| High intensity Functional exercise program to improve gait. | |
| Review paper. | |
| Emotion‐oriented care intervention providing education on dementia | |
| Overview of REACH project, site specific outcomes and future directions. | |
| Cross sectional study on caregiver characteristics and which are associated with neuropsychiatric symptoms | |
| Activities intervention, case study of three participants with dementia. | |
| Intervention specifically tailored to behaviours experienced during showering/bathing | |
| Group music with movement intervention | |
| Review paper | |
| Qualitative study reporting cases from a previous randomised controlled trial. | |
| Pharmacological intervention. | |
| Pharmacological intervention. | |
| Home hospital intervention. Reviewers could not determine a sufficient dosage of Functional Analysis to include this paper | |
| Review paper | |
| Physical activity intervention | |
| Music based exercise intervention. | |
| Snoezelen Intervention | |
| Snoezelen Intervention | |
| Role of social relationships in psychosocial and psycho‐cognitive behaviour | |
| No extractable data as only sub scale means reported. Author contacted‐ data unavailable | |
| Reality orientation and environmental intervention | |
| Psychoeducational intervention |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of problem behaviours ‐ family care only Show forest plot | 4 | 722 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.17] |
| Analysis 1.1  Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 1 Incidence of problem behaviours ‐ family care only. | ||||
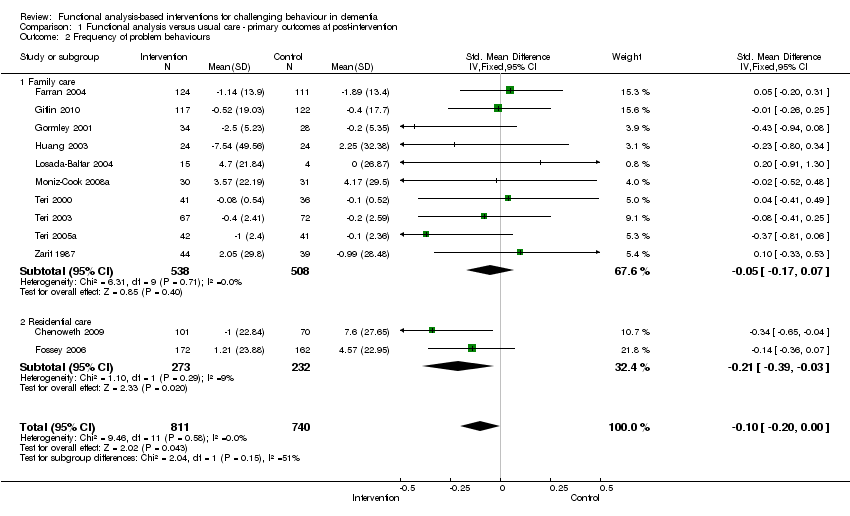
| 2 Frequency of problem behaviours Show forest plot | 12 | 1551 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.20, ‐0.00] |
| Analysis 1.2  Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 2 Frequency of problem behaviours. | ||||
| 2.1 Family care | 10 | 1046 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 2.2 Residential care | 2 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.39, ‐0.03] |
| 3 Severity of problem behaviours Show forest plot | 5 | 449 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.29, 0.08] |
| Analysis 1.3  Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 3 Severity of problem behaviours. | ||||
| 3.1 Family care | 2 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.58, 0.08] |
| 3.2 Residential care | 3 | 307 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.19] |
| 4 Patient depression Show forest plot | 3 | 480 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.33, 0.03] |
| Analysis 1.4  Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 4 Patient depression. | ||||
| 4.1 Family care | 2 | 375 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.29, 0.12] |
| 4.2 Residential care | 1 | 105 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.77, 0.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of problem behaviours ‐ family care only at 6 month follow‐up Show forest plot | 2 | 436 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.11, 0.27] |
| Analysis 2.1  Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 1 Incidence of problem behaviours ‐ family care only at 6 month follow‐up. | ||||
| 2 Frequency of problem behaviours at 6 month follow‐up Show forest plot | 4 | 627 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.16, 0.16] |
| Analysis 2.2  Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 2 Frequency of problem behaviours at 6 month follow‐up. | ||||
| 3 Frequency of problem behaviours at 12 month follow‐up Show forest plot | 3 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.27] |
| Analysis 2.3  Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 3 Frequency of problem behaviours at 12 month follow‐up. | ||||
| 3.1 Family care | 3 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caregiver reaction Show forest plot | 11 | 1259 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, ‐0.00] |
| Analysis 3.1  Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 1 Caregiver reaction. | ||||
| 1.1 Family care | 11 | 1259 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, ‐0.00] |
| 2 Caregiver burden Show forest plot | 6 | 624 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.29, 0.03] |
| Analysis 3.2  Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 2 Caregiver burden. | ||||
| 3 Caregiver well‐being (depression) Show forest plot | 5 | 473 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.30, 0.06] |
| Analysis 3.3  Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 3 Caregiver well‐being (depression). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caregiver reaction Show forest plot | 4 | 653 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.27, 0.04] |
| Analysis 4.1  Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 1 Caregiver reaction. | ||||
| 2 Caregiver burden Show forest plot | 2 | 286 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.38, 0.09] |
| Analysis 4.2  Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 2 Caregiver burden. | ||||
| 3 Caregiver well‐being (depression) Show forest plot | 2 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐2.56, 0.70] |
| Analysis 4.3  Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 3 Caregiver well‐being (depression). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Frequency of problem behaviours at post‐intervention Show forest plot | 2 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.50, 0.17] |
| Analysis 5.1  Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 1 Frequency of problem behaviours at post‐intervention. | ||||
| 2 Severity of problem behaviours at post‐intervention Show forest plot | 2 | 176 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.02, 0.63] |
| Analysis 5.2  Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 2 Severity of problem behaviours at post‐intervention. | ||||
| 3 Caregiver burden at post‐intervention Show forest plot | 2 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.46, 0.21] |
| Analysis 5.3  Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 3 Caregiver burden at post‐intervention. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
![Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.1 Incidence of problem behaviours ‐ family care only. [Instruments used: RMPBC]](/es/cdsr/doi/10.1002/14651858.CD006929.pub2/media/CDSR/CD006929/image_n/nCD006929-AFig-FIG02.png)
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.1 Incidence of problem behaviours ‐ family care only. [Instruments used: RMPBC]
![Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.2 Frequency of problem behaviours. [Instruments used: PC, RAGE, RMBPC, CMAI and MBCL]](/es/cdsr/doi/10.1002/14651858.CD006929.pub2/media/CDSR/CD006929/image_n/nCD006929-AFig-FIG03.png)
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.2 Frequency of problem behaviours. [Instruments used: PC, RAGE, RMBPC, CMAI and MBCL]
![Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.3 Severity of problem behaviours. [Instruments used: PAS, NPI, Behave‐AD and Crichton Royal Behavioural Scale].](/es/cdsr/doi/10.1002/14651858.CD006929.pub2/media/CDSR/CD006929/image_n/nCD006929-AFig-FIG04.png)
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.3 Severity of problem behaviours. [Instruments used: PAS, NPI, Behave‐AD and Crichton Royal Behavioural Scale].
![Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.4 Patient depression. [Instruments used: RMPBC Depression sub scale, AGECAT and CDDS]](/es/cdsr/doi/10.1002/14651858.CD006929.pub2/media/CDSR/CD006929/image_n/nCD006929-AFig-FIG05.png)
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.4 Patient depression. [Instruments used: RMPBC Depression sub scale, AGECAT and CDDS]
![Forest plot of comparison: 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, outcome: 3.1 Caregiver reaction. [Instruments used: PC, RMBPC ‐reaction, NPI ‐distress and ABID ‐reaction].](/es/cdsr/doi/10.1002/14651858.CD006929.pub2/media/CDSR/CD006929/image_n/nCD006929-AFig-FIG06.png)
Forest plot of comparison: 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, outcome: 3.1 Caregiver reaction. [Instruments used: PC, RMBPC ‐reaction, NPI ‐distress and ABID ‐reaction].

Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 1 Incidence of problem behaviours ‐ family care only.
Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 2 Frequency of problem behaviours.

Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 3 Severity of problem behaviours.

Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 4 Patient depression.

Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 1 Incidence of problem behaviours ‐ family care only at 6 month follow‐up.
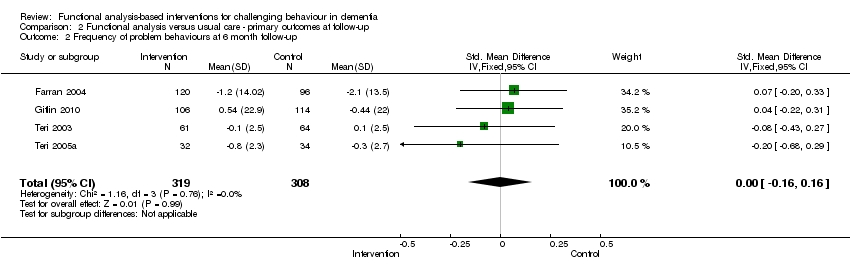
Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 2 Frequency of problem behaviours at 6 month follow‐up.
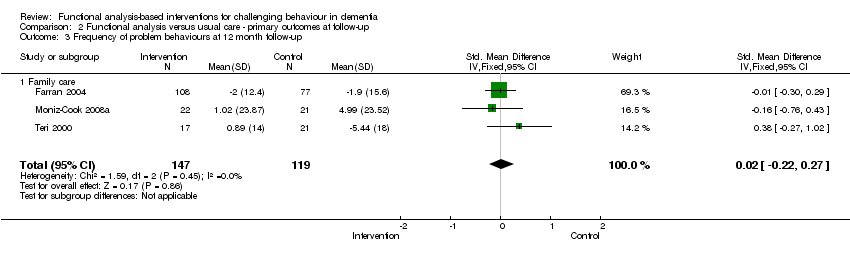
Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 3 Frequency of problem behaviours at 12 month follow‐up.

Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 1 Caregiver reaction.
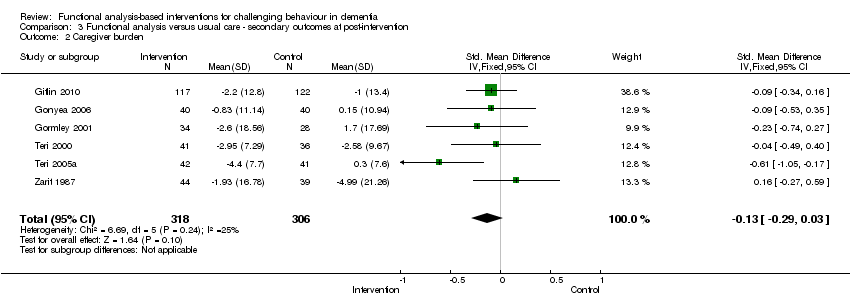
Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 2 Caregiver burden.

Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 3 Caregiver well‐being (depression).

Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 1 Caregiver reaction.
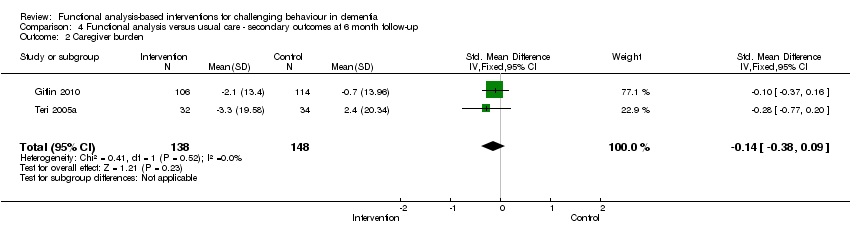
Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 2 Caregiver burden.

Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 3 Caregiver well‐being (depression).

Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 1 Frequency of problem behaviours at post‐intervention.

Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 2 Severity of problem behaviours at post‐intervention.

Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 3 Caregiver burden at post‐intervention.
| Table 1:Description of primary and secondary outcome measures | ||||||
| Outcome
| Name of measure
| Source
| Description
| Eighteen trials | ||
| Family | Residential /Assisted Living/Hospital | |||||
| Primary outcomes: Care recipient | ||||||
| Patient behaviour | Revised Memory & Behaviour Problem Checklist (RMBPC) | Assessment of behavioural problems in people with dementia. A 24‐item checklist which provides one total score and 3 sub scores for the following problems: memory (7 items), depression (9 items) and disruption (8) items. Measures caregiver reports of Incidence (0‐24), Frequency and Reaction (0‐96) to each of the 24 problems. It was developed to measure reports of behavioural concerns by family caregivers in the US.
| Frequency: Farran 2000 Gitlin 2010 (2 items) Zarit 1987(non revised version) Incidence: Gitlin 2003 (disruptive behaviour only) | |||
| Rating Scale for Aggressive Behaviour in the Elderly (RAGE) | Measures aggressive behaviours in the elderly ranging from being uncooperative to physical violence. A 21‐items scale where for 17 items ratings are made for the frequency of behaviour over the past 3 days on a Likert scale of 0 (never) to 3 (more than once every day in past 3 days). Items 18‐21 have descriptions for severity ratings of 0‐3 or yes /no. Scores range from 0‐62. Developed for staff working on psycho‐geriatric wards. |
| ||||
| Cohen Mansfield Agitation Inventory (CMAI) | Measures reported agitated behaviours in patients with cognitive impairment. A 29‐item scale of verbally/physically aggressive behaviour and verbal/physical non–aggressive behaviour. Each item is rated for frequency ‘since the last visit’ on a 7 point scale (1–7) ranging from ‘‘never’’ to ‘‘several times an hour.’’ A total score is obtained by summing the 29 individual frequency scores, yielding a total score that ranges from 29 to 203. Developed in care home settings. Chinese version: assess 43 behavioural problems; each item is scored according to the frequency ranging from 1 (never happened) to 7 (several times an hour). Scores can range from 42‐294. |
Huang 2003 (Chinese Version) | ||||
| Problem Checklist (PC) | Assessment of problems experienced by family carers of patients with dementia. The 34‐Item Problem Checklist (Gilleard 1984) was adapted to include a further 5 items. Ratings are made for reported frequency (0‐2) ‐ scores ranging 0 ± 78 and management difficulty/coping (0‐2) ‐ score ranging 0 ± 78. Developed with family caregivers in the UK. |
| ||||
| Severity of Problem Behaviours | Crichton Royal Behavioural Scale (CRBRS) | Assessment of psycho‐geriatric patients. The 11‐item scale requires ratings for each item on a 1‐5 point scale where each point has a severity description. Items are: mobility, memory, orientation, cooperation, restlessness, dressing, feeding, hearing, continence, sleep and subjective and objective mood. Scores range from 0‐55 |
| |||
| Neuropsychiatric Inventory (NPI) | Assessment of Behavioural and Psychological Symptoms of Dementia (BPSD) using a caregiver interview, with ratings of the frequency and severity of 10 or 12 neuropsychiatric domains (according to the version). Available versions include for Family / community settings and Nursing homes. Both the frequency (F) and severity (S) of each symptom are rated on a four ‐ (1–4) and three‐point (1–3) Likert scale, respectively. A separate score can be calculated for each symptom by multiplying the frequency and severity scores, resulting values ranging from 0 to 12 for each symptom. A total score can be obtained by summing the 12 F_S scores, yielding total scores that range from 0 to 144. A separate rating of caregiver distress can be made on a five point scale from 0 ‐ no distress, 1 ‐ minimal, 2 ‐ mild, 3 ‐ moderate, 4 ‐ moderately severe, 5 ‐ very severe or extreme; distress ranges 0‐60. | |||||
| Pittsburgh Agitation Scale (PAS) | Measures the severity of disruptive behaviours within four behavioural groups: aberrant vocalisations; motor agitation, aggressiveness & resisting care. Scored from 0‐4 with a maximum score 16. The score reflects the most disruptive of severe behaviour within each group. |
| ||||
| Behavioural Pathology in Alzheimer’s Disease Rating Scale (Behave‐AD) | Assessment of behavioural symptoms in Alzheimer’s disease. A 25‐item scale with Likert scale of 0‐4 covering paranoid and delusional ideation (7 items), hallucination (5 items), activity disturbances (3 items), aggression (3 items), diurnal variation (1 item), affective disturbance (2 items), and anxieties (4 items). Ratings range (0‐75) and a global rating of the trouble that the various behaviours are to the caregiver is also recorded (0‐3). |
| ||||
| Patient mood (depression) | Cornell Scale for Depression in Dementia (CSDD) | Assessment of depression in patients with a dementia syndrome administered by a clinician. The interview takes 20 minutes with the carer and 10 minutes with the patient. A 19‐item measure covering mood (4 items), behavioural disturbance (4 items), physical signs (3 items), cyclical functions (4 items), ideational disturbance (4 items). Items are rated on a 3 point scale: absent, mild or intermittent, and severe. Ratings are based on the week prior to the interview and range from 0‐38. |
| |||
| Automatic Geriatric Examination for Computer Assisted Taxonomy (AGECAT) | Measures organic and depression symptoms. Ratings are made from 1 & 2 = subclinical to 5 = severe. It provides syndrome diagnoses of: organicity, schizophrenia, mania, depression, anxiety, obsessional disorder, phobia, and hypochondriasis. |
| ||||
| Revised Memory & Behaviour Problem Checklist (RMBPC) | Depression Subscale. Measures reported incidence (0‐9), frequency (0‐36) and caregiver reaction depression (0‐36). | |||||
| Secondary outcomes: Caregiver | ||||||
| Mood (depression) | Centre for Epidemiological Studies — Depression scale (CES‐D) | Detects depressive symptoms, particularly for use in research or screening. A 20‐item scale with scores ranging 0‐60. A score of 16 = mild depression and 23 and above is indicative of significant depression. Items are rated as occurring Rarely (< 1 day), Some (1‐2 days), Occasionally (3‐4 days) and Most (5‐7 days). |
| |||
| Hospital and Anxiety Depression Scale (HADs) | Assessment of mood. A 14 item measure with two sub scales: anxiety and depression. Each item is rated on a four‐point Likert scale, giving maximum scores of 21 each for anxiety and depression. Scores of 11 or more on either sub scale are considered to be a significant 'case' of psychological morbidity, while scores of 8–10 represents 'borderline' and 0–7 'normal' |
| ||||
| Reaction | Revised Memory & Behaviour Problem Checklist (RMBPC) | Assessment of behavioural problems in people with dementia. A 24 item checklist which provides one total score and 3 sub‐scores for the following problems: Memory (7 items), Depression (9 items) and Disruption (8 items). Measures caregiver reports of Incidence (0‐24), Frequency and Reaction (0‐96) to each of the 24 problems. Developed to measure reports of behavioural concerns by family caregivers in the US. | ||||
| Agitated Behaviour in Dementia Scale (ABID) | A measure of agitation in an outpatient sample of patients with mild to moderate Alzheimer’s disease. A 16‐item measure of frequency and caregiver reaction to common agitated behaviours in community residing dementia patients. Scored on a scale of 0‐3, rated in the past 2 weeks where: 0 = did not occur during the week, 1 = occurred once or twice, 2 = occurred 3‐6 times in the week, 3 = daily or more often. |
| ||||
| Neuropsychiatric Inventory (NPI) Distress | The NPI distress scale has an additional question on each of the 10 or 12 (depending on version) domains specifically addressing the level of distress caused to carers by each symptom. Available versions include for Family / community settings and Nursing homes. Ratings are on a five point scale from 0 ‐ no distress, 1‐ minimal, 2 ‐ mild, 3 ‐ moderate, 4 ‐ moderately severe, 5 ‐ very severe or extreme. Total distress ranges from 0‐60. |
| ||||
| Problem Checklist (PC) | Assessment of problems experienced by family carers of patients with dementia. The 34‐item Problem Checklist (Gilleard 1984) was adapted to include a further 5 items. Ratings are made for reported frequency (0‐2) ‐ scores ranging 0 ± 78 and management difficulty /coping (0‐2) ‐ score ranging 0 ± 78. Developed for use with family caregivers in the UK. |
| ||||
| Burden | Zarit Burden Interview (ZBI) First described as the Burden Interview | Assessment of the feelings of burden of caregivers in caring for an older person with dementia. A 29‐item scale where scores are interpreted as follows: 0‐21 = little or no burden, 21‐20 = mild to moderate, 21‐40 = mild to moderate, 41‐60 = moderate to severe burden and 61‐88 = severe burden. |
| |||
| The Screen for Caregiver Burden (SCB) | Assessment of perceived burden of caring for a person with Alzheimer’s disease. A 25‐item scale with scores for objective and subjective burden. Objective = the number of caregiver experiences occurring independently of their distress. Subjective = overall distress. |
| ||||
| Table 2. Description of interventions and quality of included studies | |||||||||
| Trial setting | Trial | Study duration from baseline | Intervention duration | Follow‐up assessments | Details of intervention sessions & format | Intervention type, aims and components | Delivered by | Intervention dosage¹ Minimal 1‐2 sessions Moderate 3‐5 Medium High 6‐10 High > 10
Behaviour Management² = BM | Intervention Information to enable replication of trial. 1. Procedural clarity 2. Manual /protocol 3. Treatment fidelity assessments 4. Follow‐up |
| Family Care | 24 months | 3 months | Post intervention = 3 months. Follow‐up data for: Problem Behaviour (PB) Frequency & Caregiver (CG) Reaction = 6 months; Patient Depression = 6, 12, 18 & 24. | 12 x 1 hour sessions, 2 per week for 3 weeks, Weekly for 4 weeks and biweekly for 4 weeks, plus 3 follow‐up sessions | CG Skills Training Intervention Aims: CGs taught to identify and modify patient behaviours that impaired day‐to‐day function and adversely affected CR/CG interactions. Taught how to reduce the occurrences of PB, learn skills to identify and modify precipitants of patient distress. Exercise and Education | Health care professionals delivered sessions (doesn't state how many) Trainers supervised by clinical geropsychologist (received weekly supervision). | High | 1. Reported what components were included in the intervention; but detail on which components were addressed in each hour long session is absent. 2. Treatment protocol/manual 3. Treatment adherence was monitored by weekly supervision of each trainer by a clinical geropsychologist. Protocol sessions videotaped and reviewed by independent raters 4. Followed up to 24 months. | |
|
| 24 months
| 2 months
| Post‐intervention = 2 months Follow‐up = 12 months (data not available)
| 8 sessions, the last used for Post‐intervention assessment
| CG Support Intervention Aims: Stress‐ Coping Model. Training teach CG to modify situations linked to stress, increase understanding of patient disease, improve management of PBs and identify useful formal and informal supports | 2 Therapists for each group.
| Medium High
| 1. The paper reports what usually occurred in the second session of the intervention, but does not state each session’s agenda. 2. Conceived from a stress‐management approach treatment model, but no mention of a manual. 3. Interventions monitored using audiotapes and supervision sessions to ensure therapists implemented treatment approach. 4. 2 Year longitudinal study but only post‐intervention (2 month) data available. | |
| 12 months | 6 months | Post‐intervention = 6 months Follow‐up = 12 months (data not extractable) | Active phase: First 6 months, 5 (90 min) home contacts, 1 (30 min) telephone contact. Maintenance Phase: Subsequent 6 months | CG Skills Training Intervention Problem solving Intervention Includes: modifying home environments and simplifying daily tasks to address CG concerns; Education, Problem solving, Use of environmental strategies | Occupational therapist (does not state how many) | Moderate | 1. The paper reports what happens in each intervention session as run by the OT. 2. Protocol 3. Interventions monitored using case review, feedback, checklist & telephone interviews to evaluate satisfaction 4. The paper reports 6 month post‐intervention assessment, but not the results of the 12 month follow‐up. | ||
| 18 months | 3 months | Post‐intervention = 3 months Follow‐up = 6, 9, 12 & 18 months | 12 x weekly sessions (5 group, 7 individual) 2 group booster sessions at 6 & 12 months + as needed telephone contacts | CG Skills Training Intervention Aims: Improve CG skill in dealing with PB. Content included: Potential causes/contributors to behavioural symptoms, prevention & management of BPSD, building self efficacy. | Trained professionals (nurses, social workers) trained for 40 hours. 4 people functioned as intervention staff at any one time. | High | 1. Paper reports contents of intervention but not each session in detail. 2. Detailed manual of prescribed material for each session 3. Project director and principal investigator supervised implementation & provided corrective feedback on a weekly basis. Group sessions were taped and randomly selected for review. 4. All follow‐up data up to 18 months available. | ||
| 18 months | 18 months | Post‐intervention = 6 Follow‐up = 12 & 18 months | 4 consecutive weekly in home visits + clinical judgement for future contact & attend in‐service clinical supervision for the 18 month duration. (Interventions were taught prior to the study over 5 half days) | CG Support Intervention Aims: To train community mental health nurses (CMHNs) to help family carers manage behavioural changes. Includes: Problem solving approaches, Stress‐coping interventions and Functional analysis. | 9 CMHNs (usual group 20 CMHNs) ‐ 20 hrs training initially plus supervision 2 hrs per week for 1st 6 months, 1 per fortnight for next 6 months, 1 per month for last 5 months. | High | 1. The total number of sessions or content of the sessions is not reported. 2. Protocol for CMHNs to conduct 4 in‐home visits & attend supervision. No manual. 3. Only two CMHNs with dementia specific caseloads completed the ongoing supervision and adhered to the four consecutive family treatment sessions. 4. Follow‐up data for 6, 12 & 18 months | ||
| 18 months | 12 months | Post‐intervention = 6 months Follow‐up data not available | 16 in‐home treatment sessions (over 12 month period). Skill Training condition vs. Minimal Support Condition. 3 hour workshop, 4 weekly in home visits for 1 month & 2 in the second month. In the following 10 months home visits were alternated. | CG Skills Training Aims: To establish a knowledge base for CGs in behaviour management, problem solving, & cognitive restructuring. Basic information in behaviour management techniques (BMT) & support on the application of behavioural and environmental treatments. Individual behaviour prescriptions. | 11 REACH interventionists. | High
| 1. Reports the intervention procedure & components covered. 2. Manual guided intervention based on common needs and cultural preferences of American family caregivers. Manual available from authors. 3. Research personnel functioned as both interventionists and assessors. Feedback on accuracy was provided in weekly clinical case review meetings. All therapeutic contacts were audio taped to check accuracy of delivery. 4. Only 6 month data reported. | ||
| 12 months | 4 months | Post‐intervention = 4 months Follow‐up = 12 months (Weiner 2002) | BMT 8 weekly and 3 biweekly sessions. 16 week parallel design requiring 11 clinical visits. Randomisation to medication, BMT or placebo. | Behaviour Management Aims: Compare Behaviour Management Techniques – BMT‐ with pharmacological treatments for agitation. BMT included: information about AD, strategies for decreasing agitated behaviours. | Therapists with a master’s degree and 1 year clinical experience (doesn't state how many therapists) | High BM | 1. BMT intervention sessions not reported in detail. Paper only reports number and components of sessions. 2. Protocol 3. Raters participated in ongoing training to assure standardisation. All were trained prior to starting the trial. 4. Post‐treatment data only reported; Weiner 2002 reports 12 month follow‐up. | ||
| 6 months | 4 months | Post‐intervention = 4 months Follow‐up = 6 months | Up to 11 home & telephone contacts over 16 weeks. Up to 9 occupational therapy (OT) sessions, two nursing home (one home and one telephone) and a maintenance phase of 3 brief OT telephone contacts. | CG Support Intervention Aims: To help eliminate, reduce or prevent problem behaviours within 3 interacting domains: ‐ Patient based (unmet need, discomfort, pain), Caregiver based (stress & communication style) & Environment based (clutter, hazards). | 10 OTs & 2 practice nurses received 35 hours training | High | 1. Reports what took place during the intervention but not a specific outline for each session. 2. No mention of a manual. 3. Treatment fidelity maintained through twice monthly meetings & audiotapes of 10% of home sessions. Each home session was documented in terms of time spent & content covered. 4. Four and six month follow‐up. | ||
|
| 6 months
| 2 months
| Post‐intervention = 2 months Follow‐up = 6 months
| 8 weekly sessions followed by 4 monthly phone calls
| CG Support Intervention Aims: To teach family CGs a systematic behavioural approach for reducing mood and behaviour problems in persons with AD. Teaching ABC rationale and use Improving CG communication Increasing pleasant events, enhancing CG support. | 5 community consultants – trained by clinical gero‐psychologist. ‐ 2 hour orientation, 2nd training session & pilot case.
| High
| 1. Paper reports on the contents of each treatment session 2.Treatment manual 3. Protocol, Audio taped treatment sessions and rated quality 4. Post‐test and 6 month follow‐up.
| |
|
| 12 Weeks
| 3 Weeks (main phase)
| Post‐intervention = 3 weeks Follow‐up = 12 weeks | 2 in home sessions over 3 weeks, plus telephone calls every 2 weeks.
| CG Skills Training Intervention Aims: Conceptually built around the Progressively Lowered Stress Threshold (PLST) model. Helping CGs identify the timing & frequency of behavioural problems & explore the causative stressors. Plan environmental and daily schedule modifications. Nurse caregiver collaboration with individualised training to develop individual plans of care. | Investigator – Experienced Gerontological nurse
| Minimal
| 1. The paper reports what was conducted by the investigator on each visit. 2. Manual developed by research team as a guide for the training program 3. It is not reported whether there were any checks to insure adherence to the manual, however the principal investigator wrote the manual and conducted the intervention. 4. Followed 12 weeks from baseline.
| |
| 10 Weeks | 8 Weeks | Post‐intervention = 10 weeks
No follow‐up | 4 sessions conducted over 8 weeks. | Behaviour Management Training Aims: To train CGs in: Dementia education & the development of behavioural interventions by behavioural analysis. CGs taught to identify the precipitating & maintaining factors of behaviour. | Conducted by author. | Moderate BM | 1. The paper reports what the 1st, 2nd and subsequent sessions focused on. 2. No mention of manual, the program was developed following a review of guidelines and descriptive studies 3. The paper does not report information on treatment fidelity checks. 4. No follow‐up | ||
| 5 months | 2months | Post‐intervention = 2 months Follow‐up = 5 months | 8 Sessions, 2 hours per week (16 hour in total) | CG Skills Training Intervention Aims: To train CGs in modifying behavioural problems of their relative through: Managing challenging behaviours, defining & identifying the problems, possible causes (ABC) and develop strategies and solutions. | Two psychologists | Medium High | 1. States the components of the intervention but not which components were implemented in each session. 2. Due to difficulty translating the paper we are unsure if a manual was used. 3. Unsure regarding treatment fidelity checks 4. Followed up 5 months from Baseline. | ||
| 6 Weeks | 5 Weeks | Post‐intervention = 6 weeks No follow‐up | 5 weekly group sessions (90 mins) including 15 minutes of individual time. | CG Support Intervention Aims: CG multi‐component behavioural intervention to reduce CG distress through: Behavioural management (identifying ABC), Pleasant events & Relaxation. | Therapists (16‐20 hours training). | Moderate | 1. Session topics outlined 2. Highly structured groups with 5 main themes documented in the paper. 3. To monitor treatment fidelity the principle investigator consulted with therapists on a regular basis to review the group session experience and assess group progress. 4. No follow‐up | ||
| Assisted Living
| 2 months | 2 months | Post‐intervention = 2 months No follow‐up | 2 half day group workshops and 4 individualised sessions | CG Skills Training Intervention Aims: To reinforce values of dignity and respect for residents, improve staff responsiveness to resident needs, build specific staff skills to enhance resident care, improve job skill and satisfaction. | Clinical psychologist & graduate student in nursing. | Medium High
| 1. The paper reports all the essential components and features of the intervention. 2. Manual detailing all specific aspects of training. 3. Three separate meetings were held to discuss site specific issues that might hinder implementation or sustainability. 4. No follow‐up. | |
| Residential Care | 12 months | 10 months | Post‐intervention = 12 months
No follow‐up | Trial clinician worked with homes 2 days a week over 10 months | CG Skills Training Intervention Aims: Training in the delivery of Person‐centred care and Skills development training. Included: skills training, behavioural management techniques (ABC) and ongoing training and support | Psychologist, occupational therapist or nurse – supervised weekly by authors. | High
| 1. Reports the components of the intervention but detail of each session. 2. No mention of a manual just reference to a specific ‘package’ of components. 3. Staff offered supervision but no report assessing treatment fidelity. Reports the intervention took a consultation approach. 4. 10 month intervention with 12 month follow‐up (for the purposes of this review classed as post‐intervention assessment). No other follow‐ups. | |
| 8 months | 4 months | Post‐intervention = 4 months Follow‐up = 8 months | Training was delivered to 2 care staff selected by managers for 6 hours per day over 2 days, trained staff then helped their colleagues to implement care plans over the 4 month intervention period | Dementia Care Mapping and Caregiver Skills Training Aims: Person centred care Need‐driven behaviour model. where staff are educated to Included: Understand behaviour as a form of communication; recognise that feelings persist despite cognitive impairment; behaviour is a way of expressing needs; understand the impact of staff actions and use of ABC | 3 authors trained by Bradford University led training. | High
| 1. Details of the interventions components are reported, but additional information was required from the author to clarify the intervention content before this trial could be included into the review. 2. Bradford University training manual 3. No detail on checking adherence to the manual or treatment fidelity. 4. Follow‐up at 8 months from baseline. | ||
| 6 months | 6 months | Post‐intervention = 6 months No follow‐up | 7 x 1 hour seminars delivered by hospital outreach team. An experienced psychiatric nurse visited every week to give advice and support individual workers in care planning. | Behaviour Management Aims: Staff training and psychosocial management of residents PB. Includes: Formulation of detailed and specific care plans & increasing the interval between non‐contingent interactions (not in response to need) | Hospital outreach team & psychiatric nurse | Medium High BM
| 1. The paper reports only the components of each of the seminars 2.No report of a manual 3. No reports of checking treatment fidelity or adherence. 4. No follow‐up. | ||
| Hospital Care | 9 Days | 9 Days | Post‐intervention = 9 days No follow‐up | Extended Practice Nurse (EPN) saw patients within 24 hours of randomisation and formulation of a non‐pharmacological management plan of strategies to manage challenging behaviour. Assumption that Control condition Geriatric assessment was also | Behaviour Management Aims: Specialist support and education to the ward nursing staff to enable them to facilitate behaviour strategies. Included: Understanding patients needs, patient safety, minimising restraint usage, communication, nursing care & targeted behavioural strategies. | ? Geriatrician review as in Control Group + Extended practice nurse and ward staff. | High BM | 1. The paper reports the components of the intervention only. 2. No mention of a manual 3. No reporting of assessments of treatment fidelity and adherence 4. No long‐term follow‐up | |
| ¹ = Intervention dosage is based on the number of contact sessions, not the amount of functional analysis | |||||||||
| ² = Intervention focused on Behaviour Management with relatively few other components | |||||||||
| Table 3. Overview of outcome measures | |||
| Trial | Setting
| Outcomes Author’s description of care recipient (CR) & caregiver (CG) outcomes
| Assessment Tools ◊ Measure abbreviated after one full description ∞ Outcome measure not a rating scale ∆ Inadequate number of equivalent instruments for data aggregation □ Instrument not relevant or alternative measure used |
| Family | Care Recipient (CR) Behaviour & Caregiver (CG) Reaction | Revised Memory and Behaviour Problem Checklist (RMBPC) (incidence only) & RMBPC ‘bother or upset’ | |
| CG Appraisal of benefits from Caregiving | ∆ Positive Aspects of Caregiving (PAC) (developed by REACH investigators) | ||
| CG Social Support | ∆ Lubben Social Network Index (LSNI) 28 item measure (Berkman 1979 adapted scale) | ||
| CG Leisure Time satisfaction | ∆ 6‐item scale developed by interventionists | ||
| CG Mood | ∆ State‐trait personality inventory (anxiety sub scale 10 items) | ||
| The Centre for Epidemiologic studies –Depression Scale (CES‐D) | |||
| CG Desire to institutionalise | □ 7 Item scale by Morycz 1985 | ||
| Family | CR Behaviour/CG Depression | ◊ RMBPC | |
| CG Mood | ◊ CES‐D | ||
| CG Skill | ∆ Behavioural Management Skill –Revised (BMS‐R) | ||
| Time to institutionalisation | ∞ Interval from Baseline to initial entry into long‐term care Facility | ||
|
| Family | CR Behaviour | ◊ RMBPC (incidence only) |
| CR Level of ADL assistance required | □ Functional Independence Measure (FIM) | ||
| CG Objective & Subjective Burden | ∞ Vigilance, Total hours of ADL help & Help received for ADLs. | ||
| ◊ RMBPC (upset sub scale) | |||
| CG Perceived Mastery | ∆ Care‐giving Mastery Index (CMI) | ||
| CG Skill Enhancement | ∆ Task Management Strategy Index (TMSI) | ||
| CG Wellbeing | ∆ Perceived Change Index (PCI) | ||
| CR Cognitive Ability | □ Mini Mental State Exam (MMSE) | ||
| Family | CR Behaviour & CG Reaction (upset) | 16‐item Agitated Behaviors in Dementia Scale and 2 items (repetitive questioning, hiding/hoarding) from RMBPC, plus 3 other items (wandering, incontinence, shadowing). | |
| CG Mood | ◊ CES‐D | ||
| CG Burden | Zarit Burden Interview (ZBI) | ||
| CG Skill enhancement | ∆ ◊ TMSI | ||
| CG Perceived Benefits | ∆ 11 item survey developed by investigators. | ||
| CG change | ∆ ◊ PCI | ||
| Family | CR behaviour (Severity & Frequency) & CG Distress | Neuropsychiatric Inventory (NPI) | |
| CG Burden | ◊ ZBI | ||
| CR Functional Impairment | □ Activities of Daily Living (ADL) | ||
| Family | CR Behaviour (Severity & Frequency) | Behavioural Pathology in Alzheimer’s disease scale (BEHAVE‐AD) | |
| Rating Scale for Aggressive Behaviour in the Elderly (RAGE) | |||
| CG Burden | ◊ ZBI | ||
| CR Cognitive Ability | □ ◊ MMSE | ||
| CR Functional Ability | □ Blessed Dementia Rating Scale | ||
| Family | CR Behaviour | Cohen Mansfield Agitation Inventory (CMAI) | |
| CG self efficacy for managing agitation | ∆ Agitation Management Self Efficacy Scale (AMSS) | ||
| CR Cognitive Ability | □ ◊ MMSE | ||
| CR Dementia Severity | □ Clinical Dementia Rating (CDR) | ||
| CR Activities of Daily Living | □ Barthel Index | ||
| Family | CR Behaviour & CG reaction (upset) | Memory & Behaviour Checklist (MBCL‐A & MBCL‐B) | |
| CG Dysfunctional thoughts about care | ∆ Beliefs about Care‐giving Questionnaire (BACS) | ||
| CG Mood | ◊ CES‐D | ||
| CG Perceived Support | ∆ Perceived Support Questionnaire (PSQ) | ||
| CG Perceived Stress | ∆ Perceived Stress Scale (PSS) | ||
| Family | CR Behaviour & CG Management/difficulty coping | Problem Checklist (PC) | |
| CR Global Dependency | □ Global Deterioration Scale (GDS) | ||
| CG psychiatric morbidity | ∆ General Health Questionnaire (GHQ) | ||
| CG Mood | Hospital Anxiety and Depression Scale (HADS) | ||
| Family | Clinically meaningful change in CR | □ ADCS Clinical Global Impression of Change scale (ADCS‐CGIC) | |
| CR function (physical and cognitive) | □ Physical Self maintenance (PSM) | ||
| □ Instrumental activities of daily living (IADL) | |||
| □ MMSE | |||
| CG Burden & Reactivity to specific disruptive behaviours | Screen for Caregiver Burden (SCB) | ||
| ◊ RMBPC reaction (not reported) | |||
| CR behaviour | □ Consortium to establish a registry for Alzheimer’s disease (CERAD) | ||
| □ Behavioural Rating scale for Dementia (BRSD) | |||
| ◊ RMBPC (Frequency) | |||
| □ ◊ CMAI (Frequency) | |||
| Agitated behaviour in dementia scale (ABID) (Frequency & Reaction) | |||
| Family | CR Behaviour & CG distress | ◊ RMBPC | |
| CR Physical Health and Function | □ Short Form Health Survey (SF‐36) | ||
| □ Sickness Impact Profile Mobility (SIP) | |||
| CR Mood | Cornell Depression in Dementia Scale (CDDS) | ||
| □ Hamilton Depression Scale (HDRS) | |||
| CR Cognitive Ability | ◊ MMSE | ||
| Other outcomes: | ∞ CR walking speed, functional reach and standing balance. | ||
| Family | CR Behaviour | ◊ RMBPC | |
| ◊ NPI | |||
| CR Quality of life | □ Quality of Life in Alzheimer’s disease scale (QOL‐AD) | ||
| CG Mood | ◊ CES‐D | ||
| CG Mood | □ ◊ HDRS | ||
| CG Perceived Stress | ∆ ◊ PSS | ||
| CG Burden | ◊ SCB | ||
| CG Sleep Problems | ∆ Caregiver Sleep questionnaire | ||
| CG Feelings of Competence | ∆ Short sense of Competence Questionnaire (SSCQ) | ||
| CR Cognitive status | □ ◊ MMSE | ||
| Adverse reactions | ∞ | ||
| Family | CR Behaviour & CG distress | Memory and Behaviour Problem Checklist (MBPC) | |
| CG Stress associated with care giving | Burden Interview (BI) | ||
| CR Frequency of psychiatric symptoms | □ Brief Symptom Inventory (BSI) | ||
| Social Support | ∞ Amount of interaction with informal support network, amount of assistance by others & caregiver rating of adequacy of social support. | ||
| Therapeutic dimensions of Intervention | ∆ Caregiver Change Interview (CCI) | ||
| CG Perception of intervention | ∆ Global rating of situation improvement | ||
| CR Cognitive Ability | □ ◊ MMSE | ||
| Residential | CR Behaviour | ◊ CMAI | |
| ◊ NPI | |||
| CR Quality of life in later stage dementia | □ Quality of Life Index (QUALID) | ||
| Amount of physical restraint | □ Quality of Interaction Schedule (QUIS) observations | ||
| CR Global Dependency | □ ◊ GDS | ||
| Other outcomes: | ∞ Antipsychotics & benzodiazepine doses, incidents and admissions to hospital. Also conducted an economic analysis. | ||
| Residential | CR Behaviour | ◊ CMAI | |
| CR Dementia Severity | □ ◊ CDR | ||
| Neuroleptic use | ∞ Daily chlorpromazine amounts to national formulary | ||
| CR Falls | ∞ Observations | ||
| CR Quality of life and well‐being | Measurement scale not reported. | ||
| Residential | CR Behaviour | Crichton Royal Behavioural Rating Scale | |
| CR Organic and Depressive symptoms | Automatic Geriatric Examination for Computer assisted taxonomy (AGECAT) | ||
| CR Activities of daily living | □ Barthel Index | ||
| Assisted Living | CR Behaviour & CG Reaction
| ◊ RMBPC | |
| □ ◊ ABID | |||
| ◊ NPI | |||
| CR Mood | RMBPC sub scale | ||
| □ Geriatric Depression Scale | |||
| □ Clinical Anxiety Scale (CAS) | |||
| Staff feelings on capability to provide care for a person with dementia | ∆ ◊ SSCQ | ||
| CR Cognitive ability | □ ◊ MMSE | ||
| Hospital | CR Behaviour (severity) | Pittsburgh Agitation Scale (PAS) | |
| Appropriateness of psychotropic medication | □ Medication Appropriateness Index (MAI) | ||
| Other outcomes | ∞ Total daily doses of benzodiazepines and antipsychotics administered, length of stay, discharge destination, number of falls, nursing satisfaction, next of kin (NOK) satisfaction with care. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of problem behaviours ‐ family care only Show forest plot | 4 | 722 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.17] |
| 2 Frequency of problem behaviours Show forest plot | 12 | 1551 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.20, ‐0.00] |
| 2.1 Family care | 10 | 1046 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 2.2 Residential care | 2 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.39, ‐0.03] |
| 3 Severity of problem behaviours Show forest plot | 5 | 449 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.29, 0.08] |
| 3.1 Family care | 2 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.58, 0.08] |
| 3.2 Residential care | 3 | 307 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.19] |
| 4 Patient depression Show forest plot | 3 | 480 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.33, 0.03] |
| 4.1 Family care | 2 | 375 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.29, 0.12] |
| 4.2 Residential care | 1 | 105 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.77, 0.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of problem behaviours ‐ family care only at 6 month follow‐up Show forest plot | 2 | 436 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.11, 0.27] |
| 2 Frequency of problem behaviours at 6 month follow‐up Show forest plot | 4 | 627 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.16, 0.16] |
| 3 Frequency of problem behaviours at 12 month follow‐up Show forest plot | 3 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.27] |
| 3.1 Family care | 3 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caregiver reaction Show forest plot | 11 | 1259 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, ‐0.00] |
| 1.1 Family care | 11 | 1259 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, ‐0.00] |
| 2 Caregiver burden Show forest plot | 6 | 624 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.29, 0.03] |
| 3 Caregiver well‐being (depression) Show forest plot | 5 | 473 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.30, 0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caregiver reaction Show forest plot | 4 | 653 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.27, 0.04] |
| 2 Caregiver burden Show forest plot | 2 | 286 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.38, 0.09] |
| 3 Caregiver well‐being (depression) Show forest plot | 2 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐2.56, 0.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Frequency of problem behaviours at post‐intervention Show forest plot | 2 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.50, 0.17] |
| 2 Severity of problem behaviours at post‐intervention Show forest plot | 2 | 176 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.02, 0.63] |
| 3 Caregiver burden at post‐intervention Show forest plot | 2 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.46, 0.21] |

