Protokollgestütztes Weaning im Vergleich zu Weaning ohne Protokoll zur Verkürzung der Dauer mechanischer Beatmung bei schwerkranken erwachsenen Patienten
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006904.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 06 noviembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Atención crítica y de emergencia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: B Blackwood (BB)
Co‐ordinating the review: BB
Undertaking manual searches: BB, P O'Halloran (POH)
Organizing retrieval of papers: BB, POH
Screening retrieved papers against inclusion criteria: BB, POH
Appraising quality of papers: BB, KEA Burns (KB)
Abstracting data from papers: BB, KB
Writing to authors of papers for additional information: BB
Providing additional data about papers: BB
Obtaining and screening data on unpublished studies: BB, KB
Data management for the review: BB
Entering data into Review Manager (RevMan 2014): BB
Review Manager statistical data: BB, CR Caldwell (CC)
Other statistical analysis not using Review Manager: BB, CC
Checking entry of data: (data entered by person one: BB; data checked by person two: POH)
Interpretation of data: CC, BB, POH, KB
Statistical analysis: CC, BB
Writing the review: BB, POH, KB, CC
Performing previous work that was the foundation of the present study: BB
Guarantor for the review (one author): BB
Person responsible for reading and checking review before submission: BB
Sources of support
Internal sources
-
Critical Care Translational Research Group, Northern Ireland, UK.
External sources
-
Research and Development Office, Northern Ireland and the Health Research Board, Ireland.
Cochrane Fellowship
Declarations of interest
Bronagh Blackwood: none known.
Karen EA Burns: holds a CAD 5000 travel bursary from Draeger Medical Inc. (Canada) for the purpose of conducting site visits to participating centres in the WEAN Study. The WEAN study is not included in this Cochrane review. (The WEAN Study is an investigator‐initiated trial comparing SmartCare™ and protocolized weaning, for which the co‐principal investigator (Dr Burns) obtained funding from peer review, non‐industry sources for implementation. Draeger Medical Inc. provided ventilators and ventilator upgrades for the WEAN study and a central randomization system using electronic mail correspondence (Draeger Medical, Germany). Draeger Medical was not involved in any aspects of study design and oversight, data management or data analysis).
Chris R Cardwell: none known.
Peter O'Halloran: none known.
Acknowledgements
We would like to thank Harald Herkner (content editor), Nathan Pace (statistical editor), Louise Rose, Paolo Pelosi, Iain McCullagh (peer reviewers), and Robert Wyllie (consumer editor/referee) for their help and editorial advice during the preparation of this updated systematic review. We would also like to acknowledge the contribution of Professor Fiona Alderdice and Dr Gavin Lavery to the original review (Blackwood 2010).
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Nov 06 | Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients | Review | Bronagh Blackwood, Karen EA Burns, Chris R Cardwell, Peter O'Halloran | |
| 2010 May 12 | Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients | Review | Bronagh Blackwood, Fiona Alderdice, Karen EA Burns, Chris R Cardwell, Gavin Lavery, Peter O'Halloran | |
| 2008 Jan 23 | Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients | Protocol | Bronagh Blackwood, Fiona Alderdice, Karen E. A. Burns, Chris R Cardwell, Gavin Lavery, Peter O'Halloran | |
Differences between protocol and review
There are four differences between the published protocol (Blackwood 2008) and this updated review.
-
We included quasi‐randomized controlled trials, that is trials that prospectively assigned patients to groups using a quasi‐random method such as alternation or hospital number. We included these studies because we felt that the rule‐based system reduced investigator bias to a certain degree. Nevertheless, we assessed risk of bias in a similar manner to randomized controlled trials and conducted a sensitivity analysis excluding quasi‐randomized trials.
-
We used The Cochrane Collaboration's new domain‐based evaluation to assess the validity and quality of the included studies because this was released after publication of the protocol.
-
We included neurosurgical units in the subgroup analysis of type of unit as there are specific differences in weaning this group of patients because of their neurological impairment.
-
We included one further sensitivity analysis to explore the impact on the findings before log transforming the variables to approximate normality.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Updated study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG04.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG05.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].
![Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG06.png)
Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].
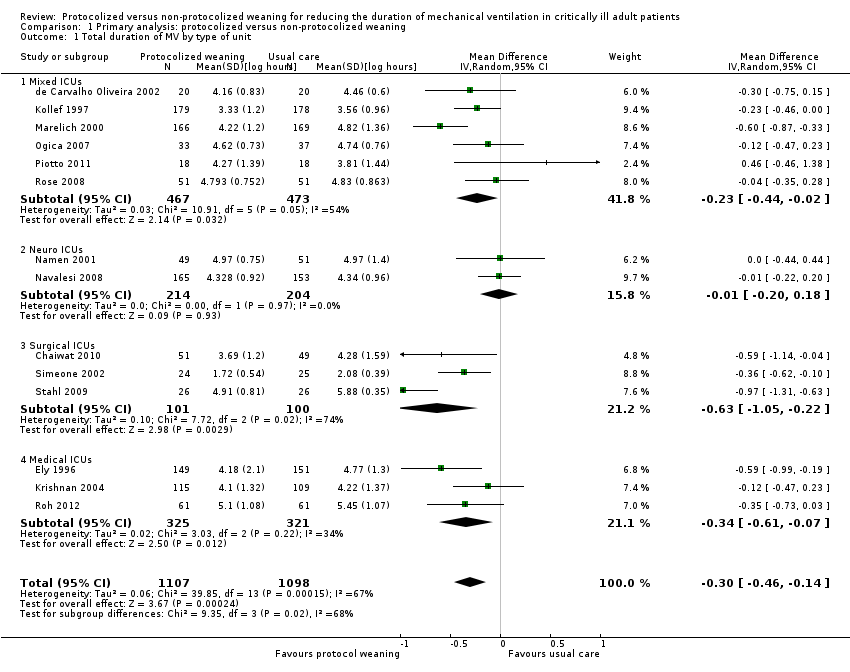
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 1 Total duration of MV by type of unit.
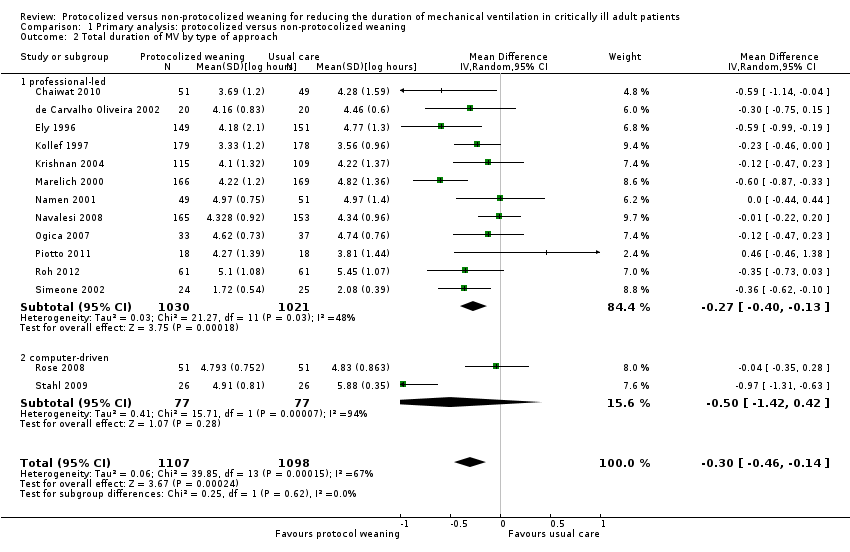
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 2 Total duration of MV by type of approach.
![Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 3 Total duration of MV by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-CMP-001-03.png)
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 3 Total duration of MV by type of protocol [log hours].

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 4 Mortality.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 5 Reintubation.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 6 Self extubation.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 7 Tracheostomy.
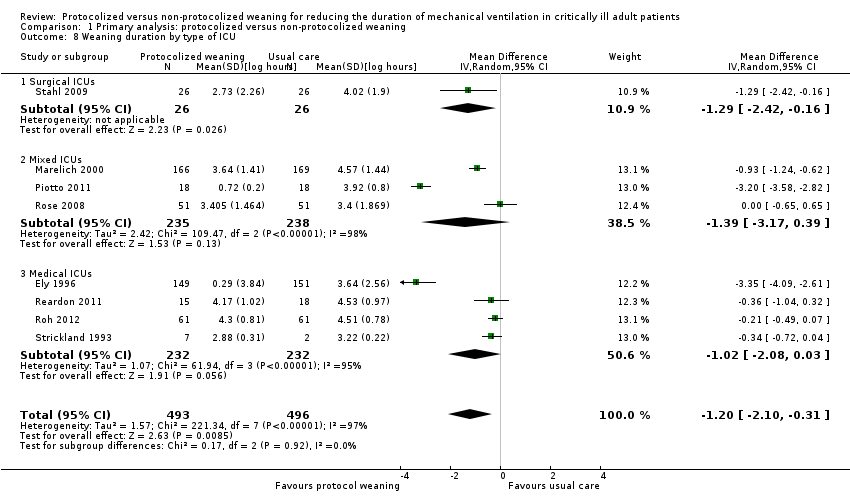
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 8 Weaning duration by type of ICU.
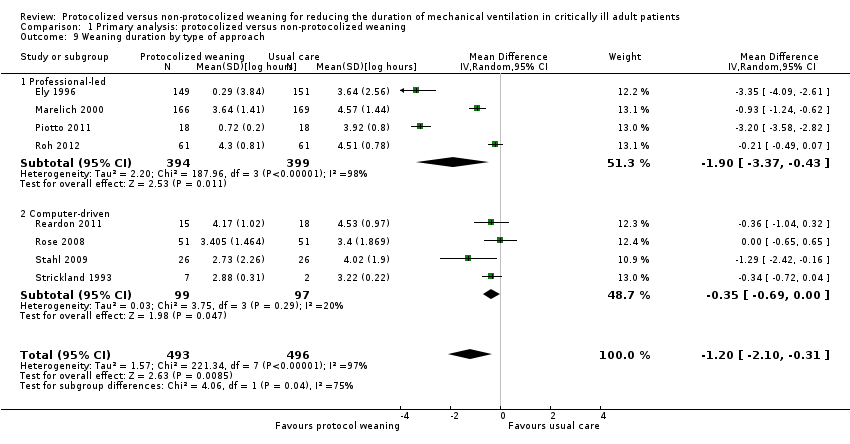
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 9 Weaning duration by type of approach.
![Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 10 Weaning duration by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-CMP-001-10.png)
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 10 Weaning duration by type of protocol [log hours].

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 11 ICU length of stay.
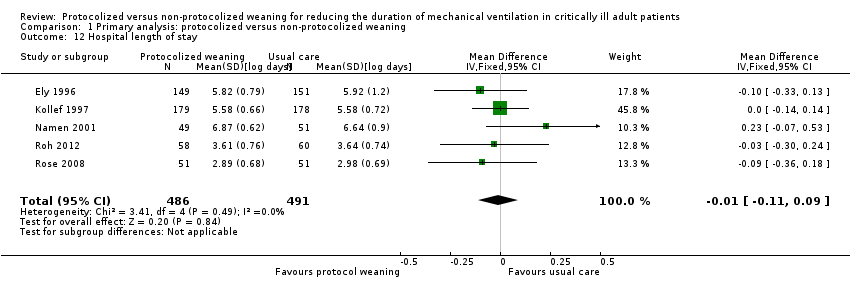
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 12 Hospital length of stay.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 13 ICU costs.
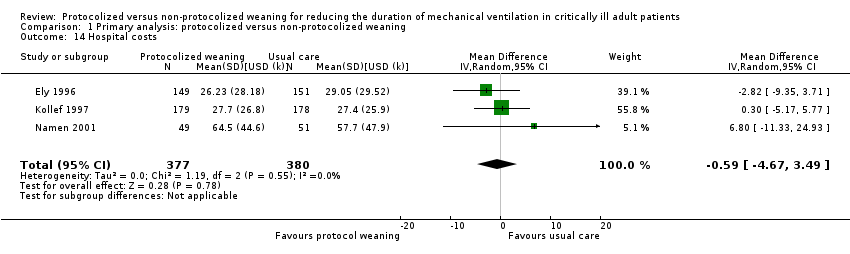
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 14 Hospital costs.
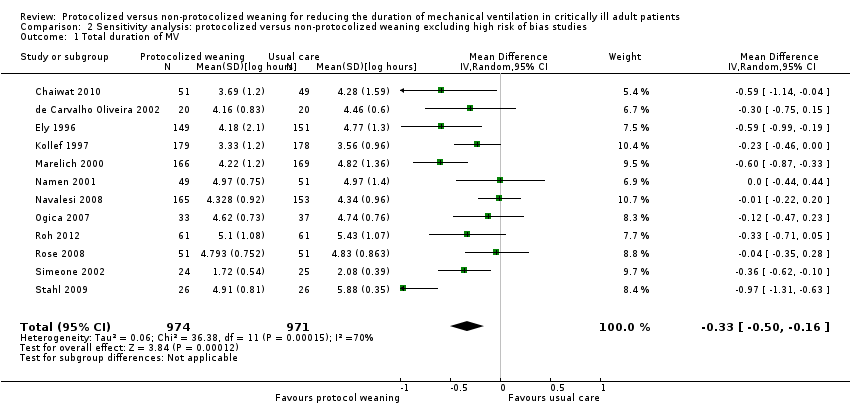
Comparison 2 Sensitivity analysis: protocolized versus non‐protocolized weaning excluding high risk of bias studies, Outcome 1 Total duration of MV.
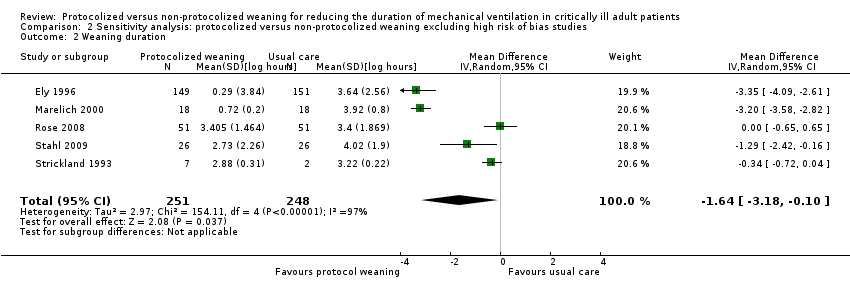
Comparison 2 Sensitivity analysis: protocolized versus non‐protocolized weaning excluding high risk of bias studies, Outcome 2 Weaning duration.
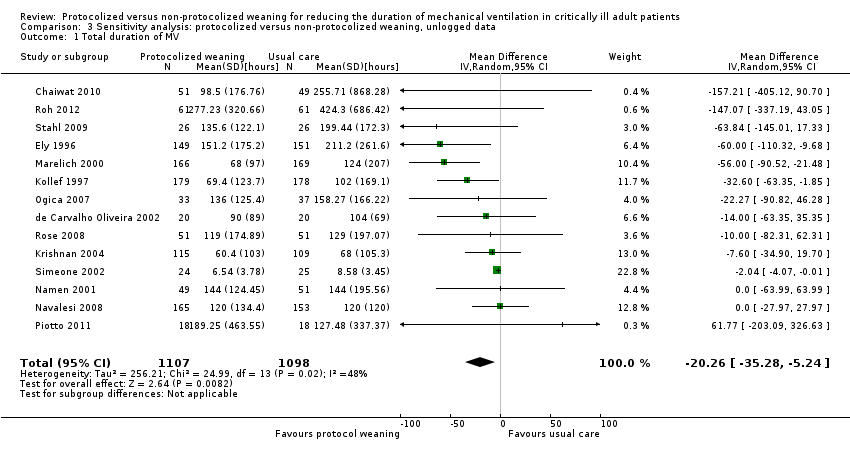
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 1 Total duration of MV.
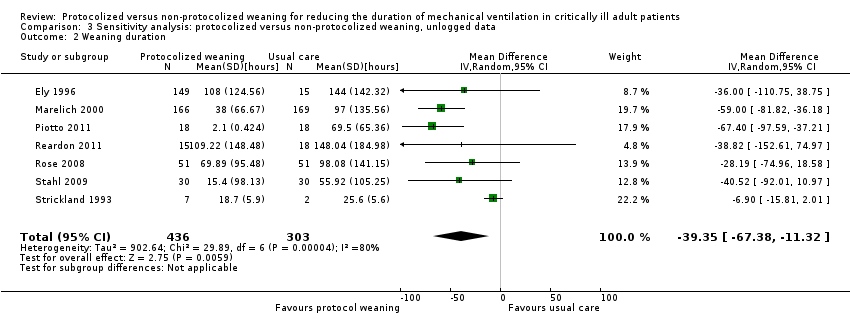
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 2 Weaning duration.

Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 3 ICU length of stay.
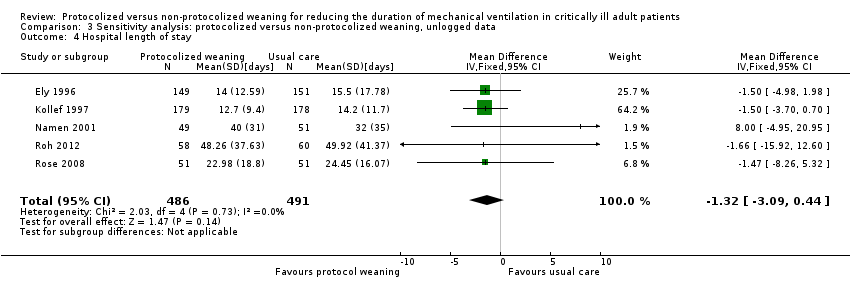
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 4 Hospital length of stay.
| Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients | |||||
| Patient or population: mechanically ventilated adult patients Settings: intensive care units Intervention: protocolized weaning Comparison: non‐protocolized weaning | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect Estimates (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk non‐protocolized weaning | Corresponding risk protocolized weaning | ||||
| Total duration of mechanical ventilation (hours) | Mean 96 hours1 | Mean 71 hours (60.5 to 83.5 hours) | Geometric mean difference ‐26% (‐37% to ‐13%) | 2205 | +++O |
| Weaning duration (hours) | Mean 24 hours1 | Mean 7 hours (2.8 to 17.5 hours) | Geometric mean difference ‐70% (‐88% to ‐27%) | 989 | ++OO |
| ICU length of stay (days) | Mean 8 days1 | Mean 7 days (6.5 to 7.8 days) | Geometric mean difference ‐11% (‐19% to ‐3%) | 1378 [9 studies] | ++OO |
| ICU mortality | 31%1 | 30% (20% to 42%) | OR 0.97 (0.57 to 1.63) | 651 [6 studies] | +++O |
| Reintubation | 10%1 (following deliberate extubation) | 8% (5% to 12%) | OR 0.74 (0.44 to 1.23) | 1487 [11 studies] | ++OO |
| *The basis for the assumed risk (e.g. the mean control group risk) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the effect estimate of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The assumed risk is derived from the median reported in a large epidemiological study of characteristics and outcomes in patients (N = 4968) receiving mechanical ventilation by Esteban 2008. The reported medians were used as an approximation for the means used for illustrative comparisons of all continuous variables. The table shows the mean duration of mechanical ventilation, weaning and ICU length of stay if patients are not weaning by protocol (non‐protocolized weaning) and what would be expected with protocolized weaning based on the effect estimates from our review. 2 There was considerable variability in effect estimates (I2 = 67%) that could not be explained by subgroup analysis although variability was lower than the previous review. The confidence interval was narrower in this review and the difference at the lower limit would still be clinically significant. 3 There was considerable variability in effect estimates (I2 = 97%) and the wide confidence intervals indicate imprecision in results. The lower limit suggests a one hour difference in weaning that is not clinically significant. 4 There was no heterogeneity among trials effects estimates, but wide confidence intervals indicate imprecision in results. 5 There was moderate variability in effect estimates (I2 = 50%). 6 There was moderate variability in effect estimates (I2 = 43%). | |||||
| Study | Assessment frequency | Oxygenation | Other respiratory factors | Cardiovascular | Neurological | Inflammatory response | Medication | Other |
| Daily screen | PaO2/FiO2 >/= 200 on FiO2 </= 0.4 SpO2 >/= 94% | PEEP </= 5 Respiratory rate < 35 Rapid Shallow breathing index </= 105 Static lung compliance >/= 25 mL/cmH2O Minute volume </= 10L/min | HR < 120 b/min | Awake and easily rousable | Not included | Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Pain score < 4 | |
| Not reported | PaO2 < 90 on FiO2 </= 0.4 | PEEP < 5 Pimax < ‐ 25 cm H2O | Not included | GCS > 8 | Not included | No sedation No vasopressors | Cause of MV resolved No planned surgery | |
| Daily screen
| PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Protocol entry criteria
| PaO2/FiO2 > 200 | PEEP </= 5 RR </= 35 b/min
| HR < 140 b/min | Awake and orientated | Not included | No vasoactive or inotropic agents | Not included | |
| Daily screen
| SpO2 >/= 92% FiO2 </= 0.5 | PEEP </=5
| Stable CAD HR < 140 b/min | No raised ICP | Not included | No paralytics | Cough and gag reflex present Responsive to stimuli
| |
| x 2 daily screen
| PaO2/FiO2 >/= 200 | Not included | MAP >/= 60 mmHg | GCS >/= 10 or tracheostomy | Not included | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough not limited by pain
| |
| Daily screen | PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | PaO2/FiO2 > 200 FiO2 </= 0.4 pH >/= 7.35 PaCO2 </= 50 mmHg | PEEP </= 5
| HR </= 125 b/min SBP >/= 90 mmHg | GCS >/= 8 | T < 38.5oC | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough Suctioning < 2/hr Normal Na blood values
| |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Daily screen | PaO2/FiO2 150‐300 FiO2 </= 0.4 PaO2 >/= 60 mmHg Hb = 8 ‐ 10 g/L
| Not included | MAP >/= 60 mmHg HR </= 140 b/min | Awake GCS >/= 9 | T < 37.8oC | Minimum sedation No or low vasopressors | Cause of MV resolved Effective cough Metabolic stability No hydroelectrolyte disorders
| |
| Daily screen | SaO2 > 90% or PaO2 > 60 mmHg on FiO2 </= 0.5 | Respiratory rate < 35 pH > 7.20 Triggering breaths | SBP > 90 and < 180 HR > 50 and < 130 No cardiac ischaemia | GCS > 8 | Not included | Minimal pressure requirements | Improving condition Absence of excessive secretions Suctioning < hourly Deemed ready to wean | |
| Not reported | FiO2 </= 0.5 | RR </= 35 PEEP </= 8 Triggering breaths | SBP >/= 90 mmHg HR </= 150 b/min | Not included | Not included | No paralytics No vasopressors Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Not included | |
| Inclusion criteria | PaO2/FiO2 > 150 or SaO2 >/= 90% on FiO2 0.5 | PEEP </= 8 Plateau pressure </= 30 cmH2O Successful 30 min SBT using PS 20 cm H2O to achieve TV > 200 mL
| Haemodynamically stable | GCS > 4 | T = 36 ‐ 39oC | Not included | No surgery anticipated MV > 24 hr
| |
| Inclusion criteria | PaO2/FiO2 >/= 200 FiO2 < 0.5 pH 7.3 ‐ 7.5 PaO2 30 ‐ 50 mmHg SaO2 > 90% Hb > 8 mg/dL Pulse oximeter oxygenation stable Cardiopulmonary bypass time < 150 min | PEEP < 4 RR < 35 b/min Dynamic compliance > 22 mL/cmH2O Compliance statica >33 mL/cmH2O Vital capacity >10 mL/kg MIP >/= ‐15 cmH2O
| Haemodynamically stable | Awake and conscious | T > 35 < 38oC | Not included | Urine output > 100 mL/hr Normal CXR | |
| Inclusion criteria | FiO2 </= 0.5 PaO2 > 75 mmHg or SaO2 > 90% pH </= 7.2 Hb >/= 7g/dL | PEEP </= 10 | Haemodynamically stable | Not included | Not included | Dopamine </= 5 ug/kg/min | MV > 24 hr Breathing spontaneously Ramsey sedation score =/< 3
| |
| Inclusion criteria | FiO2 </= 0.4 pH >/= 7.3 </= 7.5 PCO2 >/= 30 </= 50 SaO2 >/= 90% on SIMV rate 6 ‐ 10 PS 20 cmH2O | NIF </= ‐ 20 cmH2O FVC >/= 10 mL/kg TV 10 ‐ 15 mL/kg
| Haemodynamically stable | Not included | T </= 37oC | Not included | Judged ready to wean by physician Feeding ‐ parenteral or tube Stable renal function Normal electrolytes
| |
| CAD = coronary artery diease; CXR = chest X‐ray; GCS = Glasgow Coma Scale; FVC = forced vital capacity; Hb = haemoglobin; HR ‐ heart rate; MAP = mean arterial pressure; MIP = maximal inspiratory pressure; MV = mechanical ventilation; NIF = negative inspiratory force; PEEP = positive end expiratory pressure; Pimax = maximal inspiratory mouth pressure; PS = pressure support; RR = respiratory rate; SBP = systolic blood pressure; SIMV = synchronized intermittent mechanical ventilation; T = temperature; TV = tidal volume; f/VT = ratio of respiratory frequency to tidal volume. | ||||||||
| Study | Time of randomization | Intervention protocol | Extubation criteria | Comparator (usual practice) |
| ICU admission | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Notify MD | Not reported | |
| Not reported | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Yes | Not reported | |
| Enrolment, time not reported | SBT 2 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| Not reported | a) SBT 30 minutes and extubation if passed b) If failed, daily SBT and stepwise reduction in SIMV and PS until 4 breaths/min and PS 7 cmH2O | Not reported | Not reported | |
| ICU admission | a) SBT 30 to 60 min on CPAP 5 cmH2O, PS 6 cmH2O b) PS stepwise reduction to 6 cmH2O c) IMV stepwise reduction to 0 breaths/min, on PEEP 5 cmH2O and PS 6 cmH2O for 30 to 60 min | a) Yes
b) Yes c) Yes | Not reported | |
| Not reported | SBT 1 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| On meeting weaning criteria | a) < 72‐hour admissions: SBT 30 min on PS </= 8 cmH2O & PEEP </= 8 cmH2O b) > 72‐hour admissions: PEEP, IMV and PS stepwise reductions to achieve FiO2 0.5, PEEP </= 8 cmH2O, IMV </= 6 breaths/min, PS </= 8 cmH2O then SBT as above | a) Notify MD
b) Notify MD | Not reported | |
| On meeting weaning criteria | SBT 2 hours on CPAP 5 cmH2O | Notify MD | Not reported | |
| Enrolment, time not reported | SBT 1 hour on CPAP 2 to 3 cmH2O, FiO2 0.4 | Yes | Not reported | |
| Not reported | SBT (details not reported) | Not reported | Not reported | |
| Not reported | SBT 2 hours on PS 7 cmH2O, PEEP 5 cmH2O, FiO2 0.4, RR 1 breath/min | Yes | Stepwise reduction in PS and IMV | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | Notify MD | Stepwise reduction in PS and SBT | |
| On meeting weaning criteria | CPAP trial on 5 cmH2O, then stepwise reductions in PS to 5 cmH2O, then SBT on T‐piece for 30 minutes | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | No | Stepwise reduction in PS and PEEP | |
| Not reported | SIMV and PS stepwise reductions to SIMV 0 breath/min and PS 4 cmH2O | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS stepwise reductions to PS | Yes | Spepwise reduction in PS and CPAP | |
| On meeting weaning criteria | Computer automated Supersport model 2 stepwise reductions in SIMV and PS to RR 2 breaths/min and PS 5 cmH2O | Not reported | Stepwise reduction in IMV and PS | |
| CPAP = continuous positive airway pressure; IMV = intermittent mechanical ventilation; MD = Medical Doctor; PEEP = positive end expiratory pressure; PS = pressure support; SBT = spontaneous breathing trial; SIMV =synchronized intermittent mechanical ventilation; RR = respiratory rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV by type of unit Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 1.1 Mixed ICUs | 6 | 940 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.44, ‐0.02] |
| 1.2 Neuro ICUs | 2 | 418 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.20, 0.18] |
| 1.3 Surgical ICUs | 3 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.05, ‐0.22] |
| 1.4 Medical ICUs | 3 | 646 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.61, ‐0.07] |
| 2 Total duration of MV by type of approach Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 2.1 professional‐led | 12 | 2051 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.40, ‐0.13] |
| 2.2 computer‐driven | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.42, 0.42] |
| 3 Total duration of MV by type of protocol [log hours] Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 3.1 SBT protocol | 8 | 1188 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, 0.00] |
| 3.2 Stepwise reduction protocol | 6 | 1017 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.66, ‐0.18] |
| 4 Mortality Show forest plot | 14 | 2234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.26] |
| 4.1 Hospital mortality | 8 | 1523 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.32] |
| 4.2 ICU mortality | 7 | 711 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.48] |
| 5 Reintubation Show forest plot | 11 | 1487 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.23] |
| 6 Self extubation Show forest plot | 3 | 433 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.34] |
| 7 Tracheostomy Show forest plot | 8 | 1346 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 8 Weaning duration by type of ICU Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 8.1 Surgical ICUs | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.42, ‐0.16] |
| 8.2 Mixed ICUs | 3 | 473 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.17, 0.39] |
| 8.3 Medical ICUs | 4 | 464 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.08, 0.03] |
| 9 Weaning duration by type of approach Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 9.1 Professional‐led | 4 | 793 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.37, ‐0.43] |
| 9.2 Computer‐driven | 4 | 196 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.69, ‐0.00] |
| 10 Weaning duration by type of protocol [log hours] Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 10.1 SBT protocol | 2 | 336 | Mean Difference (IV, Random, 95% CI) | ‐3.23 [‐3.57, ‐2.89] |
| 10.2 Stepwise reduction protocol | 6 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.81, ‐0.12] |
| 11 ICU length of stay Show forest plot | 9 | 1378 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.21, ‐0.03] |
| 12 Hospital length of stay Show forest plot | 5 | 977 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 13 ICU costs Show forest plot | 2 | 400 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐15.02, 21.76] |
| 14 Hospital costs Show forest plot | 3 | 757 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐4.67, 3.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV Show forest plot | 12 | 1945 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.50, ‐0.16] |
| 2 Weaning duration Show forest plot | 5 | 499 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.18, ‐0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐20.26 [‐35.28, ‐5.24] |
| 2 Weaning duration Show forest plot | 7 | 739 | Mean Difference (IV, Random, 95% CI) | ‐39.35 [‐67.38, ‐11.32] |
| 3 ICU length of stay Show forest plot | 9 | 1378 | Mean Difference (IV, Fixed, 95% CI) | ‐9.08 [‐15.85, ‐2.30] |
| 4 Hospital length of stay Show forest plot | 5 | 977 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐3.09, 0.44] |

