جداسازی بیمار از دستگاه ونتیلاسیون مکانیکی بر اساس پروتکلهای موجود، در مقایسه با عدم تبعیت از پروتکلها، به منظور کاهش مدت زمان استفاده از دستگاه ونتیلاسیون مکانیکی در بیماران بزرگسالان به شدت بدحال
چکیده
پیشینه
این یک بهروزرسانی از یک مرور کاکرین است که برای آخرین بار در شماره 5، سال 2010 کتابخانه کاکرین منتشر شد. کاهش زمان جداسازی بیمار از دستگاه ونتیلاتور، در به حداقل رساندن عوارض احتمالی ناشی از ونتیلاسیون مکانیکی، مطلوب است. هدف از استاندارد کردن پروتکلهای جداسازی بیمار از دستگاه ونتیلاسیون، کاهش مدت زمان وابستگی به ونتیلاسیون مکانیکی برای تنفس است. با این حال، شواهد حمایت کننده از کاربرد آنها در بالین، متناقض هستند.
اهداف
هدف اولیه این مطالعه مروری، مقایسه کل مدت زمان استفاده از ونتیلاسیون مکانیکی در بزرگسالان به شدت بدحالی بود که براساس پروتکلها از دستگاه جدا شدند، در مقابل افرادی که بر اساس روشهای متداول (بدون تبعیت از پروتکلها)، جداسازی در آنان صورت گرفت.
هدف دوم، عبارت بود از مشخص کردن تفاوتها میان جداسازی بیمار از دستگاه ونتیلاتور براساس پروتکلها و بدون رعایت پروتکلها، بر پیامدهای اندازهگیری کننده مدت زمان جداسازی از دستگاه، آسیب (عوارض جانبی) و استفاده از منابع (واحد مراقبتهای ویژه (ICU) و طول مدت زمان بستری در بیمارستان، هزینه).
هدف سوم، ارزیابی کردن، با استفاده از آنالیزهای زیرگروه، تغییرات در پیامدها بر اساس نوع ICU، نوع پروتکل و رویکرد صورت گرفته به ارائه پروتکل (توسط فرد متخصص یا توسط کامپیوتر) بود.
روشهای جستوجو
ما پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL) (کتابخانه کاکرین؛ شماره 1؛ 2014)؛ MEDLINE (1950 تا ژانویه 2014)؛ EMBASE (1988 تا ژانویه 2014)؛ CINAHL (1937 تا ژانویه 2014)؛ LILACS (1982 تا ژانویه 2014)؛ ISI Web of Science و مجموعه مقالات کنفرانس ISI (1970 تا فوریه 2014) و فهرست منابع مقالات را جستوجو کردیم. ما هیچ گونه محدودیت زبانی را اعمال نکردیم. جستوجوی اصلی در ژانویه 2010 انجام و در ژانویه 2014 بهروزرسانی شد.
معیارهای انتخاب
ما کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و شبه‐RCTهای انجام شده را در مورد جداسازی بیماران به شدت بدحال از دستگاه ونتیلاتور مکانیکی بر اساس پروتکلها در برابر عدم رعایت پروتکلها، وارد کردیم.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم کیفیت کارآزمایی را ارزیابی و دادهها را استخراج کردند. آنالیزهایی را روی زیرگروه قبلی و حساسیت انجام دادیم. برای کسب اطلاعات بیشتر با نویسندگان مطالعه تماس گرفتیم.
نتایج اصلی
در این بهروزرسانی مرور، 17 کارآزمایی (با 2434 بیمار) را وارد کردیم. مرور اصیل شامل 11 کارآزمایی بود. میانگین عددی طول مدت زمان نیاز به ونتیلاسیون مکانیکی در گروه جداسازی بر اساس پروتکلها، در مقایسه با گروه مراقبت متداول، بهطور متوسط 26% کاهش یافت (14 کارآزمایی؛ 95% فاصله اطمینان (CI): 13% تا 37%؛ P = 0.0002). موارد کاهش در مدت زمان نیاز به استفاده از دستگاه ونتیلاسیون در بخشهای مراقبت ویژه داخلی، جراحی و مختلط داخلی/جراحی با احتمال بیشتری اتفاق افتاد، اما در ICUهای جراحی اعصاب، اینگونه نبود. طول مدت زمان جداسازی بیمار از دستگاه ونتیلاتور، تا 70% (8 کارآزمایی؛ 95% CI؛ 27% تا 88%؛ P = 0.009) و طول مدت بستری در بخش مراقبتهای ویژه، تا 11% (9 کارآزمایی؛ 95% CI؛ 3% تا 19%؛ P = 0.01) کاهش یافت. ناهمگونی قابل توجهی میان مطالعات برای کل مدت زمان استفاده از ونتیلاسیون مکانیکی (I2 = 67%، P < 0.0001) و مدت زمان جداسازی از دستگاه ونتیلاتور (I2 = 97%، P < 0.00001) وجود داشت، که با آنالیزهای زیرگروه بر اساس نوع واحد یا نوع رویکرد، توجیهپذیر نبود.
نتیجهگیریهای نویسندگان
شواهدی از کاهش مدت زمان نیاز به ونتیلاسیون مکانیکی، مدت زمان جداسازی از دستگاه و طول مدت بستری در بخش مراقبتهای ویژه با استفاده از پروتکلهای استاندارد جداسازی بیمار از دستگاه وجود دارد. موارد کاهش در مدت زمان نیاز به استفاده از دستگاه ونتیلاسیون در بخشهای مراقبت ویژه داخلی، جراحی و مختلط داخلی/جراحی با احتمال بیشتری اتفاق افتاد، اما در ICUهای جراحی اعصاب، اینگونه نبود. با این حال، وجود ناهمگونی قابل توجه میان مطالعات، به این معنی است که باید در تعمیم نتایج احتیاط کرد. برخی از نویسندگان مطالعه پیشنهاد میکنند که پیشزمینه سازمانی ممکن است پیامدها را متاثر کند، اما این عوامل در همه مطالعات وارده در نظر گرفته نشدند و قابل ارزیابی نبودند. کارآزماییهای آتی باید ارزیابی روند انجام مداخله را در نظر بگیرند تا بتوانند میان تاثیرات مداخله و اجرای آنها، تمایز قایل شوند. نیاز قابلتوجهی برای توسعه و پژوهش بیشتر در جمعیت بیماران گروه جراحی اعصاب وجود دارد.
PICO
خلاصه به زبان ساده
سودمندی تبعیت از پروتکلهای جداسازی بیمار از دستگاه ونتیلاسیون مکانیکی به منظور کاهش مدت زمانی که بیماران بزرگسال به شدت بدحال نیازمند ونتیلاسیون مکانیکی هستند
سوال مطالعه مروری: ما شواهد را در مورد تاثیر پروتکلهای (دستورالعملها (guidelines)) جداسازی بیمار از دستگاه ونتیلاسیون مکانیکی که پزشکان از آنها استفاده میکنند، در کاهش مدت زمان نیاز بیماران بزرگسال به شدت بدحال به ماشین تنفس، مرور کردیم.
پیشینه: کمک به بیماران برای تنفس با استفاده از ونتیلاتور مکانیکی، میتواند نجاتبخش زندگی آنها باشد. هر چه فردی مدت زمان بیشتری را وابسته به یک دستگاه ونتیلاتور باشد، احتمال بروز اثرات مضر، مانند عفونت ریهها و عوارض ناشی از عدم تحرک طولانیمدت نظیر تشکیل لخته خونی در پاها یا ریهها، افزایش مییابد. بنابراین، تشخیص زودهنگام این که بیماران چه زمانی آماده تنفس مستقل و خودبهخودی هستند تا بتوان آنها را به تدریج از دستگاه ونتیلاتور جدا کرد (تحت عنوان فرآیند جداسازی از ونتیلاتور (weaning))، اهمیت زیادی دارد. معمولا، جداسازی بیمار از دستگاه ونتیلاتور بر اساس نظر پزشکان انجام میشود، اما پروتکلهای اخیر برای جداسازی بیماران از دستگاه ونتیلاتور، برای بیماران بیخطر و برای پزشکان کارآمد شناخته شدهاند. برخی مطالعات معتقدند که پروتکلها، موجب عملکرد بالینی بهتر میشوند، اما شواهد روشنی وجود ندارد که استفاده از آنها، واقعا نتایج مفیدی را برای بیماران به همراه داشته باشند.
تاریخ جستوجو: شواهد تا ژانویه 2014 بهروز است.
ویژگیهای مطالعه: این مرور بهروز شده کاکرین، شامل 17 مطالعه با حضور 2434 زن و مرد به شدت بدحال بود که تحت مراقبت در بخشهای مراقبت ویژه داخلی، جراحی، جراحی اعصاب و ترکیب داخلی/جراحی قرار داشتند. در این مطالعات، استفاده از پروتکلها برای جداسازی بیماران از دستگاه ونتیلاتور با مراقبت بالینی معمول مقایسه شدند. مطالعات مذکور در بخشهای مراقبتهای ویژه در آمریکا، اروپا، آسیا و استرالیا انجام شدند. بخشهای مراقبتهای ویژه، از بیماران مبتلا به بیماریهای قلبی، مشکلات تنفسی، آسیبهای سر، تروما و پس از جراحی بزرگ، مراقبت کردند. در 13 مطالعه، پزشکان از پروتکلهای جداسازی بیماران از دستگاه ونتیلاتور برای کاهش نیاز آنها به حمایت تنفسی استفاده کردند. در 4 مطالعه، حمایت تنفسی ونتیلاتور توسط کامپیوترهای برنامهریزی شده و بر اساس یک پروتکل، به طور خودکار کاهش یافت.
نتایج: در مقایسه با روش معمول و بدون تبعیت از پروتکلهای جداسازی، میانگین کل زمان وابستگی به دستگاه ونتیلاتور، تا 26% کاهش یافت. مدت زمان جداسازی از دستگاه ونتیلاتور، تا 70% و مدت زمان اقامت در بخش مراقبت ویژه، تا 11% کمتر شد. استفاده از پروتکلهای جداسازی، منتهی به هیچ آسیب اضافی دیگری نشد. ما تفاوت قابل توجهی را در انواع پروتکلهای مورد استفاده، معیارهایی که باید برای زمان شروع جداسازی از دستگاه در نظر گرفته شوند، شرایط پزشکی بیماران و روش معمول جداسازی بیماران از دستگاه، یافتیم. این موضوع بدین معناست که نمیتوانیم بگوییم کدام پروتکلها برای بیماران خاص بهتر عمل خواهند کرد، اما میدانیم که این موارد در بیماران جراحی اعصاب مفید نیستند.
کیفیت شواهد: کیفیت شواهد موجود را برای طول مدت زمان نیاز به ونتیلاسیون و تاثیرات مضر آن، در سطح متوسط و برای طول مدت زمان جداسازی بیماران از دستگاه و مدت بستری در بخش مراقبتهای ویژه، در سطح پائین قرار دادیم. دلایل ما برای این رتبهبندی شواهد این بود که نتایج در طول مطالعات پیوسته و پایدار نبودند، و مطالعات جزئیات کافی را در مورد روشهای متداول درمانی توصیف نکردند.
Authors' conclusions
Summary of findings
| Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients | |||||
| Patient or population: mechanically ventilated adult patients Settings: intensive care units Intervention: protocolized weaning Comparison: non‐protocolized weaning | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect Estimates (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk non‐protocolized weaning | Corresponding risk protocolized weaning | ||||
| Total duration of mechanical ventilation (hours) | Mean 96 hours1 | Mean 71 hours (60.5 to 83.5 hours) | Geometric mean difference ‐26% (‐37% to ‐13%) | 2205 | +++O |
| Weaning duration (hours) | Mean 24 hours1 | Mean 7 hours (2.8 to 17.5 hours) | Geometric mean difference ‐70% (‐88% to ‐27%) | 989 | ++OO |
| ICU length of stay (days) | Mean 8 days1 | Mean 7 days (6.5 to 7.8 days) | Geometric mean difference ‐11% (‐19% to ‐3%) | 1378 [9 studies] | ++OO |
| ICU mortality | 31%1 | 30% (20% to 42%) | OR 0.97 (0.57 to 1.63) | 651 [6 studies] | +++O |
| Reintubation | 10%1 (following deliberate extubation) | 8% (5% to 12%) | OR 0.74 (0.44 to 1.23) | 1487 [11 studies] | ++OO |
| *The basis for the assumed risk (e.g. the mean control group risk) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the effect estimate of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The assumed risk is derived from the median reported in a large epidemiological study of characteristics and outcomes in patients (N = 4968) receiving mechanical ventilation by Esteban 2008. The reported medians were used as an approximation for the means used for illustrative comparisons of all continuous variables. The table shows the mean duration of mechanical ventilation, weaning and ICU length of stay if patients are not weaning by protocol (non‐protocolized weaning) and what would be expected with protocolized weaning based on the effect estimates from our review. 2 There was considerable variability in effect estimates (I2 = 67%) that could not be explained by subgroup analysis although variability was lower than the previous review. The confidence interval was narrower in this review and the difference at the lower limit would still be clinically significant. 3 There was considerable variability in effect estimates (I2 = 97%) and the wide confidence intervals indicate imprecision in results. The lower limit suggests a one hour difference in weaning that is not clinically significant. 4 There was no heterogeneity among trials effects estimates, but wide confidence intervals indicate imprecision in results. 5 There was moderate variability in effect estimates (I2 = 50%). 6 There was moderate variability in effect estimates (I2 = 43%). | |||||
Background
Prolonged mechanical ventilation for critically ill patients is associated with adverse clinical outcomes, including physiological and psychological experiences. Consequently, in an effort to reduce morbidity and mortality associated with mechanical ventilation, clinical and research attention, over the last 20 years, has been focused on reducing the duration of mechanical ventilation, by improving the processes of ventilator weaning. For approximately 77% of patients, resuming spontaneous, unassisted breathing is accomplished easily (Esteban 2008); for others it is more difficult. Patients who experience difficulty in discontinuing mechanical ventilation present significant challenges to clinicians involved in their care. These patients frequently require longer hospital stays and generally have a higher morbidity, including ventilator‐associated pneumonia, ventilator‐associated lung injury and mortality (Boles 2007). Moreover, ventilator‐dependent patients generally remain in an intensive care unit (ICU) setting, as they require specialized care and frequent monitoring. In the current climate of limited ICU bed availability, maximizing use of limited ICU resources (including nursing and equipment costs) is an important goal of providing care to critically ill patients. Thus, timely and safe discontinuation of mechanical ventilation is a desirable outcome for patients and clinicians alike.
Description of the condition
The process leading to discontinuation of mechanical support is known as 'weaning' and has been classically defined as follows. "Weaning from mechanical ventilation represents the period of transition from total ventilatory support to spontaneous breathing" (Mancebo 1996). However, there are many interpretations of the 'period of transition' and the endpoint of 'spontaneous breathing'.
The transition period may take many forms, ranging from abrupt to gradual withdrawal from ventilatory support (Alia 2000). Some clinicians do not view abrupt withdrawal as weaning and suggest the term 'discontinuation' as a better descriptor, with 'weaning' being used to describe the more gradual withdrawal process (Cook 2000; Hess 2011). There are differing schools of thought regarding this gradual process of weaning. Some clinicians maintain that the transition should be initiated gradually right from the outset of mechanical ventilation, with as much of the breathing workload transferred to the patient as tolerated; which obscures the onset of weaning. Other clinicians believe that the transition should only be attempted when the condition that indicated the need for respiratory support has significantly resolved. Another view is to provide full support during an initial period and then attempt to transfer the breathing workload to the patient when the patient's condition shows early signs of improvement (Marini 1995). The work of Levine and colleagues (Levine 2008) showing marked atrophy of diaphragmatic myofibrils after less than three days of ventilation would support strategies that lead to some early spontaneous breathing during the phase of mechanical ventilatory support. Gradually transferring the breathing workload requires titrating ventilatory support to the needs of the patient. Titration may mean increasing or decreasing support and may be so gradual that it leads to problems in defining the time when weaning commenced.
The end of the weaning process can be defined as the cessation of mechanical ventilation, which implies the return of spontaneous breathing, but the term spontaneous breathing is ambiguous. All forms of spontaneous breathing involve the initiation of each breath by the patient, and contraction of the respiratory muscles. If the patient is free from all respiratory support (disconnected from the ventilator and extubated, or disconnected but still intubated and breathing through a T‐piece circuit), the depth or size of the patient's breath will depend upon the strength and duration of respiratory muscle contraction, airways resistance and lung compliance. If the patient is still connected to a ventilator, the patient‐initiated breath may be augmented by mechanical (albeit minimal) assistance from the ventilator. Both these situations are considered to be spontaneous breathing. Furthermore, some clinicians view the end of the weaning process as extubation without the need for (i) reintubation and (ii) ventilatory support within the following 48 to 72 hours (MacIntyre 2001).
Identifying when the patient is ready to wean, and deciding on the most appropriate method of weaning is influenced by the judgement and experience of the clinician (Sahn 1973). In some cases, clinicians tend to underestimate the probability of successful discontinuation of mechanical ventilation (Strickland 1993) and predictions, based on judgement alone, have low sensitivity (ability to predict success) and specificity (ability to predict failure) (Stroetz 1995). Until recently, there have been few standards of care in this area based on scientifically sound data. As a result, variation exists in weaning practice. There are several options, or weaning methods, for decreasing support. They include intermittent T‐piece trials involving short time periods of spontaneous breathing through a T‐piece circuit; synchronized intermittent mechanical ventilation (SIMV) involving gradual reductions in the ventilator rate, by increments of 1 to 4 breaths/min; pressure support ventilation (PSV) involving the gradual reduction of pressure by increments of 2 to 6 cmH2O; spontaneous breathing through a ventilator circuit with the application of continuous positive airway pressure (CPAP), and combinations of these and newer options, such as bi‐level, positive airway pressure. The evidence is equivocal as to which method is superior, although it has been suggested that SIMV is the least effective method (Brochard 1994; Esen 1992; Esteban 1995).
Description of the intervention
A weaning protocol is a structured guide for reducing, or discontinuing, or both, mechanical ventilatory support, and it generally contains three components. The first component is a list of objective criteria based on general clinical factors used to help decide if a patient is ready to breathe without the help of a ventilator, often referred to as 'readiness to wean' criteria (such as that used by Ely 1996). The second component consists of structured guidelines for reducing ventilatory support. This may be abrupt (for example spontaneous breathing trials) or gradual by using a stepwise reduction in support to achieve discontinuation (for example SIMV or PSV), such as used by Brochard 1994, Esteban 1995, Kollef 1997, and Marelich 2000. The third component consists of a list of criteria for deciding if the patient is ready for extubation (such as that used by Hendrix 2006). In many ICUs, protocols are presented as written guides or algorithms and ventilator settings are manually adjusted by healthcare professionals. More recently, progress in ventilator microprocessor technology has enabled the development of computer‐assisted management of ventilation and weaning. Computer ventilatory management adapts the ventilator output to the patient's needs using closed loop systems. These systems measure and interpret respiratory data in real time and provide continual adjustment of the level of assistance within targeted values. It is suggested that through enabling 'interaction' between the patient and the ventilator, the closed loop systems may improve mechanical ventilation tolerance and reduce the work of breathing (Burns 2008). Multiple, commercial computerized ventilation and weaning programs have been developed, including adaptive support ventilation, proportional assist ventilation and PSV(SmartCareTM/pressure support) (Rose 2007).
How the intervention might work
Clinicians have different experiences, skills and weaning philosophies, thus there is potential for variation. As a result, there has been increasing interest in establishing more consistent practice in ICUs by developing and using weaning protocols that provide structured guidance. Protocols are based on the principle that the collective knowledge of a group is usually better than that of an individual. Protocols are intended to reduce variation, to improve efficiency of practice by reducing the influence of subjectivity of judgement and experience, and by seeking to apply objectivity (Murtagh 2007). Furthermore, they can empower the nurse and respiratory therapist to initiate the process of early weaning from the ventilator by identifying patients who are ready.
Why it is important to do this review
Our initial review of 11 trials concluded that weaning protocols are safe and effective in reducing the time spent on mechanical ventilation. Notwithstanding, we found considerable heterogeneity in results reporting total duration of ventilation and weaning duration. The variability may reflect the fact that protocols differ in more ways than in composition alone. While many protocols include readiness to wean criteria and guidelines for reducing ventilator support, the criteria applied and guidance used varied. Trials of protocolized weaning are continuing (Roh 2012) and the adoption of weaning protocols is growing. Surveys show reported use in ICUs of 8% in Greece, 56% in Italy, Denmark and Norway, 61% in the UK, 68% in Switzerland and the Netherlands (Rose 2011), 22% in Poland (Kubler 2013), and 71% in Canada (Ellis 2012). For these reasons. it is important that findings from recent trials are synthesized to guide future practice.
In addition to weaning protocols, another key feature in the management of weaning is the use of sedation and analgesia. Mechanical ventilation is generally accompanied by administration of high doses of sedative medications, and sedative management is known to influence the duration of mechanical ventilation. Recent clinical trials evaluating sedation management strategies (Bucknall 2008; Girard 2008; Mehta 2008; Mehta 2012) have all reported effects on the duration of mechanical ventilation and ICU stay. A systematic review of the effectiveness of protocol‐directed sedation on duration of mechanical ventilation is underway (Aitken 2012); therefore this review does not include sedation protocols.
Objectives
The first objective of this review was to compare the total duration of mechanical ventilation of critically ill adults who were weaned using protocols versus usual (non‐protocolized) practice.
The second objective was to ascertain differences between protocolized and non‐protocolized weaning in outcomes measuring weaning duration, harm (adverse events) and resource use (intensive care unit (ICU) and hospital length of stay, cost).
The third objective was to explore, using subgroup analyses, variations in outcomes by type of ICU, type of protocol and approach to delivering the protocol (professional‐led or computer‐driven).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs that compared protocolized with non‐protocolized (usual) weaning practices.
Types of participants
We included critically ill adults (at least 18 years of age and over) receiving invasive mechanical ventilation with either a nasotracheal or an orotracheal tube. We excluded studies involving children, those exploring non‐invasive ventilation as a weaning strategy and studies of tracheotomized patients only.
Types of interventions
We compared two strategies to achieve discontinuation from invasive mechanical ventilation: protocolized weaning and non‐protocolized weaning (or usual practice). For the purpose of this review, discontinuation was defined as the time when mechanical ventilatory support was discontinued and the patient was breathing spontaneously through a T‐piece circuit or following extubation. In addition, protocolized weaning was defined as a method of limiting the duration of invasive ventilation that includes at least the first two of the following three components.
-
A list of objective criteria based on general clinical factors for deciding if a patient is ready to tolerate discontinuation of mechanical ventilation.
-
Structured guidelines for reducing ventilatory support, such as a spontaneous breathing trial or a stepwise reduction in support to achieve discontinuation (e.g. synchronized intermittent mechanical ventilation (SIMV) or pressure support ventilation (PSV)).
-
A list of criteria for deciding if the patient is ready for extubation.
We did not exclude studies that did not include formal extubation criteria as not all studies included this component; and delay in extubation may be caused by organizational factors and not necessarily by delays in weaning. Usual weaning practice was defined as the usual practice in an ICU (as stated by the authors) where no written guides were applied. Where possible, usual practice was described in the review.
Types of outcome measures
Primary outcomes
-
Total duration of mechanical ventilation (time in hours, from mechanical ventilation initiation to discontinuation).
Secondary outcomes
-
Mortality (as stated by the study authors).
-
Number of patients experiencing the adverse events: reintubation; self extubation; tracheostomy; requirement for protracted mechanical ventilation (greater than 21 days).
-
Quality of life (as stated by the authors).
-
Weaning duration (time, as stated by the authors, from identification of weaning readiness to mechanical ventilation discontinuation).
-
ICU length of stay.
-
Hospital length of stay.
-
Cost.
Search methods for identification of studies
The search was performed by the Trials Search Co‐ordinator (Karen Hovhannisyan) using the standard strategy of the Cochrane Anaesthesia Review Group of The Cochrane Collaboration.
Electronic searches
In this updated review, we searched the current issue of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2014), MEDLINE (1950 to January 2014), EMBASE (1988 to January 2014), CINAHL (1937 to January 2014), ISI Web of Science (to February 2014) and LILACS (to January 2014). The search strategies for each database can be found in the appendices (Appendix 1: MEDLINE; Appendix 2: EMBASE; Appendix 3: LILACS; Appendix 4: CINAHL; Appendix 5: CENTRAL; Appendix 6: ISI Web of Science). The original search was performed in January 2010 (Blackwood 2010).
Searching other resources
In addition, we searched the reference lists of all identified study reports; we contacted authors for further information on ongoing trials; and we searched the meta‐register of controlled trials web site at http://www.controlled‐trials.com.
Data collection and analysis
BB entered the data into Review Manager 5 software (RevMan 2014) and POH checked data entry.
Selection of studies
Two authors (BB, POH) independently scanned the titles and abstracts identified by electronic searching, manual searches and contact with experts. Two authors (BB, POH) retrieved and evaluated the full text versions of potentially relevant studies.
Data extraction and management
Two authors (BB, KB) independently extracted data using a modified paper version of the Cochrane Anaesthesia Review Group's data extraction form (Appendix 7). We extracted information pertaining to the study design, method of randomization, study use of allocation concealment; and reporting of the study setting and participants, inclusion and exclusion criteria, interventions and outcomes. We contacted the authors of included studies if sufficient information was unavailable in the publications, and to obtain raw data. There were no disagreements requiring consultation with a third author.
Assessment of risk of bias in included studies
BB and KB used The Cochrane Collaboration's domain‐based evaluation tool for assessing the risk of bias in included studies (Higgins 2011a), in the following seven domains.
1. Random sequence generation
Random allocation sequence generation included any method that used an unpredictable sequence of allocating participants to groups, such as a random table; computer‐generated random numbers; throwing dice; or shuffling envelopes.
2. Allocation concealment
Adequate allocation concealment included central randomization (for example allocation by a central office unaware of participant characteristics); on‐site computer system combined with allocation kept in a locked unreadable computer file accessed only after the characteristics of an enrolled participant were entered; sequentially numbered, sealed, opaque envelopes or other similar approaches that ensured the person who generated the allocation scheme did not administer it.
3. Blinding of participants and personel
We report any attempts to blind up until the point of randomization.
4. Blinding of outcome assessment
We ascertained if study outcome assessors were independent from the clinical personnel delivering or supervising the assigned intervention.
5. Incomplete outcome data
6. Selective reporting
7. Other bias
Within each study we described what was reported for each domain and contacted the authors for additional information, where necessary. We evaluated the risk of bias for each domain as follows.
Low risk: criteria appropriately applied and described in the report or ascertained in communication with the primary author of the study.
Unclear: criteria not described and impossible to acquire from or clarify with the author.
High risk: criteria inappropriately applied.
Blinding of study personnel (domain 3) is impossible in these trials and, as a result, all studies were assessed as high risk of bias in this domain. Therefore, we amended the previous version of classification of included studies as follows.
A ‐ Low risk of bias: all criteria met, except domain 3.
B ‐ Moderate risk of bias: domain 3 not met, and one or more criteria unclear.
C‐ High risk of bias: two or more criteria not applied or met.
At each stage, BB and KB compared results.
Measures of treatment effect
We expressed treatment effect using the odds ratio (OR) for dichotomous data and mean difference (MD) for continuous data.
Unit of analysis issues
There were no cross over studies and randomization was by patient, therefore there were no unit of analysis issues.
Dealing with missing data
We contacted authors for clarification where data were missing or unclear.
Assessment of heterogeneity
We informally evaluated the degree of statistical heterogeneity by visual inspection of forest plots and more formally by measuring the impact of heterogeneity using the I2 statistic (I2 > 50%: significant heterogeneity); we tested it using the Chi2 statistic (P < 0.05) (Higgins 2002).
We evaluated clinical heterogeneity (differences in the studies in relation to type of ICU, clinician(s) involved in weaning and the protocol used to guide the weaning process) using clinical judgement. We calculated pooled summary estimates of effect only in the absence of clinical heterogeneity.
Assessment of reporting biases
We constructed funnel plots (trial effect versus standard error) to assess possible publication bias when sufficient (at least five) studies were identified (Egger 1997).
Data synthesis
For continuous variables (duration of mechanical ventilation, duration of weaning, ICU and hospital length of stay) the data were skewed; therefore, these data were log transformed for the primary analyses. In three studies the authors provided the means and standard deviations on the log scale (Ely 1996; Navalesi 2008; Rose 2008). In three studies the authors provided raw data (Ogica 2007; Reardon 2011; Roh 2012) for log transformation. In five studies where only means and standard deviations of the unlogged data were available (de Carvalho Oliveira 2002; Kollef 1997; Piotto 2011; Simeone 2002; Strickland 1993) approximations were used to calculate the mean and standard deviation on the log scale using Method 1 in Higgins (Higgins 2008). In five studies we could only obtain outcomes reported as the median and interquartile range (Chaiwat 2010; Krishnan 2004; Marelich 2000; Namen 2001; Stahl 2009): we approximated the mean using the median as suggested previously (Hozo 2005) and approximate standard deviation estimates were calculated from the interquartile range on the log scale as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The difference between the treatment and control groups in the mean of a variable on the log scale was exponentiated to give the ratio of geometric means of the variable on the unlogged scale. This was generally reported as a percentage increase (or reduction) in geometric mean in the treatment group compared with the control group for ease of understanding (see Bland 1996 for more details). One study (Fan 2013) reported the mean with no standard deviation for duration of mechanical ventilation, duration of weaning, ICU length of stay and cost; we excluded this study from meta‐analyses of these outcomes. We undertook meta‐analyses for similar comparisons and the same outcomes across studies. We calculated pooled estimates of the difference in means using either the fixed‐effect model or the random‐effects model, depending on the degree of heterogeneity.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to assess the impact of type of ICU, type of protocol and approach to delivering the protocol (physician‐led, non‐physician led or computer‐driven) on the total duration of mechanical ventilation and weaning duration. We performed subgroup analyses for type of ICU (medical, surgical, mixed, neurosurgical) and approach (professional‐led and computer‐driven) on duration of mechanical ventilation. We were unable to complete the other subgroup analyses due to the small number of studies in some subgroups and lack of clarity in studies on protocol delivery.
Sensitivity analysis
A priori, we planned a sensitivity analysis to assess the impact of excluding studies with a high risk of bias (that is those in which there was a high risk of bias in two or more of the six domains) on the total duration of mechanical ventilation and weaning duration. In addition, we conducted a further sensitivity analysis to show the results using the unlogged data.
Summary of findings
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence in our review associated with five main specific outcomes (total duration of mechanical ventilation, weaning duration, ICU length of stay, ICU mortality, and reintubation). BB and POH independently graded the evidence prior to agreement and construction of the 'Summary of findings' table using the GRADE software (Higgins 2011b). We appraised the quality of evidence based on the extent to which we were confident that an estimate of effect reflected the outcome assessed. In doing this we considered study risk of bias, directness of the evidence, heterogeneity of the data, precision of the effect estimates, and risk of publication bias.
Results
Description of studies
The studies were RCTs or quasi‐RCTs conducted on mechanically ventilated adult patients in intensive care units (ICUs). The intervention groups were weaned following written or automated weaning protocols delivered by healthcare professionals or computer systems. The control groups were weaned according to the subjective judgement of healthcare professionals without the use of written, formal guidelines.
Results of the search
The original search resulted in 11 studies being included in our review (Blackwood 2010). As a result of our updated search we retrieved a total of 3080 citations: 3077 references from the database search, including one reference missed in the original search; three relevant references from web‐based sources, including one abstract missed in the previous search. After reviewing the titles and abstracts, we identified and retrieved for review eight database references in full text, and obtained further information on three unpublished trials located on the controlled trials web site and conference proceedings (see Figure 1). We excluded four database references (Gnanapandithan 2011; Liu 2013; Ma 2010a; Ma 2010b) and one conference abstract (Vaschetto 2011) that did not meet our inclusion criteria. We included six studies with 463 participants (Chaiwat 2010; de Carvalho Oliveira 2002; Fan 2013; Ogica 2007; Reardon 2011; Roh 2012) following this search, bringing the total number included in this review to 17 studies with 2434 participants.

Updated study flow diagram.
Included studies
The 17 studies included in this updated review are described in the Characteristics of included studies tables. The individual studies involved sample sizes of 15 to 357 participants and took place in ICUs. Studies were conducted in four continents: nine American studies from the US (Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Reardon 2011; Strickland 1993) and Brazil (de Carvalho Oliveira 2002; Piotto 2011); four European studies from Italy (Navalesi 2008; Simeone 2002), Germany (Stahl 2009) and Romania (Ogica 2007); three Asian studies from China (Fan 2013), South Korea (Roh 2012) and Thailand (Chaiwat 2010); and one study from Australia (Rose 2008). Participants were recruited from a variety of ICUs, including medical (Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Reardon 2011; Roh 2012; Strickland 1993); coronary (Ely 1996; Piotto 2011); surgical (Chaiwat 2010; Kollef 1997; Stahl 2009); surgical and trauma (Marelich 2000); mixed (including medical, surgical and trauma patients) (de Carvalho Oliveira 2002; Rose 2008); neurosurgical (Fan 2013; Namen 2001; Navalesi 2008); cardiac surgical units (Simeone 2002); and two were not reported (Fan 2013; Ogica 2007). One study specified the population (neurosurgical) rather than the unit (Namen 2001). Three studies were conducted in multiple ICUs (Ely 1996; Kollef 1997; Marelich 2000), and the remaining studies were conducted in single sites.
The reported time of randomizing patients to weaning protocol or usual practice groups varied among trials. In six trials this was either not reported (de Carvalho Oliveira 2002; Fan 2013; Krishnan 2004; Ogica 2007; Piotto 2011; Simeone 2002) or reported as 'on enrolment', but the timing of enrolment was unclear (Ely 1996; Navalesi 2008). Seven trials randomized patients when they met weaning criteria (Marelich 2000; Namen 2001; Reardon 2011; Roh 2012; Rose 2008; Stahl 2009; Strickland 1993), and two trials randomized on ICU admission (Chaiwat 2010; Kollef 1997).
Five studies provided details of the ventilatory modes used as ‘usual practice’ in the control group; these were the four computer‐led studies (Reardon 2011; Rose 2008; Stahl 2009; Strickland 1993) and one professional‐led study (Piotto 2011). Usual practice involved a reduction in respiratory rate in synchronized intermittent mechanical ventilation (SIMV) and a reduction in pressure support (Piotto 2011; Strickland 1993); a reduction in positive end expiratory pressure (PEEP) and pressure support (Rose 2008); a reduction in pressure support (Stahl 2009); and a reduction in pressure support followed by a spontaneous breathing trial (SBT). The remaining 12 studies described usual practice as weaning according to the physician’s discretion but did not describe what this constituted. A printed standard approach to ventilatory management was used to guide usual practice in the surgical and trauma unit in the Marelich 2000 study; the author was unable to provide further information on the ventilatory mode used or compliance with its use.
The weaning protocol was professional‐led in 13 studies and computer‐led in four studies. Professional‐led weaning was delivered by registered nurse and respiratory therapist (Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000); by registered nurse (Chaiwat 2010; Roh 2012); by respiratory therapist (Namen 2001); by physician, registered nurse and respiratory therapist (Navalesi 2008); and unclear or not stated in five studies (de Carvalho Oliveira 2002; Fan 2013; Ogica 2007; Piotto 2011; Simeone 2002). Computer‐led weaning was delivered by Draeger EvitaXL ventilator with SmartCareTM/pressure support software that titrated pressure support and initiated SBTs (Reardon 2011; Rose 2008; Stahl 2009) or an early computer prototype (Supersport model 2) that titrated respiratory rate and pressure support (Strickland 1993).
All studies, except Ogica 2007 (reported in an abstract) and Fan 2013, reported readiness to wean criteria for protocol entry. Criteria ranged from a list of five to 19, and parameters were inconsistent. Fourteen studies included criteria that measured oxygenation; namely PaO2 and FiO2 (Chaiwat 2010; de Carvalho Oliveira 2002; Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Navalesi 2008; Piotto 2011; Reardon 2011; Rose 2008; Simeone 2002; Stahl 2009; Strickland 1993), and may or may not have included criteria relating to cardiovascular, neurological, inflammatory response, medication or other factors (see Table 1). Sedation scores were not reported as a readiness to wean criterion in any study, although awake and conscious/rousable was reported in four studies (Chaiwat 2010; Kollef 1997; Piotto 2011; Simeone 2002) and a Glasgow Coma Scale was reported in six studies with variable parameters (de Carvalho Oliveira 2002; Marelich 2000; Navalesi 2008; Piotto 2011; Rose 2008; Reardon 2011). The frequency of assessing readiness to wean was reported as twice daily (Marelich 2000); daily (Chaiwat 2010; Ely 1996; Fan 2013; Krishnan 2004; Namen 2001; Navalesi 2008; Piotto 2011; Reardon 2011); not reported (de Carvalho Oliveira 2002; Ogica 2007; Roh 2012); or only when the patient entered the study (Kollef 1997; Simeone 2002; Rose 2008; Stahl 2009; Strickland 1993).
| Study | Assessment frequency | Oxygenation | Other respiratory factors | Cardiovascular | Neurological | Inflammatory response | Medication | Other |
| Daily screen | PaO2/FiO2 >/= 200 on FiO2 </= 0.4 SpO2 >/= 94% | PEEP </= 5 Respiratory rate < 35 Rapid Shallow breathing index </= 105 Static lung compliance >/= 25 mL/cmH2O Minute volume </= 10L/min | HR < 120 b/min | Awake and easily rousable | Not included | Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Pain score < 4 | |
| Not reported | PaO2 < 90 on FiO2 </= 0.4 | PEEP < 5 Pimax < ‐ 25 cm H2O | Not included | GCS > 8 | Not included | No sedation No vasopressors | Cause of MV resolved No planned surgery | |
| Daily screen
| PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Protocol entry criteria
| PaO2/FiO2 > 200 | PEEP </= 5 RR </= 35 b/min
| HR < 140 b/min | Awake and orientated | Not included | No vasoactive or inotropic agents | Not included | |
| Daily screen
| SpO2 >/= 92% FiO2 </= 0.5 | PEEP </=5
| Stable CAD HR < 140 b/min | No raised ICP | Not included | No paralytics | Cough and gag reflex present Responsive to stimuli
| |
| x 2 daily screen
| PaO2/FiO2 >/= 200 | Not included | MAP >/= 60 mmHg | GCS >/= 10 or tracheostomy | Not included | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough not limited by pain
| |
| Daily screen | PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | PaO2/FiO2 > 200 FiO2 </= 0.4 pH >/= 7.35 PaCO2 </= 50 mmHg | PEEP </= 5
| HR </= 125 b/min SBP >/= 90 mmHg | GCS >/= 8 | T < 38.5oC | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough Suctioning < 2/hr Normal Na blood values
| |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Daily screen | PaO2/FiO2 150‐300 FiO2 </= 0.4 PaO2 >/= 60 mmHg Hb = 8 ‐ 10 g/L
| Not included | MAP >/= 60 mmHg HR </= 140 b/min | Awake GCS >/= 9 | T < 37.8oC | Minimum sedation No or low vasopressors | Cause of MV resolved Effective cough Metabolic stability No hydroelectrolyte disorders
| |
| Daily screen | SaO2 > 90% or PaO2 > 60 mmHg on FiO2 </= 0.5 | Respiratory rate < 35 pH > 7.20 Triggering breaths | SBP > 90 and < 180 HR > 50 and < 130 No cardiac ischaemia | GCS > 8 | Not included | Minimal pressure requirements | Improving condition Absence of excessive secretions Suctioning < hourly Deemed ready to wean | |
| Not reported | FiO2 </= 0.5 | RR </= 35 PEEP </= 8 Triggering breaths | SBP >/= 90 mmHg HR </= 150 b/min | Not included | Not included | No paralytics No vasopressors Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Not included | |
| Inclusion criteria | PaO2/FiO2 > 150 or SaO2 >/= 90% on FiO2 0.5 | PEEP </= 8 Plateau pressure </= 30 cmH2O Successful 30 min SBT using PS 20 cm H2O to achieve TV > 200 mL
| Haemodynamically stable | GCS > 4 | T = 36 ‐ 39oC | Not included | No surgery anticipated MV > 24 hr
| |
| Inclusion criteria | PaO2/FiO2 >/= 200 FiO2 < 0.5 pH 7.3 ‐ 7.5 PaO2 30 ‐ 50 mmHg SaO2 > 90% Hb > 8 mg/dL Pulse oximeter oxygenation stable Cardiopulmonary bypass time < 150 min | PEEP < 4 RR < 35 b/min Dynamic compliance > 22 mL/cmH2O Compliance statica >33 mL/cmH2O Vital capacity >10 mL/kg MIP >/= ‐15 cmH2O
| Haemodynamically stable | Awake and conscious | T > 35 < 38oC | Not included | Urine output > 100 mL/hr Normal CXR | |
| Inclusion criteria | FiO2 </= 0.5 PaO2 > 75 mmHg or SaO2 > 90% pH </= 7.2 Hb >/= 7g/dL | PEEP </= 10 | Haemodynamically stable | Not included | Not included | Dopamine </= 5 ug/kg/min | MV > 24 hr Breathing spontaneously Ramsey sedation score =/< 3
| |
| Inclusion criteria | FiO2 </= 0.4 pH >/= 7.3 </= 7.5 PCO2 >/= 30 </= 50 SaO2 >/= 90% on SIMV rate 6 ‐ 10 PS 20 cmH2O | NIF </= ‐ 20 cmH2O FVC >/= 10 mL/kg TV 10 ‐ 15 mL/kg
| Haemodynamically stable | Not included | T </= 37oC | Not included | Judged ready to wean by physician Feeding ‐ parenteral or tube Stable renal function Normal electrolytes
|
CAD = coronary artery diease; CXR = chest X‐ray; GCS = Glasgow Coma Scale; FVC = forced vital capacity; Hb = haemoglobin; HR ‐ heart rate; MAP = mean arterial pressure; MIP = maximal inspiratory pressure; MV = mechanical ventilation; NIF = negative inspiratory force; PEEP = positive end expiratory pressure; Pimax = maximal inspiratory mouth pressure; PS = pressure support; RR = respiratory rate; SBP = systolic blood pressure; SIMV = synchronized intermittent mechanical ventilation; T = temperature; TV = tidal volume; f/VT = ratio of respiratory frequency to tidal volume.
In addition to the wide variety in ways of assessing readiness to wean, there were considerable differences in weaning methods (see Table 2). Eleven studies used a protocolized weaning intervention that included a SBT (Chaiwat 2010; de Carvalho Oliveira 2002; Ely 1996; Fan 2013; Krishnan 2004; Marelich 2000; Namen 2001; Navalesi 2008; Ogica 2007; Piotto 2011; Roh 2012). In addition, Marelich 2000 used a stepwise reduction in PEEP, SIMV and pressure support prior to the SBT in patients ventilated for more than 72 hours, and Roh 2012 used a Continuous Positive Airway Pressure (CPAP) trial followed by gradual reduction of pressure support prior to the SBT. One trial used a weaning protocol consisting of stepwise reductions in SIMV and pressure support with extubation (Simeone 2002). Kollef 1997 implemented different protocols in four ICUs: SBT and extubation; SIMV reduction and extubation; and pressure support reduction and extubation. Weaning parameters varied among trials. SBTs ranged from 30 to 120 minutes, delivered through a T‐tube or ventilator circuit with CPAP ranging from 2 to 5 cmH2O with or without pressure support of 6 or 7 cmH2O. In pressure support weaning protocols, the pressure support was reduced to levels ranging from 4 to 8 cmH2O prior to extubation. In SIMV weaning protocols, respiratory rates were reduced to between 0 and 6 breaths/minute prior to a SBT or extubation. In automated weaning protocols, the pressure support was reduced to levels between 5 or 7 cmH2O prior to a SBT.
| Study | Time of randomization | Intervention protocol | Extubation criteria | Comparator (usual practice) |
| ICU admission | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Notify MD | Not reported | |
| Not reported | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Yes | Not reported | |
| Enrolment, time not reported | SBT 2 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| Not reported | a) SBT 30 minutes and extubation if passed b) If failed, daily SBT and stepwise reduction in SIMV and PS until 4 breaths/min and PS 7 cmH2O | Not reported | Not reported | |
| ICU admission | a) SBT 30 to 60 min on CPAP 5 cmH2O, PS 6 cmH2O b) PS stepwise reduction to 6 cmH2O c) IMV stepwise reduction to 0 breaths/min, on PEEP 5 cmH2O and PS 6 cmH2O for 30 to 60 min | a) Yes
b) Yes c) Yes | Not reported | |
| Not reported | SBT 1 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| On meeting weaning criteria | a) < 72‐hour admissions: SBT 30 min on PS </= 8 cmH2O & PEEP </= 8 cmH2O b) > 72‐hour admissions: PEEP, IMV and PS stepwise reductions to achieve FiO2 0.5, PEEP </= 8 cmH2O, IMV </= 6 breaths/min, PS </= 8 cmH2O then SBT as above | a) Notify MD
b) Notify MD | Not reported | |
| On meeting weaning criteria | SBT 2 hours on CPAP 5 cmH2O | Notify MD | Not reported | |
| Enrolment, time not reported | SBT 1 hour on CPAP 2 to 3 cmH2O, FiO2 0.4 | Yes | Not reported | |
| Not reported | SBT (details not reported) | Not reported | Not reported | |
| Not reported | SBT 2 hours on PS 7 cmH2O, PEEP 5 cmH2O, FiO2 0.4, RR 1 breath/min | Yes | Stepwise reduction in PS and IMV | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | Notify MD | Stepwise reduction in PS and SBT | |
| On meeting weaning criteria | CPAP trial on 5 cmH2O, then stepwise reductions in PS to 5 cmH2O, then SBT on T‐piece for 30 minutes | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | No | Stepwise reduction in PS and PEEP | |
| Not reported | SIMV and PS stepwise reductions to SIMV 0 breath/min and PS 4 cmH2O | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS stepwise reductions to PS | Yes | Spepwise reduction in PS and CPAP | |
| On meeting weaning criteria | Computer automated Supersport model 2 stepwise reductions in SIMV and PS to RR 2 breaths/min and PS 5 cmH2O | Not reported | Stepwise reduction in IMV and PS |
CPAP = continuous positive airway pressure; IMV = intermittent mechanical ventilation; MD = Medical Doctor; PEEP = positive end expiratory pressure; PS = pressure support; SBT = spontaneous breathing trial; SIMV =synchronized intermittent mechanical ventilation; RR = respiratory rate.
All studies, with the exception of Reardon 2011 and Strickland 1993, reported on the review’s primary outcome measure, total duration of mechanical ventilation. Strickland’s data collection was limited to 48 hours because the trial tested a computerized protocol and only one computer system was available for the study. Only one study reported time from discontinuation from mechanical ventilation to extubation (Piotto 2011), and no study reported quality of life.
Excluded studies
We excluded 14 studies. Eight studies (Beale 2008; Donglemans 2009; Lellouche 2006; Liu 2013; Ma 2010b; NCT00502489; NCT00445289; Taniguchi 2009) compared automated (computerized) protocolized weaning with standardized weaning guidelines as opposed to 'no guidelines'. Gnanapandithan 2011 compared two different weaning protocols. Ma 2010a compared the efficacy of a SBT prior to extubation; the weaning method was the same in both groups. Vaschetto 2011 included tracheotomized patients only. In addition, East 1999 and McKinley 2001 evaluated automated (computerized) protocolized weaning in a population of adult respiratory distress syndrome patients using a cluster‐RCT. From the papers, we were unable to identify the comparator or the weaning outcomes, and we were unable to contact the authors to obtain further information. One registered trial was not completed due to recruitment problems, and the data were unobtainable (NCT00157287). See the Characteristics of excluded studies tables.
Risk of bias in included studies
We used The Cochrane Collaboration's domain‐based evaluation table provided in Review Manager 5 (RevMan 2014) to assess included trials for risk of selection, performance, detection, attrition, reporting and other bias (see Figure 2 and Figure 3).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
In 11 (69%) studies, we assessed risk of selection bias as low because the allocation and concealment of participants to groups was adequately conducted (Chaiwat 2010; Ely 1996; Kollef 1997; Marelich 2000; Navalesi 2008; Reardon 2011; Roh 2012; Rose 2008; Simeone 2002; Stahl 2009; Strickland 1993). Three studies (de Carvalho Oliveira 2002; Namen 2001; Ogica 2007) did not report their methods and two studies used inadequate methods: Krishnan 2004 allocated using odd and even hospital numbers; and Piotto 2011 allocated sequentially on recruitment. One study (Fan 2013) used a random numbers table to generate the sequence, but it was unclear how allocation was concealed.
Blinding
Blinding of study participants and personnel from intervention allocations after inclusion of participants was not possible in these studies, thus we assessed the risk of performance bias as high for all studies. Eleven (65%) studies were assessed as being at unclear risk because they did not report if outcome assessors were blinded (Chaiwat 2010; de Carvalho Oliveira 2002; Fan 2013; Krishnan 2004; Namen 2001; Ogica 2007; Piotto 2011; Reardon 2011; Roh 2012; Rose 2008; Simeone 2002) and the remaining six studies had low risk of detection bias.
Incomplete outcome data
Eleven (65%) studies reported complete outcome data (Chaiwat 2010; Ely 1996; Fan 2013; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Navalesi 2008; Rose 2008; Stahl 2009; Strickland 1993) and the remaining six studies reported insufficient information on the recruitment, attrition and exclusion numbers to permit a judgement (de Carvalho Oliveira 2002; Ogica 2007; Piotto 2011; Reardon 2011; Roh 2012; Simeone 2002).
Selective reporting
Eleven studies provided a description or algorithm for their intervention, ventilator weaning protocol (Chaiwat 2010; de Carvalho Oliveira 2002; Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Navalesi 2008; Piotto 2011; Roh 2012; Strickland 1993) and three described the automated computer system (Reardon 2011; Rose 2008; Stahl 2009). Eleven (63%) studies reported prespecified outcomes and we assessed these at low risk of reporting bias. We assessed six studies (Chaiwat 2010; de Carvalho Oliveira 2002; Fan 2013; Reardon 2011; Roh 2012; Simeone 2002) at unclear risk because they did not prespecify outcomes, or did not report usual outcomes of interest in protocolized weaning trials (duration of mechanical ventilation, mortality, ICU length of stay).
Other potential sources of bias
Nine studies appeared free from 'other sources of bias' as determined in The Cochrane Collaboration's domain‐based evaluation (Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Navalesi 2008; Piotto 2011; Roh 2012; Rose 2008; Strickland 1993). Four studies were stopped early for futility (Chaiwat 2010; Namen 2001; Reardon 2011; Stahl 2009); Simeone 2002 reported unsubstantiated findings; Fan 2013 reported insufficient information to permit judgement; and the Ogica 2007 study was published as an abstract so there was insufficient information to permit judgement.
Effects of interventions
See: Summary of findings for the main comparison
All trials presented data suitable for inclusion in the meta‐analyses. All study authors were contacted to confirm and supplement, where needed, information related to study methods and data. Fourteen study authors responded (Ely 1996; de Carvalho Oliveira 2002; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Navalesi 2008; Ogica 2007; Piotto 2011; Reardon 2011; Roh 2012; Rose 2008; Simeone 2002; Stahl 2009), although not all were able to supply information. Three study authors could not be contacted (Chaiwat 2010; Fan 2013; Strickland 1993). We converted all reported durations of mechanical ventilation and weaning to hours; ICU and hospital length of stay are reported in days. Fan 2013 reported the mean only for these outcomes; these data were not included in meta‐analyses and are reported in the text. We present the results in three sections. In section one, we present the primary analysis for total duration of mechanical ventilation, weaning duration, ICU and hospital length of stay using log‐transformed data due to the skewed distribution of these outcomes. We also present subgroup analyses for type of ICU and approach on the durations of mechanical ventilation and weaning. In section two, we present a sensitivity analysis of the logged data for duration of mechanical ventilation and weaning duration that excludes studies judged at high risk of bias (Krishnan 2004; Piotto 2011). In section three, we present a further sensitivity analysis using the mean and standard deviation prior to log‐transformation for total duration of mechanical ventilation, weaning duration, ICU and hospital length of stay for all studies. We present this sensitivity analysis to show the effects without log‐transformation.
Section 1. Primary analysis: comparison of protocolized versus non‐protocolized weaning
Total duration of mechanical ventilation
Fourteen trials reported the total duration of mechanical ventilation and we included them in the meta‐analysis (Chaiwat 2010; de Carvalho Oliveira 2002; Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Navalesi 2008; Ogica 2007; Piotto 2011; Roh 2012; Rose 2008; Simeone 2002; Stahl 2009). Strickland 1993 did not measure this outcome as the trial duration was 48 hours for each individual patient, and Reardon 2011 did not report the outcome. Pooled data, using the random‐effects model because of significant (P < 0.0001) and substantial heterogeneity (I² = 67%), showed a significant reduction in duration of mechanical ventilation in the protocolized weaning group (mean log ‐0.30, 95% confidence interval (CI) ‐0.46 to ‐0.14, P = 0.0002) equivalent to a reduction of 26% (95% CI 13% to 37%) in the geometric mean. Fan 2013 reported a non‐significant reduction of 151.52 hours for the protocolized weaning group (mean 272.01 versus 423.53, P = 0.20).
We performed a subgroup analysis to assess the impact of type of ICU on the total duration of mechanical ventilation. The ICU subgroups included: mixed ICUs that incorporated medical, surgical and trauma patients (de Carvalho Oliveira 2002; Kollef 1997; Marelich 2000; Ogica 2007; Piotto 2011; Rose 2008); neurosurgical ICUs (Namen 2001; Navalesi 2008); surgical ICUs (Chaiwat 2010; Simeone 2002; Stahl 2009); and medical ICUs (Ely 1996; Krishnan 2004; Roh 2012). Pooled data from the neurosurgical subgroup showed no difference in duration of mechanical ventilation (mean log ‐0.01, 95% CI ‐0.2 to 0.18, P = 0.93; equivalent to a 1% reduction, 95% CI 20% reduction to 18% increase in geometric mean). Pooled data in the other three subgroups showed a significant reduction in duration of mechanical ventilation in the protocolized weaning arm: mixed ICU subgroup (N = 6 trials, mean log ‐0.23, 95% CI ‐0.44 to ‐0.02, P = 0.03, equivalent to a 21% 95% CI 2% to 36% reduction in geometric mean); surgical ICU subgroup (N = 3 trials, mean log ‐0.63, 95% CI ‐1.05 to ‐0.22, P = 0.003 equivalent to a 47%, 95% CI 20% to 65% reduction in geometric mean); and medical ICU subgroup (N = 3, mean log ‐0.34, 95% CI ‐0.61 to ‐0.07, P = 0.01 equivalent to a 29%, 95% CI 7% to 46% reduction in geometric mean). There was evidence of a difference in estimates between the four subgroups (P for subgroup differences = 0.02). See Analysis 1.1.
We performed a subgroup analysis to assess the impact of type of approach: professional‐led or computer‐driven. Pooled data from 12 studies using a professional‐led approach (Chaiwat 2010; de Carvalho Oliveira 2002; Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Navalesi 2008; Ogica 2007; Piotto 2011, Roh 2012; Simeone 2002) showed a significant reduction in duration of mechanical ventilation favouring the protocolized weaning arm (mean log ‐0.27, 95% CI ‐0.40 to ‐0.13, P = 0.0002 equivalent to a 24% 95% CI 12% to 49%) reduction in the geometric mean; there was significant moderate heterogeneity (P = 0.03, I2 = 48%). Pooled data from the computer‐driven subgroup (Rose 2008; Stahl 2009) showed no difference in duration of mechanical ventilation (mean log ‐0.5, 95% CI ‐1.42 to 0.42, P = 0.28; equivalent to 39% reduction, 95% CI 52% reduction to 76% increase in geometric mean). There was no evidence of a difference in estimates between subgroups (P = 0.62 for subgroup differences). See Analysis 1.2.
The larger number of trials included in this updated review allowed us to perform a subgroup analysis on type of protocol (Figure 4). Protocol subgroups were a spontaneous breathing trial (SBT) (comprising daily assessment of readiness to wean followed by SBT) and stepwise reduction (comprising a gradual reduction in either intermittent mandatory ventilation or pressure support ventilation (PSV) with or without a SBT). We included automated systems in this latter subgroup as these involved stepwise reductions in support. Eight trials evaluated a SBT protocol (Chaiwat 2010; de Carvalho Oliveira 2002; Ely 1996; Krishnan 2004; Namen 2001; Navalesi 2008; Ogica 2007; Piotto 2011). Pooled data indicated a trend towards reduced duration of ventilation in the SBT subgroup with low heterogeneity (39%), but was not significant (mean log ‐0.18, 95% CI ‐0.36 to 0.00, P =0.05; equivalent to a 16%, 95% CI 0% to 30% reduction in geometric mean). Six trials evaluated a stepwise reduction protocol (Kollef 1997; Marelich 2000; Roh 2012; Rose 2008; Simeone 2002; Stahl 2009). There was a significant reduction in duration of ventilation in this protocol group (mean log ‐0.42, 95% CI ‐0.66 to ‐0.18, P = 0.0007; equivalent to a 34%, 95% CI 16% to 48% reduction in geometric mean); there was also significant heterogeneity in effect estimates (I2 = 75%, P = 0.001). See Analysis 1.3.
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].](/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG04.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].
Mortality
Fourteen trials reported ICU, or hospitality mortality, or both. Pooled data from eight trials (Ely 1996; Kollef 1997; Krishnan 2004; Marelich 2000; Namen 2001; Reardon 2011; Roh 2012; Stahl 2009) showed no difference in hospital mortality (odds ratio (OR) 1.04, 95% CI 0.82 to 1.32, P = 0.74). Pooled data from seven trials (de Carvalho Oliveira 2002; Fan 2013; Navalesi 2008; Ogica 2007; Piotto 2011; Rose 2008; Stahl 2009) showed no difference in ICU mortality (OR 0.93, 95% CI 0.58 to 1.48, P = 0.75) (Analysis 1.4).
Adverse events
Adverse events were reported in 11 trials and the OR was not significant between groups. Reintubation was reported in 11 trials (Chaiwat 2010; de Carvalho Oliveira 2002; Ely 1996; Kollef 1997; Namen 2001; Navalesi 2008; Piotto 2011; Reardon 2011; Rose 2008; Simeone 2002; Stahl 2009) with an 11% (158/1484) event rate. The pooled result was not statistically significant (OR 0.74, 95% CI 0.44 to 1.23, P = 0.25) (Analysis 1.5). Self extubation was reported in three trials (Ely 1996;Namen 2001; Reardon 2011). There was a 3% (14/433) event rate and the pooled result was not statistically significant (OR 0.43, 95% CI 0.14 to 1.34, P = 0.15) (Analysis 1.6). Tracheostomy was reported in eight trials (Ely 1996; Marelich 2000; Namen 2001; Navalesi 2008; Reardon 2011; Roh 2012; Piotto 2011; Rose 2008) with an 11% (148/1346) event rate. The pooled effect was not statistically significant (OR 0.85, 95% CI 0.51 to 1.40, P = 0.51) (Analysis 1.7). Four trials reported the requirement for protracted mechanical ventilation at three different time points: > 21 days, > 14 days and > 7 days. Ely 1996 showed a significantly reduced likelihood of protracted mechanical ventilation (> 21 days) in the protocolized group (OR 0. 42, 95% CI 0.19 to 0.96, P = 0.04). Namen 2001 showed no difference in protracted mechanical ventilation (> 21 days) (OR 0.18, 95% CI 0.02 to 1.63, P = 0.21). Rose 2008 showed no difference in protracted mechanical ventilation (> 14 days) (OR 0.68, CI 0.20 to 2.31, P = 0.54); and Kollef 1997 showed no difference in protracted weaning (> 7 days) (OR 0.63, 95% CI 0.35 to 1.15, P = 0.13).
Quality of life
None of the trial authors reported on quality of life.
Weaning duration (hours)
In the meta‐analysis, we included weaning duration reported in eight trials (Ely 1996; Marelich 2000; Piotto 2011; Reardon 2011; Roh 2012; Rose 2008; Stahl 2009; Strickland 1993). The pooled result, using the random‐effects model because of significant (P < 0.00001) and considerable heterogeneity (I² = 97%), showed a significant reduction in the mean log for the protocolized weaning group (mean log ‐1.20, 95% CI ‐2.10 to ‐0.31, P < 0.009), which corresponds to a reduction of 70% (95% CI 27% to 88%) in the geometric mean (Analysis 1.8). Subgroups by type of ICU were small and subgroup analyses showed no evidence of a difference in estimates (P for subgroup differences = 0.92). However, there was evidence of a significant difference among type of approaches (P for subgroup differences = 0.04) (Figure 5). Pooled results for the professional‐led approach showed a significant mean log reduction for protocolized weaning (mean log ‐1.90, 95% CI ‐3.37 to ‐0.43, P = 0.01) corresponding to an 85% reduction (95% CI 35% to 97%) in the geometric mean. The computer‐driven approach showed less of an effect (mean log ‐0.35, 95% CI ‐0.69 to ‐0.00, representing a reduction of 30%, 95% CI 0% to 50%, P = 0.05) that may be attributed to the small number of trials in this subgroup (Analysis 1.9).
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].](/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG05.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].
There was evidence of a difference in estimates between the two protocol subgroups (P for subgroup differences < 0.00001). Weaning duration was significantly reduced in the stepwise reduction protocol group (mean log ‐0.46, 95% CI ‐0.81 to ‐0.12, P = 0.009, equivalent to a 37% reduction in geometric mean, 95% CI 11% to 56%). The effect on weaning duration was, expectedly, stronger in the SBT protocol subgroup (mean log ‐3.23, 95% CI ‐3.57 to ‐2.89, P < 0.00001; equivalent to a 96% reduction in geometric mean, 95% CI 94% to 97%) (Analysis 1.10).
Fan 2013 reported a significant reduction of 188.04 hours in weaning duration in the protocolized group (55.91 versus 243.95 hours, P < 0.01).
ICU length of stay (hours)
We entered data in the meta‐analysis for ICU length of stay reported in nine trials (Ely 1996; Namen 2001; Krishnan 2004; Navalesi 2008; Piotto 2011; Roh 2012; Rose 2008; Simeone 2002; Stahl 2009). There was no statistical heterogeneity among studies (I2 = 0%). Two trials (Krishnan 2004; Simeone 2002) showed a significant reduction in ICU stay in the protocolized weaning group and the others did not, but the pooled estimate was statistically significant (Analysis 1.11) (mean log ‐0.12, 95% CI ‐0.21 to ‐0.03, P = 0.01). This corresponds to an average percentage reduction in geometric mean in the protocolized weaning group of 11% (95% CI 3% to 19%). Fan 2013 reported a non‐significant reduction of 205 hours in the protocolized weaning group (611.03 versus 816.03, P = 0.212).
Hospital length of stay (days)
Protocolized weaning produced no significant reduction (mean log ‐0.01, 95% CI ‐0.11 to 0.09, P = 0.84) in mean hospital length of stay in five trials (Ely 1996; Kollef 1997; Namen 2001; Roh 2012; Rose 2008). There was no heterogeneity (I2 = 0%) (Analysis 1.12). This corresponded to an average percentage reduction in geometric mean of 1% (95% CI 9% reduction to 10% increase).
Economic costs
Four trials reported costs; three in the US (Ely 1996; Kollef 1997; Namen 2001) and one in China (Fan 2013). Ely 1996 and Namen 2001 reported no significant differences between groups in ICU costs (Analysis 1.13) (mean difference (MD) USD 3.37k, 95% CI ‐15.02 to 21.76, P = 0.72); and Ely 1996, Kollef 1997 and Namen 2001 reported no difference in hospital costs (Analysis 1.14) (MD USD 0.59k, 95% CI ‐4.67 to 3.49, P = 0.78). Fan 2013 reported a non‐significant reduction of CNY 29,346.21 (CNY 101,642.74 versus CNY 130,988.95, P = 0.305), but it was unclear if this referred to hospital or ICU costs.
Section 2. Sensitivity analysis: comparison of protocolized versus non‐protocolized weaning excluding high risk of bias studies
This sensitivity analysis explored the effects of the intervention when high risk of bias studies (Krishnan 2004; Piotto 2011) were excluded. Excluding these studies did not change the effects observed in the primary analysis. Pooled results showed that protocolized weaning significantly reduced the mean log duration of mechanical ventilation by an average of 0.33 (Analysis 2.1) (mean log ‐0.33, 95% CI ‐0.50 to ‐0.16, P = 0.0001), which corresponds to a reduction of 28% (95% CI 15% to 39%) in the geometric mean; there was significant heterogeneity (I2 = 70%, P < 0.0001). Additionally, protocolized weaning significantly reduced the mean log weaning duration by an average of 1.64 (Analysis 2.2) (mean log ‐1.64, 95% CI ‐3.18 to ‐0.1, P = 0.04), which corresponds to a reduction of 81% (95% CI 10% to 96%) in the geometric mean; there was significant heterogeneity (I2 = 97%, P < 0.00001).
Section 3. Sensitivity analysis: protocolized versus non‐protocolized weaning for all studies, unlogged data
This sensitivity analysis explored the effects of the intervention on the data prior to log‐transformation. In 11 studies we obtained the mean and standard deviation from the authors or the published papers (de Carvalho Oliveira 2002; Ely 1996; Kollef 1997; Navalesi 2008; Ogica 2007; Piotto 2011; Reardon 2011; Roh 2012; Rose 2008, Simeone 2002; Strickland 1993). In five studies where outcomes were reported as median and interquartile ranges (Chaiwat 2010; Krishnan 2004; Marelich 2000; Namen 2001; Stahl 2009), we approximated the mean and standard deviation as described in the methods.
The pooled result for duration of mechanical ventilation, using the random‐effects model (because of significant heterogeneity) (I² = 48 %, P = 0.02), showed that protocolized weaning significantly reduced the total duration of mechanical ventilation by an average of 20.26 hours (Analysis 3.1) (MD ‐20.26 hours, 95% CI ‐5.24 to ‐35.28 hours, P = 0.008).
The pooled result for weaning duration, using the random‐effects model (because of significant heterogeneity) (I² = 80 %, P < 0.0001), showed that protocolized weaning significantly reduced the weaning duration by an average of 39.35 hours (Analysis 3.2) (N = 7 trials, MD ‐39.35 hours, 95% CI ‐11.32 to ‐67.38 hours, P = 0.006).
ICU length of stay was significantly reduced in the protocol group by an average of 9 hours (Analysis 3.3) (N = 9 trials, MD ‐9.08 hours, 95% CI ‐2.30 to ‐15.85, P = 0.009).
Pooled results for hospital length of stay showed no difference between groups (Analysis 3.4) (N = 5 trials, MD ‐1.32 days, 95% CI ‐3.09 to 0.44 days, P = 0.14).
Funnel plots
Although funnel plots did not conform to the expected shape, there was little evidence of asymmetry. As we were able to obtain published and unpublished data from studies reporting both significant and non‐significant statistical differences in the primary outcome measure, we concluded that there was no evidence of publication or reporting bias. The non‐conformity to expected shape may be due to small sample and effect sizes in some studies (see Figure 6).
![Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].](/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG06.png)
Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].
Discussion
The conclusions of our updated review remain the same as the original (Blackwood 2010). In comparison with usual (non‐protocolized) weaning practice, protocolized weaning significantly reduced the total duration of ventilation, weaning duration and intensive care unit (ICU) length of stay without impacting on mortality or adverse events. There is significant heterogeneity among effect sizes. The evidence from trials of protocolized weaning to reduce the duration of mechanical ventilation in critically ill adults is derived from 17 trials which have a variety of settings, participants, interventions and outcome measures. The main outcome, duration of mechanical ventilation, was reported in 15 trials and data were available for seven out of eight secondary outcomes. The methodological quality of the studies varied from low to high. Fifteen trials were randomized and two were quasi‐randomized.
Summary of main results
Impact on ventilation duration
In comparison with usual (non‐protocolized) weaning practice, protocolized weaning significantly impacted on ventilation durations, reducing the total duration of mechanical ventilation by an average of 26% in geometric mean and weaning duration by 70%. However, substantial heterogeneity among study effect estimates (67% and 97% respectively) indicated that findings should be interpreted with caution. Subgroup analysis of total duration of mechanical ventilation by type of ICU, showed a significant difference in effect estimates between subgroups. In comparison with usual weaning practice, protocolized weaning significantly reduced the duration of ventilation in surgical (47% reduction in geometric mean), medical (29%) and mixed ICUs (21%), but not neurological ICUs (1%). There were no significant differences between subgroup effect estimates for type of delivery (professional and automated) and type of protocol (spontaneous breathing trial (SBT) and stepwise reduction). However, protocolized weaning delivered by professionals significantly reduced the geometric mean duration of mechanical ventilation (23%), as did protocols consisting of stepwise reductions in ventilator support (34%). For weaning duration, there was no evidence of a difference in effect estimates for type of ICU subgroups, but there were significant differences in effects for the type of delivery and type of protocol subgroups. Protocols delivered by professionals significantly reduced weaning duration by 85%, and although automated systems showed a 29% reduction in geometric mean, this did not reach significance. The SBT protocol group showed an unsurprising reduction in geometric mean (96%) (unsurprising because the SBT duration is generally fixed at 2 hours duration), while protocols comprising stepwise reductions in support, also showed a significant, albeit smaller, reduction (37%).
Impact on mortality and adverse events
Protocolized weaning did not impact on ICU or hospital mortality. Neither did it impact on adverse events such as reintubation, self extubation, tracheostomy, or protracted weaning at 7, 14 and 21 days.
Impact on resource utilisation
Protocolized weaning significantly reduced the mean geometric length of stay in ICUs by 11%, but with no impact on overall hospital length of stay. Basic costing exercises undertaken in four trials showed no statistically significant differences between groups in either ICU or hospital costs. However, these fail to provide a full understanding of the true impact of protocolized weaning, including costs associated with training. A cost‐effectiveness analysis would be beneficial in enabling policymakers to compare the costs associated with protocolized weaning with the benefits gained.
Overall completeness and applicability of evidence
We are confident that our search strategy obtained all available updated studies and, through contact with experts, we were also able to obtain additional studies that did not appear in the previous search. The majority of trials evaluated spontaneous breathing trials (SBTs) or stepwise reduction in pressure support ventilation (PSV) protocols and thus provide a clear reflection of the evidence applicable to current weaning practice.
It is not easy to isolate the reasons for heterogeneity because weaning from ventilation is a complex process. It is plausible that heterogeneity may be due to contextual factors (differences in patient populations and usual practice within units); intervention factors (differences in determining readiness to wean and weaning protocols, trial fidelity); or inconsistency in measuring ventilation outcomes. A Cochrane synthesis review is in progress exploring the contribution of these factors in the trials included in this review, and on protocolized weaning in general, and may help explain the heterogeneity demonstrated in this review (Jordan 2012).
In contrast to the original review, this updated review showed a significant impact for protocolized weaning in mixed and medical ICU groups: previously protocols only impacted favourably in the surgical group. There was one additional study in a neurological intensive care unit (ICU), but as we could not include this in the meta‐analysis it is difficult to assess a change in impact. Another important contextual factor, and one that causes controversy in ICU studies of non‐pharmacological interventions, is the use of the ‘usual care’ group as a control in trials (Thompson 2007). Usual care in ICUs may encompass a wide variety of styles. For example usual care may be standardized around high level evidence and thus represent best practice, but it may also be highly variable and include unfavourable practice (Thompson 2007). Consequently, if the culture of an ICU is such that usual care is a standardized high level approach to weaning, albeit not formally laid out in guidelines, then it may not differ greatly from that delivered in a weaning protocol. Thus in a trial of effectiveness, the gap between usual care and protocolized weaning may be too narrow to show a significant difference between groups. For example, the Marelich 2000 study was conducted in one medical and one surgical and trauma ICU, and the authors reported variable practice between units. The medical ICU had no standardized approach to weaning whereas the surgical ICU had a standardized approach to ventilatory management, although extubation was based on subjective decisions. Thus, while combined data from both units demonstrated a reduction in the duration of mechanical ventilation time, when data were analysed separately for each unit, the reduction in mechanical ventilation was only statistically significant in the medical ICU, where there was variability in weaning practice. Similarly, the study by Rose 2008 attributed their lack of effect between computer‐directed weaning and non‐protocolized weaning to usual practice in their ICU. They reported unlimited assessment of weaning and readiness to wean by experienced and relatively autonomous critical care nurses, a one‐to‐one nurse‐to‐patient ratio supported by 24‐hour medical staff and twice‐daily intensivist rounds. These examples suggest that one might not find any further beneficial effect from using weaning protocols in comparison with standardized high level approaches to weaning. Twelve of the 17 trials included in this review did not describe usual care in their control group; as a result it is impossible to determine if this was a cause of heterogeneity and, further, it limits generalizability of findings.
This review has highlighted inconsistency in defining outcome measures that may have contributed to heterogeneity in effects. The review noted variation in defining the start time of randomization (unreported in 44% of included studies) and criteria for determining readiness to wean. Furthermore, although readiness to wean criteria usually involved similar indicators of oxygenation, factors such as positive end expiratory pressure (PEEP) varied from 5 to 8: as a consequence, the leniency or restrictiveness of criteria may have contributed to differences in effects across studies. Substantial variation in the selection and definition of ventilation outcomes is an ongoing issue that was highlighted in a recent review of trials published from 2007 to 2012 in eight main critical care journals. From 66 trials measuring duration of mechanical ventilation, 75% did not define start and endpoint measures, and prompted the authors to call for establishment of a core outcome set (Blackwood 2014).
Weaning of sedation is an important accompanying feature in ventilation weaning, yet only two trials included minimal sedation as a criterion for weaning, making it difficult to ascertain if sedation management impacted on outcomes. Concerning the weaning protocols themselves, although they mainly included gradual reductions in pressure support, or SBT, or both, reflecting contemporary weaning practice, only two studies used an identical weaning protocol (Ely 1996; Namen 2001). Even so, they reported conflicting results in the duration of mechanical ventilation and weaning that could reflect differences in the type of patient population (medical and neurosurgical) and unreported usual practice within the units.
A limitation of the review is that outcome data for duration of mechanical ventilation and weaning duration were skewed: this is likely why some authors reported median and interquartile ranges. In our primary analysis, the estimates were based on approximations of the data presented (as described in the methods) and this may have impacted on our analysis. However, we feel this is likely to have had negligible impact as we conducted a sensitivity analysis of the unlogged data and this had little effect on the main findings. Similarly, when high risk studies were removed from the analysis, heterogeneity and effect estimates were similar to those from the primary analysis, indicating that high risk of bias studies did not adversely impact on overall results.
Quality of the evidence
Overall, the quality of the evidence was low for weaning duration and ICU length of stay due to substantial variability in effect estimates for weaning duration and wide confidence intervals. The quality of evidence was moderate for duration of mechanical ventilation, mortality and reintubation. We rated the majority of trials as low risk of bias across all six domains with the exception of performance bias. The nature of protocolized weaning means it is not possible to blind clinicians involved in the weaning process. Only four studies fully reported the trial sufficiently to enable a clear rating of risk of bias in all domains.
Potential biases in the review process
We adhered closely to our protocol which outlined our procedures for minimising bias in the review: these included independent screening for trial inclusion, data extraction and assessment of risk of bias by two review authors. With assistance from the Cochrane Anaesthesia Group’s Search Trials Co‐ordinator and an experienced librarian, we conducted a thorough search strategy, and believe we have identified all relevant studies.
Agreements and disagreements with other studies or reviews
Automated computerized systems are increasingly being employed in an attempt to improve the adaptation of mechanical support to the needs of patients. Computers can continuously monitor changes in ventilation, interpret real time physiological changes and adapt ventilation in response to these changes. This is evident from the automated weaning studies included in this review (Reardon 2011; Rose 2008; Stahl 2009; Strickland 1993). However, in comparison with usual care of non‐protocolized weaning, their efficacy in reducing the duration of mechanical ventilation has yet to be demonstrated. It should be noted that the practice of protocolized weaning has increased to the point where it is 'usual practice' in many units. As one might expect, we are beginning to see studies that compare automated weaning with protocolized weaning practice and a review of automated versus non‐automated systems summarises the findings from these trials (Rose 2013). Similar to this review, automated systems also impact favourably on the duration of mechanical ventilation and weaning without causing harm, but with significant heterogeneity in effect estimates.
A review of protocolized weaning in children (Blackwood 2013) highlighted the paucity of trials in the paediatric population. Three trials reported findings from three different interventions. Only one large trial was adequately powered to detect an effect, therefore the benefits and harms of protocolized weaning on children could not be determined.
The paediatric review and this updated review of protocolized weaning are being augmented by a systematic review of qualitative evidence to identify contextual factors and processes that might explain the observed heterogeneity (Jordan 2012). The qualitative review will enhance these reviews by synthesizing trial‐related qualitative evidence to help explain the observed heterogeneity. It will extend the reviews by undertaking a specific search for and synthesis of evidence from relevant qualitative research to address questions raised by the review. These questions concern the contextual factors (for example, ICU culture, organization, staffing levels and extent of collaboration), and their interplay, that may impact on the effective use of weaning protocols in mechanical ventilation. Both the enhancing and extending reviews of qualitative evidence will add value to the reviews of protocolized weaning by exploring questions to do with the development, delivery, uptake, implementation, experience and evaluation of weaning protocols.

Updated study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG04.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG05.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].
![Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG06.png)
Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].
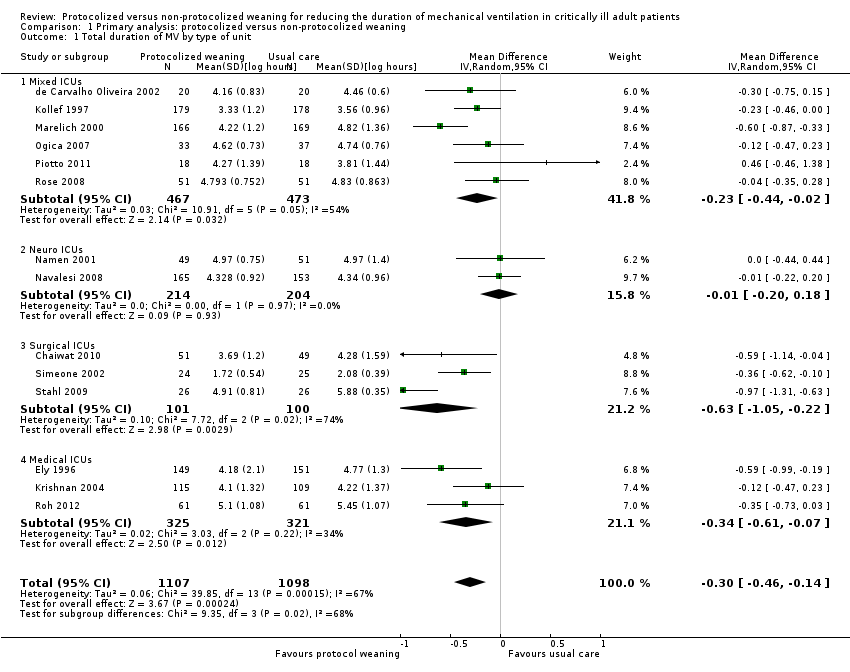
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 1 Total duration of MV by type of unit.
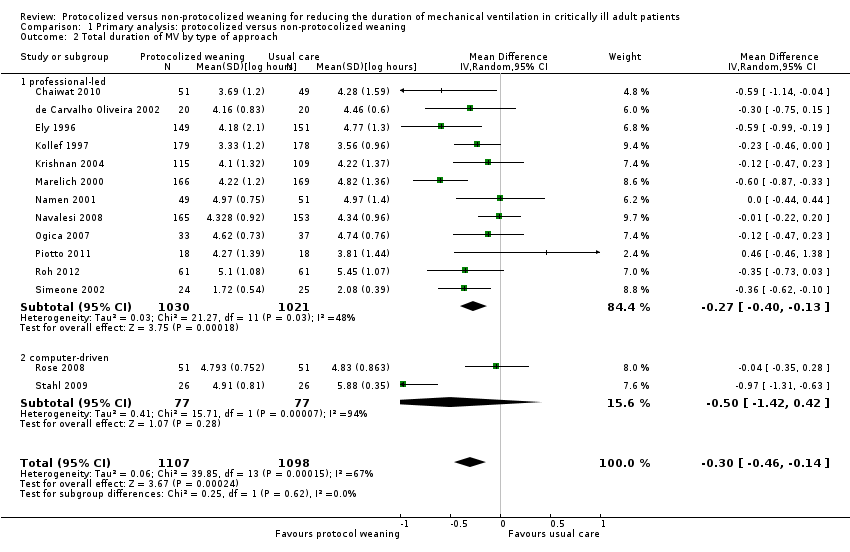
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 2 Total duration of MV by type of approach.
![Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 3 Total duration of MV by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-CMP-001-03.png)
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 3 Total duration of MV by type of protocol [log hours].

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 4 Mortality.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 5 Reintubation.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 6 Self extubation.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 7 Tracheostomy.
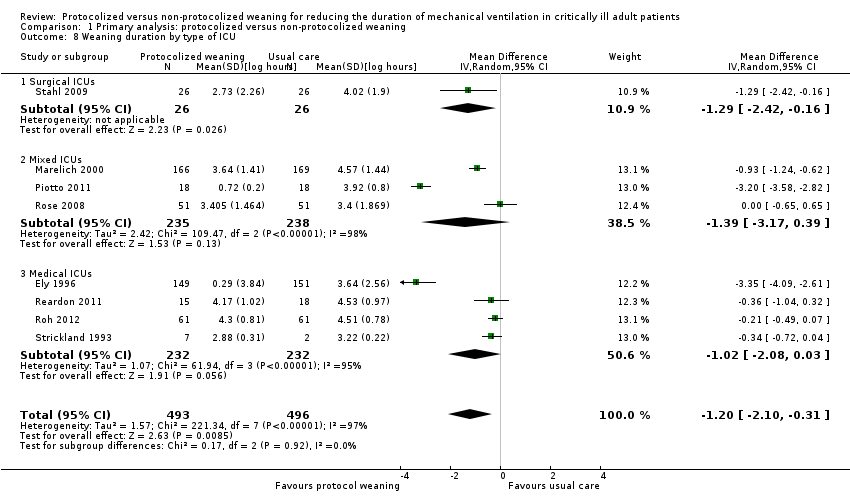
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 8 Weaning duration by type of ICU.
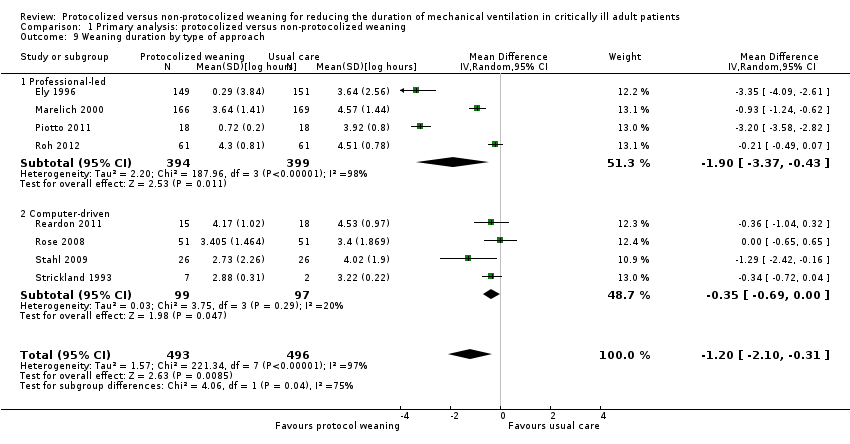
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 9 Weaning duration by type of approach.
![Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 10 Weaning duration by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-CMP-001-10.png)
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 10 Weaning duration by type of protocol [log hours].

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 11 ICU length of stay.
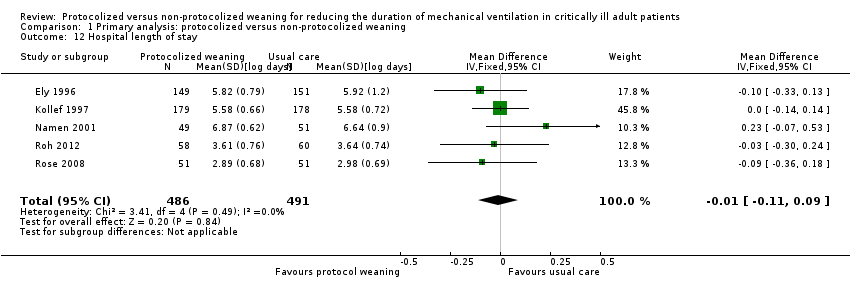
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 12 Hospital length of stay.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 13 ICU costs.
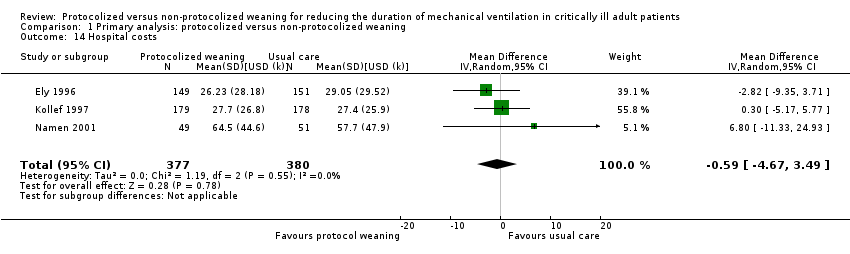
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 14 Hospital costs.
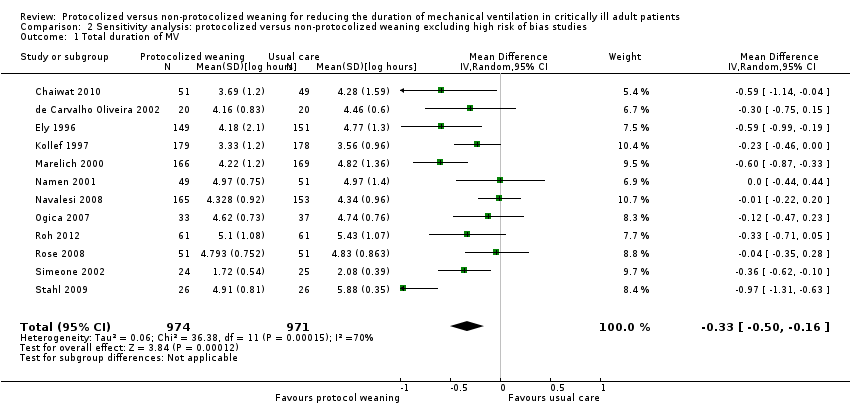
Comparison 2 Sensitivity analysis: protocolized versus non‐protocolized weaning excluding high risk of bias studies, Outcome 1 Total duration of MV.
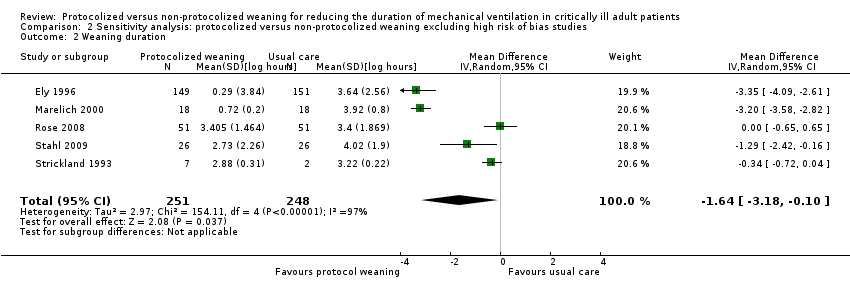
Comparison 2 Sensitivity analysis: protocolized versus non‐protocolized weaning excluding high risk of bias studies, Outcome 2 Weaning duration.
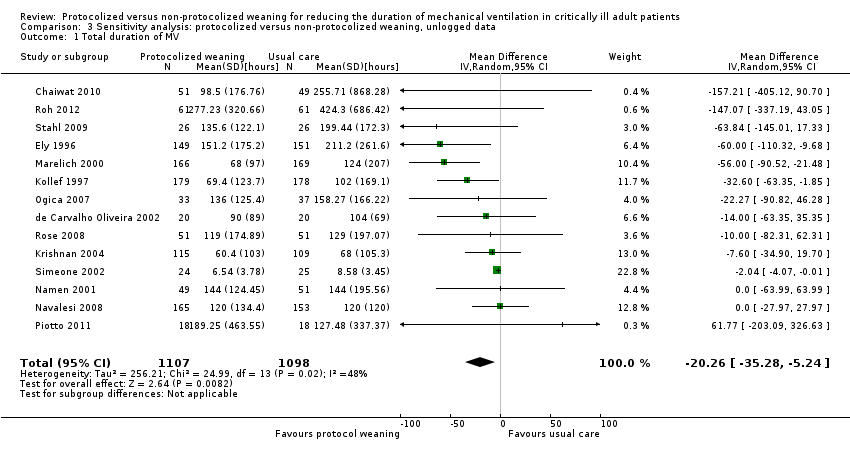
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 1 Total duration of MV.
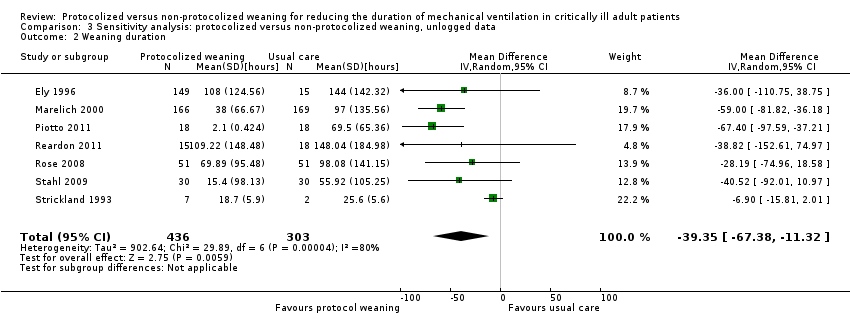
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 2 Weaning duration.

Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 3 ICU length of stay.
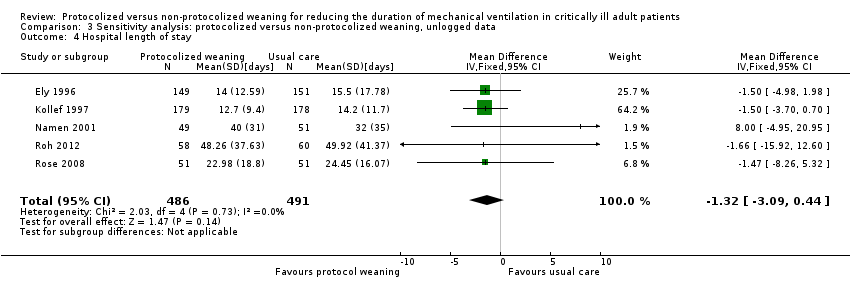
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 4 Hospital length of stay.
| Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients | |||||
| Patient or population: mechanically ventilated adult patients Settings: intensive care units Intervention: protocolized weaning Comparison: non‐protocolized weaning | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect Estimates (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk non‐protocolized weaning | Corresponding risk protocolized weaning | ||||
| Total duration of mechanical ventilation (hours) | Mean 96 hours1 | Mean 71 hours (60.5 to 83.5 hours) | Geometric mean difference ‐26% (‐37% to ‐13%) | 2205 | +++O |
| Weaning duration (hours) | Mean 24 hours1 | Mean 7 hours (2.8 to 17.5 hours) | Geometric mean difference ‐70% (‐88% to ‐27%) | 989 | ++OO |
| ICU length of stay (days) | Mean 8 days1 | Mean 7 days (6.5 to 7.8 days) | Geometric mean difference ‐11% (‐19% to ‐3%) | 1378 [9 studies] | ++OO |
| ICU mortality | 31%1 | 30% (20% to 42%) | OR 0.97 (0.57 to 1.63) | 651 [6 studies] | +++O |
| Reintubation | 10%1 (following deliberate extubation) | 8% (5% to 12%) | OR 0.74 (0.44 to 1.23) | 1487 [11 studies] | ++OO |
| *The basis for the assumed risk (e.g. the mean control group risk) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the effect estimate of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The assumed risk is derived from the median reported in a large epidemiological study of characteristics and outcomes in patients (N = 4968) receiving mechanical ventilation by Esteban 2008. The reported medians were used as an approximation for the means used for illustrative comparisons of all continuous variables. The table shows the mean duration of mechanical ventilation, weaning and ICU length of stay if patients are not weaning by protocol (non‐protocolized weaning) and what would be expected with protocolized weaning based on the effect estimates from our review. 2 There was considerable variability in effect estimates (I2 = 67%) that could not be explained by subgroup analysis although variability was lower than the previous review. The confidence interval was narrower in this review and the difference at the lower limit would still be clinically significant. 3 There was considerable variability in effect estimates (I2 = 97%) and the wide confidence intervals indicate imprecision in results. The lower limit suggests a one hour difference in weaning that is not clinically significant. 4 There was no heterogeneity among trials effects estimates, but wide confidence intervals indicate imprecision in results. 5 There was moderate variability in effect estimates (I2 = 50%). 6 There was moderate variability in effect estimates (I2 = 43%). | |||||
| Study | Assessment frequency | Oxygenation | Other respiratory factors | Cardiovascular | Neurological | Inflammatory response | Medication | Other |
| Daily screen | PaO2/FiO2 >/= 200 on FiO2 </= 0.4 SpO2 >/= 94% | PEEP </= 5 Respiratory rate < 35 Rapid Shallow breathing index </= 105 Static lung compliance >/= 25 mL/cmH2O Minute volume </= 10L/min | HR < 120 b/min | Awake and easily rousable | Not included | Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Pain score < 4 | |
| Not reported | PaO2 < 90 on FiO2 </= 0.4 | PEEP < 5 Pimax < ‐ 25 cm H2O | Not included | GCS > 8 | Not included | No sedation No vasopressors | Cause of MV resolved No planned surgery | |
| Daily screen
| PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Protocol entry criteria
| PaO2/FiO2 > 200 | PEEP </= 5 RR </= 35 b/min
| HR < 140 b/min | Awake and orientated | Not included | No vasoactive or inotropic agents | Not included | |
| Daily screen
| SpO2 >/= 92% FiO2 </= 0.5 | PEEP </=5
| Stable CAD HR < 140 b/min | No raised ICP | Not included | No paralytics | Cough and gag reflex present Responsive to stimuli
| |
| x 2 daily screen
| PaO2/FiO2 >/= 200 | Not included | MAP >/= 60 mmHg | GCS >/= 10 or tracheostomy | Not included | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough not limited by pain
| |
| Daily screen | PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | PaO2/FiO2 > 200 FiO2 </= 0.4 pH >/= 7.35 PaCO2 </= 50 mmHg | PEEP </= 5
| HR </= 125 b/min SBP >/= 90 mmHg | GCS >/= 8 | T < 38.5oC | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough Suctioning < 2/hr Normal Na blood values
| |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Daily screen | PaO2/FiO2 150‐300 FiO2 </= 0.4 PaO2 >/= 60 mmHg Hb = 8 ‐ 10 g/L
| Not included | MAP >/= 60 mmHg HR </= 140 b/min | Awake GCS >/= 9 | T < 37.8oC | Minimum sedation No or low vasopressors | Cause of MV resolved Effective cough Metabolic stability No hydroelectrolyte disorders
| |
| Daily screen | SaO2 > 90% or PaO2 > 60 mmHg on FiO2 </= 0.5 | Respiratory rate < 35 pH > 7.20 Triggering breaths | SBP > 90 and < 180 HR > 50 and < 130 No cardiac ischaemia | GCS > 8 | Not included | Minimal pressure requirements | Improving condition Absence of excessive secretions Suctioning < hourly Deemed ready to wean | |
| Not reported | FiO2 </= 0.5 | RR </= 35 PEEP </= 8 Triggering breaths | SBP >/= 90 mmHg HR </= 150 b/min | Not included | Not included | No paralytics No vasopressors Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Not included | |
| Inclusion criteria | PaO2/FiO2 > 150 or SaO2 >/= 90% on FiO2 0.5 | PEEP </= 8 Plateau pressure </= 30 cmH2O Successful 30 min SBT using PS 20 cm H2O to achieve TV > 200 mL
| Haemodynamically stable | GCS > 4 | T = 36 ‐ 39oC | Not included | No surgery anticipated MV > 24 hr
| |
| Inclusion criteria | PaO2/FiO2 >/= 200 FiO2 < 0.5 pH 7.3 ‐ 7.5 PaO2 30 ‐ 50 mmHg SaO2 > 90% Hb > 8 mg/dL Pulse oximeter oxygenation stable Cardiopulmonary bypass time < 150 min | PEEP < 4 RR < 35 b/min Dynamic compliance > 22 mL/cmH2O Compliance statica >33 mL/cmH2O Vital capacity >10 mL/kg MIP >/= ‐15 cmH2O
| Haemodynamically stable | Awake and conscious | T > 35 < 38oC | Not included | Urine output > 100 mL/hr Normal CXR | |
| Inclusion criteria | FiO2 </= 0.5 PaO2 > 75 mmHg or SaO2 > 90% pH </= 7.2 Hb >/= 7g/dL | PEEP </= 10 | Haemodynamically stable | Not included | Not included | Dopamine </= 5 ug/kg/min | MV > 24 hr Breathing spontaneously Ramsey sedation score =/< 3
| |
| Inclusion criteria | FiO2 </= 0.4 pH >/= 7.3 </= 7.5 PCO2 >/= 30 </= 50 SaO2 >/= 90% on SIMV rate 6 ‐ 10 PS 20 cmH2O | NIF </= ‐ 20 cmH2O FVC >/= 10 mL/kg TV 10 ‐ 15 mL/kg
| Haemodynamically stable | Not included | T </= 37oC | Not included | Judged ready to wean by physician Feeding ‐ parenteral or tube Stable renal function Normal electrolytes
| |
| CAD = coronary artery diease; CXR = chest X‐ray; GCS = Glasgow Coma Scale; FVC = forced vital capacity; Hb = haemoglobin; HR ‐ heart rate; MAP = mean arterial pressure; MIP = maximal inspiratory pressure; MV = mechanical ventilation; NIF = negative inspiratory force; PEEP = positive end expiratory pressure; Pimax = maximal inspiratory mouth pressure; PS = pressure support; RR = respiratory rate; SBP = systolic blood pressure; SIMV = synchronized intermittent mechanical ventilation; T = temperature; TV = tidal volume; f/VT = ratio of respiratory frequency to tidal volume. | ||||||||
| Study | Time of randomization | Intervention protocol | Extubation criteria | Comparator (usual practice) |
| ICU admission | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Notify MD | Not reported | |
| Not reported | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Yes | Not reported | |
| Enrolment, time not reported | SBT 2 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| Not reported | a) SBT 30 minutes and extubation if passed b) If failed, daily SBT and stepwise reduction in SIMV and PS until 4 breaths/min and PS 7 cmH2O | Not reported | Not reported | |
| ICU admission | a) SBT 30 to 60 min on CPAP 5 cmH2O, PS 6 cmH2O b) PS stepwise reduction to 6 cmH2O c) IMV stepwise reduction to 0 breaths/min, on PEEP 5 cmH2O and PS 6 cmH2O for 30 to 60 min | a) Yes
b) Yes c) Yes | Not reported | |
| Not reported | SBT 1 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| On meeting weaning criteria | a) < 72‐hour admissions: SBT 30 min on PS </= 8 cmH2O & PEEP </= 8 cmH2O b) > 72‐hour admissions: PEEP, IMV and PS stepwise reductions to achieve FiO2 0.5, PEEP </= 8 cmH2O, IMV </= 6 breaths/min, PS </= 8 cmH2O then SBT as above | a) Notify MD
b) Notify MD | Not reported | |
| On meeting weaning criteria | SBT 2 hours on CPAP 5 cmH2O | Notify MD | Not reported | |
| Enrolment, time not reported | SBT 1 hour on CPAP 2 to 3 cmH2O, FiO2 0.4 | Yes | Not reported | |
| Not reported | SBT (details not reported) | Not reported | Not reported | |
| Not reported | SBT 2 hours on PS 7 cmH2O, PEEP 5 cmH2O, FiO2 0.4, RR 1 breath/min | Yes | Stepwise reduction in PS and IMV | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | Notify MD | Stepwise reduction in PS and SBT | |
| On meeting weaning criteria | CPAP trial on 5 cmH2O, then stepwise reductions in PS to 5 cmH2O, then SBT on T‐piece for 30 minutes | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | No | Stepwise reduction in PS and PEEP | |
| Not reported | SIMV and PS stepwise reductions to SIMV 0 breath/min and PS 4 cmH2O | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS stepwise reductions to PS | Yes | Spepwise reduction in PS and CPAP | |
| On meeting weaning criteria | Computer automated Supersport model 2 stepwise reductions in SIMV and PS to RR 2 breaths/min and PS 5 cmH2O | Not reported | Stepwise reduction in IMV and PS | |
| CPAP = continuous positive airway pressure; IMV = intermittent mechanical ventilation; MD = Medical Doctor; PEEP = positive end expiratory pressure; PS = pressure support; SBT = spontaneous breathing trial; SIMV =synchronized intermittent mechanical ventilation; RR = respiratory rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV by type of unit Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 1.1 Mixed ICUs | 6 | 940 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.44, ‐0.02] |
| 1.2 Neuro ICUs | 2 | 418 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.20, 0.18] |
| 1.3 Surgical ICUs | 3 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.05, ‐0.22] |
| 1.4 Medical ICUs | 3 | 646 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.61, ‐0.07] |
| 2 Total duration of MV by type of approach Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 2.1 professional‐led | 12 | 2051 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.40, ‐0.13] |
| 2.2 computer‐driven | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.42, 0.42] |
| 3 Total duration of MV by type of protocol [log hours] Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 3.1 SBT protocol | 8 | 1188 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, 0.00] |
| 3.2 Stepwise reduction protocol | 6 | 1017 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.66, ‐0.18] |
| 4 Mortality Show forest plot | 14 | 2234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.26] |
| 4.1 Hospital mortality | 8 | 1523 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.32] |
| 4.2 ICU mortality | 7 | 711 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.48] |
| 5 Reintubation Show forest plot | 11 | 1487 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.23] |
| 6 Self extubation Show forest plot | 3 | 433 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.34] |
| 7 Tracheostomy Show forest plot | 8 | 1346 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 8 Weaning duration by type of ICU Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 8.1 Surgical ICUs | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.42, ‐0.16] |
| 8.2 Mixed ICUs | 3 | 473 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.17, 0.39] |
| 8.3 Medical ICUs | 4 | 464 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.08, 0.03] |
| 9 Weaning duration by type of approach Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 9.1 Professional‐led | 4 | 793 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.37, ‐0.43] |
| 9.2 Computer‐driven | 4 | 196 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.69, ‐0.00] |
| 10 Weaning duration by type of protocol [log hours] Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 10.1 SBT protocol | 2 | 336 | Mean Difference (IV, Random, 95% CI) | ‐3.23 [‐3.57, ‐2.89] |
| 10.2 Stepwise reduction protocol | 6 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.81, ‐0.12] |
| 11 ICU length of stay Show forest plot | 9 | 1378 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.21, ‐0.03] |
| 12 Hospital length of stay Show forest plot | 5 | 977 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 13 ICU costs Show forest plot | 2 | 400 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐15.02, 21.76] |
| 14 Hospital costs Show forest plot | 3 | 757 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐4.67, 3.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV Show forest plot | 12 | 1945 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.50, ‐0.16] |
| 2 Weaning duration Show forest plot | 5 | 499 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.18, ‐0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐20.26 [‐35.28, ‐5.24] |
| 2 Weaning duration Show forest plot | 7 | 739 | Mean Difference (IV, Random, 95% CI) | ‐39.35 [‐67.38, ‐11.32] |
| 3 ICU length of stay Show forest plot | 9 | 1378 | Mean Difference (IV, Fixed, 95% CI) | ‐9.08 [‐15.85, ‐2.30] |
| 4 Hospital length of stay Show forest plot | 5 | 977 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐3.09, 0.44] |

