جداسازی بیمار از دستگاه ونتیلاسیون مکانیکی بر اساس پروتکلهای موجود، در مقایسه با عدم تبعیت از پروتکلها، به منظور کاهش مدت زمان استفاده از دستگاه ونتیلاسیون مکانیکی در بیماران بزرگسالان به شدت بدحال
Appendices
Appendix 1. Ovid MEDLINE(R) in‐process and other non‐indexed citations and Ovid MEDLINE(R) (1950 to week 04 January 2014)
#1 exp Ventilator Weaning/
#2 mechanical ventilat$ weaning.mp.
#3 mechanical ventilation.mp.
#4 (protocol$ adj weaning).mp.
#5 (ventilat$ adj weaning).mp.
#6 exp Ventilators, Mechanical/
#7 exp Ventilators, Negative‐Pressure/
#8 (mechanical adj ventilat$).mp.
#9 (mechanical adj weaning).mp.
#10 ventilat$.ab,ti.
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 protocol$.mp.
#13 exp Clinical Protocols/
#14 exp Patient Care Management/
#15 Practice Guidelines/
#16 #12 or #13 or #14 or #15
#17 #11 and #16
#18 clinical trial.pt.
#19 randomized.ab.
#20 placebo.ab.
#21exp Clinical Trials/
#22 randomly.ab.
#23 trial.ti.
#24 #18 or #19 or #20 or #21 or #22 or #23
#25 Animals/
#26 Humans/
#27 #25 not (#25 and #26)
#28 #24 not #27
#29 #17 and #28
Appendix 2. EMBASE (1988 to week 04 January 2014)
#1 exp Ventilator Weaning/
#2 mechanical ventilat$ weaning.mp.
#3 mechanical ventilation.mp.
#4 (protocol$ adj weaning).mp.
#5 (ventilat$ adj weaning).mp.
#6 exp Ventilators, Mechanical/
#7 exp Ventilators, Negative‐Pressure/
#8 (mechanical adj ventilat$).mp.
#9 (mechanical adj weaning).mp.
#10 ventilat$.ab,ti.
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 protocol$.mp.
#13 exp Clinical Protocols/
#14 exp Patient Care Management/
#15 Practice Guidelines/
#16 #12 or #13 or #14 or #15
#17 #11 and #16
#18 clinical trial.pt.
#19 randomized.ab.
#20 placebo.ab.
#21exp Clinical Trials/
#22 randomly.ab.
#23 trial.ti.
#24 #18 or #19 or #20 or #21 or #22 or #23
#25 Animals/
#26 Humans/
#27 #25 not (#25 and #26)
#28 #24 not #27
#29 #17 and #28
Appendix 3. LILACS (via BIREME interface) (1982 to January 2014)
# 1 "WEANING" or "MECHANICAL VENTILATION" or “VENTILATOR" or "NEGATIVE‐PRESSURE" [Words] or "ventilat* weaning" or "mechanical ventilator*" or "destetar mecánico" or "desmamar mecânico" [Words]
Appendix 4. CINAHL Plus EBSCO host (1937 to January 2014)
#1 (MM "Ventilators, Mechanical") or (MM "Ventilator Weaning") or (MH”Respiration, artificial+”)
#2 (“mechanical ventilat$ weaning”) or (“MH Ventilator Weaning”) or (MH “Mechanical Ventilatory Weaning (Iowa NIC)”) or (MH “Ventilatory Weaning Impairment (Saba CCC)”)
#3 “mechanical ventilation”
#4 “weaning protocol”
#5 #1 or #2 or #3 or #4
#6 (“protocol$”) or (MM “Nursing Protocols+”)
#7 (MM “Practice Guidelines”)
#8 #6 or #7
#9 #5 and #8
#10 (MM “Clinical Trials+”)
#11 (MH “Random Assignment”)
#12 “randomly”
#13 “trial”
#14 #10 or #11 or #12 or #13
#15 #9 and #14
Appendix 5. CENTRAL (The Cochrane Library Issue 1, 2014)
#1 MeSH descriptor Ventilator Weaning explode all trees
#2 mechanical ventilat* weaning
#3 protocol* near weaning
#4 ventilat* near weaning
#5 MeSH descriptor Ventilators, Mechanical explode all trees
#6 MeSH descriptor Ventilators, Negative‐Pressure explode all trees
#7 (mechanical ventilat*):ab
#8 mechanical near weaning
#9 ventilat*:ti
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 protocol*:ti,ab
#12 MeSH descriptor Clinical Protocols explode all trees
#13 MeSH descriptor Patient Care Management explode all trees
#14 MeSH descriptor Practice Guidelines explode all trees
#15 (#11 OR #12 OR #13 OR #14)
#16 (#10 AND #15)
Appendix 6. ISI Web of Science with Conference Proceedings (1970 to February 2014)
#1 TS=mechanical ventilat*
#3 TS=(ventilat* SAME weaning)
#2 TS=(protocol* SAME weaning)
#4 TS=Ventilator* Negative‐Pressure
#5 TS=(mechanical SAME weaning)
#6 TS=ventilat*
#7 #6 OR #5 OR #4 OR #3 OR #2 OR #1
#8 TS=protocol*
#9 TS=(Care SAME Manag*)
#10 TS=(Patient* SAME Management )
#11 TS=(Practice Guideline*)
#12 #11 OR #10 OR #9 OR #8
#13 #12 AND #7
#14 TS=clinical trial*
#15 TS=random*
#16 TS=placebo*
#17 #16 OR #15 OR #14
#18 #17 AND #13
Appendix 7. Data extraction form
Study Selection, Quality Assessment & Data Extraction Form
Name of author extracting data: ____________________________
Date form completed: ____________________________
Study ID
| Title | |
| Study ID for RevMan (Family name of first author and year of publication + letter if more than one per year, e.g. Smith2001b) | |
| Are there other articles of same study? (YES, NO, Unclear. If Yes, write Study IDs) |
Study Eligibility
| (please circle) | ||
| Type of study Can the study be described as randomized? | Yes, Unclear, No | |
| Participants 1. Were the participants adults (at least 18 years & over) and in ICUs? 2. Were participants intubated (naso/orotracheal) and receiving invasive mechanical ventilation (MV)? | Yes, Unclear, No Yes, Unclear, No | |
| Interventions 1. Was one group weaned using a formal weaning protocol1? 2. Was the other group weaned without reference to a formal protocol? | Yes, Unclear, No Yes, Unclear, No | |
| Outcomes: Did the study report any one of – 1. Total duration of MV (time from initiation of MV to MV discontinuation)? 2. Weaning duration (time from identification of weaning readiness to MV discontinuation)? 3. ICU length of stay | Yes, Unclear, No Yes, Unclear, No Yes, Unclear, No | |
| Conclusion: Do not proceed if any of the above answers are ‘No’. If study to be ‘included’ or ‘excluded & listed in excluded table’, record below the information to be inserted into tables. If included – continue to page 2 Included, or Excluded and should be listed in the excluded table More information needed before inclusion decision (specify): Record for tables: | ||
1Protocol = a written set of rules, criteria, guidelines or algorithm for deciding if a patient is ready to tolerate MV discontinuation & for reducing ventilatory support.
| PARTICIPANTS | |||||||||||||||||
| Inclusion/Exclusion Criteria | |||||||||||||||||
| INTERVENTION | CONTROL | ||||||||||||||||
| Number randomized | |||||||||||||||||
| Number analyzed | |||||||||||||||||
| Age, mean (SD) med (IQR) | Age, mean (SD) | ||||||||||||||||
| Male n (%) | Male n (%) | ||||||||||||||||
| Name severity of illness measure (e.g. APACHE, SAPS, PELOD) mean (SD) med (IQR) | Name severity of illness measure (e.g. APACHE, SAPS, PELOD) mean (SD) med (IQR) | ||||||||||||||||
| Setting | Participating site country(ies): | ||||||||||||||||
| Academic hospital | Non‐teaching hospital | Not reported | |||||||||||||||
| Any other information about hospital (e.g. number of beds) | |||||||||||||||||
| Number of ICUs and types (e.g. medical; surgical; mixed; neuro. Include number of beds if reported) | |||||||||||||||||
| Closed ICU structure | Open ICU structure | Not reported | |||||||||||||||
| Nurse staffing for vent patients | 1:1 | 1:2 | 1:3 | 1:4 | Not reported | ||||||||||||
| Physician staffing (describe) Not reported | |||||||||||||||||
| INTERVENTION | |||||||||||||||||
| Describe weaning protocol and, if appropriate, who delivered it (verbatim) | |||||||||||||||||
| Describe sedation strategies in intervention arm (tick all that apply): | |||||||||||||||||
| sedation score | sedation protocol | daily interruption | not reported | ||||||||||||||
| CONTROL | |||||||||||||||||
| Describe usual/standard weaning (verbatim) | |||||||||||||||||
| Describe sedation strategies in control arm (tick all that apply): as above | |||||||||||||||||
| sedation score | sedation protocol | daily interruption | not reported | ||||||||||||||
Outcomes (list & provide descriptors if they were described in the paper)
| Primary | |
| Secondary |
| Domain | Description (verbatim) | Judgement |
| Sequence generation Was the allocation sequence adequately generated? | Yes No Unclear | |
| Allocation concealment Was allocation adequately concealed? | Yes No Unclear | |
| Blinding (participants, personnel, outcome) Was knowledge of the allocated intervention adequately prevented during the study? | Yes No Unclear | |
| Incomplete outcome data Were incomplete outcome data adequately addressed? State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons | Total duration of mechanical ventilation (initiation of mechanical ventilation to discontinuation) | Not measured Yes No Unclear |
| Weaning duration (identification of weaning to mechanical ventilation discontinuation) | Not measured Yes No Unclear | |
| Mechanical ventilation time prior to weaning (initiation of mechanical ventilation to identification of weaning) | Not measured Yes No Unclear | |
| Time from mechanical ventilation discontinuation to extubation | Not measured Yes No Unclear | |
| ICU length of stay | Not measured Yes No Unclear | |
| Hospital length of stay | Not measured Yes No Unclear | |
| Cost | Not measured Yes No Unclear | |
| Mortality | Not measured Yes No Unclear | |
| Reintubation | Not measured Yes No Unclear | |
| Selfextubation | Not measured Yes No Unclear | |
| Postextubation NIV | Not measured Yes No Unclear | |
| ≥ 21 days vented | Not measured Yes No Unclear | |
| Tracheostomy | Not measured Yes No Unclear | |
| Selective outcome reporting. Are reports of the study free of suggestion of selective outcome reporting? | Yes No Unclear | |
| Other sources of bias. Study free from other bias? | Yes No Unclear |
Outcomes – Continuous Data
| Outcomes | Unit measurement | Intervention group | Control group | 95% CI or any further details if outcome only described in text | |||||
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | P‐value | |||
| Total duration of mechanical ventilation (initiation of mechanical ventilation to discontinuation) | |||||||||
| Weaning duration (identification of weaning to mechanical ventilation discontinuation) | |||||||||
| Mechanical ventilation time prior to weaning (initiation of mechanical ventilation to identification of weaning) | |||||||||
| Time from mechanical ventilation discontinuation to extubation | |||||||||
| ICU length of stay | |||||||||
| Hospital length of stay | |||||||||
| Cost (state, hospital or ICU) | |||||||||
Outcomes ‐ Dichotomous Data
| Outcomes | Intervention group (n = ) | Control group (n = ) | P value | Any further information |
| Reintubation | ||||
| Self extubation | ||||
| Tracheostomy | ||||
| Mechanical ventilation > 21 days | ||||
| Mortality | ||||
| Postextubation NIV |
Please specify number of patients in each group experiencing the specified outcomes.
Other information which you feel is relevant to the results:
| Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |

Updated study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG04.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.3 Total duration of mechanical ventilation by type of protocol [log hours].
![Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG05.png)
Forest plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.9 Weaning duration by type of approach [log hours].
![Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-AFig-FIG06.png)
Funnel plot of comparison: 1 Primary analysis: protocolized versus non‐protocolized weaning, outcome: 1.2 Total duration of MV by type of approach [log hours].
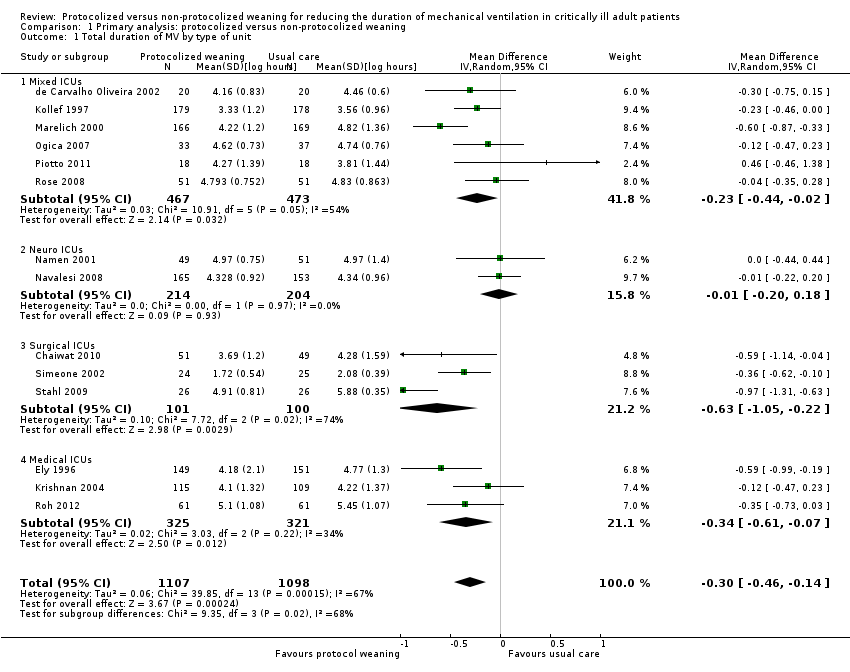
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 1 Total duration of MV by type of unit.
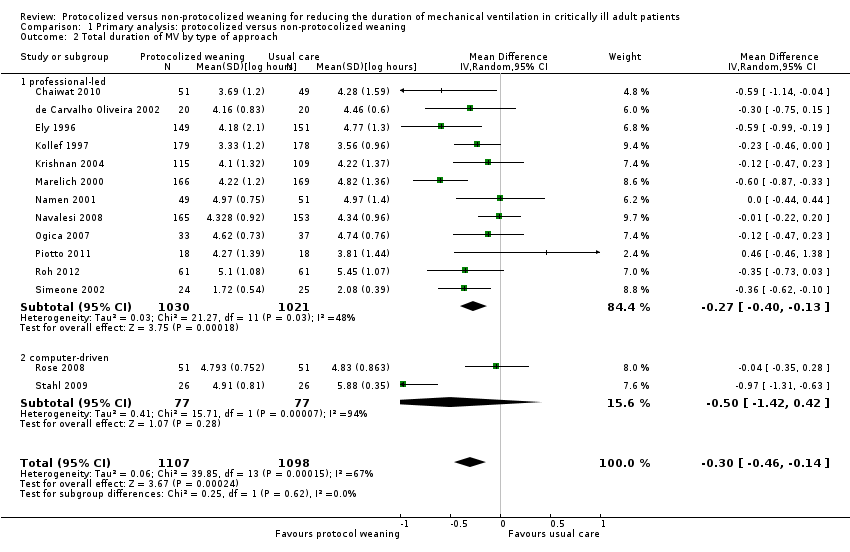
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 2 Total duration of MV by type of approach.
![Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 3 Total duration of MV by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-CMP-001-03.png)
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 3 Total duration of MV by type of protocol [log hours].

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 4 Mortality.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 5 Reintubation.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 6 Self extubation.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 7 Tracheostomy.
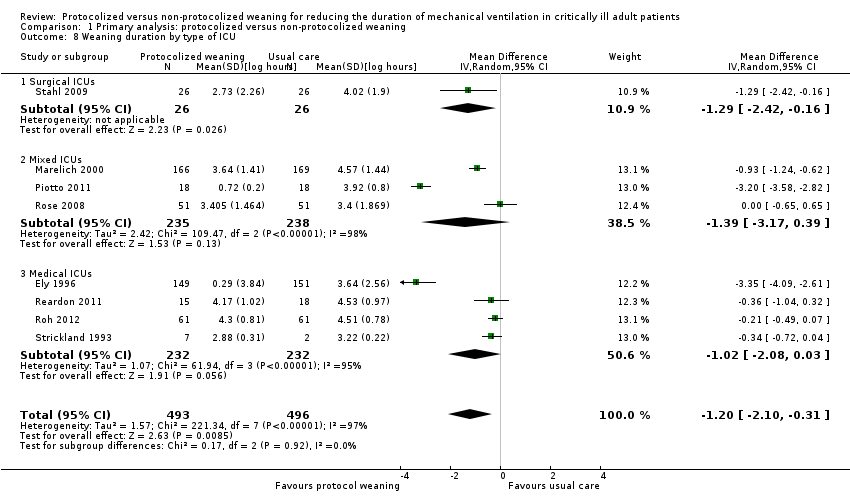
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 8 Weaning duration by type of ICU.
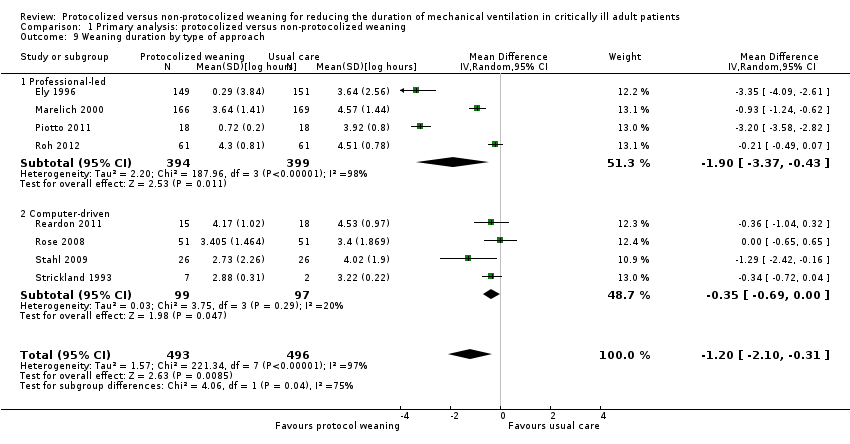
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 9 Weaning duration by type of approach.
![Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 10 Weaning duration by type of protocol [log hours].](/es/cdsr/doi/10.1002/14651858.CD006904.pub3/media/CDSR/CD006904/image_n/nCD006904-CMP-001-10.png)
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 10 Weaning duration by type of protocol [log hours].

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 11 ICU length of stay.
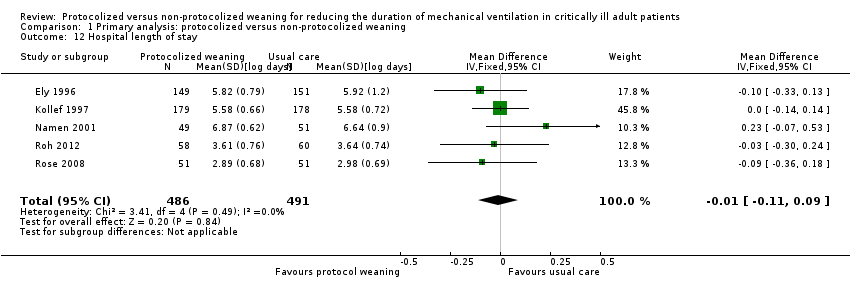
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 12 Hospital length of stay.

Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 13 ICU costs.
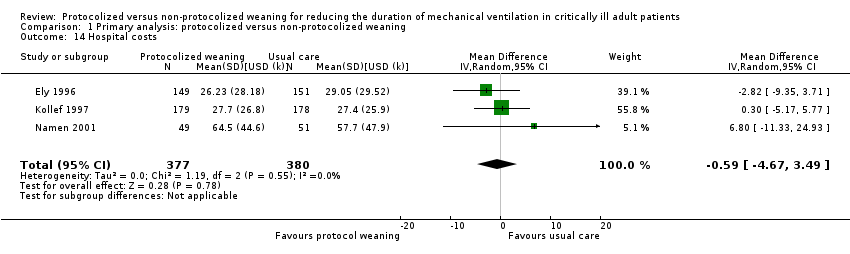
Comparison 1 Primary analysis: protocolized versus non‐protocolized weaning, Outcome 14 Hospital costs.
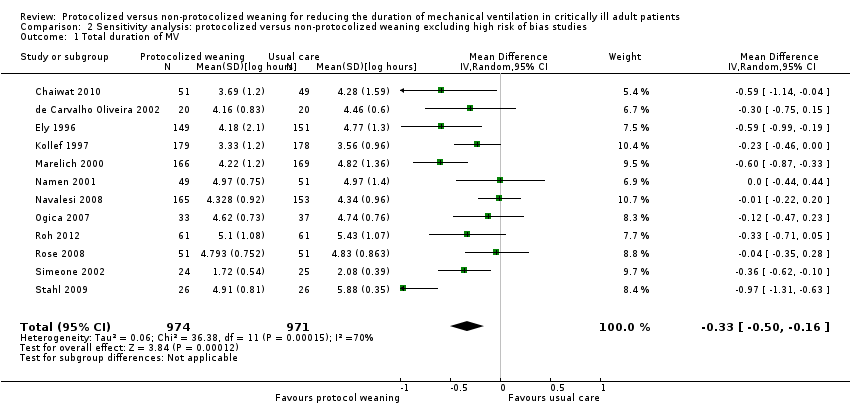
Comparison 2 Sensitivity analysis: protocolized versus non‐protocolized weaning excluding high risk of bias studies, Outcome 1 Total duration of MV.
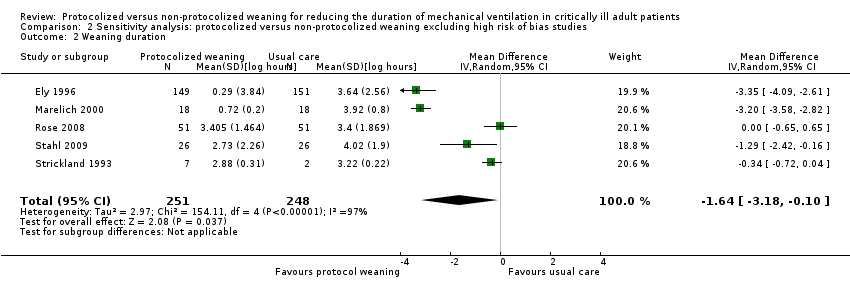
Comparison 2 Sensitivity analysis: protocolized versus non‐protocolized weaning excluding high risk of bias studies, Outcome 2 Weaning duration.
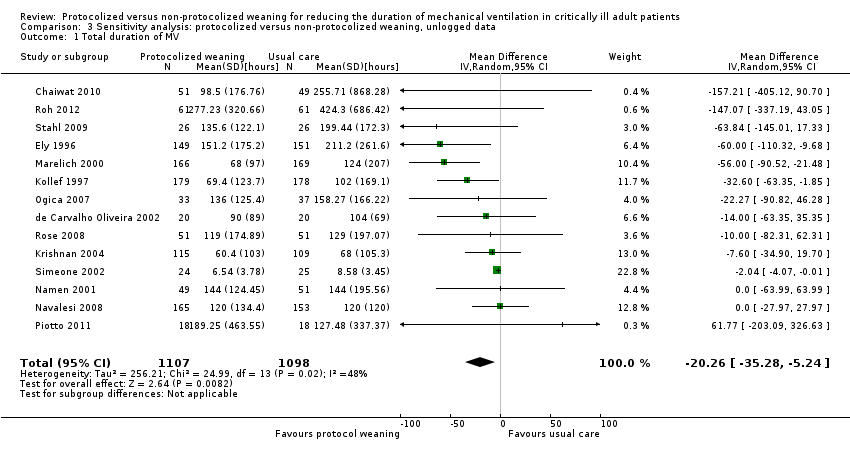
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 1 Total duration of MV.
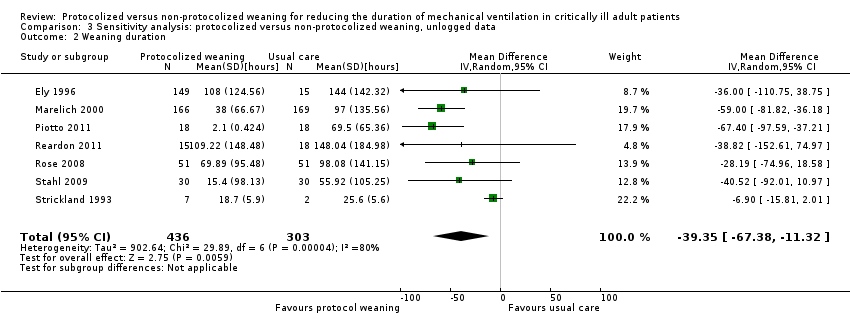
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 2 Weaning duration.

Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 3 ICU length of stay.
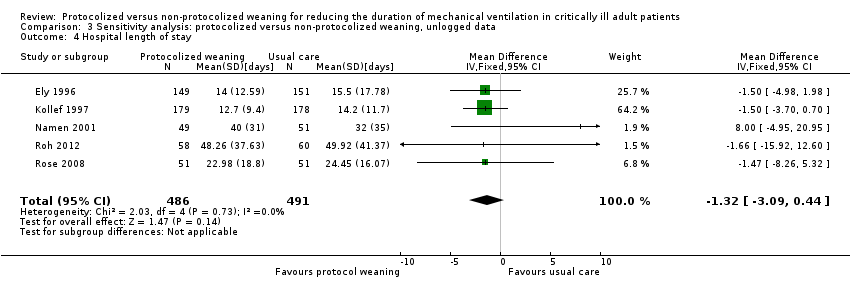
Comparison 3 Sensitivity analysis: protocolized versus non‐protocolized weaning, unlogged data, Outcome 4 Hospital length of stay.
| Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients | |||||
| Patient or population: mechanically ventilated adult patients Settings: intensive care units Intervention: protocolized weaning Comparison: non‐protocolized weaning | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect Estimates (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk non‐protocolized weaning | Corresponding risk protocolized weaning | ||||
| Total duration of mechanical ventilation (hours) | Mean 96 hours1 | Mean 71 hours (60.5 to 83.5 hours) | Geometric mean difference ‐26% (‐37% to ‐13%) | 2205 | +++O |
| Weaning duration (hours) | Mean 24 hours1 | Mean 7 hours (2.8 to 17.5 hours) | Geometric mean difference ‐70% (‐88% to ‐27%) | 989 | ++OO |
| ICU length of stay (days) | Mean 8 days1 | Mean 7 days (6.5 to 7.8 days) | Geometric mean difference ‐11% (‐19% to ‐3%) | 1378 [9 studies] | ++OO |
| ICU mortality | 31%1 | 30% (20% to 42%) | OR 0.97 (0.57 to 1.63) | 651 [6 studies] | +++O |
| Reintubation | 10%1 (following deliberate extubation) | 8% (5% to 12%) | OR 0.74 (0.44 to 1.23) | 1487 [11 studies] | ++OO |
| *The basis for the assumed risk (e.g. the mean control group risk) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the effect estimate of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The assumed risk is derived from the median reported in a large epidemiological study of characteristics and outcomes in patients (N = 4968) receiving mechanical ventilation by Esteban 2008. The reported medians were used as an approximation for the means used for illustrative comparisons of all continuous variables. The table shows the mean duration of mechanical ventilation, weaning and ICU length of stay if patients are not weaning by protocol (non‐protocolized weaning) and what would be expected with protocolized weaning based on the effect estimates from our review. 2 There was considerable variability in effect estimates (I2 = 67%) that could not be explained by subgroup analysis although variability was lower than the previous review. The confidence interval was narrower in this review and the difference at the lower limit would still be clinically significant. 3 There was considerable variability in effect estimates (I2 = 97%) and the wide confidence intervals indicate imprecision in results. The lower limit suggests a one hour difference in weaning that is not clinically significant. 4 There was no heterogeneity among trials effects estimates, but wide confidence intervals indicate imprecision in results. 5 There was moderate variability in effect estimates (I2 = 50%). 6 There was moderate variability in effect estimates (I2 = 43%). | |||||
| Study | Assessment frequency | Oxygenation | Other respiratory factors | Cardiovascular | Neurological | Inflammatory response | Medication | Other |
| Daily screen | PaO2/FiO2 >/= 200 on FiO2 </= 0.4 SpO2 >/= 94% | PEEP </= 5 Respiratory rate < 35 Rapid Shallow breathing index </= 105 Static lung compliance >/= 25 mL/cmH2O Minute volume </= 10L/min | HR < 120 b/min | Awake and easily rousable | Not included | Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Pain score < 4 | |
| Not reported | PaO2 < 90 on FiO2 </= 0.4 | PEEP < 5 Pimax < ‐ 25 cm H2O | Not included | GCS > 8 | Not included | No sedation No vasopressors | Cause of MV resolved No planned surgery | |
| Daily screen
| PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Protocol entry criteria
| PaO2/FiO2 > 200 | PEEP </= 5 RR </= 35 b/min
| HR < 140 b/min | Awake and orientated | Not included | No vasoactive or inotropic agents | Not included | |
| Daily screen
| SpO2 >/= 92% FiO2 </= 0.5 | PEEP </=5
| Stable CAD HR < 140 b/min | No raised ICP | Not included | No paralytics | Cough and gag reflex present Responsive to stimuli
| |
| x 2 daily screen
| PaO2/FiO2 >/= 200 | Not included | MAP >/= 60 mmHg | GCS >/= 10 or tracheostomy | Not included | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough not limited by pain
| |
| Daily screen | PaO2/FiO2 > 200 | PEEP </= 5 f/VT </= 105 | Not included | Not included | Not included | No vasopressors or sedation | Adequate cough
| |
| Daily screen | PaO2/FiO2 > 200 FiO2 </= 0.4 pH >/= 7.35 PaCO2 </= 50 mmHg | PEEP </= 5
| HR </= 125 b/min SBP >/= 90 mmHg | GCS >/= 8 | T < 38.5oC | No vasopressors Dopamine </= 5 ug/kg/min | Adequate cough Suctioning < 2/hr Normal Na blood values
| |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Daily screen | PaO2/FiO2 150‐300 FiO2 </= 0.4 PaO2 >/= 60 mmHg Hb = 8 ‐ 10 g/L
| Not included | MAP >/= 60 mmHg HR </= 140 b/min | Awake GCS >/= 9 | T < 37.8oC | Minimum sedation No or low vasopressors | Cause of MV resolved Effective cough Metabolic stability No hydroelectrolyte disorders
| |
| Daily screen | SaO2 > 90% or PaO2 > 60 mmHg on FiO2 </= 0.5 | Respiratory rate < 35 pH > 7.20 Triggering breaths | SBP > 90 and < 180 HR > 50 and < 130 No cardiac ischaemia | GCS > 8 | Not included | Minimal pressure requirements | Improving condition Absence of excessive secretions Suctioning < hourly Deemed ready to wean | |
| Not reported | FiO2 </= 0.5 | RR </= 35 PEEP </= 8 Triggering breaths | SBP >/= 90 mmHg HR </= 150 b/min | Not included | Not included | No paralytics No vasopressors Dopamine </= 5 ug/kg/min Noradrenaline </= 5 ug/kg/min | Not included | |
| Inclusion criteria | PaO2/FiO2 > 150 or SaO2 >/= 90% on FiO2 0.5 | PEEP </= 8 Plateau pressure </= 30 cmH2O Successful 30 min SBT using PS 20 cm H2O to achieve TV > 200 mL
| Haemodynamically stable | GCS > 4 | T = 36 ‐ 39oC | Not included | No surgery anticipated MV > 24 hr
| |
| Inclusion criteria | PaO2/FiO2 >/= 200 FiO2 < 0.5 pH 7.3 ‐ 7.5 PaO2 30 ‐ 50 mmHg SaO2 > 90% Hb > 8 mg/dL Pulse oximeter oxygenation stable Cardiopulmonary bypass time < 150 min | PEEP < 4 RR < 35 b/min Dynamic compliance > 22 mL/cmH2O Compliance statica >33 mL/cmH2O Vital capacity >10 mL/kg MIP >/= ‐15 cmH2O
| Haemodynamically stable | Awake and conscious | T > 35 < 38oC | Not included | Urine output > 100 mL/hr Normal CXR | |
| Inclusion criteria | FiO2 </= 0.5 PaO2 > 75 mmHg or SaO2 > 90% pH </= 7.2 Hb >/= 7g/dL | PEEP </= 10 | Haemodynamically stable | Not included | Not included | Dopamine </= 5 ug/kg/min | MV > 24 hr Breathing spontaneously Ramsey sedation score =/< 3
| |
| Inclusion criteria | FiO2 </= 0.4 pH >/= 7.3 </= 7.5 PCO2 >/= 30 </= 50 SaO2 >/= 90% on SIMV rate 6 ‐ 10 PS 20 cmH2O | NIF </= ‐ 20 cmH2O FVC >/= 10 mL/kg TV 10 ‐ 15 mL/kg
| Haemodynamically stable | Not included | T </= 37oC | Not included | Judged ready to wean by physician Feeding ‐ parenteral or tube Stable renal function Normal electrolytes
| |
| CAD = coronary artery diease; CXR = chest X‐ray; GCS = Glasgow Coma Scale; FVC = forced vital capacity; Hb = haemoglobin; HR ‐ heart rate; MAP = mean arterial pressure; MIP = maximal inspiratory pressure; MV = mechanical ventilation; NIF = negative inspiratory force; PEEP = positive end expiratory pressure; Pimax = maximal inspiratory mouth pressure; PS = pressure support; RR = respiratory rate; SBP = systolic blood pressure; SIMV = synchronized intermittent mechanical ventilation; T = temperature; TV = tidal volume; f/VT = ratio of respiratory frequency to tidal volume. | ||||||||
| Study | Time of randomization | Intervention protocol | Extubation criteria | Comparator (usual practice) |
| ICU admission | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Notify MD | Not reported | |
| Not reported | SBP on PS 7 cmH2O, PEEP 5 cmH2O for 2 hours | Yes | Not reported | |
| Enrolment, time not reported | SBT 2 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| Not reported | a) SBT 30 minutes and extubation if passed b) If failed, daily SBT and stepwise reduction in SIMV and PS until 4 breaths/min and PS 7 cmH2O | Not reported | Not reported | |
| ICU admission | a) SBT 30 to 60 min on CPAP 5 cmH2O, PS 6 cmH2O b) PS stepwise reduction to 6 cmH2O c) IMV stepwise reduction to 0 breaths/min, on PEEP 5 cmH2O and PS 6 cmH2O for 30 to 60 min | a) Yes
b) Yes c) Yes | Not reported | |
| Not reported | SBT 1 hour on CPAP 5 cmH2O | Notify MD | Not reported | |
| On meeting weaning criteria | a) < 72‐hour admissions: SBT 30 min on PS </= 8 cmH2O & PEEP </= 8 cmH2O b) > 72‐hour admissions: PEEP, IMV and PS stepwise reductions to achieve FiO2 0.5, PEEP </= 8 cmH2O, IMV </= 6 breaths/min, PS </= 8 cmH2O then SBT as above | a) Notify MD
b) Notify MD | Not reported | |
| On meeting weaning criteria | SBT 2 hours on CPAP 5 cmH2O | Notify MD | Not reported | |
| Enrolment, time not reported | SBT 1 hour on CPAP 2 to 3 cmH2O, FiO2 0.4 | Yes | Not reported | |
| Not reported | SBT (details not reported) | Not reported | Not reported | |
| Not reported | SBT 2 hours on PS 7 cmH2O, PEEP 5 cmH2O, FiO2 0.4, RR 1 breath/min | Yes | Stepwise reduction in PS and IMV | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | Notify MD | Stepwise reduction in PS and SBT | |
| On meeting weaning criteria | CPAP trial on 5 cmH2O, then stepwise reductions in PS to 5 cmH2O, then SBT on T‐piece for 30 minutes | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS with stepwise reductions to PS 7 cmH2O and PEEP 5 cmH2O | No | Stepwise reduction in PS and PEEP | |
| Not reported | SIMV and PS stepwise reductions to SIMV 0 breath/min and PS 4 cmH2O | Yes | Not reported | |
| On meeting weaning criteria | Computer automated SmartCareTM/PS stepwise reductions to PS | Yes | Spepwise reduction in PS and CPAP | |
| On meeting weaning criteria | Computer automated Supersport model 2 stepwise reductions in SIMV and PS to RR 2 breaths/min and PS 5 cmH2O | Not reported | Stepwise reduction in IMV and PS | |
| CPAP = continuous positive airway pressure; IMV = intermittent mechanical ventilation; MD = Medical Doctor; PEEP = positive end expiratory pressure; PS = pressure support; SBT = spontaneous breathing trial; SIMV =synchronized intermittent mechanical ventilation; RR = respiratory rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV by type of unit Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 1.1 Mixed ICUs | 6 | 940 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.44, ‐0.02] |
| 1.2 Neuro ICUs | 2 | 418 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.20, 0.18] |
| 1.3 Surgical ICUs | 3 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.05, ‐0.22] |
| 1.4 Medical ICUs | 3 | 646 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.61, ‐0.07] |
| 2 Total duration of MV by type of approach Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 2.1 professional‐led | 12 | 2051 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.40, ‐0.13] |
| 2.2 computer‐driven | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.42, 0.42] |
| 3 Total duration of MV by type of protocol [log hours] Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] |
| 3.1 SBT protocol | 8 | 1188 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, 0.00] |
| 3.2 Stepwise reduction protocol | 6 | 1017 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.66, ‐0.18] |
| 4 Mortality Show forest plot | 14 | 2234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.26] |
| 4.1 Hospital mortality | 8 | 1523 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.32] |
| 4.2 ICU mortality | 7 | 711 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.48] |
| 5 Reintubation Show forest plot | 11 | 1487 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.23] |
| 6 Self extubation Show forest plot | 3 | 433 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.34] |
| 7 Tracheostomy Show forest plot | 8 | 1346 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 8 Weaning duration by type of ICU Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 8.1 Surgical ICUs | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.42, ‐0.16] |
| 8.2 Mixed ICUs | 3 | 473 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.17, 0.39] |
| 8.3 Medical ICUs | 4 | 464 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.08, 0.03] |
| 9 Weaning duration by type of approach Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 9.1 Professional‐led | 4 | 793 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.37, ‐0.43] |
| 9.2 Computer‐driven | 4 | 196 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.69, ‐0.00] |
| 10 Weaning duration by type of protocol [log hours] Show forest plot | 8 | 989 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.10, ‐0.31] |
| 10.1 SBT protocol | 2 | 336 | Mean Difference (IV, Random, 95% CI) | ‐3.23 [‐3.57, ‐2.89] |
| 10.2 Stepwise reduction protocol | 6 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.81, ‐0.12] |
| 11 ICU length of stay Show forest plot | 9 | 1378 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.21, ‐0.03] |
| 12 Hospital length of stay Show forest plot | 5 | 977 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 13 ICU costs Show forest plot | 2 | 400 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐15.02, 21.76] |
| 14 Hospital costs Show forest plot | 3 | 757 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐4.67, 3.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV Show forest plot | 12 | 1945 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.50, ‐0.16] |
| 2 Weaning duration Show forest plot | 5 | 499 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.18, ‐0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total duration of MV Show forest plot | 14 | 2205 | Mean Difference (IV, Random, 95% CI) | ‐20.26 [‐35.28, ‐5.24] |
| 2 Weaning duration Show forest plot | 7 | 739 | Mean Difference (IV, Random, 95% CI) | ‐39.35 [‐67.38, ‐11.32] |
| 3 ICU length of stay Show forest plot | 9 | 1378 | Mean Difference (IV, Fixed, 95% CI) | ‐9.08 [‐15.85, ‐2.30] |
| 4 Hospital length of stay Show forest plot | 5 | 977 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐3.09, 0.44] |

