Irrigación nasal con solución salina para las infecciones agudas de las vías respiratorias superiores
Appendices
Appendix 1. Previous search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, issue 2), which contains the Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (1966 to May 2009), EMBASE (1974 to May 2009), CINAHL (1982 to May 2009), AMED (1985 to 2009) and LILACS (May 2009).
The following search terms were used to search MEDLINE and CENTRAL. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE; sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). The search terms were modified to search other databases. See Appendix 2 for the EMBASE search strategy.
MEDLINE (Ovid)
1 exp Respiratory Tract Infections/
2 (respiratory tract infection* or upper respiratory infection*).tw.
3 urti.tw.
4 Rhinitis/
5 rhinit*.tw.
6 Common Cold/
7 common cold*.tw.
8 exp Pharyngitis/
9 pharyngit*.tw.
10 sore throat*.tw.
11 Tonsillitis/
12 tonsillit*.tw.
13 exp Sinusitis/
14 sinusit*.tw.
15 exp Laryngitis/
16 laryngit*.tw.
17 rhinosinusit*.tw.
18 rhinorrhea*.tw.
19 Influenza, Human/
20 flu*.tw.
21 runny nose*.tw.
22 rhinorrhoea*.tw.
23 ((nasal* or nose*) adj2 congest*).tw.
24 or/1‐22
25 Sodium Chloride/
26 (saline or salt* or sodium chloride*).tw,nm.
27 or/25‐26
28 Irrigation/
29 (irrigat* or lavage* or wash* or rins* or douch* or atomis* or atomiz*).tw.
30 or/28‐29
31 (nasal* or nose*).tw.
32 Nose/
33 32 or 31
34 30 and 33
35 exp Nasal Lavage/
36 or/34‐35
37 24 and 27 and 36
Embase.com
1. 'respiratory tract infection'/de OR 'upper respiratory tract infection'/de OR 'rhinitis'/de OR 'common cold'/de OR 'pharyngitis'/de OR 'tonsillitis'/de OR 'sore throat'/de OR 'sinusitis'/de OR 'laryngitis'/de OR 'rhinosinusitis'/de OR 'influenza'/de
2. 'respiratory tract infection':ti,ab OR 'respiratory tract infections':ti,ab OR 'upper respiratory infection':ti,ab OR 'upper respiratory tract infections':ti,ab OR urti:ti,ab OR rhinit*:ti,ab OR 'common cold':ti,ab OR 'common colds':ti,ab OR pharyngit*:ti,ab OR 'sore throat':ti,ab OR 'sore throats':ti,ab OR tonsillit*:ti,ab OR sinusit*:ti,ab OR laryngit*:ti,ab OR rhinosinusit*:ti,ab OR rhinorrhea:ti,ab OR rhinorrhoea:ti,ab OR 'runny nose':ti,ab OR 'runny noses':ti,ab OR flu:ti,ab OR influenza*:ti,ab
3. #1 OR #2
4. 'nose'/de
5. nasal*:ti,ab OR nose*:ti,ab
6. #4 OR #5
7. lavage*:ti,ab OR wash*:ti,ab OR irrigat*:ti,ab OR rins*:ti,ab OR douch*:ti,ab OR atomis*:ti,ab OR atomiz*:ti,ab
8. #6 AND #7
9. 'sodium chloride'/de
10. salt*:ti,ab OR 'sodium chloride':ti,ab OR saline*:ti,ab
11. #9 OR #10
12. #8 AND #11
13. #3 AND #12
14. random*:ti,ab OR placebo*:ti,ab,de OR 'double blind':ti,ab
15. #13 AND #14
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Respiratory Tract Infections/
2 (infect* adj3 upper respiratory).tw.
3 urti.tw.
4 Rhinitis/
5 rhinit*.tw.
6 Common Cold/
7 common cold*.tw.
8 exp Pharyngitis/
9 pharyngit*.tw.
10 sore throat*.tw.
11 Tonsillitis/
12 tonsillit*.tw.
13 exp Sinusitis/
14 sinusit*.tw.
15 exp Laryngitis/
16 laryngit*.tw.
17 (rhinosinusit* or nasosinusit*).tw.
18 Influenza, Human/
19 flu*.tw.
20 (rhinorrhoea* or rhinorrhea*).tw.
21 ((nasal or nose*) adj2 (congest* or discharg* or blocked or runny or running or stuffy or stuffed)).tw.
22 (infect* adj3 (nose* or throat* or sinus* or sinonasal or sino‐nasal or pharyn* or laryn*)).tw.
23 or/1‐22
24 Therapeutic Irrigation/
25 Nose/
26 (nasal or nose*).tw.
27 25 or 26
28 24 and 27
29 ((nasal or nose*) adj5 (irrigat* or lavage* or wash* or rins* or douch* or atomis* or atomiz*)).tw.
30 Nasal Lavage/
31 or/28‐30
32 Sodium Chloride/
33 (saline or salt* or sodium chloride*).tw,nm.
34 or/32‐33
35 31 and 34
36 23 and 35
Appendix 3. Embase.com search strategy
#38. #34 AND #37
#37. #35 OR #36
#36. random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR factorial*:ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1
(blind* OR mask*)):ab,ti
#35. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp
#34. #31 AND #33
#33. #19 OR #32
#32. 'nose congestion'/de
#31. #22 AND #30
#30. #27 OR #28 OR #29
#29. ((nasal OR nose*) NEAR/5 (irrigat* OR lavage* OR wash* OR rins* OR douch* OR atomis* OR atomiz*)):ab,ti
#28. 'nasal lavage'/de
#27. #23 AND #26
#26. #24 OR #25 79,351
#25. nose*:ab,ti OR nasal:ab,ti
#24. 'nose'/de
#23. 'lavage'/de
#22. #20 OR #21 249,100
#21. saline:ab,ti OR salt*:ab,ti OR 'sodium chloride':ab,ti
#20. 'sodium chloride'/de
#19. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18
#18. influenz*:ab,ti OR flu:ab,ti
#17. 'influenza'/exp
#16. (infect* NEAR/3 (nose* OR throat* OR sinus* OR sinonasal OR 'sino‐nasal' OR pharyn* OR laryng*)):ab,ti
#15. ((nasal OR nose*) NEAR/2 (congest* OR discharg* OR blocked* OR runny OR running OR stuffy OR stuffed)):ab,ti
#14. rhinorrhoea:ab,ti OR rhinorrhea:ab,ti
#13. 'rhinorrhea'/de
#12. laryngit*:ab,ti
#11. 'laryngitis'/de OR 'laryngotracheobronchitis'/de
#10. tonsillit*:ab,ti
#9. 'tonsillitis'/de
#8. 'sore throat':ab,ti OR 'sore throats':ab,ti
#7. pharyngit*:ab,ti
#6. 'pharyngitis'/de OR 'viral pharyngitis'/de
#5. rhinit*:ab,ti OR 'common cold':ab,ti OR 'common colds':ab,ti OR rhinosinusit*:ab,ti OR nasosinusit*:ab,ti OR rhinopharyngit*:ab,ti OR nasopharyngit*:ab,ti
#4. 'rhinitis'/de OR 'common cold'/de OR 'rhinopharyngitis'/de OR 'rhinosinusitis'/de
#3. urti:ab,ti
#2. (infect* NEAR/3 'upper respiratory'):ab,ti
#1. 'upper respiratory tract infection'/exp
Appendix 4. CINAHL (Ebsco) search strategy
S42 S32 and S41
S41 S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40
S40 (MH "Quantitative Studies")
S39 TI placebo* or AB placebo*
S38 (MH "Placebos")
S37 TI random* or AB random*
S36 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or trebl* mask* or tripl* mask*) or AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or trebl* mask* or tripl* mask*)
S35 TI clinic* trial* or AB clinic* trial*
S34 PT
S33 (MH "Clinical Trials+")
S32 S21 and S31
S31 S24 and S27 and S30
S30 S28 or S29
S29 TI (nose* or nasal) or AB (nose* or nasal)
S28 (MH "Nose")
S27 S25 or S26
S26 TI ( irrigat* or lavage* or wash* or rins* or douch* or atomis* or atomiz* ) or AB ( irrigat* or lavage* or wash* or rins* or douch* or atomis* or atomiz* )
S25 (MH "Irrigation")
S24 S22 or S23
S23 TI (saline or salt* or sodium chloride) or AB (saline or salt* or sodium chloride)
S22 (MH "Sodium Chloride")
S21 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
S20 TI (influenza* or flu) or AB (influenza* or flu)
S19 (MH "Influenza, Human+")
S18 TI (infect* N3 nose* or infect* N3 throat* or infect* N3 sinus* or infect* N3 sinonasal* or infect* N3 sino‐nasal* or infect* N3 pharyn* or infect* N3 laryn*) or AB (infect* N3 nose* or infect* N3 throat* or infect* N3 sinus* or infect* N3 sinonasal* or infect* N3 sino‐nasal* or infect* N3 pharyn* or infect* N3 laryn*)
S17 TI (nose* N2 congest* or nose* N2 discharg* or nose* N2 blocked or nose* N2 runny or nose* N2 running or nose* N2 stuffy or nose* N2 stuffed) or AB (nose* N2 congest* or nose* N2 discharg* or nose* N2 blocked or nose* N2 runny or nose* N2 running or nose* N2 stuffy or nose* N2 stuffed)
S16 TI (nasal N2 congest* or nasal N2 discharg* or nasal N2 blocked or nasal N2 runny or nasal N2 running or nasal N2 stuffy or nasal N2 stuffed) or AB (nasal N2 congest* or nasal N2 discharg* or nasal N2 blocked or nasal N2 runny or nasal N2 running or nasal N2 stuffy or nasal N2 stuffed)
S15 TI (rhinorrhoea or rhinorrhea) or AB (rhinorrhoea or rhinorrhea)
S14 TI laryngit* or AB laryngit*
S13 TI sinusit* or AB sinusit*
S12 (MH "Sinusitis")
S11 TI tonsillit* or AB tonsillit*
S10 (MH "Tonsillitis")
S9 TI sore throat* or AB sore throat*
S8 TI pharyngit* or AB pharyngit*
S7 (MH "Pharyngitis")
S6 TI common cold* or AB common cold*
S5 (MH "Common Cold")
S4 TI (rhinit* or rhinosinusit* or nasosinusit*) or AB (rhinit* or rhinosinusit* or nasosinusit*)
S3 (MH "Rhinitis") OR (MH "Rhinosinusitis")
S2 TI ( upper respiratory infect* or upper respiratory tract infect* or urti ) or AB ( upper respiratory infect* or upper respiratory tract infect* or urti )
S1 (MH "Respiratory Tract Diseases+")
Appendix 5. AMED (Ovid) search strategy
1 exp respiratory tract infections/
2 (infect* adj3 upper respiratory).tw.
3 urti.tw.
4 rhinitis/
5 rhinit*.tw.
6 common cold*.tw.
7 common cold/
8 pharyngitis/
9 pharyngit*.tw.
10 sore throat*.tw.
11 tonsillitis/
12 tonsillit*.tw.
13 sinusitis/
14 sinusit*.tw.
15 laryngit*.tw.
16 (rhinosinusit* or nasosinusit*).tw.
17 (rhinorrhea or rhinorrhoea).tw.
18 influenza/
19 (influenza* or flu).tw.
20 ((nasal or nose*) adj2 (congest* or discharg* or blocked or runny or running or stuffy or stuffed)).tw.
21 (infect* adj3 (nose* or throat* or sinus* or sinonasal* or sino‐nasal* or pharyn* or laryn*)).tw.
22 or/1‐21
23 salts/
24 (salt* or saline* or sodium chloride*).tw.
25 23 or 24
26 irrigation/
27 (irrigat* or lavage* or wash* or rins* or douch* or atomis* or atomiz*).tw.
28 26 or 27
29 25 and 28
30 22 and 29
Appendix 6. LILACS (BIREME) search strategy
> Search > (MH:"Respiratory Tract Infections" OR "Infecciones del Sistema Respiratorio" OR "Infecções Respiratórias" OR MH:C01.539.739$ OR MH:C08.730$ OR "upper respiratory infection" OR "upper respiratory tract infections" OR "upper respiratory infections" OR "upper respiratory tract infection" OR MH:rhinitis OR Rinitis OR Rinite OR MH:C08.460.799 OR MH:C08.730.674 OR MH:C09.603.799 OR rhinit$ OR MH:"Common Cold" OR "common cold" OR "common colds" OR "Resfriado Común" OR "Resfriado Comum" OR coryza OR MH:C02.782.687.207 OR MH:C08.730.162 OR MH:pharyngitis OR Faringitis OR Faringite OR "sore throat" OR "sore throats" OR pharyngit$ OR MH:C07.550.781$ OR MH:C08.730.561$ OR MH:C09.775.649$ OR MH:Tonsillitis OR Tonsilitis OR Tonsilite OR MH:Sinusitis OR sinusit$ OR MH:C08.460.692.752$ OR MH:C08.730.749$ OR MH:C09.603.692.752$ OR MH:Laryngitis OR Laringitis OR Laringite OR MH:C08.360.535$ OR C08.730.368$ OR C09.400.535$ OR rhinosinusit$ OR nasosinusit$ OR MH:"Influenza, Human" OR "Gripe Humana" OR "Influenza Humana" OR Grippe OR flu* OR rhinorrhoea OR rhinorrhea) AND (MH:"Therapeutic Irrigation" OR "Irrigación Terapéutica" OR "Irrigação Terapêutica" OR douch$ OR lavage OR wash$ OR rins$ OR irrigat$ OR atomis$ OR atomiz$ OR MH:E02.533.500 OR E05.927 OR MH:"nasal lavage" OR "Lavado Nasal" OR "Lavagem Nasal" OR MH:E05.927.573) AND (MH:"sodium
chloride" OR "Cloruro de Sodio" OR "Cloreto de Sódio" OR MH:D01.857.650$ OR MH:D01.210.450.150.875 OR MH:SP4.011.097.039.729.735 OR salt$ OR salin$ OR "sodium chloride") > clinical_trials
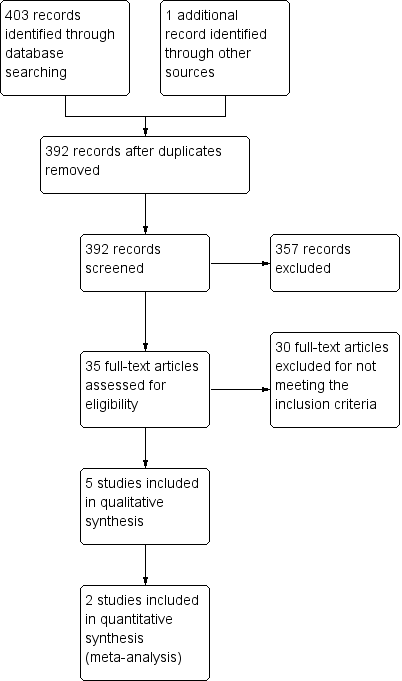
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Time to symptom resolution, Outcome 1 Mean days to wellness (normal saline plus standard therapy versus standard therapy).

Comparison 2 Antibiotic use, Outcome 1 Antibiotic usage (normal saline plus standard therapy versus standard therapy).
| Normal saline plus standard treatment compared to standard treatment alone for acute upper respiratory tract infections | ||||||
| Patient or population: patients with acute upper respiratory tract infections | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard treatment alone | Normal saline plus standard treatment | |||||
| Mean days to wellness | The mean days to wellness in the control groups was | The mean days to wellness in the intervention groups was | 111 | ⊕⊝⊝⊝ | ||
| Antibiotic usage | Study population | OR 0.65 | 422 | ⊕⊝⊝⊝ | ||
| 89 per 1000 | 60 per 1000 | |||||
| Moderate | ||||||
| 88 per 1000 | 59 per 1000 | |||||
| Sore throat | The mean sore throat in the control groups was | The mean sore throat in the intervention groups was | 390 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Bias is likely in the included studies as adequate blinding is not possible with this intervention. | ||||||
| Treatment group | Health status score |
| Symptomatic improvement compared to beginning of illness ‐ Normal treatment only | 2.60 (SD 1.02) ‐ cold 2.00 (SD 0.91) ‐ flu |
| Symptomatic improvement compared to beginning of illness ‐ Normal treatment plus isotonic saline | 1.87 (SD 0.84) ‐ cold 1.59 (SD 0.74) ‐ flu |
| Reported as significant findings (see Results section). Insufficient data to calculate confidence intervals. SD: standard deviation | |
| Treatment group | Day of well‐being |
| Hypertonic saline irrigation | 8.3 days (95% CI 6.9 to 9.7) |
| Normal saline irrigation | 8.3 days (95% CI 6.82 to 9.78) |
| Observation only | 8.0 days (95% CI 6.7 to 9.3) |
| CI: confidence interval | |
| Medication type | Use before study (%) | Use at follow‐up (%) |
| Antipyretics | 23.8 (control) 23.5 (saline wash) | 12.9 (control) 7.6 (saline wash) |
| Decongestants | 40.0 (control) 29.4 (saline wash) | 35.6 (control) 15.9 (saline wash) |
| Mucolytics | 20.0 (control) 15.6 (saline wash) | 31.7 (control) 17.3 (saline wash) |
| Systemic antibiotics | 5.0 (control) 3.1 (saline wash) | 8.9 (control) 5.5 (saline wash) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean days to wellness (normal saline plus standard therapy versus standard therapy) Show forest plot | 2 | 111 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐4.72, 3.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antibiotic usage (normal saline plus standard therapy versus standard therapy) Show forest plot | 2 | 422 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.29, 1.44] |

