Musicoterapia para la lesión cerebral adquirida
Resumen
Antecedentes
La lesión cerebral adquirida (LCA) puede ocasionar deficiencias en la función motora, el lenguaje, la cognición, el procesamiento sensorial y trastornos emocionales, lo que puede llevar a una disminución severa de la calidad de vida del superviviente. La musicoterapia se ha usado en la rehabilitación para estimular las funciones cerebrales que participan en el movimiento, la cognición, el habla, las emociones y las percepciones sensoriales. Se necesitó una actualización de la revisión sistemática publicada en 2010 para medir la eficacia de la musicoterapia en la rehabilitación de los pacientes con LCA.
Objetivos
Evaluar los efectos de la musicoterapia para los resultados funcionales en los pacientes con LCA. Se ampliaron los criterios de la revisión existente para: 1) examinar la eficacia de la musicoterapia en la recuperación de los pacientes con LCA incluida la marcha, la funcionalidad del miembro superior, la comunicación, el estado de ánimo y las emociones, las funciones cognitivas, las aptitudes sociales, el dolor, los resultados conductuales, la actividades cotidianas y los eventos adversos; 2) comparar la eficacia de la musicoterapia y la atención estándar con a) la atención estándar sola, (b) la atención estándar y un placebo, o (c) la atención estándar y otros tratamientos; 3) comparar la eficacia de diferentes tipos de musicoterapia (intervenciones con música a cargo de musicoterapeutas versus intervenciones con música a cargo de otros profesionales).
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Accidentes Cerebrales Vasculares (Cochrane Stroke Group Trials Register) (enero 2016), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL) (2015, número 6), MEDLINE (1946 hasta junio 2015), Embase (1980 hasta junio 2015), CINAHL (1982 hasta junio 2015), PsycINFO (1806 hasta junio 2015), LILACS (1982 hasta enero 2016), y en AMED (1985 hasta junio 2015). Se realizaron búsquedas manuales de revistas y resúmenes de congresos de musicoterapia en las bases de datos de disertaciones y especialistas en música, ensayos y registros de investigación y listas de referencias, y se contactó con expertos y asociaciones de musicoterapia pertinentes para identificar estudios de investigación no publicados. No se impuso ninguna restricción de idioma. Se realizó la búsqueda original en 2009.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios y ensayos clínicos controlados que compararon la musicoterapia y la atención estándar con la atención estándar sola o combinada con otros tratamientos. Se examinaron los estudios que incorporaron a pacientes mayores de 16 años de edad que presentaban una LCA de naturaleza no degenerativa y participaban en los programas de tratamiento ofrecidos en el ámbito hospitalario, ambulatorio o de la comunidad. Se incluyeron estudios en cualquier idioma, publicados e inéditos.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente extrajeron los datos y evaluaron el riesgo de sesgo de los estudios incluidos. Se contactó con investigadores de ensayos para obtener datos faltantes o para obtener información adicional cuando era necesario. Cuando era posible, se presentaron los resultados para los resultados continuos en metanálisis con las diferencias medias (DM) y las diferencias de medias estandarizadas (DME). Se utilizaron las puntuaciones posteriores a las pruebas. En los casos de diferencias iniciales significativas se utilizaron las puntuaciones de cambio. Se realizó un análisis de sensibilidad para evaluar la repercusión del método de asignación al azar.
Resultados principales
Se identificaron 22 nuevos estudios para esta actualización. Las pruebas para esta actualización se basan en 29 ensayos con 775 participantes. Un tipo de musicoterapia conocido como estimulación auditiva rítmica puede ser beneficiosa para mejorar los siguientes parámetros de la marcha después del accidente cerebrovascular. Se halló un aumento informado de la velocidad de la marcha de 11,34 metros por minuto (intervalo de confianza [IC] del 95%: 8,40 a 14,28; nueve ensayos; 268 participantes; P < 0,00001; pruebas de calidad moderada). También puede beneficiarse la longitud del paso del lado afectado, con un promedio informado de 0,12 metros más (IC del 95%: 0,04 a 0,20; cinco ensayos; 129 participantes; P = 0,003; pruebas de calidad moderada). Se encontró una mejoría promedio informada para la marcha general de 7,67 unidades en el Dynamic Gait Index (IC del 95%: 5,67 a 9,67; dos ensayos; 48 participantes; P < 0,00001). También puede haber una mejoría en la cadencia de la marcha, con un aumento promedio informado de 10,77 pasos por minuto (IC del 95%: 4,36 a 17,18; siete ensayos; 223 participantes; P < 0,001; pruebas de baja calidad).
La musicoterapia puede ser beneficiosa para mejorar la sincronización de la funcionalidad del miembro superior después del accidente cerebrovascular, según se evaluó con una reducción de 1,08 segundos en la Wolf Motor Function Test (IC del 95%: ‐1,69 a ‐0,47; dos ensayos; 122 participantes; pruebas de muy baja calidad).
La musicoterapia puede ser beneficiosa para los resultados de la comunicación en los pacientes con afasia posterior al accidente cerebrovascular. En general, la comunicación mejoró en 0,75 desviaciones estándar en el grupo de intervención; efecto moderado (IC del 95%: 0,11 a 1,39; tres ensayos; 67 participantes; P = 0,02; pruebas de muy baja calidad). Se informó una mejoría de la denominación de 9,79 unidades en la Aachen Aphasia Test (IC del 95%: 1,37 a 18,21; dos ensayos; 35 participantes; P = 0,02). La musicoterapia puede tener un efecto beneficioso sobre la repetición del habla, informada como un aumento promedio de 8,90 puntos en la Aachen Aphasia Test (IC del 95%: 3,25 a 14,55; dos ensayos; 35 participantes; P = 0,002).
Puede haber una mejoría en la calidad de vida posterior al accidente cerebrovascular con la estimulación auditiva rítmica, informada con desviaciones estándar de 0,89 en la Stroke Specific Quality of Life Scale, que se considera un efecto grande (IC del 95%: 0,32 a 1,46; dos ensayos; 53 participantes; P = 0,002; pruebas de baja calidad). No se hallaron pruebas sólidas para los efectos sobre la memoria y la atención. Los datos fueron insuficientes para examinar el efecto de la musicoterapia sobre otros resultados.
El riesgo de sesgo fue alto en la mayoría de los estudios incluidos en esta actualización de la revisión, por lo que la calidad de las pruebas es baja.
Conclusiones de los autores
La musicoterapia puede ser beneficiosa para la marcha, la sincronización de la funcionalidad del miembro superior, los resultados de la comunicación y la calidad de vida después del accidente cerebrovascular. Estos hallazgos son alentadores, pero se necesitan más ensayos controlados aleatorios de alta calidad en todos los resultados antes de poder formular recomendaciones para la práctica clínica.
PICO
Resumen en términos sencillos
Musicoterapia para la lesión cerebral adquirida
Pregunta de la revisión
Se examinaron las pruebas para los efectos de la musicoterapia sobre los resultados funcionales en adultos con lesión cerebral adquirida.
Antecedentes
La lesión cerebral adquirida (daño cerebral a causa de un accidente o una enfermedad, incluido el accidente cerebrovascular, que tiene poca probabilidad de un deterioro adicional) puede causar problemas con el movimiento, el lenguaje, los sentidos, el pensamiento o las emociones. Cualquiera de estas alteraciones puede reducir de manera significativa la calidad de vida de un paciente que sobrevive a la lesión. Se han desarrollado muchos tratamientos nuevos para ayudar a recuperar las funciones perdidas y para prevenir la depresión. La musicoterapia incluye el uso de música para ayudar en la rehabilitación. Entre los tratamientos específicos, se encuentra el uso de ritmo para ayudar con el movimiento y la marcha; la ejecución de instrumentos musicales para mejorar el movimiento; el canto para mejorar la calidad de la voz y el habla; la escucha de música para mejorar el tratamiento del dolor, el estado de ánimo o el pensamiento; y la interpretación y la composición de música para mejorar el sentido de bienestar.
Características de los estudios
El objetivo fue identificar estudios de investigación que evaluaran la musicoterapia combinada con la atención estándar para adultos con lesión cerebral adquirida, sometidos a rehabilitación en el ámbito hospitalario o la comunidad. Se buscaron estudios de investigación que analizaron los efectos de la musicoterapia en la marcha, el movimiento, la comunicación, el pensamiento, las emociones, el dolor y el bienestar. Las intervenciones fueron: moverse con la música, cantar, escuchar música, componer, tocar instrumentos musicales o una combinación de estas actividades. Se identificaron e incluyeron 29 ensayos con 775 participantes adultos. Las pruebas están actualizadas hasta junio 2015.
Resultados clave
Los resultados indican que la musicoterapia mediante el ritmo puede ser beneficiosa para mejorar la marcha en los pacientes con accidente cerebrovascular, lo que a su vez puede mejorar la calidad de vida. La musicoterapia puede ser beneficiosa para mejorar la velocidad de los movimientos repetitivos de los brazos y la comunicación en pacientes con accidente cerebrovascular. Los tipos que usan golpes fuertes junto con la música pueden ser más efectivos que las intervenciones que usan golpes fuertes sin música. El tratamiento a cargo de un musicoterapeuta podría ser más eficaz que el tratamiento a cargo de otros profesionales. La información fue insuficiente para examinar los efectos de la musicoterapia sobre otros resultados. No se encontraron estudios que informaran sobre efectos nocivos.
Calidad de la evidencia
La calidad de la investigación fue baja en general. Se encontró un solo estudio que se consideró con bajo riesgo de sesgo. La calidad de las pruebas para la velocidad de marcha y la longitud del paso fue moderada. La calidad de las pruebas para otros aspectos de la marcha fue baja. La calidad de las pruebas para la velocidad de los movimientos repetitivos de los brazos y para la comunicación general fue muy baja. La calidad de las pruebas para la calidad de vida fue baja. Se necesitan ensayos clínicos adicionales.
Authors' conclusions
Summary of findings
| Music compared with standard care for acquired brain injury | |||
| Patient or population: acquired brain injury | |||
| Outcomes | Relative effect | No of participants | Quality of the evidence |
| Gait velocity | The mean gait velocity in the intervention group was 11.34 metres more (8.4 more to 14.28 more). | 268 | ⊕⊕⊕⊝ |
| Stride length (affected side) | The mean stride length (affected side) in the intervention group was 0.12 metres more (0.04 more to 0.2 more). | 129 | ⊕⊕⊕⊝ |
| Gait cadence | The mean gait cadence in the intervention group was 10.77 steps/minute more (4.36 more to 17.18 more). | 223 | ⊕⊕⊝⊝ |
| Stride symmetry | The mean stride symmetry in the intervention group was 0.94 standard deviations more (0.32 fewer to 2.2 more). | 139 | ⊕⊕⊝⊝ |
| General upper extremity functioning assessed with: Fugl‐Meyer Assessment | The mean general upper extremity functioning in the intervention group was 3.56 units higher (0.88 lower to 8 higher). | 194 | ⊕⊝⊝⊝ |
| Overall communication | The mean overall communication in the intervention group was 0.75 standard deviations more (0.11 more to 1.39 more). | 67 | ⊕⊝⊝⊝ |
| Quality of life assessed with: Stroke Specific Quality of Life Scale | The mean quality of life in the intervention group was 0.89 standard deviations more (0.32 more to 1.46 more). | 53 | ⊕⊕⊝⊝ |
| CI: confidence interval; RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence | |||
| 1Most studies were rated as at unclear or high risk of bias | |||
Background
Description of the condition
Acquired brain damage embraces a range of conditions involving rapid onset of brain injury, including trauma due to head injury or postsurgical damage, vascular event such as stroke or subarachnoid haemorrhage, cerebral anoxia, toxic or metabolic insult such as hypoglycaemia, and infection or inflammation (RCP 2012). Acquired brain injury (ABI) can result in impairments in motor function, language, cognition, sensory processing, as well as emotional disturbances. Hemiplegia and hemiparesis are common and may severely reduce a survivor's quality of life. Consequently, a primary concern in rehabilitation for ABI is the restoration of motor function. The improvement of ambulation and upper extremity function directly affects the level of independence of the person with ABI related to activities of daily living. The affected individual is likely to be left with communication impairments, such as a severely reduced ability to understand, speak, and use spoken and written language, which can result in isolation. Furthermore, brain damage often leads to disturbances in memory, learning, and awareness. Sensory disturbances and neuropathic pain can result from damage to the nervous system. Finally, there may be behavioral implications resulting in disinhibition, apathy, and a lack of motivation. Recovery of lost functions and skills after acquired brain damage is typically incomplete, putting survivors at increased risk for depression. Poststroke depression and apathy are estimated to be as high as 33%, impeding functional recovery (Matsuzaki 2015). Mood disorders are considered to be one of the greatest barriers to reintegration back into the community, affecting motivation to engage in rehabilitation (Giles 2006). Effective treatment of depression may bring substantial benefits by improving medical status, enhancing quality of life, and reducing pain and disability (van de Port 2007; Whyte 2006).
Acquired brain injury causes significant levels of disabilities that tend to result in long‐term problems. There were an estimated 316,080 people living with disabilities stemming from stroke, and a further 170,000 people per year who sustained a traumatic brain injury in the UK in 2013 (NA 2014). Figures from the US exceed those in the UK, with an estimated 3.5 million people sustaining a traumatic brain injury each year (Coronado 2012), of whom 125,000 will be left with long‐term disability (Selassie 2008). Approximately 5.3 million Americans, or 2% of the population of all ages, have long term or lifelong needs for help in performing personal activities of daily living following traumatic brain injury (Selassie 2008; Thurman 1999; Zaloshnja 2008). In 2010, 16.9 million people had a first stroke, and the worldwide prevalence of stroke was 33 million (Mozaffarian 2015).
Global health burden attributed to ABI resulting from stroke and traumatic brain injury is considerable. Furthermore, with the population ageing, even if the stroke incidence stagnates, the number of people with stroke requiring medical and rehabilitation care will rise dramatically (WHO 2014). In Europe alone in 2010, estimated costs were EUR 64.1 billion for stroke and EUR 33.0 billion for traumatic brain injury (Gustavsson 2010). In the USA, traumatic brain injury annual costs are estimated at USD 221 billion, comprising USD 14.6 billion for medical costs, USD 69.2 billion for work loss, and USD 137 billion for lost quality of life (Orman 2011). Acquired brain injury therefore has significant effects on society in terms of human and economic costs.
Description of the intervention
Many innovative therapy methods have been developed to help restore lost functions and aid in the prevention and treatment of depression in ABI. Music therapy has been used in rehabilitation settings to stimulate brain functions involved in movement, cognition, speech, emotions, and sensory perceptions. Music interventions range from the use of rhythmic auditory stimulation (RAS) to aid in the execution of movement and normalisation of gait parameters (Thaut 1993), to music listening and singing to reduce pain (Kim 2005), to the use of music listening, music improvisations, composition, and song discussions to address emotional needs and enhance sense of well‐being (Nayak 2000). While music interventions are traditionally implemented by trained music therapists, other health professionals may also use music to facilitate therapeutic outcomes. For example, music listening has been used by other health professionals in rehabilitation settings to enhance cognitive recovery and to improve mood (Särkämö 2008). Music interventions utilised in therapy are distinguished from passive music listening or recreational music activities when the following components are present: 1) implementation of goal‐directed music interventions by a trained health professional, or 2) the use of music experiences individualised to the need of the person with ABI. In rehabilitation settings, these interventions may include 1) listening and moving to live, improvised, or pre‐recorded music as well as RAS, 2) performing or creating music on an instrument, 3) improvising music spontaneously using voice or instruments or both, 4) singing or vocal activities to music, 5) music‐based speech and language activities, 6) composing music, and 7) music combined with other modalities (e.g. imagery, art) (Dileo 2007; Magee 2006b; Magee 2009). Music therapy (in comparison with music interventions more broadly) is delivered by a professional with specific clinical training in music therapy, who offers a systematic therapeutic process including assessment, treatment, and evaluation. Music therapy treatment involves the presence of a therapeutic process and the use of personally tailored music experiences.
How the intervention might work
Biomedical theories suggest that neurophysiological processes may be activated through musical stimulation and used to affect non‐musical behaviour and encourage neuroplasticity (Thaut 2014a). Following neurological injury, major neural reorganisation is common. Music interventions aim to capitalise on this naturally occurring neuroplastic change by enriching the environment of the person with ABI to promote functional gains (Särkämö 2008).
Music is physiologically arousing, entrains movement, and can motivate exercise and override pain perception. In particular, rhythm in music is a strong driving stimulus for motor function (Clark 2016). This influence of rhythm may be useful in physical rehabilitation, for example gait retraining and upper limb co‐ordination (Thaut 1997; Thaut 2002). Speech and language skills can also be addressed using music interventions. Singing is a motivating way to practice the structured movement behaviours necessary for speech rehabilitation, as it requires controlled deep breathing, phonation, pitch control, rhythmic accuracy, controlled volume, and articulation of lyrics (Baker 2011). Furthermore, melodic intonation therapy uses the unimpaired singing ability of a person with brain injury to rehabilitate impaired language skills (Norton 2009).
Music is processed diffusely in the brain, meaning that music interventions can be targeted to address a wide range of cognitive deficits and behavioural and emotional issues. The repetitive and predictable structures in music can act as cues for learning. For example, songs can chunk information to aid in memory formation and recall (Thaut 2014b). In addition to its utility in physical rehabilitation, music has been reported to have positive effects on mood and social participation (Baker 2006). During music participation the brain releases neurochemicals that increase feelings of pleasure and alertness, and decrease anxiety and stress (Altenmuller 2013). Used in a group setting, music participation can provide opportunities for peer support and building social skills to facilitate increased independence (Nayak 2000).
Why it is important to do this review
Many research studies on the use of music in rehabilitation of ABI have suffered from small sample size, making it difficult to achieve statistically significant results. In addition, differences in factors such as study designs, methods of interventions, and intensity of treatment have led to varying results. The first edition of this review included only music therapy interventions involving a trained professional music therapist. However, in order to fully investigate the effects of music interventions in ABI rehabilitation, in this update we have included music interventions delivered by a music therapist or trainees in a music therapy programme, by other medical professionals, or by other health professionals with training in rehabilitation. This systematic review aimed to gauge more accurately the efficacy of music interventions in rehabilitation for people with ABI as well as to identify variables that may moderate any effects.
Objectives
To assess the effects of music interventions for functional outcomes in people with ABI. We expanded the criteria of our existing review to: 1) examine the efficacy of music interventions in addressing recovery in people with ABI including gait, upper extremity function, communication, mood and emotions, cognitive functioning, social skills, pain, behavioural outcomes, activities of daily living, and adverse events; 2) compare the efficacy of music interventions and standard care with a) standard care alone, b) standard care and placebo treatments, or c) standard care and other therapies; 3) compare the efficacy of different types of music interventions (music therapy delivered by trained music therapists versus music interventions delivered by other professionals).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials and controlled clinical trials with quasi‐randomised or systematic methods of treatment allocation in any language, published and unpublished. We conducted a sensitivity analysis to assess the impact of the randomisation method.
Types of participants
We included people of any gender older than 16 years of age who had acquired brain damage of a non‐degenerative nature and were participating in treatment programmes offered in hospital, outpatient, or community settings at the time that they received the music intervention. This included traumatic brain injury, stroke, anoxia, infection, and any mixed cause. We excluded any condition of a progressive nature. We did not use the site of lesion and stage of rehabilitation as inclusion or exclusion criteria.
Types of interventions
We included all studies in which standard treatment combined with music interventions was compared with: 1) standard care alone, 2) standard care with placebo, or 3) standard care combined with other therapies. We considered studies where the music interventions were delivered by a formally trained music therapist, by trainees in a formal music therapy programme, or by professionals other than trained music therapists. We included studies in which one or more of the following music interventions was used.
-
Interventions in which musical instruments are played (e.g. clinical improvisation in which participants are involved in active music making in dialogue with the therapist, therapeutic instrumental musical performance, cognitive training with drums).
-
Singing and music‐based voice interventions (e.g. song‐singing programmes, melodic intonation therapy or modified melodic intonation therapy, vocal intonation therapy, rhythmic speech cueing, and therapeutic singing).
-
RAS or rhythmic auditory cueing (RAC).
-
Receptive interventions in which participants listen to music.
-
Songwriting.
-
Any combination of the above.
Types of outcome measures
Primary outcomes
Rehabilitation of mobility is crucial in ABI rehabilitation to enhance personal independence. We therefore selected the following primary outcomes for this review.
-
Improvement in gait, measured by changes in gait velocity, cadence, stride length, stride symmetry, stride timing, general gait, balance.
-
Improvement in upper extremity function (UEF), measured by general UEF, timing of UEF, range of motion, hand function, upper limb strength, manual dexterity, and elbow extension.
Secondary outcomes
-
Communication (e.g. language production, speech production, parameters of voice production, speaking fundamental frequency).
-
Mood and emotions (e.g. depression, anger, anxiety).
-
Social skills and interactions (e.g. eye contact, non‐verbal interactions).
-
Pain.
-
Behavioural outcomes (e.g. participation in treatment, motivation, self esteem).
-
Cognitive functioning.
-
Activities of daily living.
-
Adverse events (e.g. death, fatigue, falls).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers where necessary. We imposed no language restrictions for either searching or trial inclusion.
Electronic searches
We searched the following electronic databases and trials registers. Due to our changed criteria, we updated the previously run searches from our 2010 review; however, we ran searches from the inception of each database. The original searches are detailed in the appendices.
-
Cochrane Stroke Group Trials Register (last searched by the Managing Editor on 5 January 2016).
-
Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 6, part of the Cochrane Library (www.thecochranelibrary.com); accessed 11 June 2015; Appendix 1).
-
MEDLINE (1946 to June 2015; Appendix 2).
-
Embase (1980 to June 2015; Appendix 3).
-
CINAHL (1982 to June 2015; Appendix 4).
-
PsycINFO (1806 to June 2015; Appendix 5).
-
LILACS (Latin American and Caribbean Health Sciences Literature) (1982 to January 2016; Appendix 6).
-
AMED (Allied and Complementary Medicine) (1985 to June 2015; Appendix 7).
-
CAIRSS for Music (Computer‐Assisted Information Retrieval Service System) (December 2015; Appendix 8).
-
ProQuest Digital Dissertations (1861 to August 2015; Appendix 9).
-
ClinicalTrials.gov (www.clinicaltrials.gov/) (August 2015; Appendix 10).
-
Current Controlled Trials (www.controlled‐trials.com/) (December 2015; Appendix 11).
We undertook searches of the following for our previous review; however, we could not renew the searches for this update as the databases are no longer functional, no longer maintained, or have been subsumed by other databases we searched: The National Research Register (NRR) Archive, RehabTrials.org, Indexes to Theses in Great Britain and Ireland, and Music Therapy World. We also conducted a search of the Science Citation Index for our previous review; however, we did not have access to this database for this review update and so did not update that search.
Searching other resources
We handsearched the following music therapy journals and conference proceedings:
-
Arts in Psychotherapy (1974 to 2015;46);
-
Australian Journal of Music Therapy (1990 to 2015;26);
-
Australian Music Therapy Association Bulletin (1977 to 2005; final issue);
-
British Journal of Music Therapy (1987 to 2015;29(1));
-
Canadian Journal of Music Therapy (1976 to 2015;21(1));
-
International Journal of the Arts in Medicine (1993 to 1999;6(2), final issue);
-
Journal of Music Therapy (1964 to 2015;52(4));
-
Japanese Journal of Music Therapy (2005 to 2013;13(2; latest issue available with online abstracts));
-
Music and Medicine (2009 to 2015:17(4));
-
Musik‐, Tanz‐, und Kunsttherapie (Journal for Art Therapies in Education, Welfare and Health Care) (1999 to 2014;25(3));
-
Musiktherapeutische Umschau (1980 to 2015;35(4));
-
Music Therapy (1981 to 1996;14(1), final issue);
-
Music Therapy Yearbook (1951 to 1962; final issue);
-
Music Therapy Perspectives (1982 to 2015;33(2));
-
Nordic Journal of Music Therapy (1992 to 2016;25(1));
-
Music Therapy Today (online journal of music therapy) (2000 to 2007;3, final issue);
-
New Zealand Journal of Music Therapy (1987 to 2013;11, latest issue available with online abstracts);
-
Psychomusicology (1981 to 2015:25(4));
-
Voices (online international journal of music therapy) (2001 to 2015;15(32));
-
Canadian Conference Proceedings (2004 to 2006);
-
The World Music Therapy Congress Proceedings (1993 to 2014);
-
The European Music Therapy Congress Proceedings (1992 to 1998; 2004 to 2010).
Data collection and analysis
Selection of studies
For this update, four review authors (WM, IC, JT, JB) conducted the searches as outlined in the Search methods for identification of studies. One review author (WM) and a graduate research assistant scanned titles and abstracts of each record retrieved from the search and deleted obviously irrelevant references. When we were uncertain as to whether to reject a title or abstract, we obtained the full article, which two review authors (IC and JT) independently inspected. Both review authors used an inclusion criteria form to assess the trial's eligibility for inclusion. One review author (WM) checked the inter‐rater reliability for trial selection, and in the case of disagreement or uncertainty, consulted a third review author (JB). We kept a record of both the article and the reason for exclusion for all excluded studies.
Data extraction and management
Two authors (WM and JB) independently extracted data from the selected trials using a standardised coding form. Any differences in data extraction were discussed. We extracted the following data.
General information
-
Author
-
Year of publication
-
Title
-
Journal (title, volume, pages)
-
If unpublished, source
-
Duplicate publications
-
Country
-
Language of publication
Trial information
-
Study design (parallel group, cross‐over)
-
Randomisation
-
Randomisation method
-
Allocation concealment
-
Allocation concealment method
-
Level of blinding (interventionist, objective outcomes, subjective outcomes)
-
Attrition (rate, reasons for withdrawal)
Intervention information
-
Type of intervention (e.g. clinical improvisation, therapeutic instrumental musical performance, singing or music‐based voice interventions, RAS or RAC, receptive interventions, songwriting, combination)
-
Music preference (participant preferred versus researcher selected in cases of music listening)
-
Professional delivering the intervention (music therapist or other)
-
Length of intervention
-
Intensity of intervention
-
Comparison intervention
Participant information
-
Total sample size
-
Number of experimental group
-
Number of control group
-
Gender
-
Age
-
Ethnicity
-
Diagnosis
-
Site of lesion
-
Setting
-
Country
-
Inclusion criteria
Outcomes
We planned to extract statistical information for the following outcomes (if applicable):
-
parameters of gait (e.g. velocity, cadence, stride length, stride symmetry, stride timing, general gait, balance);
-
parameters of UEF (e.g. range of movement, hand function, manual dexterity, upper limb strength, elbow extension);
-
communication outcomes (e.g. language production; parameters of voice production, speaking fundamental frequency);
-
mood and emotion outcomes (e.g. depression, anger, anxiety);
-
social interactions outcomes (e.g. eye contact, non‐verbal interactions);
-
pain;
-
cognitive functioning (e.g. memory, attention);
-
behavioural outcomes (e.g. participation in treatment, motivation);
-
activities of daily living;
-
adverse events (e.g. death, fatigue, falls).
Assessment of risk of bias in included studies
Two review authors (WM and JB) independently assessed all included trials for trial quality. We used the following criteria for quality assessment.
1. Random sequence generation
-
Low risk
-
Unclear risk
-
High risk
We rated random sequence generation as low risk if every participant had an equal chance to be selected for either condition and if the investigator was unable to predict to which treatment the participant would be assigned. Use of date of birth, date of admission, or alternation resulted in high risk of bias.
2. Allocation concealment
-
Low risk methods to conceal allocation included:
-
-
central randomisation;
-
serially numbered, opaque, sealed envelopes;
-
other descriptions with convincing concealment.
-
-
Unclear risk: authors did not adequately report on method of concealment.
-
High risk (e.g. alternation methods were used).
3. Blinding of participants and personnel
-
Low risk
-
Unclear risk
-
High risk
Participants usually cannot be blinded in a music intervention trial, with the exception of studies where pre‐recorded music is used in a comparative trial that compares different types of music. For this reason, we did not downgrade studies for not blinding the participants. As for the personnel delivering the intervention, in many music intervention studies the professional delivering the intervention cannot be blinded because they are actively making music with the participants or providing music for the intervention. We therefore applied downgrading for not blinding personnel only in studies that used interventions where blinding was possible, for example in studies in which listening to pre‐recorded music was the treatment condition and control group participants were provided with headphones but no music (such as a blank CD). This included studies that examined the use of metronome beat as part of the RAS intervention.
4. Blinding of outcome assessors
-
Low risk:
-
outcome assessors were blinded; or
-
particular outcome group (i.e. objective outcomes; subjective outcomes) was not included in the review.
-
-
Unclear risk: authors did not adequately report on method of blinding.
-
High risk:
-
outcome assessors were not blinded; or
-
self report measures were used and participants were not blinded.
-
5. Incomplete data
We recorded the proportion of participants whose outcomes were analysed. We coded losses to follow‐up for each outcome as follows.
-
Low risk: if fewer than 20% of participants were lost to follow‐up, and reasons for loss to follow‐up were similar in both treatment arms.
-
Unclear risk: if loss to follow‐up was not reported.
-
High risk: if more than 20% of participants were lost to follow‐up, or reasons for loss to follow‐up differed between treatment arms.
6. Selective reporting
-
Low risk: reports of the study were free of the suggestion of selective outcome reporting.
-
Unclear risk: unclear if reports of the study included selective outcome reporting.
-
High risk: reports of the study suggested selective outcome reporting.
7. Financial conflict of interest
We considered information on potential financial conflicts of interest as a possible source of additional bias.
-
Low risk: unlikely that other sources of bias influenced the results.
-
Unclear risk: unclear if other sources of bias may have influenced the results.
-
High risk: likely that other sources of bias influenced the results.
We used the above criteria to give each article an overall quality rating based on Section 8.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Low risk of bias: all criteria met.
-
Moderate risk of bias: one or more of the criteria only partially met.
-
High risk of bias: one or more criteria not met.
We did not exclude studies based on a low quality score.
Measures of treatment effect
We presented all outcomes in this review as continuous variables. We calculated standardised mean differences (SMDs) with 95% confidence intervals (CIs) for outcome measures using results from different scales. When sufficient data were available from various studies using the same measurement instrument, we computed a mean difference (MD) with 95% CI.
Unit of analysis issues
In all studies included in this review, participants were individually randomised to the intervention or the standard‐care control group. We collected and analysed post‐test values or change values on a single measurement for each outcome from each participant.
Dealing with missing data
We analysed data on an endpoint basis, including only participants for whom final data point measurement was obtained (available‐case analysis). We did not assume that participants who dropped out after randomisation had a negative outcome.
Assessment of heterogeneity
We investigated heterogeneity using the I2 test with I2 greater than 50% indicating significant heterogeneity.
Assessment of reporting biases
We tested for publication bias visually in the form of funnel plots (Higgins 2011).
Data synthesis
One review author (JB) entered all trials included in the systematic review into Review Manager 5 (RevMan 2014). JB conducted the data analysis, and WM reviewed the analysis for accuracy. We presented the main outcomes in this review as continuous variables. We calculated SMDs for outcome measures using the results from different scales, and computed MDs for results using the same scales. We calculated pooled estimates using the random‐effects model. We determined levels of heterogeneity using the I2 statistic (Higgins 2002). We calculated 95% CIs for each effect size estimate. This review did not include any categorical variables.
For cross‐over trials, we used the guidelines by Elbourne 2002 for the inclusion of cross‐over trials in meta‐analyses that include both parallel‐group and cross‐over trials. When statistical information regarding the within‐individual comparison of treatment was available, we used or computed estimates of the treatment effects and associated standard errors. If these data were not available, we opted to use data from the first period only if those data were reported separately. A third option was to treat the results as if they came from a study of parallel‐group design. We favoured this option the least, as according to Elbourne and colleagues it ignores the within‐patient correlation and results in an underestimate of the treatment effect (Elbourne 2002).
We made the following treatment comparison: music interventions versus standard care alone.
Subgroup analysis and investigation of heterogeneity
We planned the following subanalyses a priori as described by Deeks 2001 and as recommended in Section 8.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
-
type of music intervention;
-
interventionist (music therapist or other);
-
dosage of music intervention; and
-
diagnosis.
We performed subanalyses on intervention where possible; however, for most interventions there were not enough studies per outcome to do so. We did not perform subanalyses on diagnosis, as the populations in the studies that examined the same outcomes were heterogenous.
Sensitivity analysis
We examined the impact of group allocation method by comparing the results of including and excluding trials that used inadequate or unclear randomisation methods.
Results
Description of studies
Results of the search
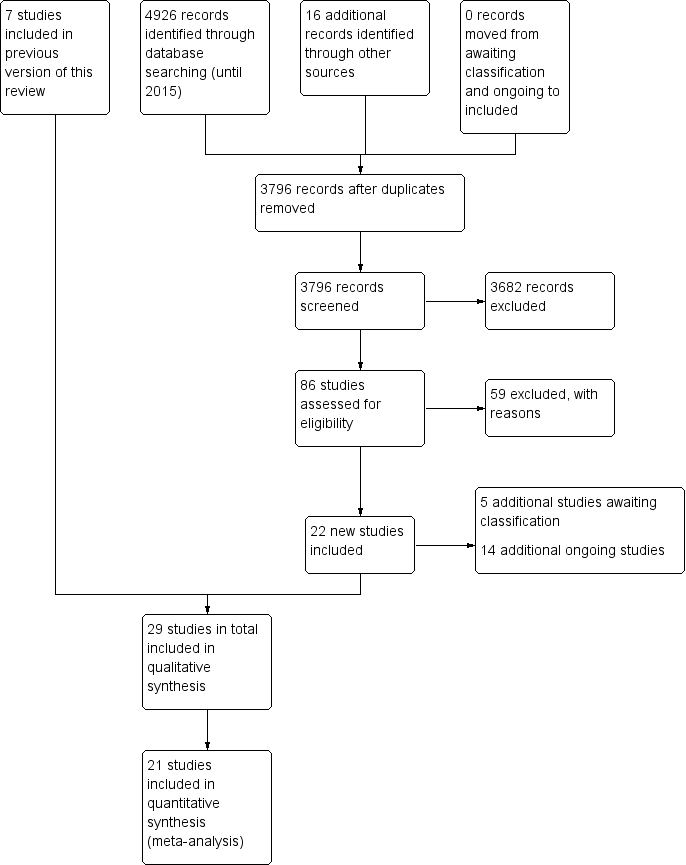
For the original review, the database searches and handsearching of conference proceedings and journals identified 3855 unique citations, of which 94 references were identified for possible inclusion. After further title and abstract scanning, 14 references to seven studies were identified that met all of the inclusion criteria (see Figure 1).
Study flow diagram for the updated review.
The 2016 update of the search, based on the revised inclusion criteria, resulted in 3796 additional citations. One review author (WM) and a graduate research assistant scanned the titles and abstracts and identified 100 references to 86 studies for possible inclusion, which two review authors (IC and JT) independently screened. We consulted another review author (JB) where needed. We included 29 references to 22 new studies in this review update (see Characteristics of included studies) (Baker 2001; Cha 2014a; Cha 2014b; Chouan 2012; Conklyn 2012; Fernandes 2014; Hill 2011; Jeong 2007; Jungblut 2004; Kim 2005; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Mueller 2013; O'Kelly 2014; Park 2010a; Paul 1998; Pool 2012; Särkämö 2008; Schneider 2007; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Tong 2015; Van Delden 2013; van der Meulen 2014; Whitall 2011). We contacted chief investigators to obtain additional information on study details and data where necessary.
The studies that had been classified in our previous review as awaiting assessment (N = 1) and ongoing (N = 3) have now been excluded. We reclassified one study that was previously excluded as included in this review update, given the revised inclusion criteria. In this update, five further studies are awaiting classification and 14 additional studies are ongoing (see Figure 1).
Included studies
We included 29 studies (24 randomised controlled trials (RCTs) and five quasi‐RCTs) with a total of 775 participants. These studies examined the effects of music interventions on gait parameters after stroke (Cha 2014a; Cha 2014b; Chouan 2012; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Park 2010a; Suh 2014; Thaut 1997; Thaut 2007), UEF following stroke (Chouan 2012; Hill 2011; Jeong 2007; Paul 1998; Schneider 2007; Thaut 2002; Tong 2015; Van Delden 2013; Whitall 2011), communication outcomes following stroke (Conklyn 2012; Jungblut 2004; Särkämö 2008; van der Meulen 2014), mood (Jeong 2007; Pool 2012; Särkämö 2008), social skills following stroke (Jeong 2007), pain during exercise following stroke (Kim 2005), behavioural outcomes (Baker 2001; Cha 2014b; Fernandes 2014; Hill 2011; Jeong 2007; O'Kelly 2014), cognitive functioning (Baker 2001; Mueller 2013; Pool 2012; Särkämö 2008), and activities of daily living (Van Delden 2013). Twenty‐five studies involved only participants with stroke (N = 698, 90% of total N). Four studies involved participants with mixed ABI aetiologies, including two studies with participants with disorders of consciousness (N = 47, 6% of total N). Fifty‐seven per cent of the participants were male. The average age of the participants was 58.27 years. We could not compute average time post incident, as times were reported in days, weeks, months, and years. The studies were conducted in 10 different countries: South Korea (Cha 2014a; Cha 2014b; Jeong 2007; Kim 2005; Kim 2011a; Kim 2012a; Kim 2012b; Park 2010a; Suh 2014), the USA (Conklyn 2012; Hill 2011; Mueller 2013; Paul 1998; Thaut 1997; Thaut 2002; Whitall 2011), Germany (Jungblut 2004; Schneider 2007), China (Lichun 2011; Tong 2015), the Netherlands (Van Delden 2013; van der Meulen 2014), the UK (O'Kelly 2014; Pool 2012), Australia (Baker 2001), Finland (Särkämö 2008), India (Chouan 2012), Spain (Fernandes 2014), and the USA and Germany (Thaut 2007). Only four studies reported on the ethnicity of the participants (Baker 2001; Hill 2011; Kim 2005; Tong 2015). Trial sample size ranged from nine to 111 participants (mean 28.3).
Types of interventions: live versus recorded music
Thirteen studies used music therapy interventions as defined by the review authors in the Background section of this review (Baker 2001; Conklyn 2012; Jungblut 2004; Kim 2005; Lichun 2011; Mueller 2013; O'Kelly 2014; Paul 1998; Pool 2012; Särkämö 2008; Thaut 1997; Thaut 2002; Thaut 2007). Nineteen studies used music that was either live or recorded (Baker 2001; Cha 2014b; Conklyn 2012; Fernandes 2014; Jeong 2007; Jungblut 2004; Kim 2005; Lichun 2011; Mueller 2013; O'Kelly 2014; Park 2010a; Paul 1998; Pool 2012; Särkämö 2008; Schneider 2007; Thaut 1997; Thaut 2007; Tong 2015; van der Meulen 2014), and 10 studies used a rhythmic stimulus only without music (Cha 2014a; Conklyn 2012; Hill 2011; Kim 2011a; Kim 2012a; Kim 2012b; Suh 2014; Thaut 2002; Van Delden 2013; Whitall 2011). Twelve studies used live music interventions, eight of which were music therapy studies (Baker 2001; Conklyn 2012; Jungblut 2004; Lichun 2011; Mueller 2013; O'Kelly 2014; Paul 1998; Pool 2012), and four involved rehabilitation professionals (Jeong 2007; Schneider 2007; Tong 2015; van der Meulen 2014). Live music interventions included receptive listening to live music, active music‐making on instruments and electronic devices, songwriting, vocalising to music, and movement to music. Seven studies used recorded music (Cha 2014b; Fernandes 2014; Kim 2005; Park 2010a; Särkämö 2008; Thaut 1997; Thaut 2007), and two used both live and recorded music (Baker 2001; O'Kelly 2014). Ten studies used a rhythmic pulse only without music, employing either a metronome (Cha 2014a; Chouan 2012; Hill 2011; Kim 2011a; Kim 2012a; Kim 2012b; Thaut 2002; Van Delden 2013; Whitall 2011), or single tone series (Suh 2014). Only three studies used participant‐preferred music (Baker 2001; O'Kelly 2014; Särkämö 2008).
Sixteen studies used rhythm‐based methods to address motor disorders including gait and UEF. Fourteen studies used RAS or RAC (Cha 2014a; Cha 2014b; Chouan 2012; Jeong 2007; Hill 2011; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Whitall 2011). RAS and RAC involve the use of rhythmic sensory cueing of the motor system, engaging entrainment principles in which "rhythmic auditory cues synchronize motor responses into stable time relationships. The fast‐acting physiological entrainment mechanisms between auditory rhythm and motor response serve as coupling mechanisms to stabilise and regulate gait patterns" or reaching arm movements (Thaut 2007, p 455). The rhythmic stimulus used in the majority of studies was a beat provided by a metronome, although one study used pitched tones (Suh 2014). Two other studies used modified versions of RAS or RAC: Park 2010a used fast‐tempo RAS, and Van Delden 2013 used modified bilateral arm training with RAC (mBATRAC), which targeted rhythmic flexion and extension movements.
Types of interventions: active versus receptive methods
Six studies evaluated the effects of active music‐making using musical instruments. Three music therapy studies used active music‐making (Mueller 2013; Paul 1998; Pool 2012). Mueller 2013 used instrument playing to train endogenous task shifting; Pool 2012 used simple instrument playing tasks to train attention; and Paul 1998 required participants to actively play electronic music devices that demanded active shoulder flexion and elbow extension and that enabled easy sound manipulation by the participants. Electronic paddle drums were individually set to the maximum range of motion of each participant. This was compared with a control intervention that involved a physical exercise group in which participants were encouraged to reach their affected extremity as far as they could in different directions. Jeong 2007 combined RAS with instrument playing using dynamic rhythmic movements; Schneider 2007 used music‐supported training that addressed fine motor skills through playing a MIDI keyboard or gross motor skills by playing an electronic drum set with eight pads, or both. Music exercises were adapted to participant need and increased incrementally over 10 levels of difficulty. Tong 2015 used an audible percussion instrument in comparison to a muted musical instrument that resembled the audible instrument, but was made of sponge. The muted musical instrument thus inhibited the participants from hearing sound during the music‐supported therapy training.
Other active methods included songwriting to address mood state (Pool 2012), and neurologic music therapy methods to address cognition (Mueller 2013; Pool 2012; Thaut 2014a).
Receptive methods are those in which the participant is directed to listen to recorded music or live music presented by the interventionist, and thus is not required to be actively involved in making the music him or herself. Five studies used receptive methods (Baker 2001; Fernandes 2014; Kim 2005; O'Kelly 2014; Särkämö 2008). Two of these studies involved heavily dependent participants emerging from coma with whom active methods would not be viable (Fernandes 2014; O'Kelly 2014).
Four trials examined the effects of music therapy on communication outcomes (Conklyn 2012; Jungblut 2004; Särkämö 2008; van der Meulen 2014). Each of these used a different music intervention. Jungblut 2004 employed SIPARI, a music therapy method to address aphasia using singing, intonation, prosody embedded in physiologically appropriate breathing. This method also employs instrumental and vocal rhythmic exercises and music improvisations to practice communication scenarios. Särkämö 2008 used receptive methods where participants listened to recordings of participant‐preferred music. Conklyn 2012 and van der Meulen 2014 used melodic intonation therapy, a method that involves repetitive singing of short phrases in conjunction with left hand tapping of the rhythm.
Dosage of interventions and trial designs
Frequency and duration of treatment sessions varied greatly among the studies. The total number of sessions ranged from one to 60. The duration of sessions varied widely due to the range of interventions being used to address a diverse set of outcomes. As interventions were so varied, it was not meaningful to provide a comparison of session durations. The frequency of sessions ranged from once to 10 times weekly. We have included details on frequency and duration of sessions for each trial in the Characteristics of included studies table.
Eight studies used cross‐over designs (Baker 2001; Cha 2014a; Kim 2005; Kim 2011a; O'Kelly 2014; Pool 2012; Thaut 2002; Tong 2015); one study used a wait‐list control design (van der Meulen 2014); and all of the other studies used a parallel‐group design. Not all studies measured all outcomes identified in this review.
Details of the studies included in the review are shown in the Characteristics of included studies table.
Excluded studies
In this update, we identified 80 additional experimental research studies that appeared to be eligible for inclusion. However, we excluded these after closer examination or after receiving additional information from the chief investigators. Reasons for exclusions were:
-
not an RCT or controlled clinical trial (48 studies);
-
insufficient data reporting (nine studies);
-
comparative study of two music interventions with no control (two studies);
-
control participants did not have ABI (seven studies);
-
could not locate published report of the research (five studies);
-
not population of interest (two studies);
-
outcomes not of interest to this review (four studies); and
-
the methodological problems employed presented a risk of bias to reported results (three studies).
We have listed details of the excluded trials in the Characteristics of excluded studies table.
Risk of bias in included studies
Only one study received a rating of low risk of bias (Thaut 1997), and two studies received a rating of unclear risk of bias (Cha 2014a; O'Kelly 2014). Twenty‐four studies received a rating of high risk of bias. 'Risk of bias' summaries are reported in Figure 2 and Figure 3, with details about each 'Risk of bias' item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We included 22 studies that used appropriate methods of randomisation (e.g. computer‐generated random number table, drawing of lots, flipping of coins) (Baker 2001; Cha 2014a; Conklyn 2012; Fernandes 2014; Jeong 2007; Kim 2005; Kim 2011a; Kim 2012a; Lichun 2011; Mueller 2013; O'Kelly 2014; Park 2010a; Pool 2012; Särkämö 2008; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Tong 2015; Van Delden 2013; van der Meulen 2014; Whitall 2011), as well as four studies that used non‐random methods of group assignment (e.g. alternate group assignment) (Hill 2011; Jungblut 2004; Paul 1998; Schneider 2007). The methods used in three studies resulted in a judgement of unclear risk of bias (Cha 2014b; Chouan 2012; Kim 2012b). We examined the impact of method of randomisation by sensitivity analyses.
Seventeen studies used allocation concealment (Cha 2014a; Cha 2014b; Chouan 2012; Kim 2005; Kim 2011a; Kim 2012a; Lichun 2011; O'Kelly 2014; Park 2010a; Pool 2012; Särkämö 2008; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Van Delden 2013; van der Meulen 2014). Allocation concealment was unclear in eight studies (Conklyn 2012; Fernandes 2014; Hill 2011; Jeong 2007; Kim 2012b; Mueller 2013; Tong 2015; Whitall 2011), and not used in the remaining four studies (Baker 2001; Jungblut 2004; Paul 1998; Schneider 2007).
Blinding
In music intervention studies, research participants and interventionists cannot be blinded, with the exception of studies that compare different types of music interventions (blinding of participant) or interventions that use headphones (blinding of outcome assessors and potentially interventionist). For this reason, we did not downgrade studies for not blinding participants. Only one study reported blinding of participants (Suh 2014). We rated one study at high risk for performance bias (Fernandes 2014); music was delivered via headphones to heavily dependent participants, however blinding of interventionists was not reported.
Thirteen studies reported blinding of the outcome assessors for objective measures (Cha 2014a; Conklyn 2012; Hill 2011; Jungblut 2004; Kim 2005; Mueller 2013; O'Kelly 2014; Paul 1998; Pool 2012; Särkämö 2008; Thaut 1997; Thaut 2007; Whitall 2011). In 14 trials the use of blinding for detection bias was unclear (Cha 2014b; Chouan 2012; Fernandes 2014; Jeong 2007; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Park 2010a; Schneider 2007; Suh 2014; Tong 2015; Van Delden 2013; van der Meulen 2014). Two studies did not blind outcome assessors (Baker 2001; Thaut 2002).
For subjective outcomes (e.g. the Profile of Mood States (POMS)) (Lorr 2003), blinding of the outcome assessor was not possible unless the participants were in studies that compared different types of music interventions. The 'Risk of bias' summary lists 20 studies at low risk of bias for outcome assessment of subjective outcomes (Figure 3). However, these studies did not include subjective outcomes and were therefore not downgraded for this 'Risk of bias' criterion. We assessed seven trials as having a high risk of bias, as subjective outcomes were used and participants were not blinded (Jeong 2007; Kim 2005; Kim 2012a; Mueller 2013; Pool 2012; Särkämö 2008; Whitall 2011). The use of blinding for subjective outcomes was unclear for two trials (Hill 2011; Thaut 2007).
Incomplete outcome data
Just under half of the trials reported attrition, at a rate of between 0% and 17%. Six studies had attrition rates of 20% or higher (20% to 29%) (Conklyn 2012; Hill 2011; Jungblut 2004; Kim 2005; Pool 2012; Thaut 2007). Nine studies did not report attrition adequately (Cha 2014a; Cha 2014b; Fernandes 2014; Jeong 2007; Kim 2012b; Lichun 2011; O'Kelly 2014; Suh 2014; Thaut 2002). We have included detailed information on dropout rates in the Characteristics of included studies table.
Selective reporting
We found evidence of selective reporting by the authors in one study (Fernandes 2014).
We examined publication bias visually in the form of funnel plots for gait velocity (Figure 4). The funnel plot did not show evidence of publication bias.
![Funnel plot of comparison: 1 Music therapy versus control, outcome: 1.1 Gait velocity [metres/min].](/cdsr/doi/10.1002/14651858.CD006787.pub3/media/CDSR/CD006787/image_n/nCD006787-AFig-FIG04.png)
Funnel plot of comparison: 1 Music therapy versus control, outcome: 1.1 Gait velocity [metres/min].
Other potential sources of bias
We assessed one study as having a potential conflict of interest (Whitall 2011).
Effects of interventions
Primary outcomes
Gait
Ten RCTs with a total of 298 participants examined the effects of RAS versus standard neurodevelopmental therapy (Kim 2012a; Suh 2014; Thaut 1997; Thaut 2007), or versus gait training without auditory stimulation on improvement in gait (Cha 2014a; Cha 2014b; Chouan 2012; Kim 2012b; Lichun 2011; Park 2010a). Improvements in gait were measured by changes in gait velocity (nine studies), cadence (seven studies), stride length (eight studies), stride symmetry (three studies), general gait (two studies), and balance (three studies).
Gait velocity
The pooled estimate of nine RCTs with 268 participants indicated that RAS improved gait velocity by an average of 11.34 metres per minute compared with the control group (95% CI 8.40 to 14.28; P < 0.00001) (Cha 2014a; Cha 2014b; Kim 2012a; Kim 2012b; Lichun 2011; Park 2010a; Suh 2014; Thaut 1997; Thaut 2007). The results were inconsistent across studies (I2 = 61%), with some studies reporting greater effect sizes than others, but all effect sizes were in the desired direction (Analysis 1.1). A subgroup analysis comparing studies conducted by a music therapist versus those conducted by non‐music therapy healthcare professionals indicated that music therapy studies (MD 14.76, 95% CI 13.84 to 15.69; P < 0.00001; I2 = 0%) resulted in a statistically significantly greater improvement (P = 0.0004) in gait velocity than the studies conducted by a non‐music therapy interventionist (MD 8.48, 95% CI 5.16 to 11.80; P < 0.00001; I2 = 11%). Results were consistent across studies within each subgroup (Analysis 1.2).
We also conducted a subgroup analysis for the type of auditory stimulation used in the study, namely music versus an auditory stimulus without music (e.g. metronome beat). Results indicated that the use of music led to greater and more consistent improvements in gait velocity (MD 14.69, 95% CI 13.77 to 15.61; P < 0.00001; I2 = 0%) than auditory stimulation without music (MD 7.7, 95% CI 3.03 to 12.38; P = 0.001; I2 = 42%), and this difference was statistically significant (P = 0.004) (Analysis 1.3).
A sensitivity analysis to examine the impact of randomisation method, excluding the data of two trials for which the randomisation method was not clear (Cha 2014b; Kim 2012b), had minimal impact on the effect size (MD 10.79, 95% CI 7.23 to 14.35; P < 0.00001; I2 = 70%; Analysis 1.1).
Stride length
RAS also resulted in significantly greater improvements in stride length of the affected side in five RCTs (MD 0.12 metres, 95% CI 0.04 to 0.20; P = 0.003; I2 = 80%; N = 129) (Analysis 1.4) (Cha 2014a; Cha 2014b; Kim 2012a; Kim 2012b; Lichun 2011), and stride length of the unaffected side in four studies (MD 0.11 metres, 95% CI 0.01 to 0.22; P = 0.03; I2 = 85%; N = 99; Analysis 1.6) (Cha 2014a; Cha 2014b; Kim 2012a; Kim 2012b). The heterogeneity across studies was due to some studies reporting greater improvements than others, but all treatment effects were in the desired direction. Three studies (186 participants) examined the effects of RAS on stride length but did not specify whether stride length was assessed for the affected or unaffected side or whether an average for both sides was computed (Suh 2014; Thaut 1997; Thaut 2007). The pooled effect size of these three studies was not statistically significant, and the results were inconsistent across studies (MD 0.16 metres, 95% CI ‐0.01 to 0.33; P = 0.07; I2 = 83%; Analysis 1.7).
Subgroup analysis per music intervention type revealed that there was no statistically significant difference (P = 0.37) between studies that used music (MD 0.08, 95% CI 0.05 to 0.12; P <0.00001; I2 = 0%) and those that used an auditory stimulus without music in terms of stride length (MD 0.14, 95% CI 0.02 to 0.25; P = 0.02; I2 = 55%) (Analysis 1.5).
A sensitivity analysis to examine the impact of randomisation method, excluding the data of two trials for which the randomisation method was not clear (Cha 2014b; Kim 2012b), resulted in a small decrease in effect size, but it greatly reduced the heterogeneity so that the treatment effect was consistent across the studies that used adequate methods of randomisation. Pooling the effects of only those studies that used adequate methods of randomisation resulted in an improvement of stride length by 0.08 metres (95% CI 0.05 to 0.11; P < 0.00001; I2 = 0%) on the affected side (Analysis 1.4) and 0.06 metres (95% CI 0.01 to 0.12; P = 0.03; I2 = 0%) on the unaffected side (Analysis 1.6).
Gait cadence
The pooled estimate of seven RCTs with 223 participants indicated that RAS improved gait cadence by 10.77 steps per minute compared with the control group (95% CI 4.36 to 17.18; P = 0.001; I2 = 83; Analysis 1.8) (Cha 2014a; Cha 2014b; Kim 2012a; Lichun 2011; Suh 2014; Thaut 1997; Thaut 2007). However, the results were inconsistent across studies, with the larger study, Thaut 2007, showing a greater cadence improvement (22.00 steps/minute, 95% CI 16.94 to 27.06; N = 78) than the other studies (ranging from 3.86 to 12.78 steps/minute).
A subgroup analysis compared studies in which the intervention was delivered by a music therapist, Lichun 2011, Thaut 1997, and Thaut 2007, with studies in which the intervention was delivered by another professional, Cha 2014a, Cha 2014b, Kim 2012a, and Suh 2014. This analysis revealed that studies with music therapist interventionists led to greater improvements (MD 11.51, 95% CI ‐2.57 to 25.60; P = 0.11) than studies with non‐music therapist interventionists (MD 7.65, 95% CI 4.43 to 10.86; P < 0.0001), but this difference was not statistically significant (P = 0.6). The effect size of the music therapist interventionist subgroup was no longer statistically significant. The heterogeneity within the music therapist interventionist subgroup (I2 = 94%) was much larger than that of the non‐music therapist interventionist group (I2 = 0%). This was due to the large effect sizes reported in the Thaut 2007 study (Analysis 1.9).
A subgroup analysis comparing studies that used music versus those that used an auditory stimulus without music indicated a larger improvement in the music group (MD 11.34, 95% CI ‐1.05 to 23.74; P = 0.07; I2 = 91%) than in the no‐music auditory stimulation group (MD 7.58, 95% CI 4.33 to 10.83; P < 0.00001; I2 = 0%), but this difference was not statistically significant (P = 0.57) (Analysis 1.10).
For gait cadence, one study used unclear randomisation methods (Cha 2014b). Excluding this study from the analysis had little impact on the pooled effect size (MD 10.80, 95% CI 4.05 to 17.56; P = 0.002; I2 = 86%) (Analysis 1.8).
Stride symmetry
Three RCTs involving 139 participants examined the effects of RAS on stride symmetry (defined as the ratio between the swing time of two consecutive steps using the longer step as the denominator) (Cha 2014a; Thaut 1997; Thaut 2007). Their pooled estimate was not statistically significant, and the results were inconsistent across studies (SMD 0.94, 95% CI ‐0.32 to 2.20; P = 0.14; I2 = 90%; Analysis 1.11).
General gait
The pooled estimate of two RCTs indicated that RAS improved general gait by 7.67 units on the Dynamic Gait Index compared with the control group (95% CI 5.67 to 9.67; P < 0.00001; I2 = 0%; N = 48; Analysis 1.12) (Chouan 2012; Kim 2012a).
Balance
Finally, there was no strong evidence for an effect of RAS on balance (SMD 0.31, 95% CI ‐0.48 to 1.09; P = 0.44; I2 = 51%). This evidence was based on three RCTs with small sample sizes resulting in a total sample size of 54 participants (Analysis 1.13) (Cha 2014b; Kim 2012a; Suh 2014). Removing one study for which the method of randomisation was not clear reduced the effect size (SMD 0.13, 95% CI ‐1.1 to 1.37) (Cha 2014b), and the effect size remained not statistically significant (P = 0.84).
Other outcomes
RAC was examined as an added music intervention to visual locomotor imagery training and kinaesthetic locomotor imagery training in an RCT with 15 stroke participants (Kim 2011a). This review included only the visual locomotor imagery training as the control condition with added RAC as the music intervention. We measured changes of peak‐to‐peak joint angular displacement using electromyographic analyses, and so we could not include these results in the meta‐analysis. Increased activation in a greater number of lower limb muscles involved in gait and an improvement in lower limb joint angular displacement were reported when auditory step rhythm was integrated into locomotor imagery. During the swing phase there were significant differences for all four muscles for the rhythm condition: quadriceps (F = 3.398; P < 0.05); hamstring (F = 9.324; P < 0.05); tibialis anterior (F = 5.089; P < 0.05); and gastrocnemius (F = 3.639; P < 0.05). Activation was increased significantly during the stance phase in the hamstring (F = 4.815; P < 0.05) and the gastrocnemius (F = 4.087; P < 0.05) for the rhythm intervention. Peak‐to‐peak joint angular displacement was significantly different for the ankle joint with rhythmic auditory cueing (F = 6.519; P < 0.05).
Upper extremity function
Nine studies, comprising six RCTs, Chouan 2012, Jeong 2007, Thaut 2002, Tong 2015, Van Delden 2013, and Whitall 2011, and three quasi‐RCTs, Hill 2011, Paul 1998, and Schneider 2007, with a total of 308 participants, examined the effects of music interventions on UEF. Improvements in UEF were measured by changes in general UEF (five studies), timing of UEF movements (two studies), range of motion (shoulder flexion) (two studies), hand function (two studies), upper limb strength (two studies), manual dexterity (two studies), and elbow extension angle (two studies).
General upper extremity function
Five studies, comprising four RCTs, Chouan 2012, Tong 2015, Van Delden 2013, and Whitall 2011, and one quasi‐RCT (Hill 2011), examined the effect of music‐based interventions on general UEF in 194 participants as measured by the Fugl‐Meyer Assessment (MD 3.56, 95% CI ‐0.88 to 8.00; P = 0.12; Analysis 1.14). Their pooled effect was not statistically significant, and the results were inconsistent across studies (I2 = 85%), with one study reporting a much greater improvement than the other studies (Chouan 2012). Whereas Chouan 2012 used RAS, Van Delden 2013 and Whitall 2011 used modified bilateral arm training with RAC (mBATRAC), and Tong 2015 used music‐supported therapy with audible and mute musical instruments.
Upper extremity function: time
Two RCTs examined the effects of music interventions on timed upper extremity movements to complete functional tasks using the Wolf Motor Function Test or a validated modified version of this measure (Tong 2015; Whitall 2011). Their pooled effect indicated a statistically significant reduction in time in the music intervention groups (MD ‐1.08, 95% CI ‐1.69 to ‐0.47; P = 0.0006; I2 = 52%; N = 122; Analysis 1.15).
Range of motion: shoulder flexion
There was no evidence of effect of RAS on range of motion (MD 9.81, 95% CI ‐12.71 to 32.33; P = 0.39; I2 = 0%). This evidence was based on only two studies, comprising one RCT, Jeong 2007, and one quasi‐RCT, Paul 1998, that used different types of music interventions to improve shoulder flexion. Jeong 2007 used an "RAS music‐exercise intervention" (p127). Paul 1998 evaluated the effects of electronic music‐making activity using "musical activities that were improvisational … requiring that the participants find a rhythm or beat that was expressive and comfortable for them. Music pieces were designed to elicit steady rhythmic pulses that were engaging to the participant." (p230). Both interventions used rhythm embedded in music as part of instrument playing activities, and thus were similar enough to warrant examination within meta‐analysis. In addition, Jeong 2007 had large standard deviations indicating significant variability in the findings (Analysis 1.16). Both studies used goniometer measures.
Hand function
The pooled estimates of two RCTs, Van Delden 2013 and Whitall 2011, with 113 participants using mBATRAC did not indicate evidence of effect for hand function as measured by the Stroke Impact Scale (MD 0.32, 95% CI ‐0.91 to 1.54; P = 0.61; I2 = 0%; Analysis 1.17) (Duncan 1999).
Upper limb strength
A pooled estimate of 6.03 (95% CI ‐2.52 to 14.59; I2 = 56%) in two RCTs with 113 participants found upper limb strength favouring the mBATRAC intervention, but this effect was not statistically significant (P = 0.17; Analysis 1.18) (Van Delden 2013; Whitall 2011).
Manual dexterity
We found no evidence of effect for manual dexterity (MD 0.47, 95% CI ‐1.08 to 2.01; P = 0.55; I2 = 52%). This evidence was based on the results of two studies, comprising one RCT, Van Delden 2013, and one quasi‐RCT, Schneider 2007, with a total of 74 participants (Analysis 1.19). The effect of music on dexterity was assessed with the Nine‐Hole Peg Test (Kellor 1971).
Elbow extension angle
Two studies, comprising one RCT, Thaut 2002, and one quasi‐RCT, Paul 1998, measured the effects of music therapy on elbow extension angle in people with hemispheric stroke. However, due to the significant clinical heterogeneity of the studies, we did not pool their effect sizes.
Thaut 2002 examined the effects of RAS on spatio‐temporal control of reaching movements of the paretic arm in 21 participants. Results indicated that RAS increased the elbow extension angle by 13.8% compared with the non‐rhythmic trial, and this difference was statistically significant (P = 0.007). Results further indicated that variability of timing and reaching trajectories were reduced significantly (35% and 40.5%, respectively; P < 0.05).
Paul 1998 evaluated the effects of music‐making activity on elbow extension in 20 participants with hemiplegia. The elbow extension (measured from 135 to 0, with negative numbers expressing limitations) postintervention was ‐29.4 (standard deviation (SD) 29.49) for the experimental group and ‐39.2 (SD 38.19) for the control group. This difference was not statistically significant. Post‐test shoulder flexion data indicated a non‐statistically significant difference (P = 0.44) between the music therapy group (85.6°, SD 26.71) and the control group (71.8°, SD 39).
Secondary outcomes
Communication
Overall communication
Music interventions significantly improved the overall communication of people with aphasia after stroke as indicated by a moderate effect size of 0.75 (95% CI 0.11 to 1.39; P = 0.02; I2 = 31%) (Cohen 1988). This included people with ischaemic stroke (Särkämö 2008; van der Meulen 2014), haemorrhagic stroke or stroke of an unknown type (van der Meulen 2014), and people with chronic expressive and global aphasia (Jungblut 2004). This evidence was based on three studies, comprising two RCTs, Särkämö 2008 and van der Meulen 2014, and one quasi‐RCT (Jungblut 2004), with a total of 67 participants (Analysis 1.20). Each of the three studies used different measures. Overall communication in Särkämö 2008 was measured using repetition and reading subtests from the Finnish version of the Boston Diagnostic Aphasia Examination (Hänninen 1989), verbal fluency and naming subtests from the Consortium to Establish a Registry for Alzheimer’s Disease (Morris 1989), and a shortened version of the Token Test (De Renzi 1978). Overall communication outcomes in van der Meulen 2014 were measured with the Amsterdam‐Nijmegen Everyday Language Test (Blomert 1995). For Jungblut 2004, we used the reported total score from the Aachen Aphasia Test (Hogrefe 1983).
Removing one study considered to be at high risk of bias for randomisation reduced the size of the effect (SMD 0.52, 95% CI ‐0.03 to 1.07), and the resulting effect size was no longer statistically significant (P = 0.06) (Analysis 1.20) (Jungblut 2004).
Naming
The pooled estimate of two small studies, comprising one RCT, van der Meulen 2014, and one quasi‐RCT (Jungblut 2004), with a total of 35 participants, suggested an improvement in naming by 9.79 units on the Aachen Aphasia Test (95% CI 1.37 to 18.21; P = 0.02; I2 = 0%) in participants who received music therapy interventions compared with training without music (Analysis 1.21).
Repetition
Music interventions also had a beneficial effect on speech repetition as measured by the Aachen Aphasia Test (MD 8.90, 95% CI 3.25 to 14.55; P = 0.002; I2 = 0%). However, this pooled estimate was based on only two studies, comprising one RCT, van der Meulen 2014, and one quasi‐RCT (Jungblut 2004), with a total of 35 participants (Analysis 1.22). A third study, Conklyn 2012, examined the effects of modified melodic intonation therapy on speech repetition using two tasks drawn from the Western Aphasia Battery (Kertesz 1982). Changes were examined over three session visits. Due to high attrition in visit three, we included change scores between visits one and two only for this review and examined total scores only rather than subscale scores. Change scores were used due to large differences in pre‐test scores between the treatment arms. Significant improvements were found in both the control group adjusted total score (change = 4.1; P = 0.03) and the treatment group adjusted total scores (change 8.1; P < 0.01). The improvement in the treatment group was not significantly greater than that in the control group. However, post‐hoc analyses suggested that the control group improved in repetition only, whereas the treatment group improved in both repetition and responsiveness, suggesting a possible carry‐over effect of the modified melodic intonation therapy intervention.
Mood
Three RCTs examined mood as measured by the Profile of Mood States (POMS) (Jeong 2007; Pool 2012; Särkämö 2008). However, we could not combine these studies in a meta‐analysis as different versions of the POMS were used, and the scores were reported inconsistently, omitting either total scores or subscale scores. Särkämö 2008 used the shortened Finnish version of the POMS (Hänninen 1989), with 38 items measuring tension, depression, irritability, vigour, fatigue, inertia, confusion, and forgetfulness in eight subscales. Subscale scores were reported, and total scores were provided by the principal investigator. Jeong 2007 reported total scores only for the 34‐item version of the POMS translated and modified into a Korean version (Shin 1996). Mood subscales of the Korean POMS were not reported. Pool 2012 used the bipolar version of the POMS (Lorr 2003), which contains 72 adjectives grouped into six bipolar mood states. Pool 2012 used a shortened version of the POMS with just four subscales (48 items) due to the cognitive deficits of the participants, including composed‐anxious, agreeable‐hostile, elated‐depressed, and energetic‐tired only. Subscale total scores only were available. Although subscale totals were provided in both Särkämö 2008 and Pool 2012, the mood states subscales were different in the two different versions of the POMS, and so these could not be combined meaningfully.
Särkämö 2008 compared the effects of music listening versus no intervention versus audio book listening (not included in this review) on mood states in 60 people in the acute stage after stroke. Significant differences were found between the music intervention and the other groups at three months' poststroke (the time frame examined in this review) for the mood states confusion (F(2, 51) = 3.3; P = 0.045) and depression (F(2, 51) = 3.7; P = 0.031). A post‐hoc test revealed significantly lower scores for depression in the music intervention group (P = 0.024). Scores for confusion were marginally lower in the music intervention group than in the control group (P = 0.061). Tendencies for less depression in the music intervention group were sustained at the six‐month poststroke stage.
Pool 2012 examined the effects of group music therapy interventions versus standard care in 10 people with chronic ABI (mixed aetiologies) on mood. Four bipolar mood states were measured: agreeable‐hostile, composed‐anxious, elated‐depressed, and energetic‐tired. No significant differences were found in mood states between conditions after eight weeks. Mean scores showed that mood states improved slightly following eight weeks of standard care (control) for each mood state but worsened slightly following music therapy intervention at the same time point. Although non‐significant, an improvement in mean mood scores for all moods states was noted after 16 weeks for music therapy intervention beyond the scores for standard care.
Jeong 2007 compared RAS with no intervention in 36 people with stroke. The Korean version of the POMS was used, in which total scores range from 0 to 60, and a higher total score indicates worse depression. There was a significant improvement in mood for both groups (post‐RAS scores: 1.56 (SD 0.82) and post‐control scores: 2.29 (SD 0.77)). However, it should be noted that baseline scores were already very low (RAS: 2.11; control: 2.81), providing a narrow window for change.
Two further RCTs examining physical functioning as the primary outcome also reported on mood subscales in their results, specifically the Stroke Impact Scale emotion subscale (Van Delden 2013; Whitall 2011). However, because mood was not identified as a primary outcome at the outset of the study or discussed in the findings, we did not include these data, as it appeared they were extraneous.
Social skills
Jeong 2007 used the Relationship Change Scale (Shannon 1973), translated into Korean and then further modified to examine the effects of music interventions on social relationships. A significant effect was found for the music intervention, showing improved interpersonal relationships compared with the control group (F = 10.087; P = 0.003), which showed a significant decrease in interpersonal relationships.
Pain
Kim 2005 examined the effects of listening to pre‐recorded music on pain in people with ABI. Pain ratings on a 0‐to‐10 numeric scale indicated no statistically significant difference in pain ratings between the music and the no‐music condition (P = 0.05).
Behavioural outcomes
Agitation
One RCT examined the effects of listening to live music and to recorded music on agitation in 22 people with a severe head injury with a diagnosis of post‐traumatic amnesia (Baker 2001). Listening to live music was effective in reducing agitation scores (as measured by the Agitation Behavior Scale (ABS)) (effect size = 5.01 ABS units; P < 0.0001) (Corrigan 1989). Agitation also decreased after listening to recorded music (6.25 ABS units; P < 0.0001). The difference in effect between live and recorded music was not statistically significant (1.2 ABS units; P = 0.8).
Other behavioural outcomes
Two studies, comprising one RCT, O'Kelly 2014, and one quasi‐RCT, Fernandes 2014, with people with disorders of consciousness reported on other behavioural outcomes. O'Kelly 2014 reported on a range of behavioural outcomes including blinks per minute, eyes closed with or without body movements, eyes open with or without body movements, and respiration rate per minute. Behaviours of 21 participants with disorders of consciousness were observed across conditions of baseline silence, non‐music therapy conditions (white noise, recordings of disliked music), and music therapy conditions (live, participant‐preferred music and live, improvised music entrained to the participant's respiration). Differences in eye blink rate in vegetative participants were significant across conditions (F(2.3, 13.9) = 3.6; P = 0.019), with a peak response during the participant‐preferred live music condition when compared with baseline silence (F(1, 11) = 8.2; P = 0.029). Fernandes 2014 also reported on changes in facial expression, including muscular facial relaxation, eye opening, mouth movements, head movements, yawning, smiling, and eyebrow movements in response to recorded music. However, insufficient data reporting by Fernandes 2014 prevented meta‐analysis on this outcome.
Quality of life
Two RCTs, Cha 2014b and Jeong 2007, looked at the impact of RAS on quality of life (N = 53) using the Stroke Specific Quality of Life Scale (Williams 1999). However, the reported means and standard deviations suggested that the authors computed the total score differently: Cha 2014b appears to have computed the total score by adding the participant's rating of each item, whereas Jeong 2007 computed the total score by averaging all the ratings. We therefore computed a SMD for this meta‐analysis. Their pooled estimate suggested a large effect on quality of life (SMD 0.89, 95% CI 0.32 to 1.46; P = 0.002; I2 = 0%; Analysis 1.25). A third quasi‐RCT examined the effects of auditory rhythmic training on quality of life using the Stroke Impact Scale (Hill 2011); however, due to large baseline differences between the groups in this study, we could not include the data from this study in the meta‐analysis. Computation of a SMD does not allow for combining post‐test scores with change scores.
Cognitive functioning
Memory
Two RCTs included memory as an outcome variable (N = 42) (Pool 2012; Särkämö 2008). Särkämö 2008 examined short‐term working memory using the digit span subtest from the Wechsler Memory Scale‐Revised (Wechsler 1987). Pool 2012 used the Rivermead Behavioural Memory Test (Wilson 2008). Their pooled estimate indicated no strong evidence of effect for music interventions on memory (SMD 0.33, 95% CI ‐0.29 to 0.95; P = 0.30; I2 = 0%; Analysis 1.23).
Attention
Two RCTs examined the effects of music on attention (N = 39), but their pooled estimate indicated no strong evidence for an effect (SMD 0.30, 95% CI ‐0.34 to 0.94; P = 0.36; I2 = 0%; Analysis 1.24). Pool 2012 used the Test of Everyday Attention (Robertson 1994). Särkämö 2008 used CogniSpeed reaction time software to measure the percentage of correct responses in the vigilance subtest and summed reaction times in the vigilance and simple reaction time subtests (Revonsuo 1995).
Mental flexibility
One RCT examined the effects of music‐based endogenous shifting training led by a music therapist on executive functioning of 14 people with stroke or ABI (Mueller 2013). The effects of music training were compared with a control group and a placebo singing group (not included in this review). Mental flexibility was tested using the Trail Making Test Part B (Reitan 1985). No difference was found between the treatment and control conditions (F = 0.81; P = 0.4717). This study also examined working memory; however, we did not include this outcome in the review due to the adapted administration of the test to determine outcomes.
Orientation
One RCT examined the effects of listening to live music and to recorded music on orientation levels in 22 participants with a severe head injury with a diagnosis of post‐traumatic amnesia (Baker 2001). Listening to live music had a significant effect on participant orientation levels (as measured by the Westmead Post‐traumatic Amnesia Scale) compared with the no‐music control condition (effect size = 0.82; P < 0.001) (Shores 1986), and this effect was slightly larger than the effect of listening to recorded music compared to the control condition (effect size = 0.72; P < 0.001).
Activities of daily living
One RCT measured the quality and quantity of spontaneous paretic upper limb use to accomplish 26 activities of daily living outside the laboratory (Van Delden 2013), using the Motor Activity Log (Uswatte 2005). No significant differences in change scores were observed between the groups for amount of use (P = 0.09) or quality of use (P = 0.27).
Adverse events
No studies included adverse event outcomes.
Discussion
Summary of main results
Gait
The results of 10 studies suggest that RAS may have a beneficial effect on gait velocity in people with stroke with an average of 11.34 metres per minute compared with standard treatment. RAS may also improve stride length by about 0.12 metres and general gait by an average of 7.67 units as measured on the Dynamic Gait Index in people with stroke compared with standard treatment. One study found significant improvement in peak‐to‐peak joint angular displacement in the lower limbs during RAC. RAS may have a beneficial effect on gait cadence for people with stroke; however, the degree of improvement across studies was inconsistent. We found no evidence of effect for music interventions on gait symmetry and balance.
Upper extremity function
The music interventions used for UEF varied across nine studies, including rhythm‐based instrument‐playing tasks in music‐making (Paul 1998), RAS within music‐making (Jeong 2007), RAS using rhythmic pulse without music (Chouan 2012; Thaut 2002), fast‐tempo auditory stimulation with and without music (Tong 2015), bilateral arm training with RAC (BATRAC) or a modified version of BATRAC (Van Delden 2013; Whitall 2011), and music‐supported training (Schneider 2007). The results of two studies indicated that music interventions may improve the timing of UEF by about one second. One study found significant improvements in elbow extension angle using RAS with reduced variability of timing (35%) and reduced reaching trajectories (45%) (Thaut 2002). We found no evidence of effect for music interventions for general UEF, range of motion (shoulder flexion), hand function, upper limb strength, and manual dexterity.
Communication outcomes
The results of this review suggest that music interventions may have a moderate effect (SMD = 0.69) on overall communication. This pooled effect size was derived from three studies. The results of two small studies suggested that music interventions may benefit the expressive language outcome of naming (9.79 units on the Aachen Aphasia Test) and the speech outcome of repetition (8.9 units on the Aachen Aphasia Test) for people following stroke (Jungblut 2004; van der Meulen 2014). The studies that examined communication outcomes used diverse music interventions encompassing both receptive (listening) and active (singing and playing) methods.
Mood
Three studies included in our review suggested positive effects of music interventions on mood (Jeong 2007; Pool 2012; Särkämö 2008). Meta‐analysis of these three studies was not possible due to: 1) the use of different versions of the same measure (POMS), and 2) reporting of selected subscales or total score only. Two studies found significant improvements in mood states. One music‐listening study found improvements in depression and confusion, with the positive effects on depression sustained at six months' follow‐up (Särkämö 2008). One study found significant improvements in mood following rhythmic movement to music and active music‐making (Jeong 2007).
Quality of life
Based on the results of Cha 2014b and Jeong 2007, we found a large effect for music interventions on quality of life (SMD = 0.89). The music intervention used in both studies was RAS. A third study that we could not include in the meta‐analysis also used auditory rhythmic training (Hill 2011). More research examining the effects of a wider range of music interventions on quality of life is needed.
Other secondary outcomes
The primary reason noted for referral to music therapy in rehabilitation settings is the rehabilitation of social skills (Magee 2007). However, we identified only one study that measured this as an outcome. Jeong 2007 reported significant improvements in social skills following rhythmic movement to music and active music‐making with stroke participants.
Based on the results of one study, we found no evidence for the effect of music listening on pain for people with ABI (Kim 2005).
One trial reported positive effects for reducing agitation in people with post‐traumatic amnesia following a severe head injury, using both live and recorded music (Baker 2001). Two studies examined the effects of music interventions on a range of behavioural outcomes in people with disorders of consciousness (Fernandes 2014; O'Kelly 2014). We could not combine the results for meta‐analysis due to insufficient data reporting. The severity of injury in this population means that participants are heavily dependent, and only receptive methods can be used. One study reported significant changes in behaviours to music conditions compared with baseline silence (O'Kelly 2014).
Based on two trials, we found no strong evidence for the effect of music interventions on cognitive functioning, specifically memory or attention (Pool 2012; Särkämö 2008). One trial found significant effects for orientation in response to listening to live or recorded music in comparison with no music in participants with post‐traumatic amnesia (Baker 2001). We found no studies that examined activities of daily living or adverse events as outcomes.
More research is needed for all secondary outcomes before reliable conclusions can be drawn.
Overall completeness and applicability of evidence
This review included 29 studies with a total of 775 participants. The results suggest that music interventions may improve gait, communication, and quality of life in people with ABI. While there is much cross‐over in treatments for people with ABI resulting from stroke and traumatic injury, 90% of participants included in this review were stroke survivors, and thus our findings may be more relevant for this population.
Subgroup analyses for gait velocity provide important information about the impact of the type of music intervention and the professional delivering the intervention on the treatment effect. Studies that used trained music therapists to deliver the music interventions resulted in significantly greater improvements in gait velocity than studies in which the intervention was delivered by a non‐music therapy healthcare professional. It should be noted that the subgroup analysis reflects the results of different trials and not direct comparisons of interventionists within a trial. The results of studies that used a trained music therapist were consistent across studies. Furthermore, the subgroup analyses indicated that interventions that use RAS (e.g. metronome beat) embedded within music may be more effective than using non‐music RAS alone. These results provide support for using professionals who are trained in delivering music interventions, such as music therapists, rather than just a metronome. Subanalyses for gait cadence suggested greater improvements when the intervention was delivered by a music therapist, and also when the music was combined with auditory stimulation. Although we had planned to complete a subanalysis for dosage of intervention, there was too much heterogeneity amongst the RAS studies in terms of the number of treatment sessions, the frequency of sessions, the duration of individual sessions, and the total course of treatment to complete this analysis; therefore, recommendations for dosage could not be made. Reporting was problematic for several studies included in this review, particularly concerning blinding of the outcome assessor. The results indicate that interventions implemented by a trained music therapist may result in greater treatment benefits than those delivered by other professionals. This could be explained by the training that music therapists have in delivering interventions using live music that matches the participant's in‐the‐moment physical responses. However, we acknowledge that other factors may have confounded this comparison.
Music interventions may improve the timing of UEF. The findings of this review were influenced by the large variance in the number of participants within studies examining UEF and the variance in reported improvements. Furthermore, one large study reported that there was a large variance in deficit severity of participants (Whitall 2011, N = 92). All of these factors may have contributed to the non‐significant results for general UEF, hand function, and upper limb strength. Rhythmic stimulation appears to induce temporal stability and enhance motor control in walking. It could be that rhythmic cueing has a similar effect on some aspects of UEF, such as timing of movements. Even though functional arm movements, unlike gait, are "discrete, biologically non‐rhythmic, and volitional" (Thaut 2002, p1074), rhythmic stimuli are successfully used to enhance the execution of motor skills in non‐rehabilitation areas such as music performance and sports (Karageorghis 2012a; Karageorghis 2012b).
Although this review included more studies with an increased number of speech and language outcomes than our previous review, the selected subdomains in speech and language outcomes were inconsistent across music intervention studies. This prevented more outcomes being examined in a meta‐analysis. Standardised communication‐specific measures included the Aachen Aphasia Test (Jungblut 2004; van der Meulen 2014), the Amsterdam‐Nijmegen Everyday Language Test (van der Meulen 2014) and the Sabadell (van der Meulen 2014). However, all of these studies examined slightly different subdomains, preventing meta‐analysis of a greater number of outcomes. Similarly, although we were able to report on the effects of music interventions on four cognitive outcomes (memory, attention, mental flexibility, and orientation), we were unable to report on a further 13 cognitive outcomes examined in research studies due to the lack of agreement between studies in the subdomains examined and outcome measures used.
We identified only three studies of sufficient methodological quality that included mood as an outcome. This is surprising given the high incidence of depression following stroke (Matsuzaki 2015), and that mood disorders can affect motivation to engage in rehabilitation and impede re‐integration back into the community (Giles 2006). Two of the three studies reported greater improvements in mood in the music intervention group compared with the control group. However, inconsistent reporting of results prevented meta‐analysis.
Given the importance of improving and maintaining mood after ABI, it is also important to examine the relationship between functional gains and mood during rehabilitation. Several studies tested the effects of music interventions on a functional outcome as well as mood (N = 3) or quality of life (N = 3). Two trials examined cognitive and mood outcomes (Pool 2012; Särkämö 2008). Three trials examined the effects on motor function (gait) and quality of life (Cha 2014b; Hill 2011; Jeong 2007), and one trial examined motor function (gait) and mood (Jeong 2007). Effects on combined domains also reflect clinical practice, which typically aims to address function in combination with mood rather than individual domains alone. Motivating interventions are important for brain‐injured populations, who may experience a loss of motivation due to brain injury.
The benefit of using music as a medium for addressing human function is its flexibility and the range of activities it offers, such as singing, playing, composing, and listening. The music used in therapeutic interventions also can be adapted through varying its multiple components, such as rhythm, tempo, articulation, melodic contour, dynamic range, and harmonic progression, to meet a person’s specific needs (Schneck 2006). This flexibility enables music to be applied in a number of ways within tasks, and it can also be adapted within that task to match or drive the person's level of functioning. Music also provides a motivational force to enhance engagement and participation through stimulating the pleasure and reward networks in the brain (Schneck 2006). However, this flexibility is not advantageous when trying to make meaningful comparisons of interventions and dosage. Given the heterogeneity of interventions across the range of domains that are targeted in ABI rehabilitation, recommendations for dosage cannot be made based on this review. Interventions for motor outcomes (gait and UEF) were relatively homogenous, using rhythm‐based interventions (RAS, variations of RAS, or instrument playing to rhythmic music). However, other interventions for any one outcome were more varied. For example, the interventions addressing mood illustrate the heterogeneity of treatments, ranging from rhythm‐based movement to music (Jeong 2007), receptive listening to participant‐selected recorded music (Särkämö 2008), and active music‐making through songwriting methods (Pool 2012). In order to generate high‐quality evidence, future trials need to standardise and clearly describe details of music‐based methods so that meta‐analysis provides more meaningful information about interventions and dosage.
Quality of the evidence
Overall, the quality of reporting was poor. We judged only one study to be at low risk of bias (Thaut 1997), and two studies as at unclear risk of bias (Cha 2014a; O'Kelly 2014). We judged all of the other studies to be at high risk of bias (N = 26). We have detailed risk of bias for each study in the 'Risk of bias' tables included in the Characteristics of included studies table. Three studies reported the methods of randomisation and allocation concealment, and detailed all levels of blinding (Cha 2014a; O'Kelly 2014; Thaut 1997). We needed to contact the chief investigators of many studies to request more information about methodological issues.
The findings of this review should be interpreted with caution due to the large number of trials rated as having a high risk of bias. We downgraded the quality of many studies because of unclear reporting. We downgraded O'Kelly 2014 and Cha 2014a for not reporting attrition. Four studies reported inadequate methods of randomisation (Hill 2011; Jungblut 2004; Paul 1998; Schneider 2007), and a further three were unclear in reporting randomisation (Cha 2014b; Chouan 2012; Kim 2012b). Four studies did not use allocation concealment (Baker 2001; Jungblut 2004; Lichun 2011; Schneider 2007), and a further seven were unclear in reporting on this criterion (Conklyn 2012; Fernandes 2014; Hill 2011; Jeong 2007; Mueller 2013; Tong 2015; Whitall 2011). Reporting the blinding of participants, interventionists, and outcome assessors needs improving in research trials using music interventions. Blinding of participants in music intervention studies is usually not possible unless two music interventions are being compared (e.g. music listening and music‐making). The lack of participant blinding is problematic when studies examine subjective outcomes such as mood or quality of life. Blinding of interventionists is often not possible in music intervention studies when active music‐making is examined. Where interventionists cannot be blinded, they should be blinded to the purpose of the study where possible. In either case, blinding should be reported or discussed. We found attrition to be problematic, rating it inadequate in six studies and not adequately reported in a further nine studies.
Most of the included trials used small sample sizes (average N = 28; range of sample size 9 to 111), except for Whitall 2011 (N = 111). For the majority of the outcomes measured, results were inconsistent across studies. However, this was due to some studies reporting much larger treatment benefits than other studies. All treatment benefits were in the desired direction. In summary of findings Table for the main comparison, large confidence intervals were reported for gait velocity, gait cadence, general UEF, and overall communication. Small sample sizes, combined with high risk of bias and wide confidence intervals, require that the results of this review be interpreted with caution. In summary, the quality of the evidence was low (summary of findings Table for the main comparison).
Potential biases in the review process
The strength of this review is based in the search of all available databases and a comprehensive number of music therapy journals (English, German, and Japanese). This update omitted an updated search of the Science Citation Index from August 2009; however, given the extensive cross‐referencing between databases, it is unlikely that potential studies would be cited on this database alone. We also checked the reference lists of all relevant trials, contacted relevant experts in order to identify unpublished trials, and included publications in any language. In spite of such a comprehensive search, it is still possible that we missed some published and unpublished trials. We requested additional data for all trials we considered for inclusion where necessary, which allowed us to obtain accurate information on the trial quality and data for most trials, assisting us in making well‐informed trial selection decisions.
It is possible that we did not identify some grey literature; however, it is doubtful that this would have had a significant impact on our results. Grey literature tends to include trials with relatively small numbers of participants and inconclusive results (McAuley 2000).
Agreements and disagreements with other studies or reviews
The aim of this review was to update the previous version examining the effects of music therapy on adults with ABI (Bradt 2010). In this update, we expanded our criteria to include trials that examined the effects of music interventions more broadly, including music interventions delivered by professionals other than trained music therapists, such as other medical or health professionals with training in rehabilitation. This revision enabled the inclusion of a greater number of studies.
In our previous review, we could include only two studies for meta‐analysis. This previous analysis showed significant improvements in gait cadence, stride length, and symmetry. A recent review by Yoo 2016 detailed the findings of 11 trials examining the effects of RAS on motor rehabilitation in people with stroke. Meta‐analyses of outcomes from seven trials examining gait function demonstrated large effect sizes for gait parameters (walking velocity, cadence, and stride length) and UEF. Another recent review by Nascimento 2015 compared the effects of cadence cueing and walking training alone following stroke (seven trials, 211 participants). Meta‐analyses of six trials with 171 participants also demonstrated improvements in walking velocity, cadence, stride length, and gait symmetry. The positive effects of RAS on gait in the current review are consistent with previous reviews (Bradt 2010; Nascimento 2015; Yoo 2016). Our review also provided evidence to support previous findings from Yoo 2016 indicating greater effects from rhythmic cueing combined with music in comparison with metronome cueing alone.
Yoo 2016 also examined the effects of RAS on UEF. Meta‐analysis of Fugl‐Meyer Assessment outcomes reported in three studies yielded large effect sizes for UEF. In our updated review, the pooled effect of five studies examining the effect of music‐based interventions on UEF using the Fugl‐Meyer Assessment was not statistically significant, nor were there significant pooled effects for shoulder flexion, hand function, upper limb strength, manual dexterity, or elbow extension angle.
We also included one additional outcome that is important in brain injury rehabilitation, namely cognitive functioning. However, there were not enough studies at this time to provide strong evidence for an effect of music interventions on cognitive outcomes.
In summary, the results of this review provide new insights and further evidence of the effects of music‐based interventions in ABI rehabilitation.
Study flow diagram for the updated review.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
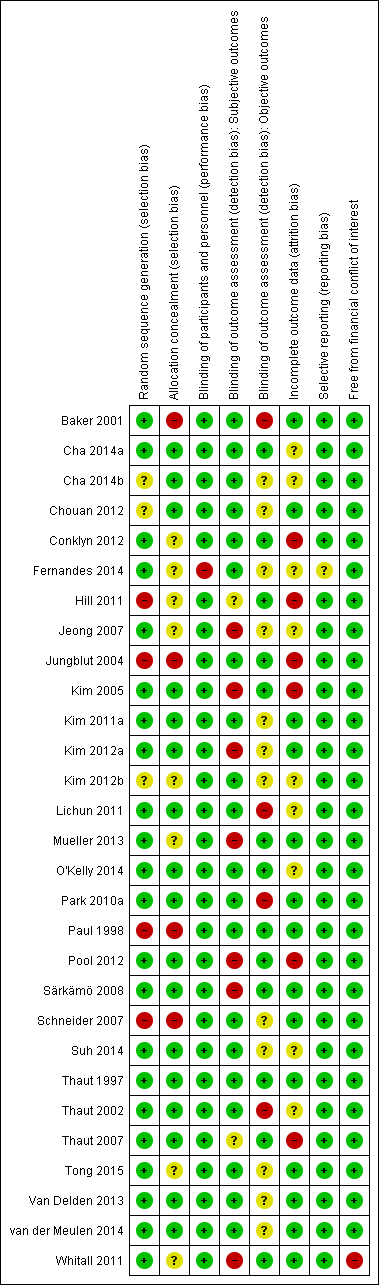
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
![Funnel plot of comparison: 1 Music therapy versus control, outcome: 1.1 Gait velocity [metres/min].](/es/cdsr/doi/10.1002/14651858.CD006787.pub3/media/CDSR/CD006787/image_n/nCD006787-AFig-FIG04.png)
Funnel plot of comparison: 1 Music therapy versus control, outcome: 1.1 Gait velocity [metres/min].

Comparison 1 Music therapy versus control, Outcome 1 Gait velocity.

Comparison 1 Music therapy versus control, Outcome 2 Gait velocity ‐ interventionist.

Comparison 1 Music therapy versus control, Outcome 3 Gait velocity ‐ music type.

Comparison 1 Music therapy versus control, Outcome 4 Stride length (affected side).

Comparison 1 Music therapy versus control, Outcome 5 Stride length (affected side) ‐ music type.
![Comparison 1 Music therapy versus control, Outcome 6 Stride length (unaffected side) [metres].](/es/cdsr/doi/10.1002/14651858.CD006787.pub3/media/CDSR/CD006787/image_n/nCD006787-CMP-001-06.png)
Comparison 1 Music therapy versus control, Outcome 6 Stride length (unaffected side) [metres].
![Comparison 1 Music therapy versus control, Outcome 7 Stride length (unspecified) [metres].](/es/cdsr/doi/10.1002/14651858.CD006787.pub3/media/CDSR/CD006787/image_n/nCD006787-CMP-001-07.png)
Comparison 1 Music therapy versus control, Outcome 7 Stride length (unspecified) [metres].
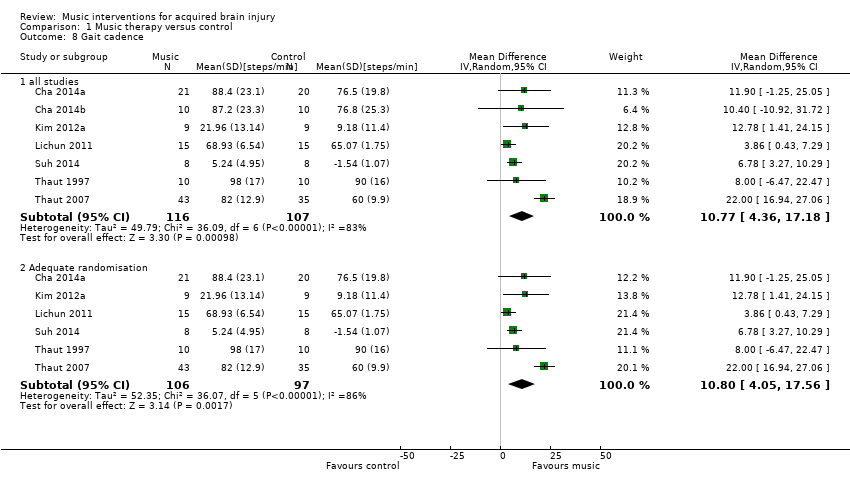
Comparison 1 Music therapy versus control, Outcome 8 Gait cadence.

Comparison 1 Music therapy versus control, Outcome 9 Gait cadence ‐ interventionist.

Comparison 1 Music therapy versus control, Outcome 10 Gait cadence ‐ music type.
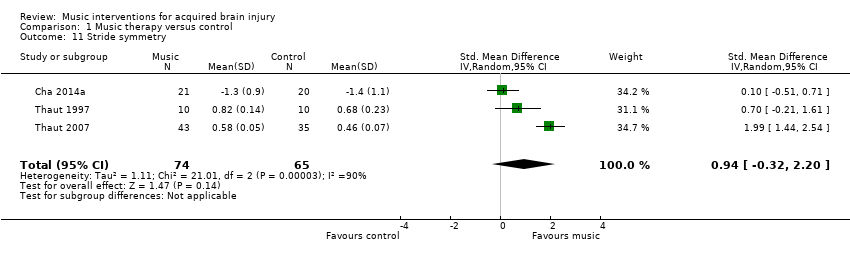
Comparison 1 Music therapy versus control, Outcome 11 Stride symmetry.
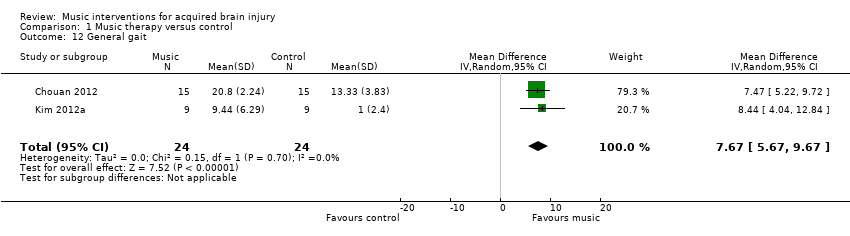
Comparison 1 Music therapy versus control, Outcome 12 General gait.

Comparison 1 Music therapy versus control, Outcome 13 Balance.

Comparison 1 Music therapy versus control, Outcome 14 Upper extremity functioning (general).
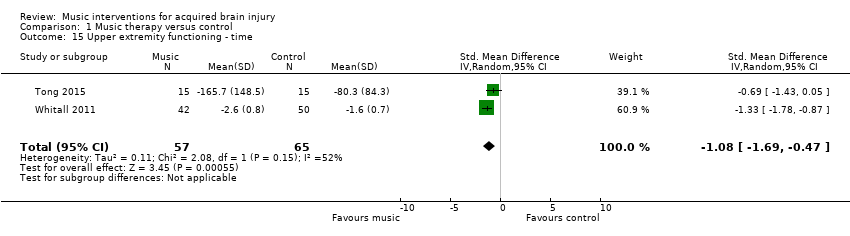
Comparison 1 Music therapy versus control, Outcome 15 Upper extremity functioning ‐ time.
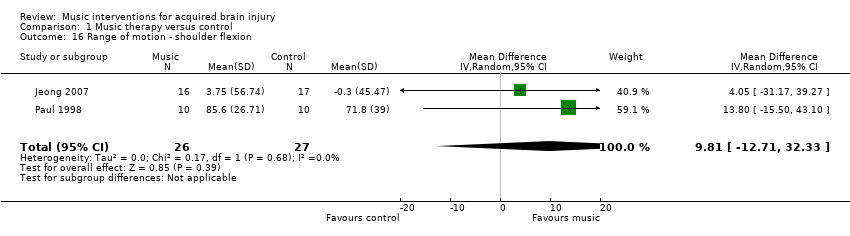
Comparison 1 Music therapy versus control, Outcome 16 Range of motion ‐ shoulder flexion.
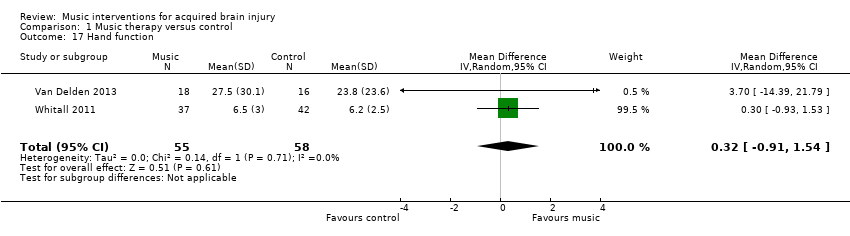
Comparison 1 Music therapy versus control, Outcome 17 Hand function.
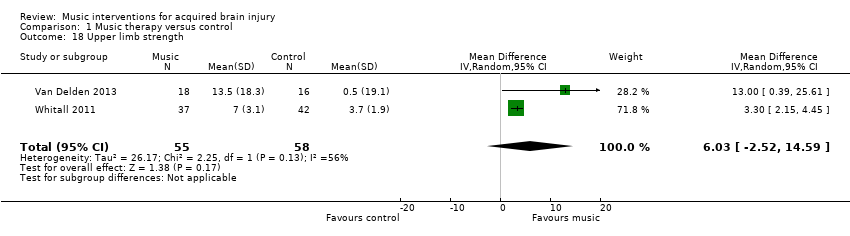
Comparison 1 Music therapy versus control, Outcome 18 Upper limb strength.
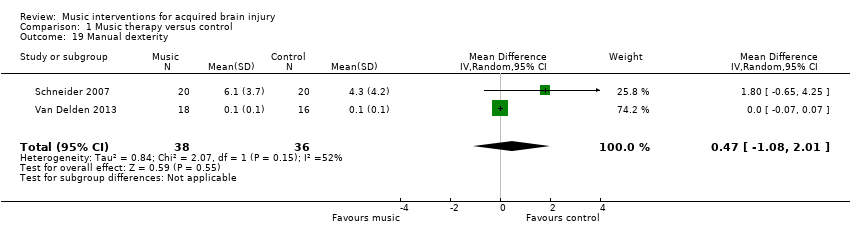
Comparison 1 Music therapy versus control, Outcome 19 Manual dexterity.
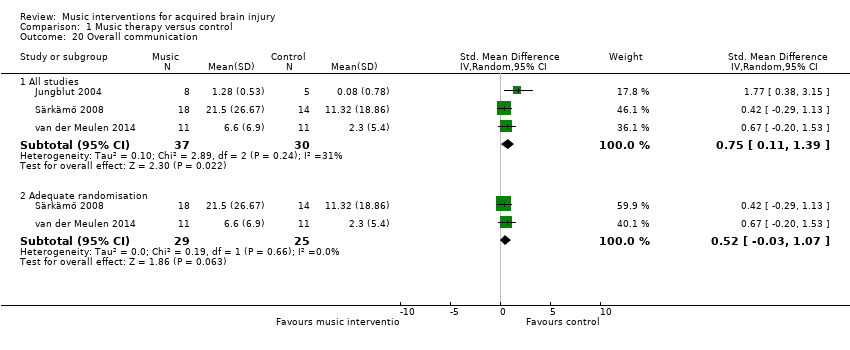
Comparison 1 Music therapy versus control, Outcome 20 Overall communication.
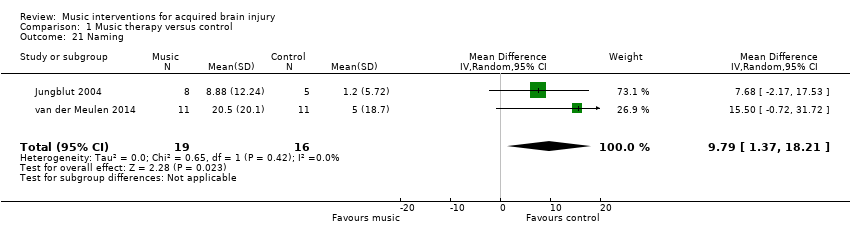
Comparison 1 Music therapy versus control, Outcome 21 Naming.
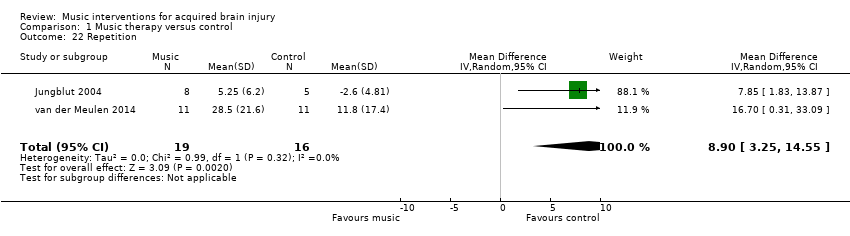
Comparison 1 Music therapy versus control, Outcome 22 Repetition.

Comparison 1 Music therapy versus control, Outcome 23 Memory.

Comparison 1 Music therapy versus control, Outcome 24 Attention.
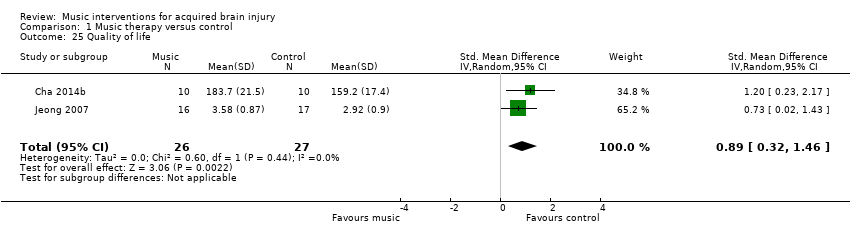
Comparison 1 Music therapy versus control, Outcome 25 Quality of life.
| Music compared with standard care for acquired brain injury | |||
| Patient or population: acquired brain injury | |||
| Outcomes | Relative effect | No of participants | Quality of the evidence |
| Gait velocity | The mean gait velocity in the intervention group was 11.34 metres more (8.4 more to 14.28 more). | 268 | ⊕⊕⊕⊝ |
| Stride length (affected side) | The mean stride length (affected side) in the intervention group was 0.12 metres more (0.04 more to 0.2 more). | 129 | ⊕⊕⊕⊝ |
| Gait cadence | The mean gait cadence in the intervention group was 10.77 steps/minute more (4.36 more to 17.18 more). | 223 | ⊕⊕⊝⊝ |
| Stride symmetry | The mean stride symmetry in the intervention group was 0.94 standard deviations more (0.32 fewer to 2.2 more). | 139 | ⊕⊕⊝⊝ |
| General upper extremity functioning assessed with: Fugl‐Meyer Assessment | The mean general upper extremity functioning in the intervention group was 3.56 units higher (0.88 lower to 8 higher). | 194 | ⊕⊝⊝⊝ |
| Overall communication | The mean overall communication in the intervention group was 0.75 standard deviations more (0.11 more to 1.39 more). | 67 | ⊕⊝⊝⊝ |
| Quality of life assessed with: Stroke Specific Quality of Life Scale | The mean quality of life in the intervention group was 0.89 standard deviations more (0.32 more to 1.46 more). | 53 | ⊕⊕⊝⊝ |
| CI: confidence interval; RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence | |||
| 1Most studies were rated as at unclear or high risk of bias | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gait velocity Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies | 9 | 268 | Mean Difference (IV, Random, 95% CI) | 11.34 [8.40, 14.28] |
| 1.2 Adequate randomisation | 7 | 228 | Mean Difference (IV, Random, 95% CI) | 10.79 [7.23, 14.35] |
| 2 Gait velocity ‐ interventionist Show forest plot | 9 | 268 | Mean Difference (IV, Random, 95% CI) | 11.34 [8.40, 14.28] |
| 2.1 Music therapist | 3 | 128 | Mean Difference (IV, Random, 95% CI) | 14.76 [13.84, 15.69] |
| 2.2 Non‐music therapist | 6 | 140 | Mean Difference (IV, Random, 95% CI) | 8.48 [5.16, 11.80] |
| 3 Gait velocity ‐ music type Show forest plot | 9 | 268 | Mean Difference (IV, Random, 95% CI) | 11.34 [8.40, 14.28] |
| 3.1 Music | 5 | 173 | Mean Difference (IV, Random, 95% CI) | 14.69 [13.77, 15.61] |
| 3.2 Auditory stimulation (no music) | 4 | 95 | Mean Difference (IV, Random, 95% CI) | 7.70 [3.03, 12.38] |
| 4 Stride length (affected side) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All studies | 5 | 129 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.04, 0.20] |
| 4.2 Adequate randomisation | 3 | 89 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.05, 0.11] |
| 5 Stride length (affected side) ‐ music type Show forest plot | 5 | 129 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.04, 0.20] |
| 5.1 Music | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.05, 0.12] |
| 5.2 Auditory stimulation (no music) | 3 | 79 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.02, 0.25] |
| 6 Stride length (unaffected side) [metres] Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 All studies | 4 | 99 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.01, 0.22] |
| 6.2 Adequate randomisation | 2 | 59 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.12] |
| 7 Stride length (unspecified) [metres] Show forest plot | 3 | 186 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.01, 0.33] |
| 8 Gait cadence Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 all studies | 7 | 223 | Mean Difference (IV, Random, 95% CI) | 10.77 [4.36, 17.18] |
| 8.2 Adequate randomisation | 6 | 203 | Mean Difference (IV, Random, 95% CI) | 10.80 [4.05, 17.56] |
| 9 Gait cadence ‐ interventionist Show forest plot | 7 | 223 | Mean Difference (IV, Random, 95% CI) | 10.77 [4.36, 17.18] |
| 9.1 Music therapist | 3 | 128 | Mean Difference (IV, Random, 95% CI) | 11.51 [‐2.57, 25.60] |
| 9.2 Non‐music therapist | 4 | 95 | Mean Difference (IV, Random, 95% CI) | 7.65 [4.43, 10.86] |
| 10 Gait cadence ‐ music type Show forest plot | 7 | 223 | Mean Difference (IV, Random, 95% CI) | 10.77 [4.36, 17.18] |
| 10.1 Music | 4 | 148 | Mean Difference (IV, Random, 95% CI) | 11.34 [‐1.05, 23.74] |
| 10.2 Auditory stimulus (no music) | 3 | 75 | Mean Difference (IV, Random, 95% CI) | 7.58 [4.33, 10.83] |
| 11 Stride symmetry Show forest plot | 3 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.94 [‐0.32, 2.20] |
| 12 General gait Show forest plot | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 7.67 [5.67, 9.67] |
| 13 Balance Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All studies | 3 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.48, 1.09] |
| 13.2 Adequate randomisation | 2 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐1.10, 1.37] |
| 14 Upper extremity functioning (general) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All studies | 5 | 194 | Mean Difference (IV, Random, 95% CI) | 3.56 [‐0.88, 8.00] |
| 14.2 Adequate randomisation | 3 | 156 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐2.33, 4.12] |
| 15 Upper extremity functioning ‐ time Show forest plot | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.69, ‐0.47] |
| 16 Range of motion ‐ shoulder flexion Show forest plot | 2 | 53 | Mean Difference (IV, Random, 95% CI) | 9.81 [‐12.71, 32.33] |
| 17 Hand function Show forest plot | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.91, 1.54] |
| 18 Upper limb strength Show forest plot | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 6.03 [‐2.52, 14.59] |
| 19 Manual dexterity Show forest plot | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐1.08, 2.01] |
| 20 Overall communication Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 All studies | 3 | 67 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.11, 1.39] |
| 20.2 Adequate randomisation | 2 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.03, 1.07] |
| 21 Naming Show forest plot | 2 | 35 | Mean Difference (IV, Random, 95% CI) | 9.79 [1.37, 18.21] |
| 22 Repetition Show forest plot | 2 | 35 | Mean Difference (IV, Random, 95% CI) | 8.90 [3.25, 14.55] |
| 23 Memory Show forest plot | 2 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.29, 0.95] |
| 24 Attention Show forest plot | 2 | 39 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.34, 0.94] |
| 25 Quality of life Show forest plot | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | 0.89 [0.32, 1.46] |

