Surgery for cataracts in people with age‐related macular degeneration
Abstract
Background
Cataract and age‐related macular degeneration (AMD) are common causes of decreased vision that often occur simultaneously in people over age 50. Although cataract surgery is an effective treatment for cataract‐induced visual loss, some clinicians suspect that such an intervention may increase the risk of worsening of underlying AMD and thus have deleterious effects on vision.
Objectives
The objective of this review was to evaluate the effectiveness and safety of cataract surgery compared with no surgery in eyes with AMD.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 11), Ovid MEDLINE, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily (January 1946 to December 2016), Embase (January 1980 to December 2016), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to December 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 December 2016.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐randomized trials that enrolled participants whose eyes were affected by both cataract and AMD in which cataract surgery was compared with no surgery.
Data collection and analysis
Two review authors independently evaluated the search results against the inclusion and exclusion criteria. Two review authors independently extracted data, assessed risk of bias for included studies, and graded the certainty of evidence. We followed methods as recommended by Cochrane.
Main results
We included two RCTs with a total of 114 participants (114 study eyes) with visually significant cataract and AMD. We identified no ongoing trials. Participants in each RCT were randomized to immediate cataract surgery (within two weeks of enrollment) or delayed cataract surgery (six months after enrollment). The risk of bias was unclear for most domains in each study; one study was registered prospectively.
In one study conducted in Australia outcomes were reported only at six months (before participants in the delayed‐surgery group had cataract surgery). At six months, the immediate‐surgery group showed mean improvement in best‐corrected visual acuity (BCVA) compared with the delayed‐surgery group (mean difference (MD) ‐0.15 LogMAR, 95% confidence interval (CI) ‐0.28 to ‐0.02; 56 participants; moderate‐certainty evidence). In the other study, conducted in Austria, outcomes were reported only at 12 months (12 months after participants in the immediate‐surgery group and six months after participants in the delayed‐surgery group had cataract surgery). There was uncertainty as to which treatment group had better improvement in distance visual acuity at 12 months (unit of measure not reported; very low‐certainty evidence).
At 12 months, the mean change from baseline between groups in cumulated drusen or geographic atrophy area size was small and there was uncertainty which, if either, of the groups was favored (MD 0.76, 95% CI ‐8.49 to 10.00; 49 participants; low‐certainty evidence). No participant in one study had exudative AMD develop in the study eye during 12 months of follow‐up; in the other study, choroidal neovascularization developed in the study eye of 1 of 27 participants in the immediate‐surgery group versus 0 of 29 participants in the delayed‐surgery group at six months (risk ratio 3.21, 95% CI 0.14 to 75.68; 56 participants; very low‐certainty evidence). Quality of life was measured using two different questionnaires. Scores on the Impact of Vision Impairment (IVI) questionnaire suggested that the immediate‐surgery group fared better regarding vision‐related quality of life than the delayed‐surgery group at six months (MD in IVI logit scores 1.60, 95% CI 0.61 to 2.59; low‐certainty evidence). However, we could not analyze scores from the Visual Function‐14 (VF‐14) questionnaire from the other study due to insufficient data. No postoperative complication was reported from either study.
Authors' conclusions
At this time, it is not possible to draw reliable conclusions from the available data as to whether cataract surgery is beneficial or harmful in people with AMD after 12 months. Although cataract surgery provides short‐term (six months) improvement in BCVA in eyes with AMD compared with no surgery, it is unclear whether the timing of surgery has an effect on long‐term outcomes. Physicians must make recommendations to their AMD patients regarding cataract surgery based on experience and clinical judgment until large controlled trials are conducted and their findings published.
There is a need for prospective RCTs in which cataract surgery is compared with no surgery in people with AMD to better evaluate whether cataract surgery is beneficial or harmful in all or a subset of AMD patients. However, ethical considerations preclude withholding surgery, or delaying it for several years, if it may be a potentially beneficial treatment. Designers of future trials are encouraged to utilize existing standardized systems for grading cataract and AMD and for measuring key outcomes: visual acuity, change in visual acuity, worsening of AMD, quality of life measures, and adverse events.
PICO
Plain language summary
Cataract surgery in people with age‐related macular degeneration
What is the aim of this review?
The aim of this Cochrane Review was to determine whether cataract surgery is safe and improves vision in eyes with age‐related macular degeneration (AMD) compared with no surgery. Cochrane researchers collected and analyzed all relevant studies to answer this question and included two studies.
Key messages
Although data from two small studies suggest that surgery to remove cataracts in eyes with AMD may improve vision without worsening of AMD, it is not possible to draw reliable conclusions from the available data at this time. Physicians must decide whether to recommend cataract surgery to their AMD patients based on clinical judgment until larger studies have been conducted and their findings published.
What was studied in this review?
Both cataract and AMD are common causes of poor vision; they often occur together in people over age 50. Cataract occurs when the clear lens in the front of the eye becomes cloudy. Removing the cloudy lens (cataract surgery) restores good vision for many eyes that do not have other eye conditions. AMD is disease in which the macula (the area in the back of the eye that is responsible for central vision) deteriorates. Some physicians believe that cataract surgery may put eyes that have AMD at higher risk of more vision loss than leaving the cloudy lens in the eye.
What are the main results of this review?
This review included two studies comparing immediate cataract surgery (within two weeks) with delayed cataract surgery (at six months) for people who had both cataract and AMD. In one study, the group that received immediate surgery had better vision at six months than the group scheduled for delayed surgery. In the other study, it was unclear which group had better vision improvements at 12 months. No participant in one study had the AMD get worse; AMD got worse in only one person in the immediate‐surgery group in the other study. Two studies measured quality of life: one study suggested that the immediate‐surgery group had a better quality of life than the delayed‐surgery group, and the second study did not report enough information to allow us to analyze the data. Neither study reported adverse events.
How up‐to‐date is the review?
Cochrane researchers searched for studies that had been published up to 2 December 2016.
Authors' conclusions
Summary of findings
| Immediate cataract surgery compared with delayed cataract surgery in eyes with age‐related macular degeneration | ||||||
| Population: people with cataract and age‐related macular degeneration Settings: ophthalmology clinics Intervention: immediate cataract surgery (within 2 weeks) Comparison: delayed cataract surgery (after 6 months) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed cataract surgery | Immediate cataract surgery | |||||
| Mean best‐corrected visual acuity (BCVA) (measured on the LogMAR scale; 0 = good vision, higher scores = worse vision) | At 6 months' follow‐up | — | 56 | ⊕⊕⊕⊝ | Neither trial reported visual acuity outcomes as dichotomous or categorical outcomes. At 12 months, 1 trial reported mean distance visual acuity, but the unit of measure was not reported. | |
| Mean BCVA in the delayed‐cataract surgery group was 0.28 LogMAR units. | Mean BCVA in the immediate‐cataract surgery group was 0.15 LogMAR units lower (better) (0.28 lower to 0.02 lower). | |||||
| At 12 months' follow‐up | ||||||
| See comment | ||||||
| Mean change in cumulated drusen or geographic atrophy area size (CDGAS) | At 6 months' follow‐up | 1 trial did not report any outcome related to drusen or geographic atrophy. | ||||
| Not reported | ||||||
| At 12 months' follow‐up | — | 49 | ⊕⊕⊝⊝ | |||
| The mean change in CDGAS in the delayed‐cataract surgery group was ‐1.125 CDGAS units. | The mean change in CDGAS in the immediate‐cataract surgery group was 0.76 CDGAS units higher (8.49 lower to 10.00 higher). | |||||
| Development of choroidal neovascularization | At 6 months' follow‐up | RR 3.21 (0.14 to 75.68) | 56 | ⊕⊝⊝⊝ | At 6 months, none of 29 participants in the delayed‐cataract surgery group compared with 1 of 27 participants in the immediate‐cataract surgery group developed choroidal neovascularization. The other trial reported that no participant in either group developed exudative AMD up to 12 months' follow‐up. | |
| Not estimated (see comment) | ||||||
| At 12 months' follow‐up | ||||||
| See comment | ||||||
| Quality of life (measured by the Impact of Vision Impairment questionnaire; higher mean scores represent better quality of life in the analyses, scale of 0 to 5) | At 6 months' follow‐up | — | 56 | ⊕⊕⊝⊝ | The other trial measured quality of life using the Visual Function‐14 questionnaire to assess patient satisfaction at baseline and 12 months' follow‐up; however, no between‐group analysis of results could be performed. | |
| Mean overall score in the delayed‐cataract surgery group was 1.8. | Mean overall score in the immediate‐cataract surgery group was 1.60 higher (0.61 to 2.59 higher). | |||||
| At 12 months' follow‐up | ||||||
| See comment | ||||||
| Complications | Not reported | |||||
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded (‐1) for unclear risks of bias such as selection bias (methods of randomization and allocation concealment not reported), performance and detection bias (effect of lack of masking on outcome unclear), and attrition bias (nine of 114 participants not included in analyses). | ||||||
Background
Description of the condition
Age‐related cataract
Cataract is an opacification of the crystalline lens that most often occurs with age (AAO 2016). According to the World Health Organization (WHO), cataract accounts for 48% of world blindness, affecting nearly 18 million people (WHO 2011). The WHO estimates that there will be 54 million people aged 60 years or older who will be blind from cataract by the year 2020, given projected increases in the elderly population in low‐, middle‐, and high‐income countries.
Age‐related cataract is a term used to describe any idiopathic lens opacification that occurs in people over 50 years of age. In its early stages, symptoms may be absent or minimal, but progression of lens opacification with time usually causes varying levels of gradual, progressive, painless loss of vision. People with cataract may have increasing difficulty with near or distance vision, or both. Glare may reduce vision in bright daylight and cause trouble with night driving. Other aspects of vision, such as contrast sensitivity, may be affected.
Cataract is diagnosed and assessed with a comprehensive eye exam. Reduction in best‐corrected visual acuity is the standard tool used to estimate visual impairment; slit‐lamp biomicroscopy allows classification and grading of cataract severity. A dilated fundus examination is performed to identify retinal disease that may complicate or exacerbate the cataract‐related impairment. The American Academy of Ophthalmology recognizes the primary indication for cataract surgery as "visual function that no longer meets the affected person's needs and for which cataract surgery provides a reasonable likelihood of improved vision" (AAO 2016). Cataract removal also is indicated when the lens opacity inhibits the proper management of posterior segment disease due to inability to view the fundus is sufficient detail (AAO 2016).
Age‐related macular degeneration
Age‐related macular degeneration (AMD) is the leading cause of irreversible legal blindness in people 65 years or older; the incidence is expected to increase further with the continued aging of the population. In Americans 40 years or older, the total prevalence of any AMD has been estimated as 9.2%, and the overall prevalence of neovascular AMD or geographic atrophy has been reported to be 1.47% (EDPRG 2004; Klein 1995).
Numerous grading systems have been proposed to classify AMD, but no universal consensus exists. The International Epidemiological Age‐related Maculopathy Study Group defined age‐related maculopathy (ARM) as the presence of drusen larger than 63 microns and retinal pigment epithelium abnormalities, whereas AMD was reserved for late stages of ARM defined by the presence of geographic atrophy (dry AMD) or choroidal neovascularization (CNV; wet AMD) (Bird 1995). Although neovascular disease constitutes only 15% of AMD, it is responsible for the majority of vision loss (Ferris 1984).
Age‐related macular degeneration may be asymptomatic in the early stages when only drusen are present (AAO 2016). Further worsening of the disease and increasing pigment alteration can be associated with a gradual visual acuity loss, diminished contrast sensitivity, and a need for increased background illumination. Central geographic atrophy causes irreversible loss of central vision. Choroidal neovascularization may cause scotoma, metamorphopsia, and varying degrees of loss of vision.
Although non‐neovascular AMD has no treatment, high‐dose vitamin supplementation was shown to reduce the incidence rate of advanced AMD (CNV or central geographic atrophy) in high‐risk participants in the Age‐Related Eye Disease Study (AREDS 2001). Antioxidant vitamin and mineral supplements were shown in a systematic review to slow the progression of AMD (Evans 2006). Large, well‐designed clinical trials have shown people with CNV to benefit when treated with laser photocoagulation (MPS Group 1982; MPS Group 1991), photodynamic therapy with verteporfin (TAP Study Group 1999; TAP Study Group 2001), or intravitreal pegaptanib (V.I.S.I.O.N. Clinical Trial Group 2006), ranibizumab (Brown 2006; Rosenfeld 2006), bevacizumab (CATT Research Group 2011), and aflibercept (Ohr 2012). Visual acuity may continue to decline despite appropriate treatment, however.
Description of the intervention
For age‐related cataract, surgery is currently the only treatment option once the lens has opacified enough to cause a significant decrease in vision (AAO 2016; Ang 2012; Riaz 2006). There are four main forms of cataract extraction surgery: intracapsular (ICCE), conventional extracapsular (ECCE), extracapsular phacoemulsification, and manual small incision (MSICS). Cochrane systematic reviews have examined various surgical interventions for eyes with age‐related cataract (Ang 2012; Riaz 2006).
How the intervention might work
Cataract surgery in middle‐ and high‐income countries most commonly involves small‐incision phacoemulsification removal of the lens and insertion of a capsule‐supported intraocular lens implant. Vision‐limiting operative complications are uncommon. Pooled results of cataract surgery prior to 1992 showed that 95% of participants without underlying ocular comorbidity obtained best‐corrected vision of 20/40 or better (Powe 1994). When all participants were included, the probability of obtaining 20/40 or better vision was still greater than 90%. Those with underlying ocular conditions such as AMD may experience limited visual improvement. Visual outcomes for various surgical intervention techniques have been systematically reviewed (Riaz 2006).
Why it is important to do this review
Our understanding of the interaction of cataract and macular degeneration is still evolving. There is uncertainty regarding the possible benefits or risks of cataract surgery in eyes with AMD. Investigators of some studies have suggested that cataract surgery may hasten the progression of AMD (Cugati 2006; Pollack 1996), but others have reported that cataract surgery may be beneficial in this group of patients (Armbrecht 2000; Kuo 2011; Shuttleworth 1998). There are many limitations to these studies. Specifically, in none of these studies was fluorescein angiography performed immediately after surgery to permit determination of whether pre‐existing subtle or obvious CNV or central geographic atrophy that already was present but not recognized prior to surgery. One prospective study in which fluorescein angiography was performed immediately prior to cataract surgery and at one week, three months, and one year postoperatively found no evidence to suggest that surgery increased the risk of AMD worsening. Most cases of neovascular AMD not identified pre‐operatively were believed to have been obscured by the cataract; however, this was a cohort study that did not compare surgery to no surgery (Dong 2009).
There are several scenarios in which cataract surgery might worsen the progression of AMD. Cataract and AMD share common risk factors, such as smoking and inadequate nutrition, that could cause them to progress simultaneously (Hiller 1997; Jacques 2005; Seddon 2006). In addition, inflammatory factors have been implicated in the causation of AMD (Donoso 2006); it is feasible that inflammation occurring after cataract surgery could cause worsening of existing macular degeneration. Moreover, the replacement of the natural lens with an artificial lens may increase exposure to light and damaging ultraviolet rays. Clinicians who believe that cataract surgery increases the risk of AMD worsening may discourage cataract surgery for patients with AMD despite visual loss and lens opacity. On the other hand, CNV or central geographic atrophy may be unrecognized prior to cataract surgery and account for some of the vision loss, thus prompting an ophthalmologist to proceed with cataract surgery and then to conclude that the surgery had an effect on progression to advanced AMD, when in reality cataract surgery merely revealed the advanced stage of AMD. In this review, we analyzed the available evidence from randomized clinical trials regarding the effectiveness and safety of cataract surgery in eyes known to have AMD.
Objectives
The objective of this review was to evaluate the effectiveness and safety of cataract surgery compared with no surgery in eyes with AMD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). In anticipation of not finding many RCTs relevant to the review objective, we also included quasi‐randomized trials (controlled clinical trials [CCTs]) in which the method of allocating participants to a treatment arm was not random but used information such as date of birth or day of the week to make assignments.
Types of participants
We included trials of participants whose eyes with AMD also had cataract that required cataract surgery. We excluded trials in which eyes required cataract surgery for angle‐closure glaucoma, lens subluxation, or clear lens extraction for refractive error.
Types of interventions
We included trials where cataract surgery was compared with no or delayed surgery. We imposed no restrictions based on type of cataract surgery.
Types of outcome measures
Primary outcomes
The primary outcome for this review was visual acuity in the operated eye at one‐year follow‐up, measured as follows.
-
Best‐corrected visual acuity dichotomized as:
-
0.3 LogMAR (20/40 Snellen equivalent) or better;
-
worse than 0.3 LogMAR.
-
-
Change in visual acuity categorized by:
-
three or more lines improvement on a LogMAR chart (improvement by 0.3 LogMAR units) from baseline;
-
within three lines of baseline visual acuity;
-
loss of three or more lines of visual acuity from baseline.
-
When continuous LogMAR data were available, we analyzed the visual acuity and degree of change as continuous data. We analyzed visual acuity at other follow‐up times (six months, two and three years) when possible.
Secondary outcomes
The secondary outcomes for this review included the following.
-
Progression of AMD in the operated eye as measured by:
-
incident geographic atrophy during follow‐up;
-
incident CNV during follow‐up;
-
increase in the number of medium‐ or large‐sized drusen (> 63 microns in size);
-
increase of the total area occupied by drusen;
-
progression of non‐central geographic atrophy to central geographic atrophy.
-
-
Vision‐related quality of life as measured by the methods applied in each trial.
We analyzed secondary outcomes at six months' and one, two, and three years' follow‐up when possible.
Adverse events
We compared vision‐threatening complications from cataract surgery, including but not limited to cystoid macular edema and retinal detachment, between groups.
We analyzed adverse events at six months' and one, two, and three years' follow‐up when possible.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 11), Ovid MEDLINE, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily (January 1946 to December 2016), Embase (January 1980 to December 2016), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to December 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 December 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), LILACS (Appendix 4), the ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6), and the WHO ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of included studies and related observational studies and reviews for possibly eligible trials.
Data collection and analysis
Selection of studies
Two review authors independently selected the studies for inclusion. Two review authors examined the titles and abstracts of all records identified by the electronic and manual searches and classified each as (a) definitely relevant, (b) possibly relevant, or (c) definitely not relevant. We obtained the full‐text reports of those records classified as (a) definitely relevant and (b) possibly relevant. Two review authors assessed each full‐text report and classified the study reported as (1) include, (2) awaiting assessment, or (3) exclude. For studies awaiting assessment, we contacted primary investigators for further information or clarification and reassessed the study when additional information became available. We documented studies that both review authors excluded and reported the reasons for exclusion in the review. The review authors were unmasked to the report authors, institutions, and trial results during this assessment. A third review author resolved any disagreements between the two review authors.
Data extraction and management
Two review authors independently extracted study characteristics and data for primary and secondary outcomes from the included study onto paper data extraction forms developed by Cochrane Eyes and Vision. Any discrepancies were resolved by discussion. One review author entered data into Review Manager 5 (RevMan 2014), and a second review author verified the data entry.
Assessment of risk of bias in included studies
Two review authors independently assessed the included trial for bias according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following parameters for risk of bias:
-
generation of random allocation sequence and allocation concealment before randomization (selection bias);
-
masking of study personnel (performance bias);
-
masking of outcome assessors (detection bias);
-
completeness of follow‐up and intention‐to‐treat analysis (attrition bias); and
-
selective outcome reporting (reporting bias).
As masking of participants is uncommon and often impossible in surgical trials, we did not assess participant masking as a measure of risk of bias in individual trials.
We classified each included study for each type of bias as low risk, unclear risk, or high risk of bias. For example, any method of allocation concealment, such as sequentially numbered, opaque envelopes or central allocation, conferred low risk of bias. When no approach to allocation concealment was reported, we considered the risk of bias to be unclear. We considered trial investigators to have conducted an intention‐to‐treat analysis only when all participants who were randomized, including those who were randomized but not treated, excluded after randomization for other reasons, or lost to follow‐up, were reported and accounted for in the data analysis. When the information available in the published trial reports was inadequate to assess the risk of bias, we contacted the trial authors for clarification. Whenever they did not respond within four weeks, we classified the trial based on the available information.
Measures of treatment effect
We analyzed data according to the guidelines set forth in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). For dichotomous outcomes, we calculated a risk ratio with 95% confidence intervals. We calculated a mean difference with standard deviations for continuous outcomes.
Unit of analysis issues
The unit of analysis was the individual participant (one study eye per person).
Dealing with missing data
We contacted the primary investigators of included studies for additional information when statistics such as standard deviations or outcome data were not clearly reported or when results were not reported for all randomized participants. When after four weeks of contacting the primary investigators we had not received the additional statistical information or outcome data, we used the data as reported. When we were unable to obtain results for all randomized participants, we used the results reported by the authors as well as reporting the loss to follow‐up for each group when available. We did not impute data for the purposes of this review.
Assessment of heterogeneity
We assessed for heterogeneity by comparing study methods, participant characteristics, interventions, and outcomes among included trials. Due to the heterogeneity among the study outcomes, we did not perform meta‐analysis. If meta‐analysis is appropriate in future updates of this review, we will assess statistical heterogeneity among effect estimates from individual trials using the Chi2 test and the I2 statistic. We will consider a P value from the Chi2 test less than 0.1 or I2 values of more than 50%, or both, to suggest substantial statistical heterogeneity.
Assessment of reporting biases
We did not assess publication bias, as fewer than 10 trials were included in the review. If in the future additional studies are included in this review, we will use asymmetry of the funnel plot as a method to identify potential publication bias. We assessed selective outcome reporting at the trial level as part of the 'Risk of bias' assessment.
Data synthesis
We did not conduct meta‐analysis due to heterogeneity among included trials. If in the future data synthesis is possible, we will combine study results when appropriate. If we detect no substantial statistical heterogeneity, and if there is no clinical heterogeneity between the trials, we will combine the results in a meta‐analysis using a random‐effects model. We will use a fixed‐effect model when the number of trials is three or fewer. In case of substantial statistical or clinical heterogeneity, we will not combine study results, but rather present a narrative or tabular summary of findings from individual trials. We will calculate a standardized mean difference whenever different scales are used to measure related continuous outcomes.
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analysis due to insufficient data. If in the future additional studies are included in this review, subgroup analyses may be performed. Subgroups of interest include types of cataract surgery and presence of CNV or central geographic atrophy in the unoperated fellow eye.
Sensitivity analysis
We did not conduct sensitivity analysis as no meta‐analysis was performed. If in the future additional studies are included in this review, we will conduct sensitivity analyses to determine the impact of exclusion of studies with high risk of bias, exclusion of unpublished studies, and exclusion of industry‐funded studies.
Summary of findings
We present a 'Summary of findings' table of the main outcomes assessed in this review. We evaluated the certainty of evidence for each outcome using the GRADE approach (GRADEpro 2014). Two review authors independently assessed each outcome as very low, low, moderate, or high according to five criteria: risk of bias in individual trials, indirectness, heterogeneity, imprecision of estimate (wide confidence intervals), and publication bias. Any discrepancies were resolved by discussion.
Results
Description of studies
Results of the search
The original electronic searches conducted in November 2008 yielded 1184 distinct titles and abstracts, of which 10 appeared to be potentially relevant but were excluded after further analysis. Updated searches as of April 2012 yielded 482 additional titles and abstracts. For the 2012 update, we modified the eligibility criteria for this review to include studies with less than one year of follow‐up for secondary outcomes. We assessed all 1666 distinct titles and abstracts from the original and updated searches using the modified eligibility criteria and classified 19 as potentially relevant. Of these 19 records, we excluded 15 records (reporting 14 studies), included two reports from one trial (Lamoureux 2007), and identified one ongoing study from two reports.
We updated the electronic searches on 2 December 2016 (Figure 1). Of 788 unique records newly identified, we excluded 787 records by screening the titles and abstracts and included one study report (Brunner 2013). This study report included the results from the ongoing study previously identified. We did not identify any eligible trials by searching the reference lists of possibly relevant articles. We did not conduct a comprehensive search for observational studies. Non‐randomized studies and observational studies known to the authors of this review were cited in the Discussion, although they were not the purpose of the systematic search.

Study flow diagram.
Included studies
The two trials (114 eyes of 114 participants) included in this review enrolled participants with cataract and AMD; however, Brunner 2013 included only participants with non‐exudative AMD, and Lamoureux 2007 included only participants with Age‐Related Eye Disease Study (AREDS) category 3 AMD in the study eye. In both trials, participants were randomized to immediate cataract surgery (within two weeks of enrollment) (n = 57) or delayed cataract surgery (six months after enrollment) (n = 57). One trial (54 participants) was conducted in Vienna, Austria and the other study (60 participants) was conducted in Melbourne, Australia. The trials included adult participants (18 years of age and older). There were no statistically significant baseline imbalances reported between groups with regard to age, gender, race, risk factors for AMD, or stage of AMD. Lamoureux 2007 assessed outcomes at six months (six months after surgery for participants in the immediate‐cataract surgery group and just before surgery for participants in the delayed‐cataract surgery group). At six months after enrollment, nine participants had been lost to follow‐up, three in the immediate‐surgery group and six in the delayed‐surgery group. Brunner 2013 assessed outcomes at 12 months (12 months after surgery for participants in the immediate‐cataract surgery group and six months after surgery for participants in the delayed‐cataract surgery group). At 12 months, five participants were lost to follow‐up, one in the immediate‐surgery group and four in the delayed‐surgery group.
Excluded studies
We excluded 14 studies from the review after assessment of the full‐text report. The excluded studies and reasons for exclusion are reported in the Characteristics of excluded studies table. Briefly, 13 studies were neither randomized nor quasi‐randomized trials, and one study included cataract surgery in eyes with or without AMD.
Risk of bias in included studies
Details of the 'Risk of bias' assessment are provided in the Characteristics of included studies table; a summary is presented in Figure 2.
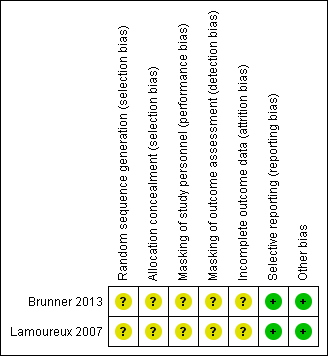
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Neither trial reported methods of randomization (sequence generation) and allocation concealment; we therefore judged the risk of selection bias as unclear for both trials.
Masking (performance bias and detection bias)
Participants randomized to either immediate surgery or delayed surgery could not be masked. Investigators of neither trial reported whether study personnel such as operating room personnel had been masked to treatment groups. Both trials reported that outcome assessors were masked; however, those refracting participants and evaluating visual acuity at follow‐up visits may or may not have known whether surgery had been performed. Participants were not masked when completing quality of life questionnaires. For these reasons, we assessed the risks of both performance bias and detection bias as unclear.
Incomplete outcome data
The trial investigators did not include all randomized participants in the analyses; we judged the risk of attrition bias as unclear for both trials. Lamoureux 2007 reported that of the four participants not included in the analysis, "one participant died, two emigrated and one refused continued participation." Brunner 2013 reported that of the five participants not included in the analysis, three developed asymptomatic occult choroidal neovascularization (CNV); no reason was provided for two participants lost to follow‐up. The remaining participants in both trials were analyzed in the group to which they were assigned at baseline.
Selective reporting
We assessed the risk of reporting bias as low for both trials. Outcomes specified in the trial registry record, which was made available prior to the publication of trial results, matched published outcomes for Brunner 2013. No protocol was available for Lamoureux 2007; however, outcomes for all assessed measures were reported.
Other potential sources of bias
We noted no other potential sources of bias for either trial.
Effects of interventions
Visual acuity
Neither trial reported visual acuity outcomes as dichotomous or categorical outcomes; however, both trials reported mean visual acuity. Lamoureux 2007 reported mean LogMAR best‐corrected distance visual acuity (BCVA) at six months' follow‐up. Brunner 2013 reported mean distance visual acuity at 12 months' follow‐up, but it was not clear how visual acuity was measured (e.g. LogMAR or decimal value, BCVA or uncorrected visual acuity). Due to the different measures of visual acuity and follow‐up times between trials, we did not combine these data in a meta‐analysis.
At six months, the immediate‐surgery group had better mean BCVA when compared with the delayed‐surgery group (mean difference (MD) ‐0.15 LogMAR, 95% confidence interval (CI) ‐0.28 to ‐0.02) in Lamoureux 2007 (Analysis 1.1). We graded the certainty of evidence as moderate for mean BCVA at six months, downgrading for unclear risks of bias in the trial.
At 12 months, distance visual acuity in the immediate‐surgery group and the delayed‐surgery group was reported as similar in Brunner 2013: distance visual acuity (mean Early Treatment Diabetic Retinopathy Study (ETDRS) values +/‐ standard deviation) was 0.70 +/‐ 0.35 in the immediate‐surgery group (n = 27) and 0.64 +/‐ 0.35 in the delayed‐surgery group (n = 22). We graded the certainty of evidence as very low for this outcome, downgrading for unclear risks of bias in the trial and indirectness (measure of visual acuity not clear).
Progression of age‐related macular degeneration
Geographic atrophy and drusen
Brunner 2013 estimated the cumulated size of the area occupied by drusen or geographic atrophy on fundus photographs (cumulated drusen or geographic area size [CDGAS]) using a computer‐assisted quantification algorithm. At 12 months, 33.3% of participants in the immediate‐surgery group compared with 26.1% of participants in the delayed‐surgery group showed an absolute increase in pixel values (proportions of participants not reported). The mean difference in CDGAS values when comparing the immediate‐surgery group with the delayed‐surgery group was 0.76 (95% CI ‐8.49 to 10.00; Analysis 1.2). No units for the CDGAS values were reported; however the trial authors interpreted this finding as equivalency between groups. We graded the certainty of evidence for this outcome as low, downgrading for unclear risks of bias in the trial and imprecision.
Lamoureux 2007 did not report outcomes related to geographic atrophy or drusen.
Choroidal neovascularization
At six months, choroidal neovascularization (CNV) had developed in the study eye of 1 out of 27 participants (3.7%) in the immediate‐surgery group compared with 0 out of 29 participants in the delayed‐surgery group (risk ratio (RR) 3.21, 95% CI 0.14 to 75.68; Analysis 1.3) in the Lamoureux 2007 trial. We graded the certainty of evidence for this outcome as very low, downgrading for unclear risks of bias in the trial and imprecision.
Brunner 2013 reported that exudative AMD had developed in no participant in either group during 12 months of follow‐up.
Quality of life
Both trials measured vision‐related quality of life outcomes; however, the trials used different questionnaires.
Brunner 2013 used the Visual Function‐14 (VF‐14) questionnaire to assess patient satisfaction at baseline and at the 12‐month follow‐up visit. We were unable to estimate between‐group effects, as only mean scores at baseline and 12 months were reported, and the baseline scores differed between groups (mean baseline score of 74.6% in the immediate‐surgery group and 65.4% in the delayed‐surgery group). At 12 months, the mean score was 78.4% in the immediate‐surgery group and 79.9% in the delayed‐surgery group.
Lamoureux 2007 used the Impact of Vision Impairment (IVI) questionnaire, which is an interviewer‐administered questionnaire where respondents rate the effect of vision on various activities as "not at all" (0), "rarely" (1), "a little" (2), "a fair amount" (3), "a lot" (4), or "can't do because of my eyesight" (5). Although higher scores represented poorer quality of life on the questionnaire, data were analyzed using logit units, which resulted in higher mean scores representing better quality life in the analyses. At six months, the immediate‐surgery group had higher overall mean IVI scores compared with the delayed‐surgery group (MD 1.60, 95% CI 0.61 to 2.59). We graded the certainty of evidence for this outcome as low, downgrading for unclear risks of bias in the trial and imprecision.
Complications
Neither Brunner 2013 nor Lamoureux 2007 reported vision‐threatening complications from cataract surgery.
Discussion
Summary of main results
The primary outcome for this review was best‐corrected visual acuity (BCVA) in the operated eye at one‐year follow‐up. One study reported this outcome; however, there was uncertainty as to how visual acuity was measured and whether the immediate‐surgery group or the delayed‐surgery group had a better visual acuity at this time point (Brunner 2013). A summary of the main findings of this review are presented in summary of findings Table for the main comparison. In Lamoureux 2007, the control group (delayed cataract surgery) participants were scheduled to receive surgery at six months after randomization, therefore outcomes at six months were assessed before participants in the delayed surgery had cataract surgery. At six months, better mean BCVA was observed in the immediate‐surgery group compared with the delayed‐surgery control group (MD ‐0.15 LogMAR, 95% CI ‐0.28 to ‐0.02). Progression of AMD was a secondary outcome. One study reported that exudative AMD had not developed in any participant in either study group (Brunner 2013); in the other study, choroidal neovascularization developed in the study eye of one participant in the immediate surgery group versus none in the delayed surgery group (Lamoureux 2007). Brunner 2013 also reported on the size of the cumulated area occupied by drusen and geographic atrophy, and concluded that there was no difference the sizes between the two groups of study eyes (MD 0.76, 95% CI ‐8.49 to 10.00). Both studies measured vision‐related quality of life, but used different instruments and reported data on different scales. Brunner 2013 reported the mean scores on the Visual Function‐14 (VF‐14) scale that were similar in the immediate surgery and delayed surgery groups at 12 months; however, imbalance between groups with respect to baseline scores precluded valid comparison of 12‐month scores. Lamoureux 2007 used the IVI questionnaire; the results at 6 months' follow‐up suggested that the immediate‐surgery group fared better regarding quality of life outcomes than the delayed‐surgery group (MD 1.60 logits, 95% CI 0.61 to 2.59). Neither of the studies reported adverse outcomes.
Overall completeness and applicability of evidence
The relationship between cataract surgery and AMD has been the subject of much debate in recent years. Both conditions are common in the elderly and have overlapping symptoms and risk factors; deciding when or whether to perform cataract surgery in people with AMD can be difficult at best. Some clinicians believe that cataract surgery is beneficial in AMD patients by giving them the best chance of recovering some of the lost vision, even if only for a few months. Other clinicians fear that surgery could exacerbate AMD and lead to worse vision than if the cataract had been left in place. Conflicting reports from retrospective studies have created confusion regarding this issue (Kaiserman 2007; Sutter 2007).
In this review we have analyzed the available evidence from prospective randomized controlled trials (RCTs); although we also sought quasi‐randomized clinical trials regarding the effectiveness and safety of cataract surgery in eyes with AMD, none was found. Although we defined outcomes at one year as those of primary interest, only one RCT with one‐year follow‐up was identified from a systematic literature search (Brunner 2013). The next best available evidence from the published literature was one RCT with six‐month follow‐up (Lamoureux 2007). As these studies had different follow‐up time points and used different methods, we could not combine the data in meta‐analyses.
Quality of the evidence
Both studies appeared to be well designed; however, details of methods required to assess potential risks of bias either were not reported or reported unclearly in the study reports. Selection bias (allocation of group assignments and concealment before assignment) was of particular concern. Due to the nature of the surgical interventions, masking of study participants and personnel was not feasible. We could not ascertain whether outcome assessors who refracted patients and measured visual acuity at follow‐up time points knew whether surgery had been performed. Further four participants and five participants respectively were lost to follow‐up and not included in analyses in Lamoureux 2007 and Brunner 2013.
Potential biases in the review process
We developed and implemented a systematic search and screening strategy to identify eligible RCTs and CCTs for this review. We did not conduct a comprehensive search for observational studies. Non‐randomized studies and observational studies known to the authors of this review are cited in this discussion, although they were not the purpose of the systematic search.
We contacted primary investigators of included studies for clarification or for additional information not provided in their published reports. Although we received responses from investigators, not all requests for information were satisfied. We will update the review with information from these studies as it becomes available.
Agreements and disagreements with other studies or reviews
Evidence from non‐randomized clinical trials
The best available evidence for long‐term outcomes appears to be from non‐randomized clinical trials and prospective observational studies (Bockelbrink 2008). Armbrecht and colleagues performed a prospective study in which participants were grouped based on the presence or absence of AMD and cataract surgery. The three groups comprised (1) people with AMD who did not have surgery, (2) people with AMD who underwent cataract surgery, and (3) a control group of people who underwent cataract surgery. Initial results based on five‐month data suggested that cataract surgery was most beneficial for people with moderate cataract irrespective of the degree of AMD (Armbrecht 2000). Further analysis of people with AMD found that visual acuity and quality of life benefits were maintained at one year (Armbrecht 2003). This finding contrasted with previous reports published by Pollack and colleagues, who detected an increased rate of CNV after unilateral cataract surgery in a non‐randomized trial (Pollack 1996).
Evidence from prospective cohort and case‐control studies
Several well‐designed epidemiologic studies have addressed the relationship amongst cataract, cataract surgery, and AMD. The Copenhagen City Eye Study, the Beaver Dam Eye Study conducted in the USA, and the Blue Mountains Eye Study conducted in Australia are large cohort studies that have addressed this relationship.
In the Copenhagen City Eye Study, the presence of cataract increased the subsequent incidence of early AMD, whereas cataract surgery increased the incidence of late AMD, defined as geographic atrophy or CNV in the study (Buch 2005). Although these findings confirm that the two conditions share common risk factors, it is not possible to state whether surgery itself caused an increased incidence of late AMD. People with neovascular AMD that was not apparent to the cataract surgeon prior to surgery may have undergone cataract surgery more often than those with known AMD.
During ten‐year follow‐up of the Beaver Dam Eye Study cohort, baseline cataract was found to be associated with early age‐related maculopathy (ARM) and progression of ARM, but not with late ARM (Klein 2002). Cataract surgery, in contrast, was associated with progression of ARM and late ARM, but not with early ARM. Eyes of participants in the similarly designed Blue Mountains Eye Study had a higher 10‐year risk of developing late ARM (geographic atrophy or neovascular AMD) in the presence of previous cataract surgery (Cugati 2006). In addition, analysis of combined five‐year data from the Beaver Dam Eye Study and the Blue Mountains Eye Study estimated an approximate 10‐fold increase in risk of late‐stage ARM (geographic atrophy or neovascular AMD) in people with a baseline history of cataract surgery (Wang 2003). However, it is not possible to infer a causal relationship between cataract surgery and progression of ARM or the presence of late ARM from a cohort study.
A case‐control study using the Age‐Related Eye Diseases Study (AREDS) database found an increased risk of lens opacities or cataract surgery in participants with large drusen and participants with neovascular AMD (AREDS 2000). There was no association between lens opacities or previous cataract surgery and geographic atrophy in AREDS. A more recent AREDS report addressed the risk of developing advanced AMD after cataract surgery based on review of annual fundus photographs, but no clear effect of cataract surgery on progression of AMD was found (Chew 2009). An earlier publication based on data from a Chesapeake Bay waterman cohort reported a higher incidence of AMD in the presence of nuclear (but not cortical) opacity (West 1989).

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
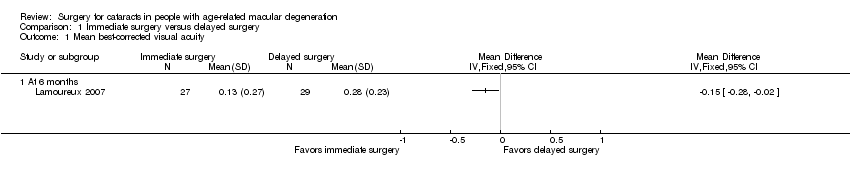
Comparison 1 Immediate surgery versus delayed surgery, Outcome 1 Mean best‐corrected visual acuity.

Comparison 1 Immediate surgery versus delayed surgery, Outcome 2 Mean change in cumulative drusen and geographic atrophy size at 12 months.
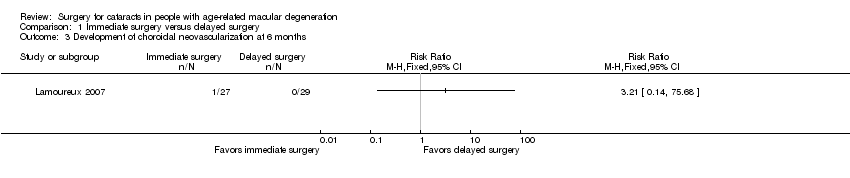
Comparison 1 Immediate surgery versus delayed surgery, Outcome 3 Development of choroidal neovascularization at 6 months.

Comparison 1 Immediate surgery versus delayed surgery, Outcome 4 Quality of life scores.
| Immediate cataract surgery compared with delayed cataract surgery in eyes with age‐related macular degeneration | ||||||
| Population: people with cataract and age‐related macular degeneration Settings: ophthalmology clinics Intervention: immediate cataract surgery (within 2 weeks) Comparison: delayed cataract surgery (after 6 months) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed cataract surgery | Immediate cataract surgery | |||||
| Mean best‐corrected visual acuity (BCVA) (measured on the LogMAR scale; 0 = good vision, higher scores = worse vision) | At 6 months' follow‐up | — | 56 | ⊕⊕⊕⊝ | Neither trial reported visual acuity outcomes as dichotomous or categorical outcomes. At 12 months, 1 trial reported mean distance visual acuity, but the unit of measure was not reported. | |
| Mean BCVA in the delayed‐cataract surgery group was 0.28 LogMAR units. | Mean BCVA in the immediate‐cataract surgery group was 0.15 LogMAR units lower (better) (0.28 lower to 0.02 lower). | |||||
| At 12 months' follow‐up | ||||||
| See comment | ||||||
| Mean change in cumulated drusen or geographic atrophy area size (CDGAS) | At 6 months' follow‐up | 1 trial did not report any outcome related to drusen or geographic atrophy. | ||||
| Not reported | ||||||
| At 12 months' follow‐up | — | 49 | ⊕⊕⊝⊝ | |||
| The mean change in CDGAS in the delayed‐cataract surgery group was ‐1.125 CDGAS units. | The mean change in CDGAS in the immediate‐cataract surgery group was 0.76 CDGAS units higher (8.49 lower to 10.00 higher). | |||||
| Development of choroidal neovascularization | At 6 months' follow‐up | RR 3.21 (0.14 to 75.68) | 56 | ⊕⊝⊝⊝ | At 6 months, none of 29 participants in the delayed‐cataract surgery group compared with 1 of 27 participants in the immediate‐cataract surgery group developed choroidal neovascularization. The other trial reported that no participant in either group developed exudative AMD up to 12 months' follow‐up. | |
| Not estimated (see comment) | ||||||
| At 12 months' follow‐up | ||||||
| See comment | ||||||
| Quality of life (measured by the Impact of Vision Impairment questionnaire; higher mean scores represent better quality of life in the analyses, scale of 0 to 5) | At 6 months' follow‐up | — | 56 | ⊕⊕⊝⊝ | The other trial measured quality of life using the Visual Function‐14 questionnaire to assess patient satisfaction at baseline and 12 months' follow‐up; however, no between‐group analysis of results could be performed. | |
| Mean overall score in the delayed‐cataract surgery group was 1.8. | Mean overall score in the immediate‐cataract surgery group was 1.60 higher (0.61 to 2.59 higher). | |||||
| At 12 months' follow‐up | ||||||
| See comment | ||||||
| Complications | Not reported | |||||
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded (‐1) for unclear risks of bias such as selection bias (methods of randomization and allocation concealment not reported), performance and detection bias (effect of lack of masking on outcome unclear), and attrition bias (nine of 114 participants not included in analyses). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean best‐corrected visual acuity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean change in cumulative drusen and geographic atrophy size at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Development of choroidal neovascularization at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Quality of life scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Overall scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Emotional well‐being scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Mobility and independence scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Reading and accessing information scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

