Intervenciones para el tratamiento de la compresión medular extradural metastásica en adultos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | "Pilot randomized comparison" | |
| Participants | Australia, September 2001 to November 2003, multi‐institutional | |
| Interventions | High dose dexamethasone 96 intravenous on days 0 to 2; n = 9 Radiotherapy 30 Gray in 10 fractions in both arms | |
| Outcomes | Outcomes of interest reported and used: | |
| Notes | No provision for rehabilitation was reported Outcomes of interest not reported: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized controlled trial. | |
| Participants | Italy, February 1998 to November 2002, multicenter trial | |
| Interventions | Two fractions: "Short course regimen"( 8 Gray, 6‐days rest, and then 8 Gray, to a total of 16 Gray in 1 week ), n = 153 | |
| Outcomes | Outcomes of interest reported and used : | |
| Notes | The author's analysis excluded 8% of participants (seven lost to follow up and seventeen deaths that occurred within the first ten days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized trial | |
| Participants | United States of America, September 1992 to December 2002, multi‐institutional | |
| Interventions | Surgery with radiotherapy: n = 50 | |
| Outcomes | Outcomes of interest reported and used : Outcomes reported but not used: | |
| Notes | Eighteen participants with unstable spine were randomized to radiotherapy alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized trial | |
| Participants | Denmark, May 1987 to April 1989 | |
| Interventions | Dexamethasone 96 mg intravenous stat and per oral for 3 days and taper over 15 days ‐ n = 27 | |
| Outcomes | Outcomes of interest reported and used: | |
| Notes | No provision for rehabilitation was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized trial | |
| Participants | Netherlands, multi‐institutional | |
| Interventions | Dexamethasone 100 mg (n = 22) versus 10 mg (n = 15) intravenous followed by 16 mg orally | |
| Outcomes | Outcomes of interest reported and used: | |
| Notes | No provision for rehabilitation was reported Stratification for carcinoma versus lymphoreticular malignancy. Outcomes of interest not reported: survival, quality of life, participant and caregiver satisfaction and characteristics of participants who benefit from treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized prospective comparison | |
| Participants | United States of America. | |
| Interventions | Laminectomy with radiotherapy: 30 Gray in ten fractions over 14 days, n = 16 | |
| Outcomes | Outcomes of interest reported and used: Outcomes reported but not used: | |
| Notes | Radiotherapy alone: Mortality ‐ 24% (due to underlying disease) No provision for rehabilitation was reported Outcomes of interest not reported: quality of life, participant and caregiver satisfaction and characteristics of patients who benefit the treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
MRI‐ magnetic resonance imaging
CT‐ computed tomography
ECOG‐ Eastern Co‐operative Oncology Group
SD ‐ Standard Deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Abstract described it as randomized, but on reviewing the full paper we found it to be a retrospective rather than a prospective randomized comparison |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised phase III trial of two Ffactionation schemes in the treatment of malignant spinal cord compression |
| Methods | |
| Participants | Inclusion:
Exclusion:
|
| Interventions | Radiotherapy (single or multiple fractions): |
| Outcomes | Primary outcome measure(s): Secondary outcome measure(s):
Median survival ‐ calculated on the basis of time from date of randomisation to death. |
| Starting date | February 2007 |
| Contact information | Dr Joe O'Sullivan Senior Lecturer and Consultant in Clinical Oncology |
| Notes |
| Trial name or title | A randomised feasibility study of single fraction radiotherapy compared to multi‐fraction radiotherapy in patients with metastatic spinal cord compression |
| Methods | |
| Participants | Inclusion: 1. Proven diagnosis of spinal cord compression on Magnetic Resonance Imaging (MRI) Exclusion : 1. Patients for whom surgery or chemotherapy treatment is more appropriate |
| Interventions | Radiotherapy (single or multiple fractions): |
| Outcomes | Primary outcome measure(s) |
| Starting date | November 2007 |
| Contact information | Prof Peter J Hoskin |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ambulation (short term) Show forest plot | 1 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| Analysis 1.1  Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 1 Ambulation (short term). | ||||
| 1.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 1.2 Pretreatment non‐ambulant subgroup ‐ regaining ambulation | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.88] |
| 2 Reduction in analgesic use Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.96, 1.67] |
| Analysis 1.2  Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 2 Reduction in analgesic use. | ||||
| 3 Urinary continence (short term) Show forest plot | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.02] |
| Analysis 1.3  Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 3 Urinary continence (short term). | ||||
| 3.1 Proportion maintaining urinary continence | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.00] |
| 3.2 Proportion regaining urinary continence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.20, 7.58] |
| 4 Gastrointestinal adverse effects Show forest plot | 1 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.43, 7.25] |
| Analysis 1.4  Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 4 Gastrointestinal adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ambulation (short term) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.59, 2.43] |
| Analysis 2.1  Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 1 Ambulation (short term). | ||||
| 1.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.84, 4.00] |
| 1.2 Pretreatment non‐ambulant subgroup ‐ regaining ambulation | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.15, 2.59] |
| 2 Ambulation (intermediate term) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.70, 2.24] |
| Analysis 2.2  Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 2 Ambulation (intermediate term). | ||||
| 3 Survival Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 3 Survival. | ||||
| 3.1 Short term survival | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.06] |
| 3.2 Intermediate term survival | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.70] |
| 4 Reduction in analgesic use Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.42, 1.81] |
| Analysis 2.4  Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 4 Reduction in analgesic use. | ||||
| 5 Urinary continence (short term) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.50, 1.77] |
| Analysis 2.5  Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 5 Urinary continence (short term). | ||||
| 5.1 Proportion maintaining urinary continence | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.42, 1.52] |
| 5.2 Proportion regaining urinary continence | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.23, 30.40] |
| 6 Urinary continence (intermediate term) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.87, 2.35] |
| Analysis 2.6  Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 6 Urinary continence (intermediate term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ambulation (short term) Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.86] |
| Analysis 3.1  Comparison 3 Decompressive surgery plus radiotherapy versus radiotherapy alone, Outcome 1 Ambulation (short term). | ||||
| 1.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.98] |
| 1.2 Pretreatment non‐ambulant subgroup regaining ambulation | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.10, 0.89] |
| 2 Survival (short term) Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.05] |
| Analysis 3.2  Comparison 3 Decompressive surgery plus radiotherapy versus radiotherapy alone, Outcome 2 Survival (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall ambulation (short term) Show forest plot | 3 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.23] |
| Analysis 4.1  Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 1 Overall ambulation (short term). | ||||
| 1.1 High dose versus no corticosteroids | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.08] |
| 1.2 High versus moderate corticosteroids | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.68, 2.12] |
| 2 Participants maintaining or regaining ambulation (short term) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 2 Participants maintaining or regaining ambulation (short term). | ||||
| 2.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.75, 1.08] |
| 2.2 Pretreatment non‐ambulant subgroup regaining ambulation | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.47] |
| 3 Survival (long term) Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.20, 4.09] |
| Analysis 4.3  Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 3 Survival (long term). | ||||
| 4 Pain reduction Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.61] |
| Analysis 4.4  Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 4 Pain reduction. | ||||
| 5 Urinary continence (short term) Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.52] |
| Analysis 4.5  Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 5 Urinary continence (short term). | ||||
| 6 Serious drug related adverse effects Show forest plot | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.97] |
| Analysis 4.6  Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 6 Serious drug related adverse effects. | ||||
| 6.1 High dose versus no corticosteroids | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.78] |
| 6.2 High dose versus moderate dose corticosteroids | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.08] |

Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 1 Ambulation (short term).

Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 2 Reduction in analgesic use.

Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 3 Urinary continence (short term).
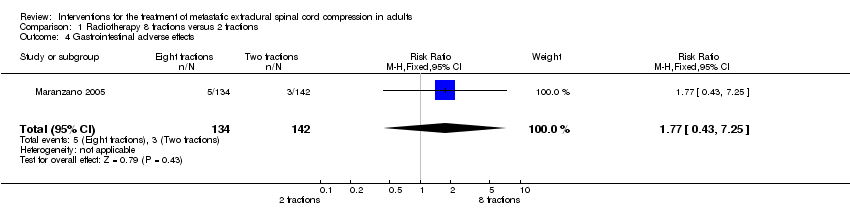
Comparison 1 Radiotherapy 8 fractions versus 2 fractions, Outcome 4 Gastrointestinal adverse effects.
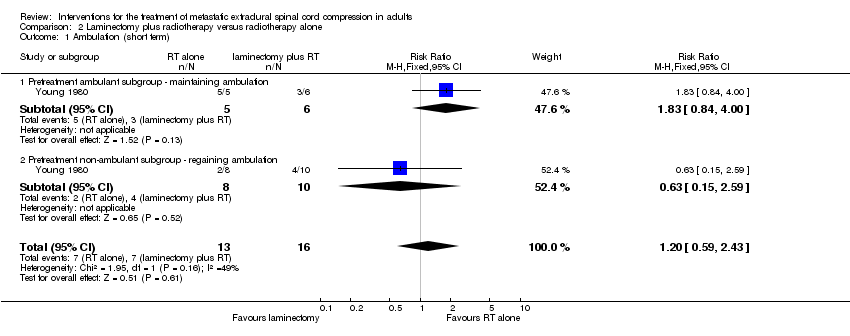
Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 1 Ambulation (short term).
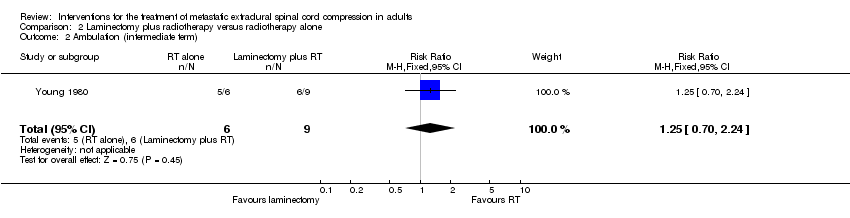
Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 2 Ambulation (intermediate term).

Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 3 Survival.

Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 4 Reduction in analgesic use.

Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 5 Urinary continence (short term).

Comparison 2 Laminectomy plus radiotherapy versus radiotherapy alone, Outcome 6 Urinary continence (intermediate term).

Comparison 3 Decompressive surgery plus radiotherapy versus radiotherapy alone, Outcome 1 Ambulation (short term).

Comparison 3 Decompressive surgery plus radiotherapy versus radiotherapy alone, Outcome 2 Survival (short term).

Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 1 Overall ambulation (short term).

Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 2 Participants maintaining or regaining ambulation (short term).
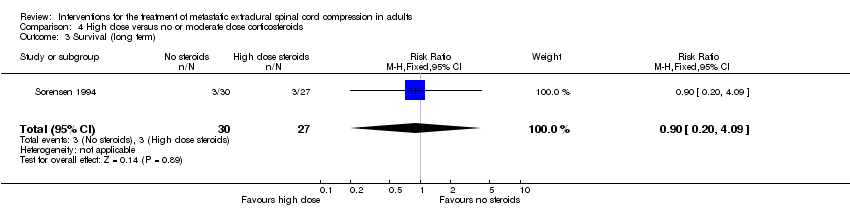
Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 3 Survival (long term).

Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 4 Pain reduction.

Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 5 Urinary continence (short term).

Comparison 4 High dose versus no or moderate dose corticosteroids, Outcome 6 Serious drug related adverse effects.
| Parameters | Results |
| Different radiotherapy schedules (Eight versus two fractions) | |
| Overall ambulatory rates (short term) | 95/134 (71%) versus 97/142 (68%) RR 1.02; (95% CI 0.90 to 1.15) (n = 276). |
| Pretreatment ambulant participants maintaining ambulation (short term) | 83/91 (91%) versus 83/93 (89%) RR 1.02; (95% CI 0.93 to 1.12) (n = 184). |
| Pretreatment non‐ambulant participants regaining ambulation (short term) | 12/43 (28%) versus 14/49 (29%) RR 0.98; (95% CI 0.51 to 1.88) (n = 92) |
| Median duration of ambulation | 3.5 months in both arms (excluding 17 early deaths) |
| Survival | Four months in both arms (excluding 17 early deaths), five months in pretreatment ambulant participants and three months in pretreatment non‐ambulant participants. |
| Pain relief (short term) | 61/126 (48%) versus 52/136 (38%) RR 1.24; (95% CI 0.94 to 1.64) (n = 262) |
| Urinary continence (short term) | 118/134 (89%) versus 128/142 (90%) RR 0.97; (95% CI 0.93 to 1.02) (n = 275) |
| Participants maintaining urinary continence (short term) | 116/120 (97%) versus 126/126 (100%) RR 0.97; (95% CI 0.93 to 1.00) (n = 246) |
| Participants regaining urinary continence (short term) | 2/13 (15%) versus 2/16 (13%) RR 1.23; (95% CI 0.20 to 7.58) (n = 29) |
| Adverse effects (early) | Grade three acute gastrointestinal mucositis attributable to radiation ‐ 5/134 (4%) versus 3/142 (2%) RR 1.77; (95% CI 0.43 to 7.25) 6/276 participants had Grade three vomiting; the incidence was similar in both the arms. Grade three nausea was present in 5/276 participants (n = 276). |
| Adverse effects (late) | No documented late radiation myelopathy or serious adverse effects |
| Outcomes not reported | Survival rates, quality of life, participant and caregiver satisfaction. |
| Laminectomy plus radiotherapy versus radiotherapy alone | |
| Overall ambulatory rates (short term) | 7 /16 (44%) versus 7/13 (54%) RR 1.20; (95% CI 0.59 to 2.43) (n = 29) |
| Pretreatment ambulant participants maintaining ambulation (short term) | 3/6 (50%) versus 5/5 (100%) RR 1.83; (95% CI 0.84 to 4.00) (n = 11) |
| Pretreatment non‐ambulant participants regaining ambulation (short term) | 4/10 (40%) versus 2/8 (25%) RR 0.63; (95% CI 0.15 to 2.59) (n = 18) |
| Overall ambulatory rates (intermediate term) | 6/9 (67%) versus 5/6 (83%) RR 1.25; (95% CI 0.70 to 2.24) (n = 15) |
| Survival (short term) | 16/16 (100%) versus 10/13 (76%) RR 0.77; (95% CI 0.56 to 1.06) (n = 29) |
| Survival (Intermediate term) | 9/16 (56%) versus 6/13 (46%) RR 0.82; (95% CI 0.40 to 1.70) (n = 29) |
| Pain relief | 8/14 (57%) versus 6/12 (50%) RR 0.88; (95% CI 0.42 to 1.81) (n = 26) |
| Overall urinary continence (short term) | 7/16 (44%) versus 7/13 (54%) 95% CI RR 0.94; (95% CI 0.50 to 1.77) (n = 29) |
| Proportion of participants maintaining urinary continence (short term) | 6/8 (75%) versus 6/10 (60%) RR 0.80; (95% CI 0.42 to 1.52) (n = 18) |
| Proportion of participants regaining urinary continence (short term) | 1/8 (13%) versus 1/3 (33%) RR 2.67; (95% CI 0.23 to 30.40) (n = 11) |
| Overall urinary continence (intermediate term) | 6/9 (67%) versus 6/6 (100%) RR 1.43; (95% CI 0.87 to 2.35) (n = 15) |
| Adverse effects | There were no surgery or radiotherapy related complications |
| Outcomes not reported | Quality of life, participant and caregiver satisfaction. |
| Direct decompressive surgery with radiotherapy versus radiotherapy | |
| Overall ambulatory rates (short term) | 29/51 (57%) versus 42/50 (84%), RR 0.67; (95% CI 0.53 to 0.86) (n = 101), NNTB 3.70 (95% CI 2.38 to 7.69) |
| Proportion of pretreatment ambulant participants maintaining ambulation (short term) | 26/35 (74%) versus 32/34 (94%) RR 0.79; (95% CI 0.64 to 0.98) (n = 69), NNTB 5.00 (95% CI 2.78 to 33.33) |
| Proportion of pretreatment non‐ambulant participants regaining ambulation (short term) | 3/16 (19%) versus 10/16 (63%) RR 0.30; (95% CI 0.10 to 0.89) (n = 32), NNTB 2.27 (95% CI 1.35 to 7.69) |
| Median duration of ambulation | The median duration of ambulation was 13 days versus 122 days, (those maintaining ambulation 54 days versus 153 days and regaining ambulation was 0 versus 59 days) |
| Survival (short term) | 44/51 (86%) versus 47/50 (94%) RR 0.92; (95% CI 0.81 to 1.05) (n = 101) |
| Median survival | 100 days versus 126 days |
| Outcomes not reported | Quality of life, participant and care giver satisfaction were not assessed. Participant rated pain relief , adverse effects and dichotomous data for analgesic reduction and urinary continence |
| High dose corticosteroids versus no or moderate dose corticosteroids | |
| Overall ambulatory rates (short term) | RR 0.91; (95% CI 0.68 to 1.23) (n = 105, three trials) |
| Proportion of pretreatment ambulant participants maintaining ambulation (short term) | 17/17 (100%) versus 17/19 (90%) RR 0.90; (95% CI 0.75 to 1.08) (n = 36, one trial) |
| Proportion of pretreatment non‐ambulant participants regaining ambulation (short term) | 5/10 (50%) versus 2/11 (18%) RR 0.36; (95% CI 0.09 to 1.47) (n = 21, one trial) |
| Survival (long term) | 5/10 (50%) versus 2/11 (18%) RR 0.36; (95% CI 0.09 to 1.47) (n = 21, one trial) |
| Pain relief | 11/14 (79%) versus 10/11(91%) RR 1.16; (95% CI 0.83 to 1.61) (n = 25, one trial) |
| Urinary continence | 12/19 (63%) versus 8/15 (53%) RR 0.84; (95% CI 0.47 to 1.52) (n = 34, one trial) |
| Adverse effects | High dose corticosteroids versus no or moderate dose corticosteroids RR 0.12; (95% CI 0.02 to 0.97) (n = 77, two trials) High dose versus no corticosteroids RR 0.10; (95% CI 0.01 to 1.78) (n = 57, one trial) High dose versus moderate dose corticosteroids RR 0.17; (95% CI 0.01 to 3.08) (n = 20, one trial) |
| Outcomes not reported | Quality of life, participant rated and care giver satisfaction. Intermediate term outcomes from Sorensen 1994 could not be calculated as survival rates were not available. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ambulation (short term) Show forest plot | 1 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 1.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 1.2 Pretreatment non‐ambulant subgroup ‐ regaining ambulation | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.88] |
| 2 Reduction in analgesic use Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.96, 1.67] |
| 3 Urinary continence (short term) Show forest plot | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.02] |
| 3.1 Proportion maintaining urinary continence | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.00] |
| 3.2 Proportion regaining urinary continence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.20, 7.58] |
| 4 Gastrointestinal adverse effects Show forest plot | 1 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.43, 7.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ambulation (short term) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.59, 2.43] |
| 1.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.84, 4.00] |
| 1.2 Pretreatment non‐ambulant subgroup ‐ regaining ambulation | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.15, 2.59] |
| 2 Ambulation (intermediate term) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.70, 2.24] |
| 3 Survival Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short term survival | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.06] |
| 3.2 Intermediate term survival | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.70] |
| 4 Reduction in analgesic use Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.42, 1.81] |
| 5 Urinary continence (short term) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.50, 1.77] |
| 5.1 Proportion maintaining urinary continence | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.42, 1.52] |
| 5.2 Proportion regaining urinary continence | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.23, 30.40] |
| 6 Urinary continence (intermediate term) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.87, 2.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ambulation (short term) Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.86] |
| 1.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.98] |
| 1.2 Pretreatment non‐ambulant subgroup regaining ambulation | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.10, 0.89] |
| 2 Survival (short term) Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall ambulation (short term) Show forest plot | 3 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.23] |
| 1.1 High dose versus no corticosteroids | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.08] |
| 1.2 High versus moderate corticosteroids | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.68, 2.12] |
| 2 Participants maintaining or regaining ambulation (short term) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Pretreatment ambulant subgroup ‐ maintaining ambulation | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.75, 1.08] |
| 2.2 Pretreatment non‐ambulant subgroup regaining ambulation | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.47] |
| 3 Survival (long term) Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.20, 4.09] |
| 4 Pain reduction Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.61] |
| 5 Urinary continence (short term) Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.52] |
| 6 Serious drug related adverse effects Show forest plot | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.97] |
| 6.1 High dose versus no corticosteroids | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.78] |
| 6.2 High dose versus moderate dose corticosteroids | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.08] |

