Hierbas medicinales chinas para pacientes con intolerancia a la glucosa o alteración de la glucemia en ayunas
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Outpatients at Guangxi TCM College Affiliated No.1 Hospital, China WHO PARTICIPATED: 45 (M/F 21/24; 23 in the treatment group, age 54.6 yrs; 22 in the control, age 57.45 yrs) INCLUSION CRITERIA: IGT diagnosed by WHO criteria (WHO 1999) EXCLUSION CRITERIA: <35 yrs, BMI <19 kg/m, serious liver or kidney disorders, hypertension, IGT induced by other organic diseases, drugs or stress. CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Jiangtang bushen tang (gou qi 10g, chuan duan xu 10g, nu zhen zi 15g, han lian cao 15g, di gu pi 15g, sheng huang qi 15g, sheng di huang 15g, ge gen 12g, huang lian 5g, sang bai pi 10g, zhi mu 6g) plus diet and exercise; Dosage: 1 decoction every two days CONTROL: lifestyle modification | |
| Outcomes | FBG (mmol/L), 2hr‐GTT (mmol/L), Triglycerides (mmol/L), total cholesterol (mmol/L), BMI (kg/m2), fasting insulin (mmol/L), TCM symptoms; Outcomes assessed at baseline, 3 months, 6 months and 12 months. | |
| Study details | DURATION OF INTERVENTION: 12 months DURATION OF FOLLOW‐UP: 12 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: No NON‐COMMERCIAL FUNDING: Not reported PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): No PUBLICATION STATUS (ABSTRACT): Yes | |
| Stated aim of study | "To evaluate the intervention effect of diet, exercise and Jiangtang Bushen Recipe (JBR, a Chinese herbal recipe) in preventing the progress of patients with impaired glucose tolerance (IGT) to diabetes mellitus (DM) type 2." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote from phone call: "numbers randomisation from random table" |
| Allocation concealment? | Unclear risk | Phone call: Participants did not know the group to which they were to be allocated. No information provided about whether researchers knew the allocation. |
| Blinding? | High risk | No blinding of participants, intervention provider or outcomes assessor. |
| Incomplete outcome data addressed? | Low risk | All participants are reported. Six withdrawals are explained. One participant left the intervention as they did not want to take the decoction, the other withdrawal did not give a reason. There were four withdrawals from the control where contact was lost. |
| Free of selective reporting? | Unclear risk | No protocol provided but all nominated and expected outcomes are reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel placebo controlled randomised trial | |
| Participants | SETTING: Outpatient and inpatients, No 1 Affiliated Hospital of An Hui University of Chinese Medicine, China WHO PARTCIPATED: 62 (in treatment group M/F 18/14; age 40‐67 yrs; in the control group M/F 17/13, age 39‐65 yrs) INCLUSION CRITERIA: IGT diagnosed by ADA criteria (ADA 1997) and traditional Chinese medicine (TCM) diagnosis of qi and yin deficiency or blood stagnation EXCLUSION CRITERIA: None reported CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none report | |
| Interventions | INTERVENTION: Dan zhi jiang tang jiao capsules: mu dan pi, shui zhi, tu si zi, ze xie, huang jing, tai zi shen plus liu wei di huang tang oral, 5 capsules (0.35g per capsule) 3 time per day after meals plus lifestyle modification (diet & lifestyle advice) CONTROL: placebo plus lifestyle modification (diet & lifestyle advice) | |
| Outcomes | FBG (mmol/L), 2hr‐GTT (mmol/L), insulin (mu/L), triglycerides (mmol/L), traditional Chinese medicine patterns and symptoms. Outcomes were assessed at baseline and trial completion (12 wks). | |
| Study details | DURATION OF INTERVENTION: 12 weeks DURATION OF FOLLOW‐UP: 12 weeks RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | To observe the intervention effects of Dan zhi jiang tang jiao on IGT. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote (from the report): "the randomly divided into two groups". Quote (from phone interview): numbers generated through a random table |
| Allocation concealment? | Low risk | Concealed envelopes with random numbers were used. |
| Blinding? | Low risk | From phone interview: participants were blinded with the use of a placebo and provided with the same diet & lifestyle advice as the treatment group; clinicians were blinded also; not known if the assessors were blinded. |
| Incomplete outcome data addressed? | Low risk | No missing participants or withdrawals. |
| Free of selective reporting? | Unclear risk | No protocol provided but all nominated outcomes reported, reported TCM patterns and symptoms also. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel placebo‐controlled randomised trial | |
| Participants | SETTING: Outpatients at the General Hospital of the People's Liberation Army, China WHO PARTCIPATED: n=168 (in treatment group M/F 62/24; mean age 55.6 yrs, duration of disease 1mth ‐2yrs; in the control group M/F 59/23, mean age 53.8, duration of disease 1mth‐2yrs). INCLUSION CRITERIA: IGT (WHO 1999) and hypertension plus high total cholesterol or high triglycerides or low HDL. EXCLUSION CRITERIA: IGT due to endocrinological disorders, liver disease, drugs, stress. CO‐MORBIDITIES: hypertension, hypercholestemia or high triglycerides or low high density lipoprotein (HDL). CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Xiaoke huaya tablet (Zhi My, Gui JIan Yu etc), dosage: 0.5g three times per day plus lifestyle modification (diet & exercise) CONTROL: lifestyle modification (diet & exercise alone) | |
| Outcomes | FBG (mmol/L), 2hr‐GTT (mmol/L), HbA1c (%), triglycerides (mmol/L), total cholesterol (mmol/L), normalisation of FBG (n), incidence of diabetes (n) Outcomes were measured at baseline and trial completion (8 wks). | |
| Study details | DURATION OF INTERVENTION: 8 weeks DURATION OF FOLLOW‐UP: 8 weeks RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: No NON‐COMMERCIAL FUNDING: Yes (Translational Funding Project) PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): No PUBLICATION STATUS (ABSTRACT): Yes | |
| Stated aim of study | Quote "to validate the therapeutic effects of Xiaoke Huaya tablet in the impaired glucose tolerance population " | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomisation method not mentioned in paper and unable to contact author. |
| Allocation concealment? | Unclear risk | Allocation concealment not mentioned in paper and unable to contact author. |
| Blinding? | High risk | Participants were not blinded. It is not clear if the outcome assessor or intervention providers were blinded. Unable to contact author. |
| Incomplete outcome data addressed? | Low risk | All participants reported. No withdrawals. |
| Free of selective reporting? | Unclear risk | No protocol but all nominated and expected outcomes reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel placebo‐controlled randomised trial | |
| Participants | SETTING: Outpatients at the Obesity Clinic of Kyoto Prefectual University, Japan WHO PARTCIPATED: n= 81 (44 in treatment group mean age 52.6 yrs, mean weight 90.8 kg, mean BMI 36.7; in the 41 in control group mean age 54.8, mean weight 90.3, mean BMI 36.1) INCLUSION CRITERIA: IGT (WHO 1999) and obese EXCLUSION CRITERIA: People with kidney, heart and/or liver disease, any metabolic or endocrine disease, psychiatric disorders and cancer. CO‐MORBIDITIES: obesity CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Bofu‐tsusho‐san (Scutellariae Radix, Glycyrrhizae Radix, Platycodi Radix, Gypsum Fibrosum, Atractylodis Rhizoma, Rhei Rhizoma, Schizonepetae Spica, Gardeniae Fructus, Paeoniae Radix, Cnidium Rhizoma, Angelicae Radix, Menthae Herba, Ledebouriellae Radix, Ephedrae Herba, Forsythiae Fructus, Zingiberis Rhizoma, Talcum, Natrium Sulphuricum), dry extract, three times a day (t.i.d) 30 mins before meals, for 24 wks plus lifestyle modification (diet & exercise). CONTROL: placebo three times a day (t.i.d) 30 mins before meals, for 24wks plus lifestyle modification (diet & exercise) Lifestyle modification for all participants involved a diet of 1200 kcal/day, analysed based on food ingestion records, and exercise (5000 steps/day) determined by pedometer recordings. | |
| Outcomes | FBG (mg/dL), 2hr‐GTT (mg/dL), HbA1c (%), triglycerides (mg/dL), HDL (mg/dL), LDL (mg/dL), total cholesterol (mg/dL), fasting insulin (μU/mL), 2hr insulin (μU/mL), insulin AUC, HOMA‐IR. Outcomes for all measures were assessed at baseline, 12 weeks, and 24 weeks. Note: For FBG mg/dL & 2hr‐GTT conversion to mmol/L: mg/dl of glucose to mmol/l, divided by 18. For total cholesterol, HDL, LDL mg/dL conversion to mmol/L: convert mg/dl of HDL or LDL cholesterol to mmol/l, divided by 38.67. For triglycerides mg/dL conversion to mmol/L: mg/dl of triglycerides to mmol/l, divide by 89. | |
| Study details | DURATION OF INTERVENTION: 24 weeks DURATION OF FOLLOW‐UP: 24 weeks RUN‐IN PERIOD: after 2 months of lifestyle modification (diet and exercise therapy as described above), the active drug or placebo was introduced. | |
| Publication details | LANGUAGE OF PUBLICATION: English and Japanese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: This study was supported, in part, by a Grant‐in‐Aid (No.14571106; to TY) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | The aim of the study was to determine whether BF was effective in decreasing visceral adiposity and insulin resistance. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by random number table |
| Allocation concealment? | Low risk | As a placebo controlled study it is likely that allocation was concealed. |
| Blinding? | Low risk | Participants were blinded (placebo controlled), outcome assessors blinded. |
| Incomplete outcome data addressed? | Low risk | All withdrawals are explained and all participant data included. Four withdrawals; 3 from treatment group for non‐compliance because of loose bowels; 1 withdrew from control for non‐compliance. The data of these subjects was excluded from the analysis. The baseline data of all 85 subjects was not significantly different from those of the 81 women. |
| Free of selective reporting? | Unclear risk | No protocol provided but all nominated and expected outcomes are reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: inpatients and outpatients at Nanning TCM hospital, China WHO PARTCIPATED: n= 64; (28/36 (M/F); mean age 50.9yrs; 31 in treatment group; 33 in control group) INCLUSION CRITERIA: IGT (WHO 1999) EXCLUSION CRITERIA: People <40yrs, BMI <19kg/m, hypertension, IGT induced by other organic diseases, drugs or stress. CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Qimai jiangtang yin (huang qi 20g, ge gen 20g, mai dong 10g, nu zhen zi 10g, san qi 10g, yu jin 10g, sheng di huang 15g) decoction taken 1 dose (100ml) every two days in 1st and 2nd months plus lifestyle modification (diet & lifestyle advice) CONTROL: Lifestyle modification (diet & lifestyle advice) | |
| Outcomes | FBG (mmol/L), 2hr GTT (mmol/L), fasting insulin, IAI, normalisation rate of IGT (n), incidence of diabetes Outcomes were measured at baseline and at trial completion (12 months). | |
| Study details | DURATION OF INTERVENTION: 12 months DURATION OF FOLLOW‐UP: 12 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | To observe the effect of Qimai Jingtang yin on impaired glucose tolerance. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not reported in published article and unable to contact authors to get further information. |
| Allocation concealment? | Unclear risk | Unable to contact authors to get further information. |
| Blinding? | High risk | Probably not as the control group were not taking a placebo. |
| Incomplete outcome data addressed? | Low risk | All participant data reported. |
| Free of selective reporting? | Unclear risk | No protocol provided, but all nominated and expected outcomes reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial (block randomisation, 3 participants in the intervention group : 2 in the control group) | |
| Participants | SETTING: Outpatients presenting at Guigang City TCM Hospital, China WHO PARTCIPATED: 80 (48 in treatment group, M/F 29/19, mean age 48.08 yrs; 32 in control group, M/F 19/13, mean age 47.62 yrs) INCLUSION CRITERIA: IGT (ADA 1997): FBG ≥ and less than 7.0 AND 2‐hr TT (75g) ≥7.8<11.1 PLUS either hypertension systolic ≥18.7kpa and/or diastolic ≥12.0; OR total cholesterol ≥5.7mmol/L OR triglycerides ≥2.26 mmol/L OR HDL ≤1.04 mmol/L. EXCLUSION CRITERIA: none reported CO‐MORBIDITIES: primary hypertension CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Yi qi yang yin huo xue (Huang qi, Dang gui, Shan yao, Sang bai pi, Sang ye, Sang zhi) 100 ml per dose, 3 times per day, plus Beijing Jiang Ya No. 0 tablets (Blood pressure lowering medication: Pterofen 12.5 mg, Dihydralazine Sulfate 12.5 mg, reserpine 0.1 mg) CONTROL: Beijing Jiang Ya No. 0 tablets (Blood pressure lowering medication: Pterofen 12.5 mg, Dihydralazine Sulfate 12.5 mg, reserpine 0.1 mg) | |
| Outcomes | Blood pressure, fasting blood glucose (mmol/L), 2hr‐GTT (mmol/L), total cholestrol (mmol/L), triglycerides (mmol/L), HDL (mmol/L), and quality of life assessment (Chinese scale, Du 1994). Outcomes were measured at baseline and trial completion (28d) This study reported that there were no adverse findings in renal, liver, and ECG tests. | |
| Study details | DURATION OF INTERVENTION: 28 days DURATION OF FOLLOW‐UP: 28 days RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: No NON‐COMMERCIAL FUNDING: No PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | "To observe the influences of combination of Chinese and western therapies on the life quality and blood‐fat [lipids] of primary hypertension patients with declined glucose tolerance." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Phone call: random table used randomisation sequence |
| Allocation concealment? | Unclear risk | No information in the report or from phone call. |
| Blinding? | High risk | Noticeably different interventions provided, blinding is unlikely and not mentioned. |
| Incomplete outcome data addressed? | Low risk | No withdrawals or exclusions, all participant data reported. |
| Free of selective reporting? | Unclear risk | Nominated and expected outcomes were reported but no protocol was available. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: inpatients and outpatients at Huaian County Hospital WHO PARTCIPATED: n=60 (30 in treatment group, M/F 21/9, mean age 48yrs and 30 in control group, M/F 17/13, mean age 49yrs) INCLUSION CRITERIA: IGT (ADA 1997) EXCLUSION CRITERIA: none reported CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Tang ping san (Huang Qi 30g, Shan Yao 10g, Sheng Di Huang 10g, Shu Di Huang 10g, Gou Qi Zi 10g, He Shou Wu 10g, Xian Ling Pi 10g, Dan Shen 30g, Ze Xie 10g, Sang Ye 10g), oral, decoction, once per day (b.i.d), plus lifestyle modification (diet & lifestyle advice) CONTROL: Metformin, oral, 0.25g per dose, three times a day (t.i.d), plus lifestyle modification (diet & lifestyle advice) | |
| Outcomes | 2hr‐GTT (mmol/L) measured at baseline, at trial completion (3 months) and at follow‐up (6 months) | |
| Study details | DURATION OF INTERVENTION: 12 weeks DURATION OF FOLLOW‐UP: 3 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: No NON‐COMMERCIAL FUNDING: Yes (Scientific research funding by Municipal Government). PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): no | |
| Stated aim of study | Quote "to observe the treatment of impaired glucose with Tangping san". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | The method of randomisation was not stated. We were unable to contact the author about the method used. |
| Allocation concealment? | Unclear risk | Comment: unable to contact the author about the method used. |
| Blinding? | High risk | Comment: both received an intervention but of a different nature (powder vs pill). It's likely that participants and intervention providers knew the intervention they were receiving. |
| Incomplete outcome data addressed? | Low risk | All participants data reported. |
| Free of selective reporting? | Unclear risk | No protocol but all expected and nominated outcomes reported. |
| Free of other bias? | Low risk | None reported. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Inpatients and outpatients at Liyuan Hospital, China WHO PARTCIPATED: 62 people (32 in intervention group, M/F 17/15, mean age 65.3 yrs, mean disease duration 3.1yrs, mean weight 82.3kg; 30 in control group, M/F 15/15, mean age 66.1 yrs, mean disease duration 3.6yrs, mean weight 82.1kg) INCLUSION CRITERIA: IGT according to WHO,1998 EXCLUSION CRITERIA: none reported CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Fufang cangzhu decoction (cang zhu 15g, yi yi ren 24g, sang shen 20g, shan yao 30g, huang bai 10g, li zhi hue 20g, di long 10g) oral, decoction, 150ml, bid CONTROL: metformin 0.25g, oral, tablet, tid | |
| Outcomes | FBG (mmol/L), 1hr‐GTT (mmol/L), 2hr‐GTT (mmol/L), weight (kg), waist‐hip ratio (WHR), triglycerides (mmol/L), total cholesterol (mmol/L), fasting insulin (mU/L) All outcomes measured at baseline and at trial completion (8 weeks). | |
| Study details | DURATION OF INTERVENTION: 8 weeks DURATION OF FOLLOW‐UP: 8 weeks RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote "To observe therapeutic effect of Fufang Cangzhu Decoction on senile obesity or overweight with impaired glucose tolerance (IGT)". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The authors were interviewed by phone and stated that the randomisation was done by use of a random table. |
| Allocation concealment? | Unclear risk | No details provided on allocation concealment. |
| Blinding? | High risk | Assessors blinded; participants could not be blinded as one group was taking capsule and the other a decoction. Intervention provider not blinded. |
| Incomplete outcome data addressed? | Low risk | All participants reported. No withdrawals. |
| Free of selective reporting? | Unclear risk | All expected and nominated outcomes reported. |
| Free of other bias? | Low risk | None reported. |
| Methods | Parallel randomised controlled trial, three arms: Chinese herbal medicines plus diet & lifestyle vs acabose plus diet & lifestyle vs diet & lifestyle | |
| Participants | SETTING: outpatients at the Intergrated Medicine Hospital of Guangdong Province, China WHO PARTCIPATED: 120 participants (40 in intervention I, M/F 24/16, mean age 53.5yrs; 40 in intervention II, M/F 20/20, mean age 54.8yrs; 40 in the control, M/F 22/18, mean age 50.6yrs) INCLUSION CRITERIA: IGT WHO 1999 EXCLUSION CRITERIA: <35yrs, BMI <19, hypertension level 3, severe liver or kidney diseases, other endocrine diseases, any IGT caused by medication or high levels of stress. CO‐MORBIDITIES: none stated CO‐MEDICATIONS: none stated | |
| Interventions | INTERVENTION I: Jian Pi Zhi Shen Huo Xue (shan yao 30g, shan zha 30g, huang qi 20g, fu ling 20g, shan zhu yu 15g, tao ren 10g) plus diet & lifestyle once per day. INTERVENTION II: Acarbose 50mg per dose, three times a day plus diet & lifestyle CONTROL: diet & lifestyle alone | |
| Outcomes | BMI (kg/m2), FBG (mmol/L), 2hr‐GTT (mmol/L), HbA1c (%), trigylcerides (mmol/L), total cholesterol (mmol/L), HDL (mmol/L0, LDL (mmol/L), FINS (mmol/L), ISI, normalisation of blood glucose (n), incidence of diabetes (n) All outcomes assessed at baseline, 6 months and at trial completion (12 months). | |
| Study details | DURATION OF INTERVENTION: 12 months. DURATION OF FOLLOW‐UP: 12 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote "to study the method of strengthen the spleen, nourish the kidney and activate the blood method for the treatment of impaired glucose tolerance." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A random table was used to divide the sample into 3 groups (40 in each group). |
| Allocation concealment? | Unclear risk | No information provided in the report on allocation concealment. |
| Blinding? | High risk | Not reported, unlikely given the design of the trial consisting of a chinese herbal powder, western pharmaceutical tablet and a group taking no medication. |
| Incomplete outcome data addressed? | Low risk | No missing data. 5 withdrawals: 2 from intervention I; reason given: could not continue with medication; 2 from intervention II reason given: could not continue with medication; 1 from the control; reason given: participant left the area. |
| Free of selective reporting? | Unclear risk | No protocol provided and all nominated outcomes reported. |
| Free of other bias? | Low risk | None reported. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: China WHO PARTCIPATED: 95 participants (48 in intervention, M/F 32/16, mean age 53.5yrs; 47 in control, M/F 29/18, mean age 53.5yrs) INCLUSION CRITERIA: IGT (WHO 1999); >30yrs<60yrs; not taking any medication that influences glucose levels for at lease one month; willingness to comply with the trial and examination; EXCLUSION CRITERIA: Level 3 hypertension; serious heart, liver or kidney dysfunction; mental diseases; allergic condition; pregnancy or lactation. DIAGNOSTIC CRITERIA: IGT WHO 1999 CO‐MORBIDITIES: none stated CO‐MEDICATIONS: none stated | |
| Interventions | INTERVENTION: Qiweitangping capsule (huang qi, huang qin, zi su zi, dang shen, da huang, da zao, shu di huang, chai hu, dan shen, yu jin, yin chen, tian hua fen, shi gao, che qian zi, shan yao, we wei zi, shan zhu yu, zhi mu, gou qi zi, ge gen, bai he, gua lou, wu yao, di huang, hua jiao, wang bu liu xing, gan cao) 3 capsule twice daily CONTROL: placebo 3 capsules twice daily oral | |
| Outcomes | FBG (mmol/L), 2hr‐GTT (mmol/L), BMI (kg/m2), WHR All outcomes were measured at baseline and trial completion (24 months). Adverse effects: one participant from treatment and one from the control developed abdominal discomfort. Both were resolved without any treatment | |
| Study details | DURATION OF INTERVENTION: 24 months DURATION OF FOLLOW‐UP: 24 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote "To observe the intervention effects of Qiweitangping capsule on impaired glucose tolerance (IGT) and on the morbidity of diabetes mellitus (DM)and the effect of conversing IFF to normal glucose tolerance (NGT) " | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | SAS was used to generate the allocation sequence. |
| Allocation concealment? | Low risk | Both the participant and the intervention provider did not know the allocation groups. |
| Blinding? | Low risk | Participants, doctor and outcomes assessor all blinded. |
| Incomplete outcome data addressed? | Low risk | All participant data reported. 5 withdrawals. |
| Free of selective reporting? | Unclear risk | No protocol reported but all expected and nominated outcome measures were reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Tianjin No. 1 Zhong Xin Hospital outpatients, China WHO PARTCIPATED: n=159 (81 in the intervention group, M/F 40/41; 78 in the control group, M/F 37/41) INCLUSION CRITERIA: IGT (WHO 1999) and/or IFG (ADA 1997) EXCLUSION CRITERIA: None reported CO‐MORBIDITIES: None reported CO‐MEDICATIONS: None reported | |
| Interventions | INTERVENTION: Jinqi Jiangtang 4‐7 tablets each dose, three times a day plus basic education (no diet or exercise) CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): basic education alone (no diet or exercise) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr‐GTT (mmol/L), normalisation of glucose tolerance (n), total cholesterol (mmol/L), triglycerides (mmol/L), systolic blood pressure (mm/Hg), diastolic blood pressure (mm/Hg) Adverse effects: 3 cases in the intervention group developed mild GIT symptoms in the early stage of taking the Chinese herbal medicine. These resolved after one to two weeks. No other adverse effects were observed. | |
| Study details | DURATION OF INTERVENTION: 2 years DURATION OF FOLLOW‐UP: 2 years RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): no | |
| Stated aim of study | Quote "To observe the effect of Jinqi Jiangtang tablet on preventing patients with impaired glucose becoming diabetes" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised but unable to contact the authors. |
| Allocation concealment? | Unclear risk | No information available in report. |
| Blinding? | High risk | Unlikely due to the nature of the medication intervention (tablets vs no medication). |
| Incomplete outcome data addressed? | Low risk | Three withdrawals reported in the control group. All data for the other participants reported. |
| Free of selective reporting? | Unclear risk | No protocol provided but all expected and nominated outcomes reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Outpatients at the Foshan TCM Hospital, Guangdong Province, China WHO PARTCIPATED: n=72 (36 in the intervention group, M/F 19/13, mean age 46.3yrs; 36 in the control group, M/F 20/13, mean age 47.1yrs) INCLUSION CRITERIA: IGT (WHO 1999) EXCLUSION CRITERIA: none reported CO‐MORBIDITIES: none reported CO‐MEDICATIONS none reported | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Xiaoke Yuye (Huang qi, huang jing, he shou wu, zhi mu) 10ml three times per day, plus lifestyle modification (diet and exercise) CONTROL: lifestyle modification (diet and exercise) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr‐GTT (mmol/L), HbA1c (%), total cholesterol (mmol/L), BMI, normalisation of fasting blood glucose (n), incidence of diabetes (n) All outcomes were measured at baseline and at trial completion (24 months) No adverse effects in the treatment group. | |
| Study details | DURATION OF INTERVENTION: 2 years DURATION OF FOLLOW‐UP: 2 years RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote "To observe the effects of Xiaoke yuye on patients with impaired glucose tolerance." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Authors were interviewed by phone and stated that a random table was used. |
| Allocation concealment? | Low risk | Allocation concealment not mentioned in the report. Information obtained from phone interview that numbers drawn from a sealed box designated the random number allocation. |
| Blinding? | High risk | No placebo so probably not blinded. |
| Incomplete outcome data addressed? | Low risk | 7 withdrawals reported, no reason given. Four in the intervention group and 3 in the control group. Baseline data reported included participants only. No difference at baseline. |
| Free of selective reporting? | Unclear risk | No protocol provided but all nominated outcomes reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Hospital outpatients and patients recruited from general company check‐up, Huabei Petroleum Two Hospital, Hebei, China WHO PARTCIPATED: n=76 (40 in the intervention group M/F 31/19, mean age 44.2yrs; 36 in the control M/F 23/13, mean age 43.9yrs) INCLUSION CRITERIA: IGT (ADA 1997): FBG <7.0 and 2hr‐GTT (75g) ≥7.8 <11.1 EXCLUSION CRITERIA: <20yrs <65yrs; IGT induced by other disorders, drugs or stress, pregnancy, serious liver, kidney or heart disorders. CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Tang Kang Yin decoction (ren shen 6g, huang lian 10g, nu zhen zi 15g, xia ku cao 30g, fan shi liu ye 30g) oral, 200ml, 2x/day, plus lifestyle modification (diet & exercise) CONTROL: lifestyle modification (diet & lifestyle) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr‐GTT (mmol/L), insulin (ng/ml), triglycerides (mmol/L), total cholesterol (mmol/L), BMI, normalisation of fasting bloood glucose (n), incidence of diabetes (n) All outcomes were assessed at baseline and trial completion (30 days). | |
| Study details | DURATION OF INTERVENTION: 30 days DURATION OF FOLLOW‐UP: 30 days RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): no PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote "To observe the clinical effect of intervention of Tangganyin decoction on patients with Impaired glucose tolerance ( IGT) ." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The authors were interviewed by phone and stated that randomisation was conducted by using a random table from the internet. |
| Allocation concealment? | High risk | The intervention provider knew the which group each participant was to be allocated to. |
| Blinding? | High risk | Not stated in the report. From phone interview "participants and intervention provider were not blinded". |
| Incomplete outcome data addressed? | Low risk | All participants reported. No withdrawals. |
| Free of selective reporting? | Unclear risk | No protocol provided but all expected and nominated outcomes reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Hospital outpatients and patients recruited from general company check‐up; Shugang Hospital, Shanghai, China WHO PARTCIPATED: 42 (22 in the intervention group, M/F 8/14; and 20 in the control group M/F 8/12) INCLUSION CRITERIA: IGT (WHO 1985) EXCLUSION CRITERIA: disorders that interfere with glucose metabolism CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Tangheng I, 2 bags twice a day for 3 mth splus lifestyle modification (diet & exercise) CONTROL: lifestyle modification (diet and exercise) for 3 months | |
| Outcomes | FBG (mmol/L), 2hr‐GTT (mmol/L), insulin (ng/mL), BMI, systolic blood pressure (mmHg), diastolic blood pressure (mmHg) All outcomes were assessed at baseline and trial completion (3 months) | |
| Study details | DURATION OF INTERVENTION: 3 months DURATION OF FOLLOW‐UP: 3 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote "To observe the intervention on 42 cases of impaired glucose with Tangheng I." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not reported and unable to contact authors. |
| Allocation concealment? | Unclear risk | Method not reported and unable to contact authors. |
| Blinding? | High risk | No placebo so probably not blinded. |
| Incomplete outcome data addressed? | Low risk | No missing data. No withdrawals. |
| Free of selective reporting? | Unclear risk | No protocol provided. All expected and nominated outcomes appropriately reported. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Hospital outpatients, Guangdong Province People's Hospital, China WHO PARTCIPATED: n=166 (I: n=56, 29/27 m/f, mean age 53.12yrs; II: n=55 m/f 29/26, mean age 52.68yrs; Control: n=55, m/f 30/25, mean age 52.45yrs) INCLUSION CRITERIA: IGT (WHO 1985) EXCLUSION CRITERIA: none reported CO‐MORBIDITIES: none reported CO‐MEDICATIONS:none reported | |
| Interventions | INTERVENTION 1: Liu wei di huang capsule, oral, 2 capsules bid, plus lifestyle modification involving a strict diet prescription, frequent diabetes lectures, moderate exercise. INTERVENTION 2: lifestyle modification as per intervention 1. CONTROL: pamphlet on diabetes only (no diet or exercise). | |
| Outcomes | FBG (mmol/L), 2‐hr GTT (mmol/L), HbA1c (%), total cholesterol (mmol/L), triglycerides (mmol/L), BMI (kg/m2), blood pressure | |
| Study details | DURATION OF INTERVENTION: 18 months DURATION OF FOLLOW‐UP: 18 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote "To study the effect of Liu wei di huang pill for the treatment of IGT to reduce the risk of CVD". | |
| Notes | Two articles about this study, one reports only two groups (2000) and the other three groups (2006). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The authors were interviewed by phone and advised that randomisation by a random table. |
| Allocation concealment? | Low risk | Allocation unknown to groups and intervention provider until after allocation. |
| Blinding? | High risk | No placebo so not blinded. |
| Incomplete outcome data addressed? | High risk | HbA1c was nominated as a collected outcome but not reported. No withdrawals. |
| Free of selective reporting? | Unclear risk | A study protocol is not available but the published data includes all nominated and expected outcomes. |
| Free of other bias? | Low risk | None identified. |
| Methods | Parallel randomised controlled trial | |
| Participants | SETTING: Outpatients, Zhejiang Province, Hangzhou Red Cross Hospital, China WHO PARTCIPATED: n=88 (46 in the intervention group M/F 18/28, mean age 55.6 yrs; 42 in the control M/F 17/25, mean age 54.0 yrs) INCLUSION CRITERIA: IGT (WHO 1985) EXCLUSION CRITERIA: none reported CO‐MORBIDITIES: none reported CO‐MEDICATIONS:none reported | |
| Interventions | INTERVENTION: Jinqi Jiangtang tablet, oral, 7 tablets, 0.42g per tablet, three times per day, 30mins before meals plus lifestyle modification (diet and exercise) CONTROL: lifestyle modification (diet and exercise) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr‐GTT (mmol/L), fasting insulin, 2hr‐insulin, triglycerides, total cholesterol, HDL, systolic blood pressure (kpa), diastolic blood pressure (kpa), normalisation of fasting blood glucose (n), incidence of diabetes (n) Blood pressure measurement converted as follows: 1 mmHg = 0.133 kPa All outcomes reported at baseline, 3 months, 6 months and trial completion (12 months) | |
| Study details | DURATION OF INTERVENTION: 12 months DURATION OF FOLLOW‐UP: 12 months RUN‐IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): no | |
| Stated aim of study | To observe the effect of Jingqi Jiangtang tablet on non‐overweight people with impaired glucose tolerance. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The authors were interviewed by phone and advised that randomisation by a random table |
| Allocation concealment? | High risk | Not mentioned in the report. From the phone interview: not adequate allocation concealment |
| Blinding? | High risk | No placebo so probably not blinded. |
| Incomplete outcome data addressed? | Low risk | All participant data reported, no withdrawals. |
| Free of selective reporting? | Unclear risk | No protocol provided. All expected and nominated outcomes appropriately reported. |
| Free of other bias? | Low risk | None identified. |
ADA: American Diabetes Association; AUC: area under the curve; BMI: body‐mass index; FBG: fasting blood glucose; GTT: (oral) glucose tolerance test; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density cholesterol; IGT: impaired glucose tolerance; M/F: male / female; LDL: low‐density cholesterol; TCM: Traditional Chinese Medicine; t.i.d.: three times daily
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Quasi‐randomised study comparing the effects of a Chinese herbal formula (ren shen 10g, huang qi 30g, sheng di huang 15g, shan yao 15, bai zhu 15g, ban xia 10g, fu ling 10g, cang zhu 10g, dang shen 12g, chi shao 10g, chuan xiong 10g, shan zha 10g, ge gen 10g, tian hua fen 15g) plus diet and lifestyle advice versus diet and lifestyle advice alone in 80 participants with impaired glucose tolerance over 4 months. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on clinician's decision. | |
| Quasi‐randomised study comparing the effects of a Chinese herbal medicine formula (Shu di 20g, shan zhu yu 10g, shan yao 10g, ze xie 10g, mu dan pi 10g, fu ling 10g, ban xia 10g, chen pi 10g, dan shen 10g, gou qi zi 10g, shan zha 10g) versus metformin in 90 participants with impaired glucose tolerance over 4 months. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on clinician's decision. | |
| Non‐randomised study of Ke Tang Ling in people with IGT. | |
| Non‐randomised study of Ke Tang Ling in people with IGT. Possible duplicate of Cai X 2001. | |
| Trial comparing two herbal medicines in people with IGT. This study did not meet the review criteria as it was an ineligible comparison. | |
| Case series of 48 people with IGT taking Jianpi nichan tang. | |
| Randomised study comparing the effects of Yuye Tang (Shanyao 30g, Shenghuangqi (raw Huangqi) 30g, Zhimu 10g, Jineijin 6g, Gegen 10g, Wuweizi 6g, Tianhuafen 30g, Huanqin 10g) versus dimethyldiguanide tablets in people with impaired glucose tolerance over 8 wks. The study reported that it randomly divided the sample into two groups. There is a large sampling discrepancy between the control (n = 43) and intervention group (n = 72) implying this was not random. Method of randomisation not reported. Unable to contact the authors. | |
| Non‐randomised study of Jinqi jiangtang tablets (n = 42) versus metformin (n = 38) in 80 participants over 3 months. | |
| A three‐arm study comparing the effects of Ketangling granules (sheng di 10g, shu di 15g, huang jing 10g, huang lian 5g, huang bai 6g, tian hua fen 10g, ze xie 15g, dan shen 10g, chuan xiong 10g, zhi da huang 6g) plus diet & lifestyle modification (n = 32) compared to diet & lifestyle alone (n = 14) and compared to no intervention (n = 16) in people with impaired glucose tolerance. The discrepancy in sampling numbers indicates that it is unlikely that the study was truly randomised. | |
| Quasi‐randomised three‐arm study comparing the effects of a Chinese herbal formula (huang qi, huang jing, ge gen, zhe xie, chai hui, gui jian yu etc) in 66 people with impaired glucose tolerance over 3 months. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on alternation. No blinding. | |
| Case series of a chinese herbal medicine in people with IGT. | |
| Case series of the effects of a chinese herbal medicine (ren shen 15g, ge gen 20g, bai zhu 10g, fu ling 30g, shan yao 30g, huang qi 30g, shan zhu yu 15g, shui zhi 10g, cang zhu 10, xuan shen 10g, tian hua fen 15g, huang lian 12g For obesity add ji nei jin 15g, shan zha 15g, yi mi 15g; For yin deficient heat add zhi mu 10g, sheng di huang 10g, and delete ren shen, huang qi) in 26 participants with impaired glucose tolerance. | |
| Case series of Huanyan kuguasu pills in people with IGT. | |
| Case series. | |
| Quasi‐randomised study comparing the effects of Danshen injection plus xuezhi kang versus xuezhi kang (extract of cholestin, red yeast Chinese rice) in 62 participants with IGT over 4 weeks. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on the judgement of the clinician. | |
| Case series of chinese herbal medicine in 60 people with IGT. | |
| Quasi‐randomised study comparing the effects of a Chinese herbal medicine (huang qi 20g, shan yao 20g, dan shen 20g, sheng di huang 20g, shu di huang 20g, tian hua fen 40g, fu ling 15g, chi shao 15g, chai hu 12g, shu da huang 10g, zhi shi 10g, huang qin 10g, qu mai 10g) versus metformin in 64 people over 12 months. The method of allocation to groups was inadequate. From a phone call to the author it was ascertained that participants were allocated to the treatment according to the judgement of the clinician. | |
| Quasi‐randomised study comparing the effects of Xiao yang tang (tao ren 15g, mu dan pi 15g, dan shen 15g, xuan shen 15g, da huang 10g, yu jin 10g, chuan bei mu 10g, lai fu zi 10g) versus metformin in 90 people with impaired glucose tolerance. The method of randomisation was alternation. From a phone call it was determined that the participants were randomised on the basis of alternation. | |
| Randomised study comparing yangxing tongmai tables versus metformin in 67 people with insulin resistance. The criteria for inclusion in the study included people with IGT, diabetes or abnormal ISI. These criteria do not match the required ones for this review. | |
| Non‐randomised study of Qiweibaizhu san and Gan cao shaoyao tang in 31 people with IGT. | |
| Non‐randomised study of a Chinese herbal extract (bereberine) in people with IGT and hyperlipedemia. | |
| Case series. | |
| Quasi‐randomised study comparing the effects of Hu Ben Hui Ni (shu di 20g, shan zhu yu 10g, shan yao 10g, ze xie 10g, mu dan pi 10g, fu ling 10g, ban xia 10g, chen pi 10g, dan shen 10g, gou qi zi 10g, shan zha 10g) in 90 people with impaired glucose tolerance. Report: "randomly divided into control group of 40 and treatment group of 42 cases. Phone interview: allocation was according to the preference of the participant. | |
| Duplicate of LI HB 2003 | |
| Quasi‐randomised study comparing the effect of Yiqi jianpi fang (dang shen 20g, ge gen 20g, huang qi 15g, fu ling 15g, bai zhu 15g, cang zhu 15g, tian hua fen 15g, shen qu 15g) plus captopril vs captopril alone in 64 participants with impaired glucose tolerance. The study duration was 21 days (<4wks). The report stated that participants were randomly divided into treatment group A and control group B. From phone contact with the authors the subjects were divided into groups according to ages and other characteristics by the clinician. Same study with more detailed information as Li HB 2002 | |
| Case series. | |
| Case series of Jianpi sanjing tang in 31 people with IGT. | |
| This trial compared different herbal medicines. | |
| Case series. | |
| Randomised study comparing Jiangtang bushen fang plus diet & lifestyle advice vs diet & lifestyle advice alone in 51 participants. Only TCM symptom outcomes were reported. These reported outcomes did not fall into our designated categories. | |
| Randomised study comparing Chinese herbal formula in 156 people with impaired glucose tolerance. Outcomes (FBG, 2hr‐GTT, HbA1c and clinical signs and symptoms) were reported as grouped data only: absolutely effective, effective, not effective. | |
| Quasi‐randomised study comparing Jinqi Jiangtang plus diet & exercise vs diet and exercise in 62 participants over 3 months. The report stated that the trial was "randomised". Contact with the author revealed that participants were "casually grouped". | |
| Randomised study comparing the effect of a chinese herbal decoction plus diet, exercise and education versus diet, exercise and education alone. The trial did not state if it was randomised. There is a sampling discrepancy, with 34 allocated to the treatment group and 22 to the control. Unable to contact the authors. | |
| Non‐randomised study comparing Xuexi II capsules plus diet & exercise versus diet & exercise alone in 50 people with IGT. | |
| Randomised study observing Yi ming decoction plus insulin vs insulin alone in 131 in people with type 2 diabetes. This population group did not meet the criteria of this review. | |
| Case series. | |
| The study used a mixed intervention of Chinese herbs and western medicine and was quasi randomised. | |
| Case series of 40 people with IGT. | |
| This study included subjects with both IGT and diabetes. | |
| Review of traditional methods of treating people with IGT. | |
| Participants were all diabetes type 2. This population group did not meet the criteria of this review. | |
| Non‐randomised study comparing Liu wei di huang wan plus diet & exercise versus diet & exercise alone in 64 participants. | |
| Case series. | |
| Randomised study comparing Yiqi ziyin granules (Huang qi 10g, sang shen 10g, xuan shen 10g, tai zi shen 15g) plus diet & lifestyle advice versus diet & lifestyle advice alone in 61 participants. There is a discrepancy in the sampling, 41 in the treatment group versus 20 in the control group. | |
| Randomised study comparing the effect of Fufang Yin Yang huo chongji versus rosiglitazone in 90 or 60 participants. Participant numbers reported in the tables were 45 in each group and in the text 30 in each group is reported. Authors refused to provide any further information about the study. | |
| Quasi‐randomised study of Shenqi di huang tang plus diet & exercise versus metformin plus diet & exercise in 80 participants over two and a half years. | |
| Quasi‐randomised, parallel trial of Tang No. 1 granules plus education versus education alone in 140 people for 6 months. Randomisation was based on the visiting sequence of the participants. | |
| Review of traditional methods of treating people with IGT. | |
| Review of traditional methods of treating people with IGT. | |
| A review of traditional methods of treating people with IGT, not a clinical trial. | |
| Randomised controlled trial of Jianpi bushen pill plus diet and exercise compared to multivitamin supplement and diet and exercise in 136 participants. The control intervention did not fall into the category of the inclusion criteria. | |
| Non‐randomised parallel study of Chinese herbal medicine in combinations with vitamin C or pioglitazone in 150 participants (30 participants in 5 groups). | |
| Quasi‐randomised, parallel study comparing Jianpi Jiangtang Yin (fu ling 20g, huang qi 30g, cang zhu 15g, yi yi ren 25g, ge gen 15g, mai dong 15g, xuan shen 15g, san qi 15g, dang gui 15g, huang lian 10g. Additions: 1) 5 palm sweat, excessive thirst & drinking: zhi mu 20g, tian hua fen 15g; 2) vexation and irritability, insomnia: bai zi ren 20g, ye jiao teng 15g, huang lian 10g; 3) dizziness and swollen eyes, reddish complexion & ears: mu dan pi 15g, gou teng 15g vs diet and exercise in 160 participants. Method of randomisation was odd‐even. | |
| Study of different methods of treating people with IGT. | |
| Non‐randomised. | |
| Review of traditional methods of treating people with IGT. | |
| Quasi‐randomised study comparing Jinqi jiangtang tablets plus diet & lifestyle advice vs diet & lifestyle advice in 72 participants. (Randomisation based on age). | |
| A study of Shengmai injection into elderly people. There were no blood glucose outcomes reported. | |
| Non‐randomised trial of Jinqi jiangtang pills in people with IGT. | |
| Quasi‐randomised (odd‐even method) study comparing Chinese herbal medicine (Zhu ru 10g, zhi shi 12g, chen pi 12g, fa xia 12g, fu ling 10g, sheng jiang 5g, yu zhu 15g, dan shen 15g, ze xie 12g, gan cao 3g) plus vigorous exercise, diet and education vs vigorous exercise, diet and education alone in 72 participants. | |
| Review of traditional methods of treating people with IGT. | |
| Randomised study comparing the effect of Jinqi jiangtang tablet vs placebo in 57 people. The trial objective was to assess efficacy of the herbal medicine on the excretion rate of microalbuminuria. No blood glucose outcomes were reported. | |
| Non‐randomised case series of a chinese herbal formula. | |
| Randomised study comparing Shen qi jiang tang ke li vs placebo in 60 participants with impaired glucose tolerance. The study duration was 14 days (<4 wks). | |
| Quasi‐randomised study comparing Huaqi Jiangtang vs diet and exercise in 72 participants with impaired glucose tolerance. Althought the report stated the participants were randomised, from the phone interview the author advised that the allocation to treatment group was based on the preference of the participants. | |
| Quasi‐randomised study comparing the effects of Jinqi jiangtang tablet in 46 participants with impaired glucose tolerance. The method of randomisation was alternation. From phone interview it was revealed that the allocation to treatment group was based on the judgement of the clinician. |
IGT: impaired glucose tolerance
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel, randomised controlled trial |
| Participants | SETTING: Outpatients, Hospital, China WHO PARTCIPATED: 160 (80 in treatment group, M/F 50/30, mean age 44.3 yrs; 80 in control group, M/F 48/32, mean age 44.1yrs) INCLUSION CRITERIA: IGT (WHO 1999). EXCLUSION CRITERIA: Excluded hyperthyroid, depression, cancer. CO‐MORBIDITIES: none reported CO‐MEDICATIONS: none reported |
| Interventions | INTERVENTION: Jie yu huo xue decoction (dang gui 15g, chai hu 15g, Yu Zhu 10g, huang qi 30g, chuan xiong 10g, chi shao 15g, san qi 3g, ge gen 15g, huang jing 15g, yu jin 15g, ren shen 25g, xia ku cao 10g) , oral, 100ml decoction per dose, 3 times per day; plus llifestyle modification (diet and lifestyle advice). This prescription was added to if the following symptoms were present: If heat in palms, thirst, increased drinking: + zhi mu 20g, wu wei zi 10g. if restlessness, tendency to anger, insomnia: + bai zi ren 20g, ye jiao teng 15g, huang lian 10g. if dizziness, stuffiness in eyes, reddish face & ears with heat: +mu dan pi, gou teng 15g. CONTROL: Lifestyle modification (diet and lifestyle advice). |
| Outcomes | FBG (mmol/L), 2hr‐GTT (mmol/L), normalisation of glucose levels (n). Outcomes were measured at baseline and at trial completion (12 months). |
| Notes | We are trying to contact the authors to clarify a discrepancy in the publication. The number randomised to the intervention group is reported as 80. In Table 1 of the published report, the incidence of diabetes gives different numbers for the intervention group with totals of 88 (42 normalised, 40 IGT, 6 diabetes mellitus). |
IGT: impaired glucose tolerance; FBG: fasting blood glucose; M/F: male/female
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Normalisation of fasting blood glucose at trial completion (n) Show forest plot | 8 | 625 | Risk Ratio (IV, Random, 95% CI) | 2.07 [1.52, 2.82] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 1 Normalisation of fasting blood glucose at trial completion (n). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Jiantang Bushen decoction | 1 | 45 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.04, 2.86] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Xiaoke Huaya tablet | 1 | 168 | Risk Ratio (IV, Random, 95% CI) | 2.54 [1.70, 3.79] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 Qimai Jiangtang Yin decoction | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 2.89 [1.41, 5.90] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 Jian Pi Zhi Shen Huo Xue | 1 | 77 | Risk Ratio (IV, Random, 95% CI) | 1.92 [1.28, 2.90] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.5 Tang Kang Yin | 1 | 76 | Risk Ratio (IV, Random, 95% CI) | 3.75 [1.74, 8.09] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.6 Xiaoke Yuye decoction | 1 | 65 | Risk Ratio (IV, Random, 95% CI) | 1.89 [1.14, 3.14] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.7 Tang Heng I | 1 | 42 | Risk Ratio (IV, Random, 95% CI) | 5.0 [1.26, 19.87] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.8 Jinqi jiangtang | 1 | 88 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.90, 1.53] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Incidence of diabetes (n) Show forest plot | 8 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.19, 0.58] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 2 Incidence of diabetes (n). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 Jiangtang Bushen tang | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.84] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 Xiaoke Huayu Pian | 1 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.30] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.3 Qimai Jiangtang Yin | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.39] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.4 Tang King Yin | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.46] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.5 Jian pi zhi shen huo xue | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.19, 1.84] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.6 Jinqi Jiangtang tablet | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.47] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.7 Xiaoke Yuye | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.54] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.8 Tangheng I Plus | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.43] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Fasting blood glucose (mmol/L) Show forest plot | 9 | 742 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.66, ‐0.16] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 3 Fasting blood glucose (mmol/L). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 Jiangtang Bushen decoction | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.12] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 Xiaoke Huayu tablet | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.72, ‐1.08] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 Qimai Jiangtang Yin formula | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.32, 0.26] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.4 Jinqi Jiangtang tablet | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.74, ‐0.42] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.5 Jian Pi Zhi Shen Huo Xue | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.35, 0.17] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.6 Xiaoke Yuye decoction | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.99, 0.21] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.7 Tang Kang Yin decoction | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.33, ‐0.09] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.8 Tang Heng I decoction | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.53, 0.05] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.9 Liu Wei Di Huang capsule | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.58, ‐0.04] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test] Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4 ![Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test].](/cdsr/doi/10.1002/14651858.CD006690.pub2/media/CDSR/CD006690/image_n/nCD006690-CMP-001-04.png) Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test]. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Jiangtang Bushen decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Xiaoke Huaye tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 Qimai Jiangtang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.4 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.5 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.6 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.7 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.8 Tang Heng I decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.9 Liu Wei Di Huang capsule | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 HbA1c (%) Show forest plot | 3 | 317 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.00, 0.06] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 5 HbA1c (%). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 Xiaoke Huayu tablet | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.99, ‐0.27] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.2 Jian Pi Zhi Shen Huo Xue | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.43, ‐0.31] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.3 Xiaoke Yuye decoction | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.12, 0.00] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Insulin (μU/ml) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.6  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 6 Insulin (μU/ml). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 Jiangtang Bushen decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.2 Qimai Jiangtang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.3 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.5 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.6 Tang Heng I decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 IAI (insulin sensitivity) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.7  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 7 IAI (insulin sensitivity). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 Qimai Jiangtang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Total cholesterol (mmol/L) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 8 Total cholesterol (mmol/L). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 Jiangtang Bushen tang | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.2 Xiaoke Huayu Pian | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.3 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.5 Xiaoke Yuye | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.6 Tang Kang Yin | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.7 Liu Wei Di Huang capsule | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Lipids: HDL (mmol/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.9  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 9 Lipids: HDL (mmol/L). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.2 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Trigylcerides (mmol/L) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.10  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 10 Trigylcerides (mmol/L). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.1 Jiangtang Bushen tang | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.2 Xiaoke Huayu Pian | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.3 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.5 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.6 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.7 Liu Wei Di Huang Wan | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 Body Mass Index (kg/m2) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.11  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 11 Body Mass Index (kg/m2). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.1 Jiangtang Bushen | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.2 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.3 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.4 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.5 Liu Wei Di Huang capsule | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.6 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 Diastolic blood pressure (mmHg) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.12  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 12 Diastolic blood pressure (mmHg). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.1 Liu wei di huang wan | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.2 Jinqi jiangtang tablets | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 Systolic Blood Pressure (mmHg) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.13  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 13 Systolic Blood Pressure (mmHg). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.1 Liu wei di huang wan | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.2 Jinqi jiangtang tablets | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 Main ingredient Astragalus membranecus (≥30g): Fasting blood glucose (mmol/ml) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.14  Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 14 Main ingredient Astragalus membranecus (≥30g): Fasting blood glucose (mmol/ml). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.2 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.3 Shan yao, shan zha, huang qi etc | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.4 Qimai Jiangtang Yin formula | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 Hypoglycaemic effects of herbal medicines with lifestyle modification compared lifestyle modification alone Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.15
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 15 Hypoglycaemic effects of herbal medicines with lifestyle modification compared lifestyle modification alone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting blood glucose (mmol/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 1 Reduction in fasting blood glucose (mmol/L). | ||||
| 1.1 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reduction in 2hr fasting blood glucose after oral glucose tolerance test Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 2 Reduction in 2hr fasting blood glucose after oral glucose tolerance test. | ||||
| 2.1 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Reduction in HbA1c (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 3 Reduction in HbA1c (%). | ||||
| 3.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 4 Total cholesterol (mmol/L). | ||||
| 4.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Trigylcerides (mmo/lL) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 5 Trigylcerides (mmo/lL). | ||||
| 5.1 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Insulin (mu/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 6 Insulin (mu/L). | ||||
| 6.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Lipids: HDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 7 Lipids: HDL (mmol/L). | ||||
| 7.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in 2 hr fasting blood glucose after oral glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Herbal medicine plus lifestyle modification versus metformin plus lifestyle modification, Outcome 1 Reduction in 2 hr fasting blood glucose after oral glucose tolerance test (mmol/L). | ||||
| 1.1 Tangping San | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normalisation of fasting blood glucose (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Herbal medicine versus placebo, Outcome 1 Normalisation of fasting blood glucose (n). | ||||
| 1.1 Qiwei Tang Ping capsule | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of diabetes (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Herbal medicine versus placebo, Outcome 2 Incidence of diabetes (n). | ||||
| 2.1 Qiwei Tang Ping | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Reduction in fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Herbal medicine versus placebo, Outcome 3 Reduction in fasting blood glucose (mmol/L). | ||||
| 3.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Herbal medicine versus placebo, Outcome 4 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L). | ||||
| 4.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Body mass index (kg/m2) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.5  Comparison 4 Herbal medicine versus placebo, Outcome 5 Body mass index (kg/m2). | ||||
| 5.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Waist‐to‐hip ratio (WHR) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Herbal medicine versus placebo, Outcome 6 Waist‐to‐hip ratio (WHR). | ||||
| 6.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Herbal medicine versus metformin, Outcome 1 Reduction in fasting blood glucose (mmol/L). | ||||
| 1.1 Fufang Cangzhu decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Herbal medicine versus metformin, Outcome 2 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L). | ||||
| 2.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Triglycerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Herbal medicine versus metformin, Outcome 3 Triglycerides (mmol/L). | ||||
| 3.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Total cholesterol Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Herbal medicine versus metformin, Outcome 4 Total cholesterol. | ||||
| 4.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Insulin (mU/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.5  Comparison 5 Herbal medicine versus metformin, Outcome 5 Insulin (mU/L). | ||||
| 5.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Waist‐to‐hip ratio (WHR) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.6  Comparison 5 Herbal medicine versus metformin, Outcome 6 Waist‐to‐hip ratio (WHR). | ||||
| 6.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Weight (kg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.7  Comparison 5 Herbal medicine versus metformin, Outcome 7 Weight (kg). | ||||
| 7.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 1 Reduction in fasting blood glucose (mmol/L). | ||||
| 1.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 2hr‐Glucose tolerance (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 2 2hr‐Glucose tolerance (mmol/L). | ||||
| 2.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Normalisation of blood glucose (n) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 3 Normalisation of blood glucose (n). | ||||
| 3.1 Jian pi zhi shen huo xue | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Incidence of diabetes (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 4 Incidence of diabetes (n). | ||||
| 4.1 Jian pi zhi shen huo xue | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 HbA1c (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.5  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 5 HbA1c (%). | ||||
| 6 Insulin (FINS mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.6  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 6 Insulin (FINS mmol/L). | ||||
| 6.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.7  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 7 Total cholesterol (mmol/L). | ||||
| 8 Trigylcerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.8  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 8 Trigylcerides (mmol/L). | ||||
| 8.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Lipids: LDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.9  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 9 Lipids: LDL (mmol/L). | ||||
| 9.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Lipids: HDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.10  Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 10 Lipids: HDL (mmol/L). | ||||
| 10.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 1 Reduction in fasting glucose (mmol/L). | ||||
| 1.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reduction in 2hr blood glucose after oral glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 2 Reduction in 2hr blood glucose after oral glucose tolerance test (mmol/L). | ||||
| 2.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 3 Total cholesterol (mmol/L). | ||||
| 3.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Triglycerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.4  Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 4 Triglycerides (mmol/L). | ||||
| 4.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Lipids: HDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.5  Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 5 Lipids: HDL (mmol/L). | ||||
| 5.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Systolic Blood Pressure (kpa) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.6  Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 6 Systolic Blood Pressure (kpa). | ||||
| 6.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Diastolic blood pressure (kpa) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.7  Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 7 Diastolic blood pressure (kpa). | ||||
| 7.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normalisation of fasting blood glucose at trial completion (n) Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 1 Normalisation of fasting blood glucose at trial completion (n). | ||||
| 1.1 Jinqi Jiangtang tablet | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of diabetes (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 2 Incidence of diabetes (n). | ||||
| 2.1 Jinqi Jiangtang tablet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 3 Fasting blood glucose (mmol/L). | ||||
| 3.1 06 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.4 ![Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test].](/cdsr/doi/10.1002/14651858.CD006690.pub2/media/CDSR/CD006690/image_n/nCD006690-CMP-008-04.png) Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test]. | ||||
| 4.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.5  Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 5 Total cholesterol (mmol/L). | ||||
| 5.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Trigylcerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.6  Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 6 Trigylcerides (mmol/L). | ||||
| 6.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |

PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow‐chart of study selection
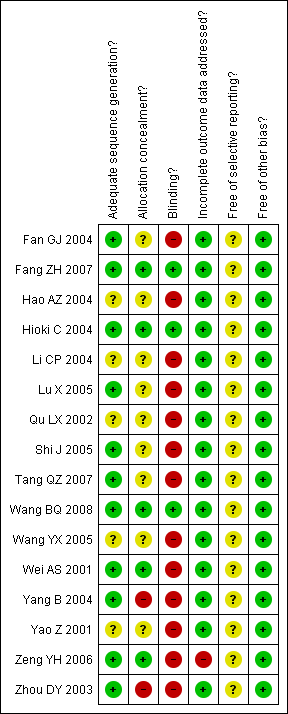
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of outcome 'normalisation of fasting blood glucose at trial completion' (herbal medicines plus lifestyle modification versus lifestyle modification alone)

Funnel plot of outcome 'diabetes incidence' (herbal medicines plus lifestyle modification versus lifestyle modification alone)

Forest plot of outcome 'normalisation of fasting blood glucose at trial completion' (herbal medicines plus lifestyle modification versus lifestyle modification alone)
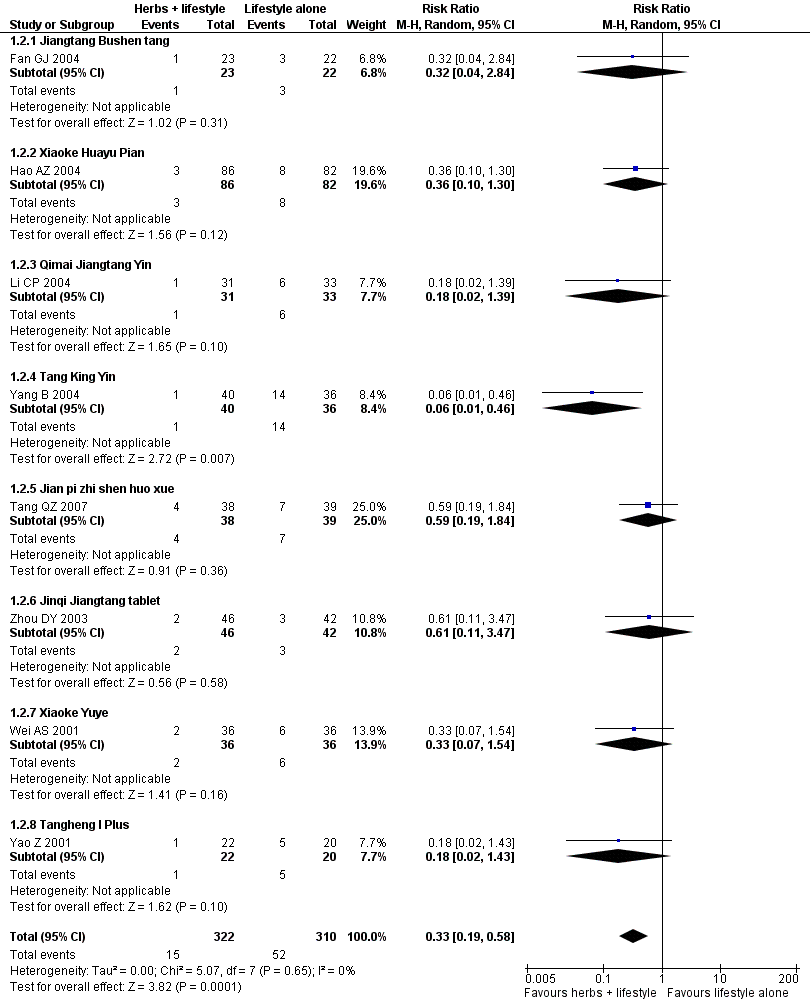
Forest plot of outcome 'diabetes incidence' (herbal medicines plus lifestyle modification versus lifestyle modification alone)

Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 1 Normalisation of fasting blood glucose at trial completion (n).
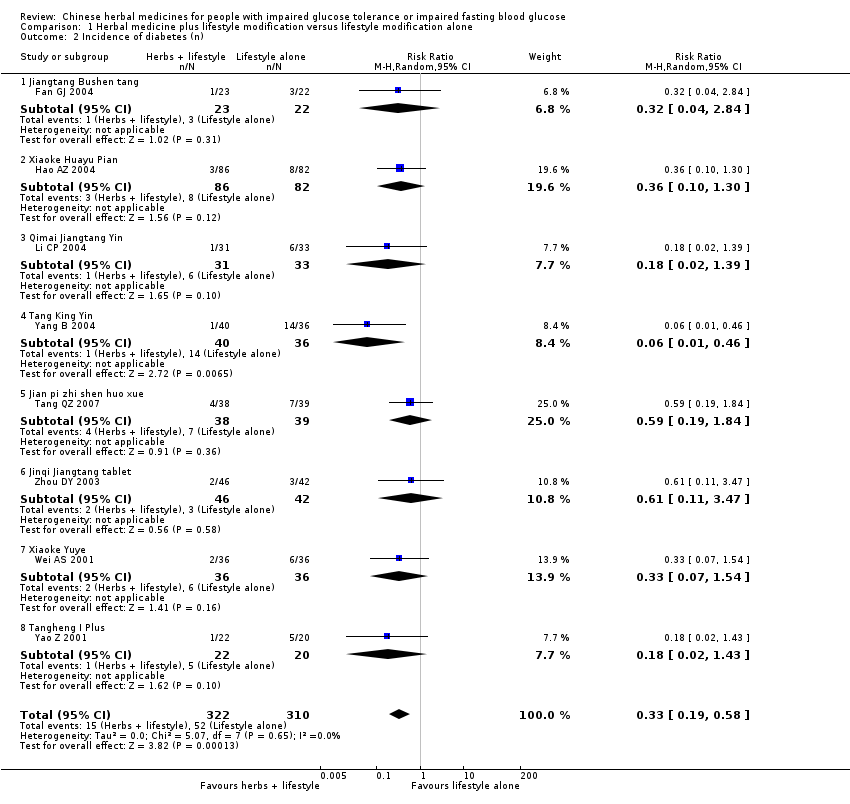
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 2 Incidence of diabetes (n).
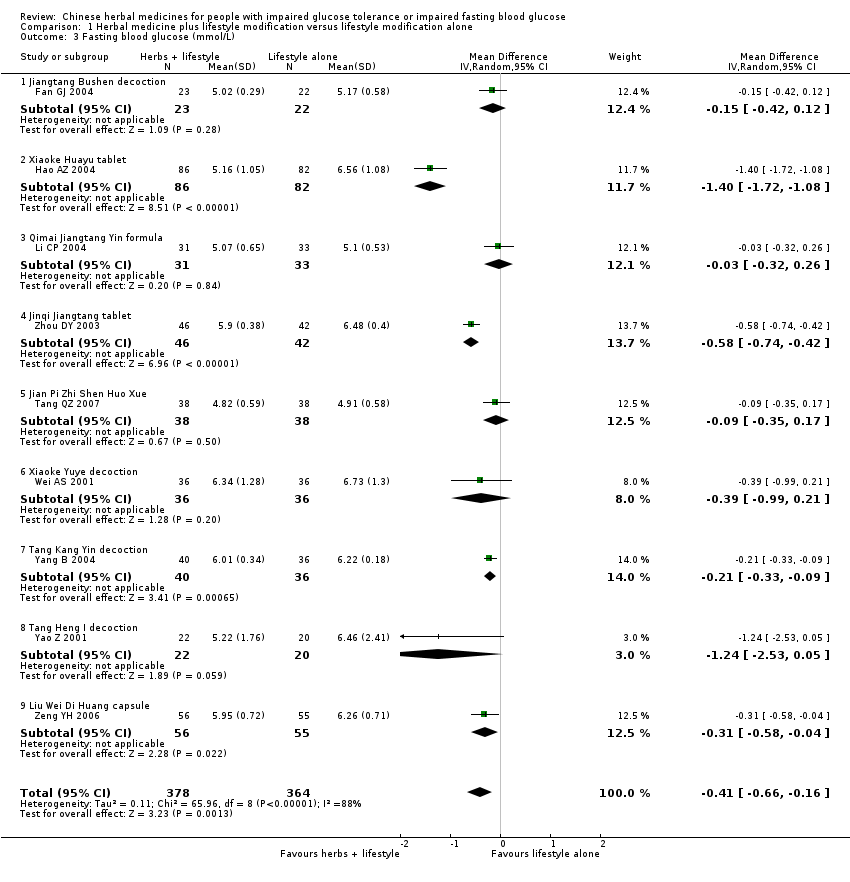
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 3 Fasting blood glucose (mmol/L).
![Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test].](/es/cdsr/doi/10.1002/14651858.CD006690.pub2/media/CDSR/CD006690/image_n/nCD006690-CMP-001-04.png)
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test].

Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 5 HbA1c (%).
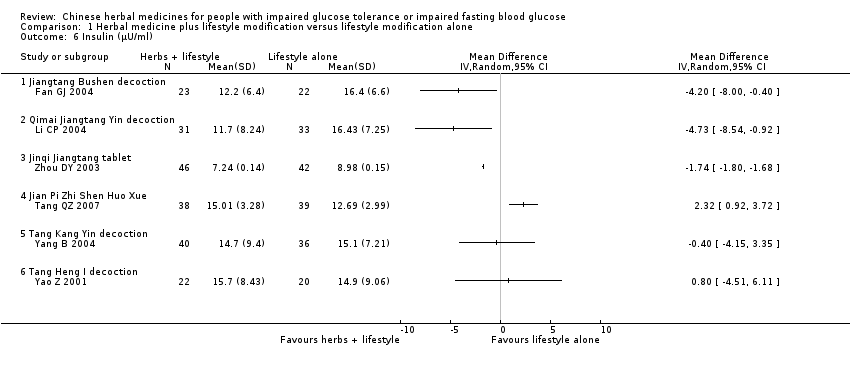
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 6 Insulin (μU/ml).
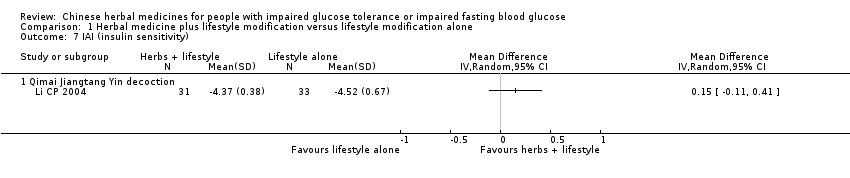
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 7 IAI (insulin sensitivity).

Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 8 Total cholesterol (mmol/L).
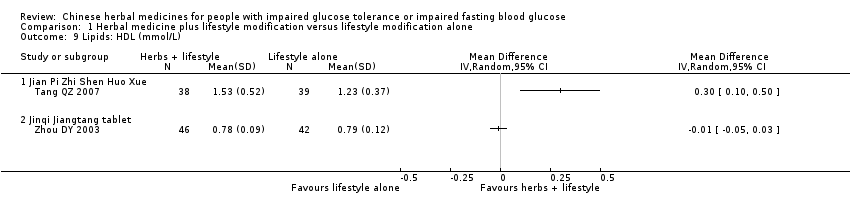
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 9 Lipids: HDL (mmol/L).

Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 10 Trigylcerides (mmol/L).

Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 11 Body Mass Index (kg/m2).

Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 12 Diastolic blood pressure (mmHg).
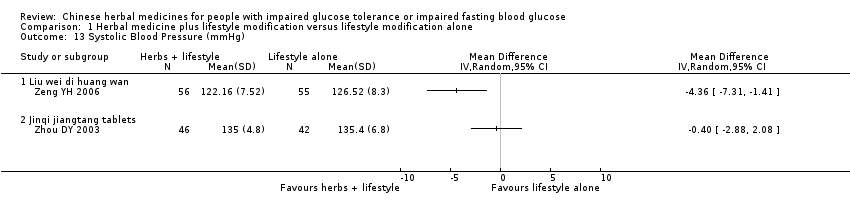
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 13 Systolic Blood Pressure (mmHg).

Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 14 Main ingredient Astragalus membranecus (≥30g): Fasting blood glucose (mmol/ml).
| Study | Intervention | FBG levels (mmol/L) | 2hr GTT FBG (mmol/L) | Normalisation of FBG: RR [95% CI] | HbA1c (%) |
| Fan GJ 2004 | Jiantang bushen Tang | ‐0.15 [0.42, 0.12] | ‐0.83 [‐1.84, 0.18] | 1.72 [1.04, 2.86] | ‐ |
| Hao AZ 2004 | Xiaoke Huaya tablet | ‐1.40 [‐1.72, ‐1.08] | ‐1.46 [‐1.83, ‐1.09] | 2.54 [1.70, 3.79] | ‐0.63 [‐0.99, ‐0.27] |
| Li CP 2004 | Qimai Jiangtang Yin formula | ‐0.03 [‐0.32, 0.26] | 2.89 [1.41, 5.90] | ‐ | |
| Tang QZ 2007 | Shan yao, Shan zha, Huang qi etc | ‐0.09 [‐0.35, 0.17] | ‐2.31 [‐3.20, ‐1.42] | 1.92 [1.28, 2.90] | ‐0.87 [‐1.43, ‐0.31] |
| Wei AS 2001 | Xiaoke Yuye decoction | ‐0.39 [0.99, 021] | ‐1.54 [‐1.65, ‐1.43] | 1.89 [1.14, 3.14] | ‐0.06 [‐0.12, 0.00] |
| Yang B 2004 | Tang Kang Yin decoction | ‐0.21 [‐0.33, ‐0.09] | ‐1.66 [‐2.21, ‐1.11] | ‐ | ‐ |
| Yao Z 2001 | Tang Heng I | ‐1.24 [‐2.53, 0.05] | ‐1.25 [‐3.30, 0.80] | 5.00 [1.26, 19.87] | ‐ |
| Zeng YH 2006 | Liu Wei Di Huang capsule | ‐1.19 [‐1.46, ‐0.92] | ‐2.77 [‐3.17, ‐2.37] | ‐ | ‐ |
| Zhou DY 2003 | Jinqi Jiangtang tablet | ‐0.58 [‐0.74, ‐0.42] | ‐0.57 [‐0.67, ‐0.47] | ‐ | ‐ |
Comparison 1 Herbal medicine plus lifestyle modification versus lifestyle modification alone, Outcome 15 Hypoglycaemic effects of herbal medicines with lifestyle modification compared lifestyle modification alone.
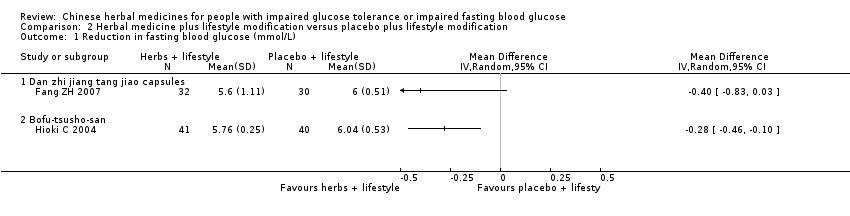
Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 1 Reduction in fasting blood glucose (mmol/L).

Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 2 Reduction in 2hr fasting blood glucose after oral glucose tolerance test.
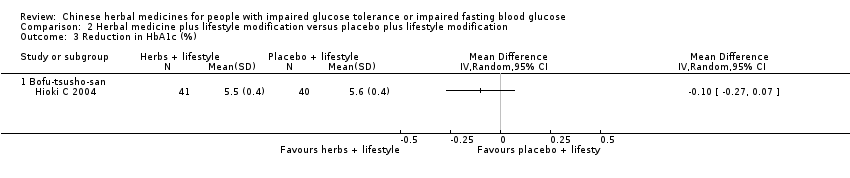
Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 3 Reduction in HbA1c (%).

Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 4 Total cholesterol (mmol/L).

Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 5 Trigylcerides (mmo/lL).

Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 6 Insulin (mu/L).

Comparison 2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification, Outcome 7 Lipids: HDL (mmol/L).

Comparison 3 Herbal medicine plus lifestyle modification versus metformin plus lifestyle modification, Outcome 1 Reduction in 2 hr fasting blood glucose after oral glucose tolerance test (mmol/L).

Comparison 4 Herbal medicine versus placebo, Outcome 1 Normalisation of fasting blood glucose (n).
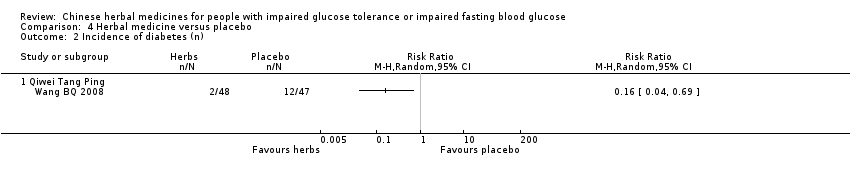
Comparison 4 Herbal medicine versus placebo, Outcome 2 Incidence of diabetes (n).

Comparison 4 Herbal medicine versus placebo, Outcome 3 Reduction in fasting blood glucose (mmol/L).

Comparison 4 Herbal medicine versus placebo, Outcome 4 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L).

Comparison 4 Herbal medicine versus placebo, Outcome 5 Body mass index (kg/m2).

Comparison 4 Herbal medicine versus placebo, Outcome 6 Waist‐to‐hip ratio (WHR).

Comparison 5 Herbal medicine versus metformin, Outcome 1 Reduction in fasting blood glucose (mmol/L).
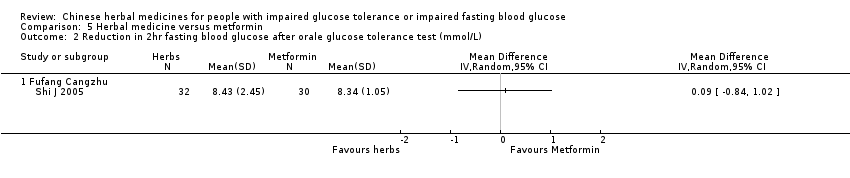
Comparison 5 Herbal medicine versus metformin, Outcome 2 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L).

Comparison 5 Herbal medicine versus metformin, Outcome 3 Triglycerides (mmol/L).

Comparison 5 Herbal medicine versus metformin, Outcome 4 Total cholesterol.

Comparison 5 Herbal medicine versus metformin, Outcome 5 Insulin (mU/L).

Comparison 5 Herbal medicine versus metformin, Outcome 6 Waist‐to‐hip ratio (WHR).

Comparison 5 Herbal medicine versus metformin, Outcome 7 Weight (kg).

Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 1 Reduction in fasting blood glucose (mmol/L).
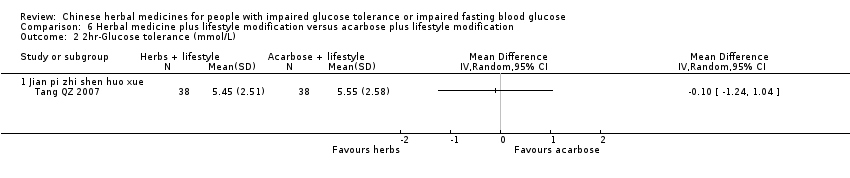
Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 2 2hr‐Glucose tolerance (mmol/L).

Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 3 Normalisation of blood glucose (n).

Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 4 Incidence of diabetes (n).
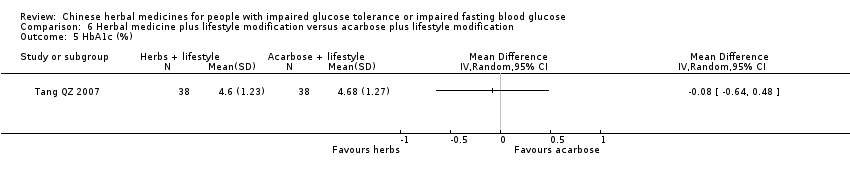
Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 5 HbA1c (%).
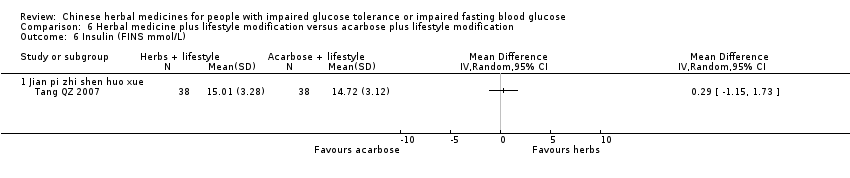
Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 6 Insulin (FINS mmol/L).

Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 7 Total cholesterol (mmol/L).

Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 8 Trigylcerides (mmol/L).
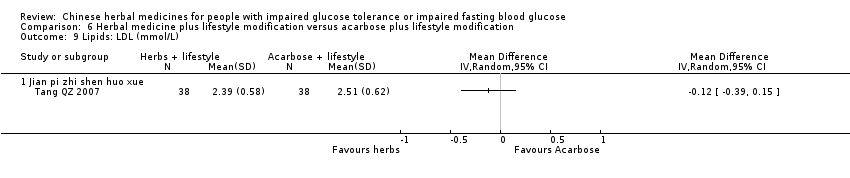
Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 9 Lipids: LDL (mmol/L).

Comparison 6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification, Outcome 10 Lipids: HDL (mmol/L).
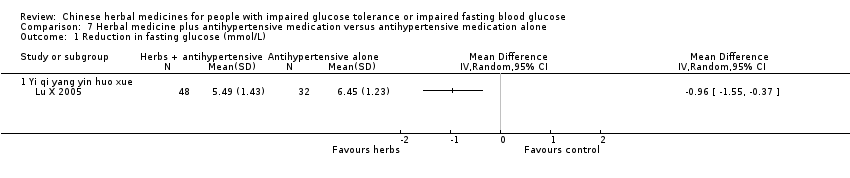
Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 1 Reduction in fasting glucose (mmol/L).

Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 2 Reduction in 2hr blood glucose after oral glucose tolerance test (mmol/L).
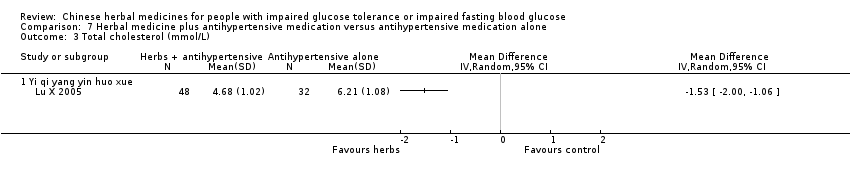
Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 3 Total cholesterol (mmol/L).

Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 4 Triglycerides (mmol/L).
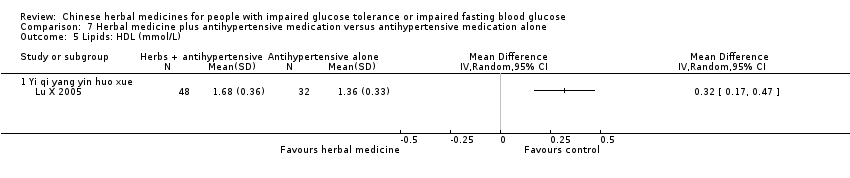
Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 5 Lipids: HDL (mmol/L).

Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 6 Systolic Blood Pressure (kpa).

Comparison 7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone, Outcome 7 Diastolic blood pressure (kpa).

Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 1 Normalisation of fasting blood glucose at trial completion (n).

Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 2 Incidence of diabetes (n).

Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 3 Fasting blood glucose (mmol/L).
![Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test].](/es/cdsr/doi/10.1002/14651858.CD006690.pub2/media/CDSR/CD006690/image_n/nCD006690-CMP-008-04.png)
Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test].

Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 5 Total cholesterol (mmol/L).

Comparison 8 Herbal medicine versus basic education (diabetes pamphlet), Outcome 6 Trigylcerides (mmol/L).
| study ID | intervention (I) | [n] screened | [n] randomised | [n] safety | [n] ITT | [n] finishing study | [%] of randomised | comments |
| Fan GJ 2004 | I: Jiang tang bu shen plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 25 C: 26 Total: 51 | I: NR C: NR | I: NR C: NR | I: 23 C: 22 Total: 45 | I: 92 C: 85 Total: 88 | |
| Fang ZH 2007 | I: Dan zhi jiang tang jiao capsule plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 32 C: 30 Total: 62 | I: NR C: NR | I: NR C: NR | I: 32 C: 30 Total: 62 | I: 100 C: 100 Total: 100 | |
| Hao AZ 2004 | I: Xiaoke Huayu tablet plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 86 C: 82 Total: 168 | I: NR C: NR | I: NR C: NR | I: 86 C: 82 Total: 168 | I: 100 C: 100 Total: 100 | |
| Hioki C 2004 | I: Bofu‐tsusho‐san plus diet & exercise C: diet & exercise | I: NR C: NR | I: 44 C: 41 Total: 85 | I: NR C: NR | I: 41 C: 40 Total: 81 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | three participants withdrew from the intervention group due to loose bowels |
| Li CP 2004 | I: Qimai jiangtang yin plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 31 C: 33 Total: 64 | I: NR C: NR | I: 31 C: 33 Total: 64 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Liu DQ 2007 | I: Jie yu huo xue tang plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 80 C: 80 Total: 160 | I: NR C: NR | I: 78 C: 79 Total: 157 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Lu X 2005 | I: Chinese herbal medicines plus antihypertensive (vasodilator) medication C: antihypertensive (vasodilator) medication | I: NR C: NR | I: 48 C: 32 Total: 80 | I: NR C: NR | I: 48 C: 32 Total: 80 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | blocked randomisation (3 participants in the intevention group : 2 participants in the control group) |
| Qu LX 2002 | I: Tang ping san plus diet & lifestyle modification C: metformin plus diet & lifestyle modification | I: NR C: NR | I: 30 C: 30 Total: 60 | I: NR C: NR | I: 30 C: 30 Total: 60 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Shi J 2005 | I: Fufang cangzhu C: metformin | I: NR C: NR | I: 32 C: 30 Total: 62 | I: NR C: NR | I: 32 C: 30 Total: 62 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Tang QZ 2007 | I: Chinese herbal medicines plus diet & lifestyle modification C1: acarbose plus diet & lifestyle modification C2: diet & lifestyle modification | I: NR C1: NR C2: NR | I: 40 C1: 40 C2: 40 Total: 120 | I: NR C1: NR C2: NR | I: 38 C1: 38 C2: 39 Total: 115 | I: NR C1: NR C2: NR Total: NR | I: ? C1: ? C2: ? Total: ? | I1, C1: drop‐outs due to not being able to continue the medication; C2: no reason was given |
| Wang BQ 2008 | I: Qiwangping capsule C: placebo | I: NR C: NR | I: 50 C: 50 Total: 100 | I: NR C: NR | I: 48 C: 47 Total: 95 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Wang YX 2005 | I: Jinqi jiangtang plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 81 C: 78 Total: 159 | I: NR C: NR | I: 81 C: 75 Total: 156 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Wei AS 2001 | I: Xiaoke Yuye plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 36 C: 36 Total: 72 | I: NR C: NR | I: 32 C: 33 Total: 65 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Yang B 2004 | I: Tang Kang Yin plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 40 C: 36 Total: 76 | I: NR C: NR | I: 40 C: 36 Total: 76 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Yao Z 2001 | I: Tangheng I plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 22 C: 20 Total: 42 | I: NR C: NR | I: 22 C: 20 Total: 42 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Zeng YH 2000 | I: Liu wei di huang capsule plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 56 C: 55 Total: 111 | I: NR C: NR | I: 56 C: 55 Total: 111 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| Zhou DY 2003 | I: Jinqi jiangtang tablet plus diet & lifestyle modification C: diet & lifestyle modification | I: NR C: NR | I: 46 C: 42 Total: 88 | I: NR C: NR | I: 46 C: 42 Total: 88 | I: NR C: NR Total: NR | I: ? C: ? Total: ? | |
| ?: unclear; ITT = intention‐to‐treat; NR: not reported | ||||||||
| name of herbal formula | preparation | composition | study ID |
| Bofu‐tsusho‐san | powder | Scutellariae Radix, Glycyrrhizae Radix, Platycodi Radix, Gypsum Fibrosum, Atractylodis Rhizoma, Rhei Rhizoma, Schizonepetae Spica, Gardeniae Fructus, Paeoniae Radix, Cnidium Rhizoma, Angelicae Radix, Menthae Herba, Ledebouriellae Radix, Ephedrae Herba, Forsythiae Fructus, Zingiberis Rhizoma, Talcum, Natrium Sulphuricum | Hioki C 2004 |
| Yi Qi Yang Yin Huo Xue | decoction | Astragali Radix 30g, Angelicae sinensis Radix 20g, Dioscoraea Rhizoma 20g, Mori Cortex 50g, Mori Folium 30g, Mori Ramulus 30g | Lu X 2005 |
| Jian Pi Zhi Shen Huo Xue | powder | Dioscoraea Rhizoma, Crategi Fructus 30g, Astragali Radix 20g, Poria 20g, Corni Fructus 15g, Persicae Semen 10g | Tang QZ 2007 |
| Dan zhi jiang tang jiao | capsules | Moutan Cortex, Hirodu, Cuscutae Semen, Polygonati Rhizoma, Pseudostellariae Radix, plus Rehmanniae glutinosae, Corni of cinalis, Dioscoreae oppositae, Alismatis orientalis, Poriae cocos, Moutan radicis. | Fang ZH 2007 |
| Fufang cangzhu | decoction | Atractylodis Rhizoma 15g, Coicis Semen 24g, Mori Fructus 20g, Dioscoraea Rhizoma 30g, Phellodendri Cortex 10g, Litchi Semen 20g, Pheretima 10g | Shi J 2005 |
| Jiangtang bushen tang | decoction | Cibotii Rhizoma 10g, Dipsaci Radix 10g, Ligustri lucidi Fructus 15g, Ecliptae Herba 15g, Lycii Cortex 15g, Astragali Radix 15g, Rehmanniae Radix 15g, Puerariae Radix 12g, Coptidis Rhizoma 5g, Mori Cortex 10g, Moutan Cortex 6g | Fan GJ 2004 |
| Jie yu huo xue | decoction | Angelicae sinensis Radix 15g, Bupleuri Radix 15g, Polygonati odorati Rhizoma 10g, Astragali Radix 30g, Chuanxiong Rhizoma 10g, Paenoiae Radix rubra 15g, Notoginseng Radix 3g, Puerariae Radix 15g, Polygonati Rhizoma 15g, Curcumae Radix 15g, Ginseng Radix 25g, Prunellae Spica 10g; If heat in palms, thirst, increased drinking: + Anemarrhenae Rhizoma 20g, Schisandrae Fructus 10g; if restlessness, tendency to anger, insomnia: + Platycladi Semen 20g, Polygoni multiflora Caulis 15g, Coptidis Rhizoma 10g; if dizziness, stuffiness in eyes, reddish face & ears with heat: + Moutan Cortex 15g, Uncariae Ramulus cum Uncis 15g | Liu DQ 2007 |
| Jinqi Jiangtang | pill | Astragali Radix, Lonicera Flos etc | Zhou DY 2003 |
| Jinqi Jiangtang | pill | Astragali Radix, Lonicera Flos etc | Wang YX 2005 |
| Liu wei di huang wan | pill | Rehmanniae glutinosae, Corni of cinalis, Dioscoreae oppositae, Alismatis orientalis, Poriae cocos, Moutan radicis. | Zeng YH 2006 |
| Qimai jiangtang yin | decoction | Astragali Radix 20g, Puerariae Radix 20g, Ophiopogonis Radix 10g, Ligustri lucidi Fructus 10g, Notoginseng Radix 10g, Curcumae Radix 10g, Rehmanniae Radix 25g | Li CP 2004 |
| Qiweitangping | capsule | Astragali Radix, Scutellariae Radix, Perillae Fructus, Codonopsis Radix, Rhei Radix et Rhizoma, Jujubae Fructus, Rehmanniae Radix preparata, Bupleuri Radix, Salviae miltorrhizae Radix, Curcumae Radix, Artemisiae scoparie Herba, Tricosanthis Radix, Gypsum fibrosum, Plantaginis Semen, Dioscoreae Rhizoma, Schisandrae Fructus, Corni Fructus, Anemarrhenae Rhizoma, Lycii Fructus, Puerariae Radix, Lilii Bulbus, Glycyrrhizae Radix, Linderae Radix, Rehmanniae Radix, Zanthoxyli Pericarpium, Vaccariae Semen, Glycyrrhizae Radix | Wang BQ 2008 |
| Tang Kang Yin | decoction | Ginseng Radix 6g, Coptidis Rhizoma 10g, Ligustri lucidi Fructus 15g, Prunellae Spica 30g, Alumen 30g | Yang B 2004 |
| Tang ping san | powder | Astragali Radix 30g, Dioscoreae Rhizoma 10g, Rehmanniae Radix 10g, Rehmanniae Radix preparata 10g, Lycii Fructus 10g, Polygoni multiflori Radix preparata 10g, Epimedii Herba 10g, Salviae miltiorrhizae 30g, Alismatis Rhizoma 10g, Mori Folium 10g | Qu LX 2002 |
| Xiaoke huayu pian | tablet | Anemarrhenae Rhizoma, Euonymi Ramulus etc | Hao AZ 2004 |
| Xiaoke yuye | decoction | Astragali Radix, Polygonati Rhizoma, Polygoni multiflori Radix preparata, Anemarrhenae Rhizoma | Wei AS 2001 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normalisation of fasting blood glucose at trial completion (n) Show forest plot | 8 | 625 | Risk Ratio (IV, Random, 95% CI) | 2.07 [1.52, 2.82] |
| 1.1 Jiantang Bushen decoction | 1 | 45 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.04, 2.86] |
| 1.2 Xiaoke Huaya tablet | 1 | 168 | Risk Ratio (IV, Random, 95% CI) | 2.54 [1.70, 3.79] |
| 1.3 Qimai Jiangtang Yin decoction | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 2.89 [1.41, 5.90] |
| 1.4 Jian Pi Zhi Shen Huo Xue | 1 | 77 | Risk Ratio (IV, Random, 95% CI) | 1.92 [1.28, 2.90] |
| 1.5 Tang Kang Yin | 1 | 76 | Risk Ratio (IV, Random, 95% CI) | 3.75 [1.74, 8.09] |
| 1.6 Xiaoke Yuye decoction | 1 | 65 | Risk Ratio (IV, Random, 95% CI) | 1.89 [1.14, 3.14] |
| 1.7 Tang Heng I | 1 | 42 | Risk Ratio (IV, Random, 95% CI) | 5.0 [1.26, 19.87] |
| 1.8 Jinqi jiangtang | 1 | 88 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.90, 1.53] |
| 2 Incidence of diabetes (n) Show forest plot | 8 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.19, 0.58] |
| 2.1 Jiangtang Bushen tang | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.84] |
| 2.2 Xiaoke Huayu Pian | 1 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.30] |
| 2.3 Qimai Jiangtang Yin | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.39] |
| 2.4 Tang King Yin | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.46] |
| 2.5 Jian pi zhi shen huo xue | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.19, 1.84] |
| 2.6 Jinqi Jiangtang tablet | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.11, 3.47] |
| 2.7 Xiaoke Yuye | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.54] |
| 2.8 Tangheng I Plus | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.43] |
| 3 Fasting blood glucose (mmol/L) Show forest plot | 9 | 742 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.66, ‐0.16] |
| 3.1 Jiangtang Bushen decoction | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 3.2 Xiaoke Huayu tablet | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.72, ‐1.08] |
| 3.3 Qimai Jiangtang Yin formula | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.32, 0.26] |
| 3.4 Jinqi Jiangtang tablet | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.74, ‐0.42] |
| 3.5 Jian Pi Zhi Shen Huo Xue | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.35, 0.17] |
| 3.6 Xiaoke Yuye decoction | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.99, 0.21] |
| 3.7 Tang Kang Yin decoction | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.33, ‐0.09] |
| 3.8 Tang Heng I decoction | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.53, 0.05] |
| 3.9 Liu Wei Di Huang capsule | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.58, ‐0.04] |
| 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test] Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Jiangtang Bushen decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Xiaoke Huaye tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Qimai Jiangtang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Tang Heng I decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Liu Wei Di Huang capsule | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 HbA1c (%) Show forest plot | 3 | 317 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.00, 0.06] |
| 5.1 Xiaoke Huayu tablet | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.99, ‐0.27] |
| 5.2 Jian Pi Zhi Shen Huo Xue | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.43, ‐0.31] |
| 5.3 Xiaoke Yuye decoction | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.12, 0.00] |
| 6 Insulin (μU/ml) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Jiangtang Bushen decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Qimai Jiangtang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Tang Heng I decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 IAI (insulin sensitivity) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Qimai Jiangtang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Total cholesterol (mmol/L) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Jiangtang Bushen tang | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Xiaoke Huayu Pian | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Xiaoke Yuye | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Tang Kang Yin | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Liu Wei Di Huang capsule | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Lipids: HDL (mmol/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Trigylcerides (mmol/L) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Jiangtang Bushen tang | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Xiaoke Huayu Pian | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.6 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.7 Liu Wei Di Huang Wan | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Body Mass Index (kg/m2) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Jiangtang Bushen | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Tang Kang Yin decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Liu Wei Di Huang capsule | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Diastolic blood pressure (mmHg) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Liu wei di huang wan | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Jinqi jiangtang tablets | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Systolic Blood Pressure (mmHg) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Liu wei di huang wan | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Jinqi jiangtang tablets | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Main ingredient Astragalus membranecus (≥30g): Fasting blood glucose (mmol/ml) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Xiaoke Yuye decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Shan yao, shan zha, huang qi etc | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 Qimai Jiangtang Yin formula | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Hypoglycaemic effects of herbal medicines with lifestyle modification compared lifestyle modification alone Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting blood glucose (mmol/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reduction in 2hr fasting blood glucose after oral glucose tolerance test Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Reduction in HbA1c (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Trigylcerides (mmo/lL) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Insulin (mu/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Dan zhi jiang tang jiao capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Lipids: HDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Bofu‐tsusho‐san | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in 2 hr fasting blood glucose after oral glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Tangping San | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normalisation of fasting blood glucose (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Qiwei Tang Ping capsule | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of diabetes (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Qiwei Tang Ping | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Reduction in fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Body mass index (kg/m2) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Waist‐to‐hip ratio (WHR) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Qiwei tangping capsules | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Fufang Cangzhu decoction | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Triglycerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Total cholesterol Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Insulin (mU/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Waist‐to‐hip ratio (WHR) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Weight (kg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Fufang Cangzhu | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 2hr‐Glucose tolerance (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Normalisation of blood glucose (n) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Jian pi zhi shen huo xue | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Incidence of diabetes (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Jian pi zhi shen huo xue | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 HbA1c (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Insulin (FINS mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Trigylcerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Lipids: LDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Lipids: HDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Jian pi zhi shen huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in fasting glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reduction in 2hr blood glucose after oral glucose tolerance test (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Triglycerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Lipids: HDL (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Systolic Blood Pressure (kpa) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Diastolic blood pressure (kpa) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Yi qi yang yin huo xue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normalisation of fasting blood glucose at trial completion (n) Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Jinqi Jiangtang tablet | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of diabetes (n) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Jinqi Jiangtang tablet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fasting blood glucose (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 06 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Total cholesterol (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Trigylcerides (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Jinqi Jiangtang tablet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |

