Profilaxis con fármacos antiinflamatorios no esteroideos para la prevención del edema macular después de la cirugía de cataratas
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: Parallel group RCT Open‐label | |
| Participants | Country: Canada Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Clinic patient having phacoemulsification with IOL implantation in their first eye; agreed to participate. Exclusion criteria: Hypersensitivity to the NSAID drug class; aspirin/NSAID‐induced asthma; pregnancy in the third trimester. Pretreatment: More women in control group (70%) versus ketorolac group (51%), but unclear of importance of this difference. Eyes: 106 eyes of 98 patients enrolled but clinical trials registry specifies first eye surgery only. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
All participants also received gatifloxacin 0.3% (Zymar) 4 times a day for 1 week Type of surgery: phacoemulsification | |
| Outcomes | Follow‐up: 1 month
| |
| Contact details | Authors name: Sherif El‐Defrawy Institution: Queen’s University, Ontario, Canada Email: [email protected] Address: Department of Ophthalmology, Queen’s University, Hotel Dieu Hospital, Brock Wing 230A, 166 Brock Street, Kingston, Ontario K7L 5G2, Canada | |
| Notes | Funding sources: "Funded by a Queen’s University grant, Kingston, Ontario, Canada" Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: June 2006 to May 2007 (from clinical trials registry entry) Trial registration number: NCT00335439 Contacting study investigators: Not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | High risk | Quote: "open‐label non‐masked." Judgement comment: High risk of bias, given open‐label nature of trial. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: Open‐label study. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: Open‐label study. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "98 were assessed at 1 week and 80 at 1 month." Judgement comment: 38/53 (72%) in ketorolac group seen at 1 month versus 42/53 (79%) of non‐treated group. One case of CMO excluded in non‐treated group; 3 ketorolac‐related AEs excluded. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Only one outcome specified on clinical trials registry and this outcome was the main focus of the published report. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Canada Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: 18 years of age or older; cataract and were expected to have phacoemulsification with implantation of a posterior chamber IOL. Exclusion criteria: Pre‐existing retinal disease (e.g. diabetic retinopathy, vein occlusion, exudative macular degeneration); previous uveitis, previous intraocular surgery; allergy or hypersensitivity to NSAIDs. "Enrolled patients who had complicated cataract surgery (e.g. significant corneal edema, posterior capsule rupture, vitreous loss, dropped nuclear material, retained cortical material, or an IOL not placed in the capsular bag) were subsequently excluded." Pretreatment: "There were no differences in age, sex, or operative eye between the 3 groups." Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. | |
| Interventions | Intervention 1: NSAIDs plus steroids
Intervention 2: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received gatifloxacin 0.3% drops 4 times a day starting 3 days before surgery and continued for 1 week after surgery. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 1 month
| |
| Contact details | Authors name: David RP Almeida Institution: Queen's University, Ontario, Canada Email: dalmeida@evolation‐medical.com Address: Department of Ophthalmology, Queen’s University, Hotel Dieu Hospital, 166 Brock Street, Eye Centre (Johnson 6), Kingston, Ontario K7L 5G2, Canada | |
| Notes | Funding sources: "Funded by an unrestricted Queen’s University educational research grant." Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: March 2010 to May 2011 Trial registration number: NCT01395069 Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive a placebo (sterile saline drops), nepafenac 0.1%, or ketorolac 0.5%." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles that were individually made by the Kingston General Hospital Investigational Pharmacy division. Bottles concealed medication information and were labelled with study identification number, patient identification number, expiration date, and emergency contact information only." Judgement comment: Unclear if investigators involved in the treatment allocation were masked. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles that were individually made by the Kingston General Hospital Investigational Pharmacy division. Bottles concealed medication information and were labelled with study identification number, patient identification number, expiration date, and emergency contact information only." Judgement comment: Placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles that were individually made by the Kingston General Hospital Investigational Pharmacy division. Bottles concealed medication information and were labelled with study identification number, patient identification number, expiration date, and emergency contact information only." Judgement comment: Placebo‐controlled study which probably means that the outcome assessors were masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "One hundred sixty‐two patients, 54 in each arm, made up the intent‐to‐treat data set." Quote: "Ninety‐seven patients (35 placebo, 32 ketorolac, 30 nepafenac) completed the COMTOL interview questionnaire (60.0% response rate)." Judgement comment: 84% follow‐up. Not clearly reported but no evidence for any differential drop out by intervention group. 31 patients out of 193 lost to follow‐up (16%). However, only 97 patients (60%) completed the COMTOL interview questionnaire and no further breakdown of losses to follow‐up in each group provided. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: Outcomes on clinical trial registry entry (NCT01395069) were reported but the trial was retrospectively registered. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Japan Setting: 5 Eye hospitals Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Age 55 to 75 years of age; nuclear hardness of Emery‐Little grade IV or less; surgery in 1 eye only. Exclusion criteria: Acute infection or inflammation within 1 month after initiation of the study; allergy to NSAIDs, steroids, or fluorescein; history of eye trauma or intraocular disease other than cataract; pseudoexfoliation syndrome; uveitis; glaucoma; diabetes and related complications; kidney disease;asthma or chronic airway disease; uncontrolled hypertension;severe heart failure; myocardial infarction or cerebrovascular disorders; intraoperative complications such as posterior capsule rupture, vitreous loss, retained lens nucleus, or lens fragments in the vitreous. Pretreatment: None noted. Compared age, gender, duration of surgery, ultrasound time, irrigating solution and hardness of crystalline lens. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs alone
Comparator: Steroids alone
Concomitant mydriatic and antibiotic agents were permitted. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 8 weeks
| |
| Contact details | Authors name: Kensaku Miyake Institution: Shohzankai Medical Foundation, Miyake Eye Hospital Email: [email protected] Address: Shohzankai Medical Foundation, Miyake Eye Hospital, 3‐15‐68, Ozone, Kita‐ku, Nagoya, 462‐0825, Japan | |
| Notes | Funding sources: NR Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: April 2004 to September 2005 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Low risk | Quote: "The controller kept the assignment code until completion of the study." Judgement comment: This probably means that the allocation was concealed from the investigators although it was not clearly reported who the controller was exactly. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy. The controller kept the assignment code until completion of the study. The controller created an emergency code, which was given to the principal investigator in an envelope. The investigator could open the envelope if severe adverse effects developed. The test drugs were administered to each patient 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery." Judgement comment: Although not clearly stated that participants and personnel were unaware of which treatment received, the study was placebo‐controlled and efforts made to keep the allocation away from investigators so we assume that masking was done. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy. The controller kept the assignment code until completion of the study. The controller created an emergency code, which was given to the principal investigator in an envelope. The investigator could open the envelope if severe adverse effects developed. The test drugs were administered to each patient 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery." Judgement comment: Although not clearly stated that outcome assessors were unaware of which treatment received, the study was placebo‐controlled and efforts made to keep the allocation away from investigators so we assume that masking was done. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Of the 150 eyes initially included in this study, 75 were assigned to the diclofenac group and 75 to the betamethasone group. Four patients in each group dropped out of the study: 1 in each group due to complications; 3 in the diclofenac group and 2 in the betamethasone group due to a discontinuation proposal (there were patients who withdrew their consent during the course of this study); 1 in the betamethasone group for not returning to the hospital 2 weeks after surgery. Seventy‐one eyes in each group completed the study." Judgement comment: In the results text quoted follow‐up appeared to be high (95%) and equal between groups but in table 3 visual acuity results follow‐up was lower 58/75 (77%) versus 52/75 (69%) and unclear why. Judgement comment: Some of the exclusion criteria may have lead to bias if they occurred differently between two treatment groups: "acute infection or inflammation within 1 month after initiation of the study" and "intraoperative complications such as posterior capsule rupture, vitreous loss, retained lens nucleus, or lens fragments in the vitreous", however these exclusions were not reported. |
| Selective reporting (reporting bias) | High risk | Judgement comment: No access to protocol or trials registry entry but noted that data on CMO were reported only at 5 weeks, but other data available at 8 weeks follow‐up. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention group: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Undergoing phacoemulsification with posterior capsular opacification after lens (PCOL) implantation. Exclusion criteria: History of systemic or ocular inflammation (iritis, uveitis); taking oral or ophthalmic steroids or NSAIDs; other ocular disease such as glaucoma, corneal disease, or diabetic retinopathy. Pretreatment: Group differences not reported. Eyes: Unclear if one or both eyes included. | |
| Interventions | Intervention group: NSAIDs alone
Comparator: Steroids alone
All patients had gentamicin drops for 7 days postoperative. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 1 month
| |
| Contact details | Authors name: Rose Marie Brown Institution: New York Hospital ‐ Cornell Medical Center Email: NR Address: Cornell University Medical College, 520 E. 70th St, Starr 817, New York, NY 10021 | |
| Notes | Funding sources: "Supported in part from a grant from Ciba Vision Ophthalmics, Duluth, Ga." Declaration of interest: NR Date study conducted: 1991 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "We conducted a prospective, randomised study." "The patients were randomly assigned to receive..." Judgement comment: Not reported how list was generated. Study was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Study was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: For measurement of inflammation ‐ Quote: "Neither examiner knew which of the study groups the patient was enrolled in." But for other outcomes, masking not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. Unclear how many people seen at 1 month. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trials registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Mexico Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Adult patients 40 years of age or older; diagnosed with senile and/or metabolic cataract (according to the Lens Opacities Classification System LOCS III, with classification NO and NC 2–3); scheduled for surgery by phacoemulsification and IOL implantation inside the capsular bag; normal fundoscopy exam (if observance was possible). Exclusion criteria: Pregnancy or breastfeeding; history of ocular inflammatory or infectious eye disease; treatment for eye infection within 30 days prior to inclusion in the study;alterations on the eye surface (including dry eye); history of ocular surgery and/or trauma; knowledge or suspicion of allergy or hypersensitivity to the preservatives, steroids, topical NSAIDs, or any other component of the study medication; use of eye medications, including prostaglandin analogues; use of topical or systemic steroids within 30 days prior to inclusion in the study; use of topical or systemic NSAIDs within 14 days prior to inclusion in the study; non‐controlled diabetes mellitus, based on clinical history and blood glucose level (126 mg); proliferative diabetic retinopathy, and/or macular oedema; preoperative mydriasis less than 6 mm prior to the study; synechiae; ocular alteration preventing adequate mydriasis such as iris atrophy; macular alteration documented by OCT, including macular oedema of any etiology, macular holes, epiretinal membrane, macular degeneration related to age, and central serous chorioretinopathy; the use of contact lens in the eye involved during the study. Pretreatment: No differences noted; compared age, gender, operated eye, ocular and systemic pathology. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 6 weeks
Subgroup analysis by diabetes reported. | |
| Contact details | Authors name: Guadalupe Cervantes‐Coste Institution: Asociación Para Evitar la Ceguera en México I.A.P. Hospital Email: [email protected] Address: Av. México 85‐5, México City, 06100 México | |
| Notes | Funding sources: NR Declaration of interest: The authors have no conflicts of interest to disclose. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a prospective, randomised, single‐masked, single‐center, longitudinal, experimental and comparative study in patients undergoing phacoemulsification cataract surgery." Judgement comment: Not reported how list was generated. Trial described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The identity of patients receiving preoperative mydriatic or preoperative mydriatic and nepafenac was concealed from the surgeons." Judgement comment: Only the surgeons appeared to be masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement Comment: The study compared nepafenac versus no treatment so is essentially open‐label. No information was provided on masking. We assume that in absence of reporting on this outcome, assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients completed the follow‐up visits over a 6‐week period." Judgement comment: No patients appeared to have been excluded or lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Greece Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: NR Exclusion criteria: History of intraocular surgery on the eye to be operated; any previous episode of uveitis in the eye to be operated; severe systemic disease (heart failure of the New York Heart Association stage III of IV, endstage renal failure, pulmonary failure, receiving chemotherapy); regular, systemic use of steroid or NSAIDs during the last 3 months. Pretreatment: None noted; compared age, gender, baseline visual acuity, education, marital status, smoking, and various systemic ocular factors. Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: phacoemulsification | |
| Outcomes | Follow‐up: 6 weeks
| |
| Contact details | Authors name: Irini Chatziralli Institution: Department of Ophthalmology, Veroia General Hospital Email: [email protected] Address: Department of Ophthalmology, Veroia General Hospital, 28, Papanastasiou Street, GR–17342 Athens (Greece) | |
| Notes | Funding sources: NR Declaration of interest: NR Date study conducted: October 2009 to January 2010 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised to 1 of the 2 postoperative treatment arms." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was masked to the patients, i.e. they received unmarked bottles so as to be unaware of which treatment they received." |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: No information on masking of outcome assessors. We assume that in absence of reporting on this outcome, assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: Follow‐up high and reasonably equal between groups: 70/73 (96%) in NSAIDs group versus 68/72 (94%) in steroid group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Scheduled for phacoemulsification. Exclusion criteria: Known sensitivity to any ingredient in the study medications; monocular status; a history of previous intraocular surgery;diabetes mellitus; a history of uveitis, iritis, or intraocular inflammation; use of a systemic NSAID during the study or the week before surgery; or pupils that did not dilate to more than 5.0 mm before surgery or requiring mechanical pupil stretching; pregnant, nursing an infant, or planning a pregnancy. Pretreatment: "There were no significant between‐group differences in any demographic variable or baseline value." Eyes: Unclear if one or both eyes included. | |
| Interventions | Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received topical gatifloxacin 0.3% 4 times a day for 3 days before cataract surgery and for 1 week after surgery. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 3 months
| |
| Contact details | Authors name: Eric D. Donnenfeld Institution: Ophthalmic Consultants of Long Island Email: [email protected] Address: Ophthalmic Consultants of Long Island, Ryan Medical Arts Building, 2000 North Village Avenue, Suite 402, Rockville Centre, New York 11570, USA | |
| Notes | Funding sources: "Supported in part by an unrestricted grant from Allergan Inc., Irvine, California, and the Lions Eye Bank for Long Island, Long Island, New York, USA" Declaration of interest: "Drs. Donnenfeld, Perry, and Wittpenn are consultants to Allergan Pharmaceuticals. No other author has a financial or proprietary interest in any material or method mentioned." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignment was based on a random‐number‐generated protocol that was created before initiation of the study." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Placebo‐controlled, but not clear if masking was successful ‐ some of the groups had different schedules. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: Placebo‐controlled but not clear if masking was successful ‐ some of the groups had different schedules. Corneal endothelial cell counts and OCT scans were evaluated by masked specialists. It was unclear whether assessors of other outcomes were aware of the treatment allocation, or if only the specialists were affected. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Egypt Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Some inconsistencies in the data. Not clearly stated exactly number of people (eyes) randomly allocated to each group and followed up. Inclusion criteria: High risk characteristics for the postoperative development of CME, one of the risk factors for CME (beside diabetic retinopathy). History of retinal vein occlusion or presence of epiretinal membrane or preoperative use of prostaglandin analogues eye drops. Exclusion criteria: NR Pretreatment: Compared age, gender, type of diabetes, duration of diabetes, retinal vein occlusion, epiretinal membrane and prostaglandin drops. Some imbalances, e.g. more prostaglandin eye drop use in control group. Eyes: 86 eyes of 70 people. Type of surgery: Phacoemulsification | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 12 weeks
| |
| Contact details | Authors name: Moataz F Elsawy Institution: Menoufia University Hospital Email: [email protected] Address: Ophthalmology Department, Menoufia University Hospital, Menoufia, 53211, Egypt | |
| Notes | Funding sources: NR Declaration of interest: "The authors report no conflicts of interest in this work." Date study conducted: January 2011 to March 2012 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation process used four opaque envelopes in two containers. The first container had (1) for dexamethasone drops only, and (2) for combined drops, and the second container had the name of patients listed for cataract surgery on that day. Patients were randomised to one of the regimes by asking an independent person to choose one envelope from each container." Judgement comment: Unusual random allocation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation process used four opaque envelopes in two containers. The first container had (1) for dexamethasone drops only, and (2) for combined drops, and the second container had the name of patients listed for cataract surgery on that day. Patients were randomised to one of the regimes by asking an independent person to choose one envelope from each container. All patients underwent phacoemulsification (divide and conquer)." Judgement comment: Unusual allocation process. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT Open‐label | |
| Participants | Country: Japan Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Patients with diabetes undergoing small incision phacoemulsification with IOL implantation. Exclusion criteria: foveal thickness of 250 microns or more; severe diabetic retinopathy for which ocular surgery (including photocoagulation) indicated;use of topical medications for glaucoma, uveitis and other diseases that cause CMO; ocular allergies to bromfenac or steroids (steroid group); use of systemic steroids or NSAIDs; serious cardiac, cerebral or renal disease. Pretreatment: No major imbalances; compared age, gender, hypertension, blood urea nitrogen. HbA1c slightly higher in NSAIDs group. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs alone
Comparator: Steroids alone
Preoperatively, all participants received gatifloxacin (four times daily for 1 day preoperatively; on the day of surgery, they received 0.5% tropicamide, 0.5% phenylephrine hydrochloride every 30 mins 2 hours preoperatively. Postoperatively, gatifloxacin four times daily until week 6, and 0.5% tropicamide and 0.5% phenylephrine hydrochloride once daily for 1 week. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 6 weeks
| |
| Contact details | Authors name: Naoko Endo Institution: Tokyo Women’s Medical University Diabetes Centre Email: [email protected] Address: Tokyo Women’s Medical University Diabetes Centre, 8‐1 Kawada‐cho, Shinjuku‐ku, Tokyo 162‐0054, Japan | |
| Notes | Funding sources: NR Declaration of interest: "The authors have no financial interest in any aspect of this article." Date study conducted: March 2005 to May 2007 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective open‐label trial was conducted using the envelope method." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Although mentioned "envelope method", not enough information on how the allocation was administered. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: Open‐label study. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: Open‐label study. |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: 17% (13/75) of patients were excluded. Vague reasons were provided. Three were excluded because of difficulty with the OCT measurement. Ten people (10 eyes) dropped out of the study for the following reasons: poor health (8), posterior capsular rupture (1) and epidemic keratoconjunctivitis (1). No details were provided about the 'difficulties with OCT measurements' and 'poor health'. 31/40 (78%) in NSAIDs group and 31/35 (89%) in steroids group were followed‐up but reasons for dropout by group were not clearly reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Italy Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids plus placebo
Inclusion criteria: 45 to 75 years of age; age‐related cataract. Exclusion criteria: Ocular malformations; dry‐eye syndrome (Schirmer I < 5 mm); glaucoma or ocular hypertension (lOP > 22 mmHg); vitreoretinal pathology; surgical complications (posterior capsule rupture, Descemet's membrane detachment, vitreous loss, significant intraocular haemorrhage, IOL dislocation); severe systemic affections; ocular surgery in the previous 2 months or had had bilateral surgery; hypersensitive to one or more of the study compounds; pregnant or nursing woman. Pretreatment: No major imbalances in age, sex, IOP and operated eye. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs alone
Comparator: Steroids plus placebo
Type of surgery: ECCE | |
| Outcomes | Follow‐up: 140 days
| |
| Contact details | Authors name: Lucio Lobefalo Institution: NR Email: NR Address: via Gran Sasso 100, 1‐66100 Chieti, Italy | |
| Notes | Funding sources: NR Declaration of interest: "S. Bianco, MD, is a Ciba Vision Ophthalmics officer. None of the other authors has a proprietary or financial interest in diclofenac." Date study conducted: October 1992 to February 1994 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Placebo‐controlled but masking of participants not described specifically. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "In each center, all patients were observed by the same examiner; surgeons and examiners were masked at all postoperative visits." |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: Follow‐up: 118/140 (84%) in diclofenac group and 111/141 (79%) in dexamethasone group followed up. Follow‐up reasonably high and not very different between the two groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trials registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: South Korea Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids
Inclusion criteria: Males or non‐pregnant females aged between 20‐ to 80‐years‐old. Exclusion criteria: Poor general condition, including high blood pressure, poor blood glucose control, or Pretreatment: No major imbalances, age, sex, hypertension, diabetes, macular thickness and volume and ocular surface status compared. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
All patients received topical gatifloxacin 0.3% 4 times a day for 28 days. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 1 month
| |
| Contact details | Authors name: Dr. Tae‐im Kim Institution: Yonsei University College of Medicine Email: [email protected] Address: Department of Ophthalmology, Yonsei University College of Medicine, 50‐1 Yonsei‐ro, Seodaemun‐gu, Seoul 03722, Korea | |
| Notes | Funding sources: "This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology 2013R1A1A2058907)." Declaration of interest: "The authors have no financial conflicts of interest." Date study conducted: November 2013 to June 2014 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: Open‐label or no information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Eligible for extracapsular cataract extraction with implantation of a Shearing posterior chamber lens. Excluded criteria: NR Pretreatment: None noted; age, gender, follow‐up and endothelial cell density preoperative compared. Eyes: Unclear if one or both eyes included. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: ECCE and phacoemulsification (unplanned ICCE n = 19 were excluded). | |
| Outcomes | Follow‐up: between 2.5 and 12 months. Quote: "The mean interval between surgery and angiography was 4.1 months, with a range of 2.5 to 12 months. Ninety percent of the angiograms were performed between 2.5 and 5 months after surgery, and 10% between 6 and 12 months after surgery."
| |
| Contact details | Authors name: Manus C Kraff Institution: Abraham Lincoln School of Medicine, University of Illinois Email: NR Address: 5600 W. Addison Street, Chicago, IL 60634 | |
| Notes | Funding sources: Core Grant EY 1792 NEI Bethesda Maryland Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Randomisation was using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: Quote: "The study was double‐masked; neither the physician nor the patient knew what drops the patient was receiving." |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: Quote: "The study was double‐masked; neither the physician nor the patient knew what drops the patient was receiving." Quote: "The angiograms were read in a masked fashion by a retired specialist (LMJ) who had no knowledge of either the drug regimen or the type of surgical procedure." |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Some patients were excluded (n = 19) and not reported: two with vitreous loss, two with vitreous pressure and a shallow anterior chamber and 15 with possible rupture of the posterior capsule. Unclear which groups these were in. Follow‐up high for visual acuity (> 95%) but lower for CMO (60% in indomethacin group versus 64% in placebo). |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: China Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Included criteria: Diabetes mellitus type 2 patients who received phacoemulsification together with artificial lens implants intervention. Excluded criteria: Diabetic retinopathy, age‐related macular degeneration, epiretinal membrane and retinal vascular disorders. Pretreatment: Unclear if group differences. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 1 month
| |
| Contact details | Authors name: Min‐Chao Li Institution: Department of Ophthalmology, Affiliated Nanhai Hospital of Southern Medical University, Foshan Email: [email protected] Address: Department of Ophthalmology, Affiliated Nanhai Hospital of Southern Medical University, Foshan 528200, Guangdong Province, China | |
| Notes | Funding sources: NR Declaration of interest: NR Date study conducted: January 2009 to December 2010 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: As per translation: "Unclear, not specified if there was any participant withdrawal or lost during the study period." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Planning to have cataract surgery by KLC at the Ambulatory Care Center, the University of North Carolina Hospitals. Exclusion criteria: Medically treated diabetes mellitus; history of uveitis;use of topical prostaglandin analogues for glaucoma; history of earlier intraocular surgery in the same eye; retinal vascular disease; macular degeneration;abnormal preoperative OCT measurements. Pretreatment: Nepafenac group were slightly older, similar gender, preoperative VA, follow‐up time, slightly longer phaco time. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
All participants received nepafenac 0.01% drops in the operated eye thrice, 5 mins apart, immediately before surgery to maintain pupillary dilation and postoperatively, moxifloxacin 0.5% four times a day for 10 days. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 2 months
| |
| Contact details | Authors name: KL Cohen Institution: School of Medicine, University of North Carolina Email: [email protected] Address: Department of Ophthalmology, School of Medicine, University of North Carolina at Chapel Hill, 5100 Bioinformatics Building, 130 Mason Farm Road, CB no. 7040, Chapel Hill, NC 27599–7040, USA | |
| Notes | Funding sources: "This work was supported in part by Research to Prevent Blindness, Inc., New York, NY." Declaration of interest: "Kenneth C Mathys and Kenneth L Cohen have no financial interest." Date study conducted: June 2007 to April 2008 Trial registration number: NCT00494494 Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised according to the even/odd subject identification number, using computer‐generated random numbers, to the control group (standard of care only) or the treatment group (standard of care plus nepafenac)." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "were consecutively enrolled in this randomised, non‐masked, parallel‐group clinical trial." Judgement comment: Participants were not masked. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "At the 2 months visit, technicians, who were masked to treatment, measured ETDRS BCVA, and OCT scans were performed." Judgement comment: Experienced ophthalmic photographers, who were masked to treatment, obtained Stratus OCT (Carl Zeiss Meditec, Inc., San Francisco, CA, USA) scans using the fast macular thickness protocol. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The mean time to follow‐up was 73.31 days ( ± 21.58 SD, range 55‐146) in the treatment group and 68.98 days ( ± 13.98, range 50‐120) in the standard‐of‐ care group." Judgement comment: 39/42 (93%) of intervention group and 40/42 (95%) of comparator group followed‐up. Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Outcomes on trial registry entry were reported. |
| Methods | Study design: Randomised control trial | |
| Participants | Country: Japan Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Age 50 to 70 years; subjected for unilateral surgery or to have 6 months’ span between surgeries in patients with bilateral cataract. Exclusion criteria: Eyes encountering acute ocular infection or inflammation during the first month of the study; eyes showing sensitivity to diclofenac or fluorometholone; eyes showing sensitivity to fluorescein sodium; eyes with insufficient dilation, (pupil diameter 4 mm) and with hazy media affecting laser Doppler flowmetry (LDF); eyes with history of other ocular surgeries; eyes with pseudoexfoliation syndrome; history of trauma; uveitis, glaucoma or other disorders; complication of diabetes and kidney disorders; heart failure, cardiac infarction, and cerebrovascular disease; uncontrollable hypertension; rupture of the posterior capsule, vitreous loss, and other complications during a cataract/IOL implantation procedure. Pretreatment: No major imbalances; compared age and sex. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs alone
Comparator: Steroids alone
Quote "Other topical drugs used before and after surgery included mydriatics and antibiotics only." Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 5 weeks
| |
| Contact details | Authors name: Kensaku Miyake Institution: Shohzankai Medical Foundation, Miyake Eye Hospital Email: [email protected] Address: Miyake Eye Hospital, 3‐15‐68, Ozone, Kita‐ku, Nagoya 462‐0825, Japan | |
| Notes | Funding sources: NR Declaration of interest: Reported none for all authors. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each patient was randomly assigned to one of the two groups by one of the authors (SA), using the envelope method." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each patient was randomly assigned to one of the two groups by one of the authors (SA), using the envelope method." Judgement comment: Reported that envelopes used but unclear if they were sequentially numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Study described as being "conducted in a prospective, double‐masked, randomised manner." Patients probably masked not clearly described. |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: Fluorescein angiography and laser flarimetry assessed by masked observers and analysis was masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 25/31 (80%) of eyes in both groups were followed up and reasons for loss to follow‐up did not appear to be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Japan Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Aged over 20 years; phacoemulsification cataract extraction and IOL implantation between October 2007 and April 2008 at Shohzankai Medical Foundation, Miyake Eye Hospital. Exclusion criteria: Systemic, topical, or ointment steroidal agents within 14 days of surgery; had had an intraocular or periocular injection of steroidal agents within 90 days of surgery; had taken systemic or topical NSAIDs within 7 days of surgery; had a history of ophthalmic surgery (including laser surgery) or of ocular trauma that could affect the study results; had pseudoexfoliation syndrome; had a history of chronic or recurring ocular inflammation (e.g. uveitis or scleritis); had diabetic retinopathy; had an ocular anomaly (e.g. aniridia, congenital cataract); had iris atrophy; had disorders that would preclude improvement in visual function; had macular oedema; had severe corneal epithelial disorder (e.g. corneal ulcer); had no visual function in the contralateral eye; were scheduled to have other ocular surgery from baseline to 5 weeks after cataract surgery; had secondary IOL implantation, were allergic to or might have been sensitive to NSAIDs, amfenac, or fluorometholone; had a positive skin reaction to fluorescein; had a tendency to bleed or were currently on anticoagulants; had had prostaglandin‐type treatment for glaucoma within 4 days of surgery; had been included in a previous study of prostaglandin type antiglaucoma drugs; had joined another clinical study within 30 days of the study; had ocular infection, had uncontrollable diabetes mellitus; had severe liver, kidney, or heart disorder; might have been pregnant or were currently breastfeeding; had other factors determined to be unsuitable for the study. Pretreatment: No major imbalances. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs alone
Comparator: Steroids alone
Levofloxacin ophthalmic solution 0.5% (Cravit) was applied to each eye 5 times before surgery and 3 times a day after surgery for 2 weeks. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 5 weeks
| |
| Contact details | Authors name: K Miyake Institution: Shohzankai Medical Foundation, Miyake Eye Hospital (K.Miyake, Ota, G.Miyake), Nagoya, and TokyoMetropolitan Geriatric Hospital (Numaga), Tokyo, Japan Email: [email protected] Address: Shohzankai Medical Foundation, Miyake Eye Hospital, 3‐15‐68, Ozone, Kita‐ku, Nagoya, 462‐0825, Japan | |
| Notes | Funding sources: NR Declaration of interest: "Drs. Miyake and Numaga are consultants to Alcon Japan Ltd." Date study conducted: October 2007 to April 2008 Trial registration number: NR Contacting study investigators: Primary investigator emailed to confirm how patients allocated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The 2 drugs had identical outer appearances and could not be differentiated. The same physician (J.N.) served as the medical monitor and assigned 1 of the drugs to each patient." Judgement comment: Unclear if allocation concealed from person recruiting participants. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome: 28/30 (93%) in nepafenac group and 27/30 (90%) in the fluorometholone group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Japan Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Scheduled to undergo routine phacoemulsification combined with IOL. Exclusion criteria: Corneal disease; glaucoma; uveitis; pseudoexfoliation syndrome; diabetes; other pathologies that might affect treatment responses or evaluations; systemic or topical anti‐inflammatory therapy within 1 month prior to surgery. Pretreatment: Quote: "There were no significant differences between groups in gender or age." Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Intervention: NSAIDs alone
Comparator: Steroids alone
All participants received 0.5% levofloxacin eyedrops four times daily until 2 months after surgery, and 0.5% tropicamide and 0.5% phenylephrinehydrochloride once daily for 2 weeks. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 2 months
| |
| Contact details | Authors name: Masaru Miyanaga Institution: Miyata Eye Hospital Email: miyanaga@miyata‐med.ne.jp Address: Miyata Eye Hospital, 6‐3 Kurahara, Miyakonojo, Miyazaki 885‐0051, Japan | |
| Notes | Funding sources: NR Declaration of interest: NR Date study conducted: February 2006 to August 2006 Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Only 1 patient was withdrawn from the study from the steroid only group due to CMO 1 month postop. Otherwise follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Greece Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Patients requiring phacoemulsification cataract surgery. Exclusion criteria: Presence of corneal abnormalities; history of intraocular surgery; preoperative ECC < 1500 cells/mm2; history of uveitis, diabetes, and age‐related macular degeneration; regular, systemic use of steroid or NSAIDs during the previous 3 months; and intraoperative complications, such as posterior capsule rupture, vitreous loss, lost nucleus, zonule dehiscence, and wound leak. Pretreatment: No major imbalances noted. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 1 month
| |
| Contact details | Authors name: Irini P. Chatziralli Institution: Department of Ophthalmology University of Athens Email: [email protected] Address: Department of Ophthalmology, University of Athens, 28 Papanastasiou street 17342 Athens, Greece | |
| Notes | Funding sources: NR Declaration of interest: "No competing financial interests exist." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised (through random number generation) to 1 of the 2 postoperative treatment arms." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Germany Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: No complication during surgery; fluorescein angiography can be done; compliance of the patient is very probable. Exclusion criteria: Exudative maculopathy; diabetic retinopathy; prior uveitis; glaucoma; allergic reaction on fluorescein angiography; systemic steroid treatment; therapy with non‐steroid antiphlogistics; treatment with anticoagulation. Pretreatment: Age and gender comparable. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received antibiotic eye drops for the first 4 days after surgery. Type of surgery: ICCE | |
| Outcomes | Follow‐up: not reported, assume 180 days as this is duration of treatment.
| |
| Contact details | Authors name: CD Quentin Institution: Uni Augenklinik Göttingen Email: NR Address: Uni Augenklinik GöttingenRobert‐Koch‐Straße 40, D‐3400 Göttingen, Germany | |
| Notes | Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up missing data > 20% but follow‐up equal in both groups: 57/90 (63%) followed up in diclofenac group and 55/89 (62%) in the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Italy Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Extracapsular cataract extraction (ECCE) with implantation of an IOL. Exclusion criteria: Diabetes; glaucoma; maculopathy; on systemic steroids, acetazolamide, or NSAIDs. Pretreatment: Age, gender and preoperative visual acuity were compared. Higher proportion of women in the diclofenac group (71%) compared with the placebo group (57%). Otherwise groups were similar. Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: ECCE | |
| Outcomes | Follow‐up: 6 months
| |
| Contact details | Authors name: Nicola Orzalesi Institution: Clinica Oculistica Universitti di Milano, Istituto di Scienze Biomediche, Ospedale San Paolo Email: NR Address: Clinica Oculistica Universitti di Milano, Istituto di Scienze Biomediche, Ospedale San Paolo, Via di Rudini 8,20142 Milano, Italy | |
| Notes | Funding sources: NR Declaration of interest: None of the authors has a proprietary interest in the instruments or materials mentioned. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Randomisation was obtained using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “double‐masked” but with no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: anterior chamber cell and flare and fluorescein angiography was performed by masked evaluations. No indication if the rest of the exam (visual acuity assessment (Snellen chart), slit‐ lamp biomicroscopy, lOP measurement by applanation tonometry, and ophthalmoscopic evaluation was performed by masked evaluators. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: Follow‐up not explicitly reported. However, demonstrated in several tables (such as in Table 5 (% of patients in the calculation of mean (SD) postoperative VA)). None of these were < 80%. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Diabetic (type 1 or type 2); 18 years and older; existing diagnosis of nonproliferative diabetic retinopathy that required cataract extraction with planned implantation of a posterior chamber IOL; at least 50% of all enrolled patients were required to have moderate to severe nonproliferative diabetic retinopathy, as defined by the International Clinical Diabetic Retinopathy Disease Severity Scale 2. Exclusion criteria: Significant corneal staining scores at baseline; history of dry eye syndrome; other conditions that may have caused macular oedema, including pre‐existing histories of retinal vein occlusions, ocular surgeries, inflammatory eye diseases, ocular infections, congenital ocular anomalies, and ocular traumas; central subfield macular thickness 250 microns or more; baseline cysts, and the presence of macular traction and epiretinal membranes; use of concomitant medications such as topical or systemic NSAIDs and steroids. Pretreatment: No major group differences. Compared age, gender, ethnic group, iris colour, NPDR classification. visual acuity. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Interventions Type of surgery: NR but presumably was phacoemulsification as USA study conducted 2008. | |
| Outcomes | Follow‐up: 90 days
| |
| Contact details | Authors name: Rishi Singh Institution: Cole Eye Institute, Cleveland Clinic Foundation, Email: [email protected] Address: Cole Eye Institute, Cleveland Clinic Foundation, 9500 Euclid Avenue, i‐32 Cleveland, OH 44195, USA | |
| Notes | Funding sources: NR Declaration of interest: "RS, LA, GJJ, RPL, JL, HJR, KS, and TW are paid consultants for Alcon Research Ltd (Fort Worth, TX). DS is an employee of Alcon Research, Ltd. Medical writing support, which was funded by Alcon Research Ltd, was provided by Cullen T Vogelson and Usha Sivaprasad, of Illuminated Research LLC (Fort Worth, TX)." Date study conducted: November 2008 and July 2010 Trial registration number: NCT00782717 Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a multicenter, randomised, double‐masked, vehicle‐controlled, parallel‐group study" Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: Study was double‐masked with a placebo consisting of vehicle only. It was not clearly stated whether the masking was likely to have been effective but we have assumed that it was. |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: Study was double‐masked with a placebo consisting of vehicle only. It was not clearly stated whether the masking was likely to have been effective but we have assumed that it was. Quote: "Total macular volume was determined from a 6 mm diameter circle centered on the foveal center. Morphological features, including intraretinal cysts, were analyzed by the reading center in a masked fashion." |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 125/133 (94%) in nepafenac group included in the analysis compared with 126/130 (97%) in control group. Missing data less than 20%. 95%‐96% of patients enrolled included in final analysis. However, 8 patients in the Nepafenac group and 4 patients in the Vehicle group excluded from final analysis. Reasons not clearly explained. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Outcomes on trial registry entry were reported. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Canada (8 sites) and Germany (2 sites) Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Unilateral extracapsular cataract extraction (by manual nuclear expression) with posterior chamber lens implantation. Exclusion criteria: Taking aspirin, topical epinephrine, systemic or topical cyclo‐oxygenase inhibitors, or oral corticosteroid; allergic to cyclo‐oxygenase inhibitors; history of chronic intraocular inflammation; pre‐existing macular pathology; history of herpetic keratitis; corneal or vitreous opacity; non‐compliant patients. Pretreatment: No major imbalances in age, gender, ethnic group. Eyes: One eye, this was the eye scheduled for unilateral extracapsular cataract extraction. | |
| Interventions | Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Duration postoperative: days ‐ the investigator had the option of extending the treatment for an additional 3 months. This option was chosen for 10.9% (25/230) of vehicle‐treated patients, 8.4% (20/238) of flurbiprofen‐treated patients, and 9.7% (22/227) of indomethacin‐treated patients. Concomitant medications included aminoglycoside antibiotics (100% of patients) and topical corticosteroids (prednisolone acetate 1% or dexamethasone sodium phosphate 0.1%) in 88.7% (204/230) of vehicle treated patients, 87.8% (209/238) of flurbiprofen treated patients, and 88.1% (200/227) of indomethacin‐treated patients. Type of surgery: ECCE | |
| Outcomes | Follow‐up: 6 months
| |
| Contact details | Authors name: Leon D Solomon Institution: NR Email: NR Address: NR | |
| Notes | Funding sources: Supported by Allergan, Inc., Irvine California Declaration of interest: None of the Flurbiprofen‐CME Study Group members has a commercial or proprietary interest in 0.03% flurbiprofen or 1% indomethacin. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised, double‐masked” but with no further details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Described as “double‐masked”. Medications were masked and fluorescein angiograms were read in a masked fashion by 2 retinal specialists. Uncertain if the operating surgeons or clinicians involved in follow‐up were masked to the allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: Each fluorescein angiogram was read in a masked fashion by two retinal specialists. Unclear if treating ophthalmologists involved in other aspects of patient care were also masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up: 177/226 (78%) in flurbiprofen group, 177/234 (76%) in indomethacin group, 160/221 (72%) in placebo group. Reasons for loss to follow‐up not described. |
| Selective reporting (reporting bias) | High risk | Judgement comment: No access to protocol or trials registry entry. Not all follow‐up points were reported fully. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: NR Exclusion criteria: NR Pretreatment: Groups differences not reported. Eyes: Unclear if one or both eyes included. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: NR | |
| Outcomes | Follow‐up: 30 days (3 month follow‐up mentioned but not reported)
| |
| Contact details | Authors name: S Tauber Institution: Ophthalmology, St. John's Hospital and Clinics, Springfield, MO Email: NR Address: Ophthalmology, St. John's Hospital and Clinics, Springfield, MO | |
| Notes | Funding sources: Alcon Laboratories, Inc. Declaration of interest: "Commercial Relationships S. Tauber, Alcon, F; Alcon, R; J. Gessler, None; W. Scott, None; C. Peterson, None; P. Hamlet, None." Date study conducted: NR Trial registration number: NR Contacting study investigators: Abstract only, authors contacted by email regarding publication of full study results but no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | High risk | Judgement comment: Some outcomes not reported including 3‐month OCT outcomes. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Brazil Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Nuclear cataract density of 2 and 3 determined by LOCS II; ( > 50 years old); indication for cataract surgery with IOL implantation under local anaesthesia. Excluded criteria: Diabetes; NSAID use; use of topical eye drops (including antiglaucoma drugs); uveitis; macular disease; pseudoexfoliation syndrome; congenital ocular abnormalities; cataract density of 1 and 4 determined by LOCS II; previous intraocular surgery; previous injections; complications during cataract surgery (e.g. posterior capsule rupture, vitreous loss, retained cortical material, or an IOL not placed in the capsular bag); not follow instructions or if they did not show up for appointments. Pretreatment: No major imbalances in age, gender and visual acuity. Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 5 weeks
| |
| Contact details | Authors name: Dr. Flavia G. Ticly Institution: Department of Ophthalmology, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil Email: [email protected] Address: Department of OphthalmologyUniversity of Campinas (UNICAMP)P.O. Box 6111Campinas 13083‐970, Sao Paulo, Brazil | |
| Notes | Funding sources: NR Declaration of interest: Reported no competing financial interests exist. Date study conducted: February 2011 to March 2012 Trial registration number: NTC01542190 Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Each of the 2 intervention groups received 50 different numbers from a random number table. These numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. Unclear how this would work. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. Unclear how this concealed the allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: Placebo‐controlled study. We assume the masking was effective. |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: Placebo‐controlled study. We assume the masking was effective. It was stated that the surgeon and the ophthalmologist who collected the data were not aware of the group assignment of the patients. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 89% follow‐up. Five patients (10%) did not complete the trial in the placebo group while five patients (11%) did not complete the study in the ketorolac group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Turkey Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Patients with unilateral cataracts. Exclusion criteria: Diabetes; rheumatoid disease; immunological disease; uveitis; glaucoma; ARMD; retinitis pigmentosa; retinal detachment; NSAIDs use; corticosteroid use; diuretic use; antihistaminics; previous eye surgery; surgical complications (e.g.. posterior capsular tear, vitreous loss, iatrogenic iridodialysis); combined surgery; postoperative complications (e.g.. iris capture, retinal detachment, choroidal detachment); non‐compliance with medications; use of systemic steroids or NSAIDs during the follow‐up period; definite posterior capsule opacification. Pretreatment: No differences in age sex, and hypertension. Eyes: One eye, people with unilateral cataracts recruited. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
At the end of surgery all participants had subconjunctival injection of dexamethasone and gentamicin. All participants used 0.03% tobramycin eye drops postoperatively 4 times a day for 14 days. Type of surgery: ECCE | |
| Outcomes | Follow‐up: 2 months
| |
| Contact details | Authors name: Murat Tunc Institution: Dokuz Eylul University Medical School Email: NR Address: Dokuz Eylul University Cumhuriyet Blv No:144, 35210 Alsancak/İzmir, Turkey | |
| Notes | Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | No information on masking. We assume that in absence of reporting on this participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The angiograms were read by the retina unit (Dr Saatchi); the patients' names and treatment protocols were kept hidden. Judgement quote: No other information on other outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement Comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement Comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Brazil Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Older than 40 years; age‐related cataract; normal ophthalmological exam. Exclusion criteria: Previous ocular surgery; central endothelial cell count < 2000 cells/mm2; glaucoma or IOP > 21 mmHg; amblyopia; retinal abnormalities; steroid or immunosuppressive treatment; connective tissue diseases; allergy or hypersensitivity to NSAIDs; enrolled patients with complicated cataract surgery (e.g. posterior capsule rupture, vitreous loss or an IOL not placed in the capsular bag). Pretreatment: Group differences at baseline not reported. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received moxifloxacin 0.5% 4 times a day 2 days before surgery and 7 days postoperatively. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 12 weeks for some outcomes, 30 days for others
| |
| Contact details | Authors name: Patrick F Tzelikis Institution: Brasilia Ophthalmologic Hospital Email: [email protected] Address: Brasilia Ophthalmologic Hospital, HOB, SQN 203, bloco K, apart 502, Brasilia, DF 70833‐110, Brazil | |
| Notes | Funding sources: NR Declaration of interest: Reported no competing interests Date study conducted: June 2013 to October 2013 Trial registration number: NCT02084576. Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned in a 1:1:1 ratio to one of three treatment groups using a computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Low risk | Quote: "All investigators were masked with regard to treatment group." |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: Placebo‐controlled. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All investigators were masked with regard to treatment group." Judgement comment: Placebo‐controlled. |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up by intervention group not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: Trial study protocol registered at NCT02084576 but does not clearly define outcomes. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Switzerland Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Intracapsular cataract extraction (124 persons); 40 patients with IOL implantation after cataract extraction. Excluded criteria: Maculopathy; diabetic retinopathy; prior uveitis; systemic steroid therapy. Pretreatment: Unclear if groups comparable. Eyes: Unclear if one or both eyes included. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Additional for all participants: cycloplegics (atropine 1%); if necessary timoptic or diamox to lower eye pressure. Type of surgery: ECCE (40) ICCE (124) | |
| Outcomes | Follow‐up: 12 weeks
| |
| Contact details | Authors name: U Umer‐Bloch Institution: University Augenklink Zurich Email: NR Address: University Augenklinik, Ramistrasse 100, CH‐8091 Zurich | |
| Notes | Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation was administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: Medication placed by nurses in a bottle with suspension: one with indomethacin another with vehicle. Neither the examiner nor the patient knew the contents of the bottle. |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: Placebo‐controlled using vehicle only. Patients, nurses, physician analysing fluorescein angiography were masked. |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: For 35 patients the study was stopped before the end of the study because of intra‐operative complications or they had, as only later recognized, an exclusion criteria as defined as maculopathy, diabetic retinopathy, prior uveitis or a systemic steroid therapy. Not reported to which groups these patients belonged. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT Open label | |
| Participants | Country: China Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Age‐related cataract patients undergoing phacoemulsification with posterior chamber IOL implantation. Exclusion criteria: Any ocular diseases that might affect treatment responses or evaluations, such as corneal disease, glaucoma, uveitis, retinal detachment, optic neuropathy or amblyopia; any systemic diseases that might affect treatment responses or evaluations, such as diabetes mellitus; potentially pregnant women; systemic or topical anti‐inflammatory therapy within 1 month prior to surgery and contraindication of oral steroids, such as patients with peptic ulcer, cancer and tuberculosis; surgical complications, such as posterior capsule rupture or hyphema; special diseases which might affect surgery in the eyes, such as limitation of pupil dilation. Pretreatment: Groups were not compared. Eyes: Not clearly reported but probably one eye per person, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus (oral) steroids
Comparator: Steroids alone
All participants received levofloxacin eye drops (Santen PharmaceuticalCo., Ltd) 4 times a day for 1 day preoperatively and 7 days postoperatively. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 2 months
| |
| Contact details | Authors name: Ke Yao Institution: Medical College of Zhejiang University Email: [email protected] Address: Eye Center, 2nd Affiliated Hospital Medical College of Zhejiang University Hangzhou 310009 (China) | |
| Notes | Funding sources: "This study was supported by grants from Zhejiang Key Innovation Team Project of China (grant no. 009R50039) and Zhejiang Key Laboratory Fund of China (No.2011E10006)." Declaration of interest: NR Date study conducted: October 2010 to December 2011 Trial registration number: NR Contacting study investigators: Trial authors not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly and prospectively assigned into four groups (OBS1, OBS2, OFM and ODM) by a random‐numbers table." |
| Allocation concealment (selection bias) | High risk | Judgement comment: The drugs were applied topically to the assigned patients open‐label. The same physician served as the medical monitor and assigned one of the drugs to each patient. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: The drugs were applied topically to the assigned patients open‐label. The same physician served as the medical monitor and assigned one of the drugs to each patient. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: The drugs were applied topically to the assigned patients open‐label. The same physician served as the medical monitor and assigned one of the drugs to each patient. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up was 83/120 (69%) in NSAIDs group and 84/120 (70%) in the steroid group. Significant loss to follow‐up but similar in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Scheduled to undergo cataract surgery; 20/20 BCVA potential without any evidence of macular abnormality, including age‐related macular changes, epiretinal membranes, or other retinal‐vascular anomalies. Exclusion criteria: Systemic diseases with ocular manifestations of the disease (e.g. diabetic patients with normal retinal exams were not excluded); vitreous loss or capsular disruption/rupture occurred during surgery; postoperative day 1, the surgeon felt the amount of inflammation was greater than expected and, in his best clinical judgment, more aggressive anti‐inflammatory treatment was indicated. Pretreatment: Quote: "There were no statistically significant between‐group differences in any demographic variable." But no data reported. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
The comparator group: "...also received four drops of ketorolac 0.4% one hour prior to cataract surgery." Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 4 weeks
| |
| Contact details | Authors name: John R. Wittpenn Institution: State University of New York at Stony Brook Email: [email protected] Address: State University of New York at Stony Brook, 2500 Route 347, Building 24, Stony Brook, NY 11790 | |
| Notes | Funding sources: "This study was supported by an unrestricted education grant from Allergan Inc, Irvine, Calfiornia." Declaration of interest: "The authors indicate no financial conflict of interest." Date study conducted: June 2005 to August 2006 Trial registration number: NCT00348244 Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in a 1:1 ratio using a randomly generated list of patient identification numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "A central coordination center (IMEDS Inc, Riverside, California, USA; [M.E.]) generated the allocation sequence, enrolled participants, and assigned participants to their treatment groups." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The patients and technical staff were unmasked because regulations prevented the medications from being repackaged into similar, unmarked bottles. The labels were covered but the technicians were capable of recognizing the bottle color and shape. Patients, however, would only have been unmasked if they researched the type and shape of the different bottles." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All investigators were masked with regard to treatment group." |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: Very low follow‐up at 6 weeks. "Of the 546 patients who entered the study, 77 patients also returned for the week‐6 visit, 35 in the ketorolac/steroid group and 42 in the steroid group." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol and trial registry entry did not include outcomes. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Patients undergoing intracapsular cataract extraction. Excluded criteria: Undergone procedures other than conventional ICCE; pre‐existing macular disease predisposing to macular oedema, such as neovascular age‐related macular degeneration. Pretreatment: Baseline comparisons not reported. Eyes: 21 people had bilateral cataract surgery ‐ the second eye was randomised separately. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Quote: "Routine postoperative drops such as cycloplegics, antibiotics and steroids were also given as was the custom of the operating ophthalmologist." Type of surgery: ICCE | |
| Outcomes | Follow‐up: 1 year
| |
| Contact details | Authors name: Lawrence A Yannuzzi Institution: Manhattan Eye, Ear and Throat Hospital Email: NR Address: Manhattan Eye, Ear and Throat Hospital 210 E 64th St, New York, NY 10021, United States | |
| Notes | Funding sources: LuEster Mertz Retinal Research Fund of the Eye, Ear and Throat Hospital Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Allocation was described as being done “in a random fashion" but with no further details. |
| Allocation concealment (selection bias) | High risk | Judgement comment: Pharmacist involved in giving treatment did not appear to be masked to treatment. |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: Placebo‐controlled study described as "double‐masked". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: Placebo‐controlled study described as "double‐masked". |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: Follow‐up 59% in both groups. High loss to follow‐up at 1 year 38/100 (38%) in NSAIDs group and 50/131 (38%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Turkey Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: NR Exclusion criteria: History of intraocular surgery; any complication during cataract surgery; glaucoma; uveitis; vitreoretinal pathology; history of diabetes mellitus, hypertension, or cardiac disease; or topical or systemic drug use. Pretreatment: Some imbalances in age and sex but unclear if important. Eyes: Right eye only included. | |
| Interventions | Intervention: NSAIDsplus steroids
Comparator: Steroids alone
All participants received 1 drop of topical antibiotic (ofloxacin 0.3%) 4 times a day daily for 1 week. Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 3 months
| |
| Contact details | Authors name: Guliz Yavas Institution: Afyon Kocatepe University Email: [email protected] Address: P.K. 25, 06502 Bahcelievler, Ankara, Turkey | |
| Notes | Funding sources: NR Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised into 3 groups." Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Fluorescein angiography was performed in all patients, and fluorescein leakage to diagnose angiographic CME was evaluated by a masked observer." Judgement comment: Unclear if other outcomes were masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Diabetic patients having cataract surgery. Exclusion criteria: NR Pretreatment: Group differences not reported. Eyes: Unclear if one or both eyes included. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: NR | |
| Outcomes | Follow‐up: 12 weeks
| |
| Contact details | Authors name: C Yung Institution: Indiana University Email: NR Address: Indiana University107 S Indiana Ave, Bloomington, IN 47405, United States | |
| Notes | Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Abstract only, tried to contact authors but could not find email address. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: Placebo‐controlled but no information on who was masked. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: Placebo‐controlled but no information on who was masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: Sweden Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: 45 and 85 years of age; cataract surgery under local anaesthesia; translucent cataract for good‐quality OCT scans of the macular at baseline. Exclusion criteria: Small pupils (< 5.0 mm after pharmacologic dilation); dark brown irides; exfoliation syndrome, history of uveitis; glaucoma; macular degeneration; vision impairing eye disorder except cataract; diabetic patients; pregnant women; patients using topical or systemic anti‐inflammatory treatment; hypersensitivity to any of the given study treatments; intraoperative difficulties (e.g. loose zonular fibres, extended operating time, residual cortical material); intraoperative complications (e.g. posterior capsule rupture and vitreous loss). Pretreatment: No major imbalances, age, gener and operated eye compared. Eyes: One eye, unclear how selected. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 6 weeks
| |
| Contact details | Authors name: Anna Zaczek Institution: Scanloc Healthcare AB Email: anna. [email protected] Address: Scanloc Healthcare AB, Lilla Bommen 6, 411 04 Gothenburg, Sweden | |
| Notes | Funding sources: Supported by Alcon Research Ltd, Fort Worth, Texas, USA, and S.A. Alcon‐Couvreur N.V. Puurs, Belgium, which produced and provided the masked eyedrop bottles. Partially supported by Alcon, Inc. Sweden. Financial support was also provided through the regional agreement on Medical training and Clinical research (ALF) between Stockholm County Council and Karolinska Institutet (20120623). Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Low risk | Quote: "All products used in this clinical trial were produced, labelled, packaged, and released by S.A. Alcon‐Couvreur N.V. Puurs, Belgium. Nepafenac and placebo suspensions were supplied in identical bottles labelled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All products used in this clinical trial were produced, labelled, packaged, and released by S.A. Alcon‐Couvreur N.V. Puurs, Belgium. Nepafenac and placebo suspensions were supplied in identical bottles labelled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All products used in this clinical trial were produced, labelled, packaged, and released by S.A. Alcon‐Couvreur N.V. Puurs, Belgium. Nepafenac and placebo suspensions were supplied in identical bottles labelled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents". |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
| Methods | Study design: Parallel group RCT | |
| Participants | Country: China Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Included criteria: NR Excluded criteria: NR Pretreatment: No information on pretreatment differences. Eyes: 220 eyes of 198 people. | |
| Interventions | Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification | |
| Outcomes | Follow‐up: 1 month
| |
| Contact details | Authors name: Zhang HY Institution: Beijing Tongren Eye Center Email: NR Address: Beijing Tongren Eye Centre, Beijing Tongren Hospital, Capital Medical University; Beijing Ophthalmology and Visual Science Key Laboratory, Beijing 100730, China | |
| Notes | Funding source: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
AE: adverse events
BCVA: best corrected visual acuity
CMO: cystoid macular oedema
CRT: corneal retinal thickness
DR: diabetic retinopathy
ECCE: extracapsular cataract extraction
IOL: intraocular lens
IOP: intraocular pressure
NR: not reported
NSAID: non‐steroidal anti‐inflammatory drug
OCT: optical coherence tomography
RCT: randomised controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not topical treatment. | |
| Not RCT. | |
| Study only performed follow‐up for 2 weeks in total. | |
| Not able to source paper. | |
| Not RCT. | |
| Not able to source paper. | |
| Not relevant comparator. | |
| Study was terminated due to lack of funding. | |
| Probably not random allocation, unclear response from study author. | |
| Not relevant intervention. | |
| Not relevant intervention. | |
| Not able to source paper. | |
| Not able to source paper. | |
| Not topical treatment. | |
| Not relevant intervention. | |
| Not RCT. | |
| Not RCT. | |
| Not RCT. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel group RCT |
| Participants | Country: India 704 people aged 50 to 70 years within 40 kms of Vellore town. Exclusion criteria: Inabiltity to visualise the macula preoperatively in the eye to be operated. Ocular disease that can affect macular function. Uncontrolled diabetics defined by RBS/PP Sugars > 200 mg/dl. Diabetic maculopathy with oedema in eye to be operated. Past history of intraocular surgery in the eye under consideration. History of use of topical steroid drops or NSAID drops within the past 30 days prior to enrolment. Current use of Oral steroids. Known NSAIDs allergy. |
| Interventions | Intervention: ketorolac tromethamine Comparator: polyvinyl Alcohol |
| Outcomes | Primary outcome:
|
| Notes | September 2016: Study investigator confirms that this study is unpublished. We are awaiting a response to request for unpublished data. |
NSAID: non‐steroidal anti‐inflammatory drug
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Preoperative topic diclofenac as a prevention of postoperative macular edema in patients with diabetic retinopathy |
| Methods | Parallel group RCT |
| Participants | Country: Croatia 120 people aged 60 to 90 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: diclofenac Comparator: placebo |
| Outcomes | Primary Outcome:
Secondary Outcome:
|
| Starting date | October 2012 End date: December 2016 |
| Contact information | Ljubo Znaor, MD PhD, Clinical Hospital Center, Split |
| Notes |
| Trial name or title | PRevention of Macular EDema After Cataract Surgery (PREMED) |
| Methods | Parallel group RCT |
| Participants | Country: Netherlands 1135 people aged 21 years and older Inclusion criteria:
Exclusion criteria will be different for non‐diabetic and diabetic patients. All ophthalmic exclusion criteria are applicable to the study eye only, unless stated otherwise. General exclusion criteria for participation in this study are:
Non‐diabetic patients with a history of CME will be excluded from participation in the study. Additionally, diabetic patients will be excluded from participation in case of:
|
| Interventions | Intervention: bromfenac Intervention: bromfenac and dexamethasone Comparator: dexamethasone |
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcome measures at 6 and 12 weeks see clinicaltrials.gov/ct2/show/NCT01774474 |
| Starting date | July 2013 End date: October 2016 |
| Contact information | Prof. Rudy MM Nuijts, MD, PhD [email protected] Laura HP Wielders, MD [email protected] |
| Notes |
| Trial name or title | Effect of preoperative topical ketorolac on aqueous cytokine levels and macular thickness in cataract surgery patients |
| Methods | Parallel group RCT |
| Participants | Country: Malaysia 80 participants aged 18 to 90 years Inclusion criteria: Diabetic patient group
Non‐diabetic patient group
Exclusion criteria
|
| Interventions | Intervention: ketorolac tromethamine Comparator: no intervention |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | August 2014 End date: June 2015 |
| Contact information | Yin Peng Lai, Univerisity of Malaya |
| Notes |
CME: cystoid macular oedema (edema)
DR: diabetic retinopathy
ETDRS: early treatment diabetic retinopathy study
IOP: intraocular pressure
NSAID: non‐steroidal anti‐inflammatory drug
OCT: optical coherence tomography
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Poor vision due to MO Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 NSAIDs plus steroids versus steroids, Outcome 1 Poor vision due to MO. | ||||
| 1.1 3 months | 5 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.76] |
| 1.2 12 months | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.09, 20.37] |
| 2 Central retinal thickness Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 NSAIDs plus steroids versus steroids, Outcome 2 Central retinal thickness. | ||||
| 2.1 Change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 FInal value | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total macular volume Show forest plot | 6 | 570 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| Analysis 1.3  Comparison 1 NSAIDs plus steroids versus steroids, Outcome 3 Total macular volume. | ||||
| 4 Macular oedema Show forest plot | 21 | 3638 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.49] |
| Analysis 1.4  Comparison 1 NSAIDs plus steroids versus steroids, Outcome 4 Macular oedema. | ||||
| 5 Inflammation Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 NSAIDs plus steroids versus steroids, Outcome 5 Inflammation. | ||||
| 6 Inflammation (flare) Show forest plot | 2 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐2.30, ‐0.52] |
| Analysis 1.6  Comparison 1 NSAIDs plus steroids versus steroids, Outcome 6 Inflammation (flare). | ||||
| 7 BCVA Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 NSAIDs plus steroids versus steroids, Outcome 7 BCVA. | ||||
| 7.1 Final value | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Central retinal thickness Show forest plot | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐22.64 [‐38.86, ‐6.43] |
| Analysis 2.1  Comparison 2 NSAIDs versus steroids, Outcome 1 Central retinal thickness. | ||||
| 2 Macular oedema Show forest plot | 5 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.18, 0.41] |
| Analysis 2.2  Comparison 2 NSAIDs versus steroids, Outcome 2 Macular oedema. | ||||
| 3 Inflammation (flare) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 NSAIDs versus steroids, Outcome 3 Inflammation (flare). | ||||
| 4 BCVA Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 NSAIDs versus steroids, Outcome 4 BCVA. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
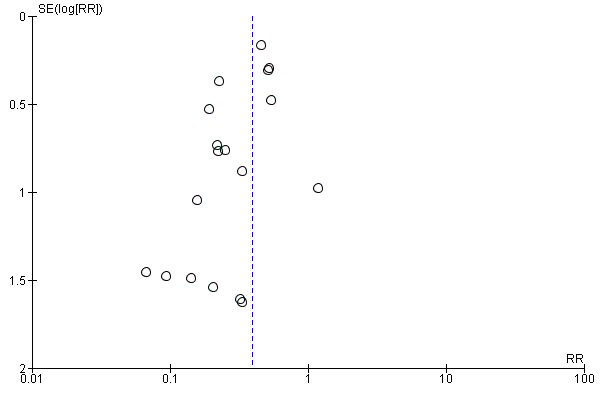
Funnel plot of comparison: 1 NSAIDs plus steroids versus steroids, outcome: 1.4 Macular oedema.

Funnel plot of comparison: 2 NSAIDs versus steroids, outcome: 2.2 Macular oedema.
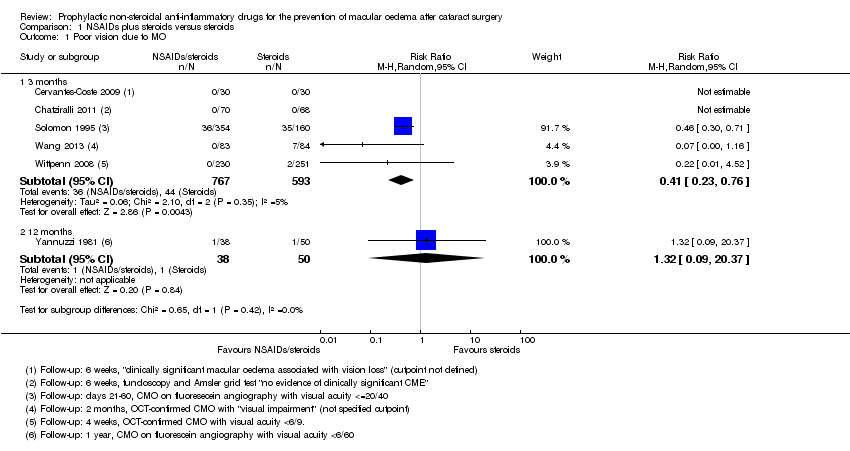
Comparison 1 NSAIDs plus steroids versus steroids, Outcome 1 Poor vision due to MO.
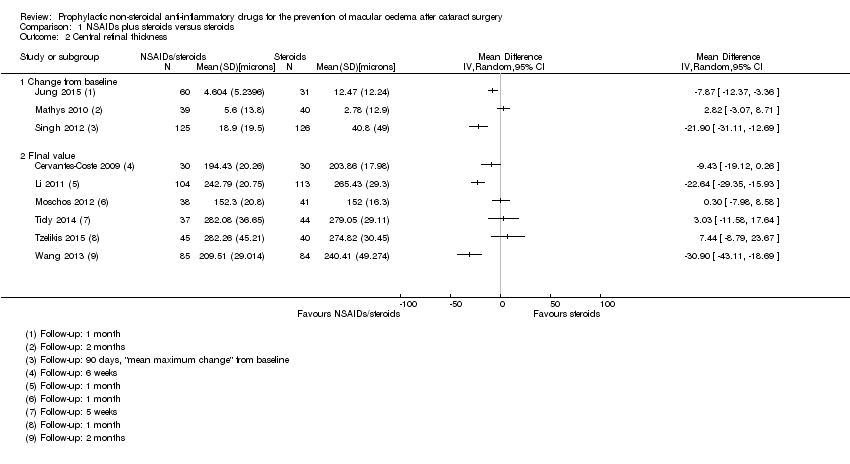
Comparison 1 NSAIDs plus steroids versus steroids, Outcome 2 Central retinal thickness.

Comparison 1 NSAIDs plus steroids versus steroids, Outcome 3 Total macular volume.
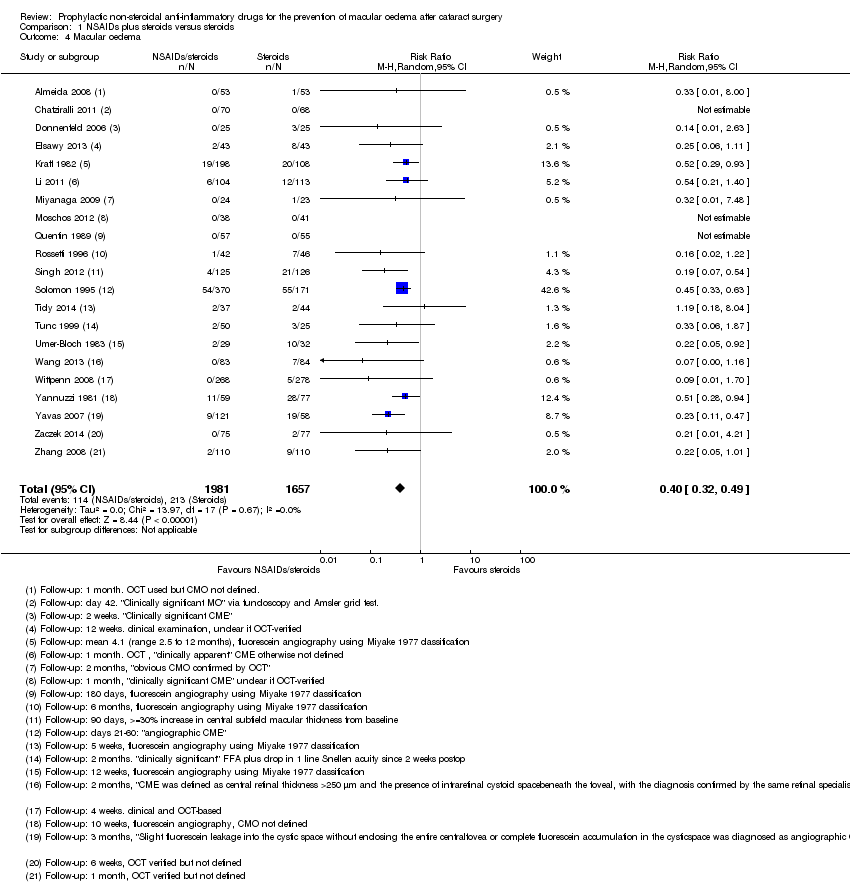
Comparison 1 NSAIDs plus steroids versus steroids, Outcome 4 Macular oedema.

Comparison 1 NSAIDs plus steroids versus steroids, Outcome 5 Inflammation.

Comparison 1 NSAIDs plus steroids versus steroids, Outcome 6 Inflammation (flare).

Comparison 1 NSAIDs plus steroids versus steroids, Outcome 7 BCVA.

Comparison 2 NSAIDs versus steroids, Outcome 1 Central retinal thickness.

Comparison 2 NSAIDs versus steroids, Outcome 2 Macular oedema.
Comparison 2 NSAIDs versus steroids, Outcome 3 Inflammation (flare).

Comparison 2 NSAIDs versus steroids, Outcome 4 BCVA.
| NSAIDs plus steroids compared with steroids for the prevention of macular oedema after cataract surgery | ||||||
| Patient or population: people having cataract surgery Setting: eye hospital | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with steroids | Risk with NSAIDs plus steroids | |||||
| Poor vision due to MO at 3 months after surgery | 74 per 1000 | 30 per 1000 | RR 0.41 | 1360 | ⊕⊕⊝⊝ | ‐ |
| Poor vision due to MO at 12 months after surgery | 20 per 1000 | 26 per 1000 | RR 1.32 | 88 | ⊕⊝⊝⊝ | ‐ |
| Quality of life at 3 months after surgery | See comment | ‐ | ‐ | 74 (1 RCT) | ‐ | Reported in 1 study only using COMTOL questionnaire. Data not fully reported but no significant differences in terms of quality of life, compliance and satisfaction scores. |
| Central retinal thickness at 3 months after surgery; | See comment | ‐ | ‐ | 1021 | ‐ | Trial results were inconsistent (I2 = 87%). Results ranged from ‐30.9 microns in favour of NSAIDs plus steroids to +7.44 microns in favour of steroids alone. |
| Adverse effects | See comment | ‐ | ‐ | (18 RCTs) | ‐ | In general, no major adverse effects were noted. The main consistent observation was burning or stinging sensation with use of NSAID drops. |
| MO at 3 months after cataract surgery, clinically symptomatic, | 130 per 1000 | 52 per 1000 (42 to 64) | RR 0.40 (CI 0.32 to 0.49) | 3638 (21 RCTs) | ⊕⊕⊝⊝ | |
| BCVA at 3 months after surgery; | See comment | ‐ | ‐ | 1158 | ‐ | Trial results were inconsistent (I2 = 70%), but all except one study found differences less than 0.1 logMAR, i.e. clinically indistinguishable from no difference. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded 1 level for risk of bias: studies at unclear or high risk of bias. 5 We considered downgrading an additional 1 level for indirectness as the MO was not always OCT‐verified and it was not always clear if the MO was clinically symptomatic. However, we did not do so partly because the size of the effect was quite strong. | ||||||
| NSAIDscompared with steroids for the prevention of macular oedema after cataract surgery | ||||||
| Patient or population: people having cataract surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with steroids | Risk with NSAIDs | |||||
| Poor vision outcome due to MO at 3 months after surgery | ‐ | ‐ | ‐ | ‐ | ‐ | No data were available for this outcome. |
| Poor vision outcome due to MO at 12 months after surgery | ‐ | ‐ | ‐ | ‐ | ‐ | No data were available for this outcome. |
| Quality of life at 3 months after surgery | No data were available for this outcome. | |||||
| Central retinal thickness at 3 months after surgery; | The mean central retinal thickness at 3 months after surgery was 228 microns | MD 22.64 microns lower | ‐ | 121 | ⊕⊕⊝⊝ | ‐ |
| Adverse effects | ‐ | ‐ | ‐ | 488 | ‐ | 1 study had 2 unspecified complications in 142 participants, 2 studies reported that no adverse events were noted in either group, 1 study (55 people) mentioned 15 mild adverse effects but unclear if related to treatment. |
| MO at 3 months after cataract surgery; clinically symptomatic | 130 per 1000 | 35 per 1000 (23 to 53) | RR 0.27 (0.18 to 0.41) | 520 (5 RCTs) | ⊕⊕⊝⊝ | |
| BCVA at 3 months after surgery; | See comment | ‐ | ‐ | 220 | ‐ | Trial results were inconsistent (I2 = 84%), but all studies found differences less than 0.1 logMAR, i.e. clinically indistinguishable from no difference. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded 1 level for risk of bias: studies at unclear or high risk of bias. 3 We considered downgrading 1 level for indirectness as the MO was not always OCT‐verified and not always clear if it was clinically symptomatic however we did not do so, partly because the effect was strong. | ||||||
| Domain | Risk of bias | ||
| Low | Unclear | High | |
| Sequence generation | Computer‐generated list, random table, other method of generating random list. | Not reported how list was generated. Trial may be described as “randomised” but with no further details. | Alternate allocation, date of birth, records (these RCTs should be excluded). |
| Allocation concealment | Central centre (web/telephone access), sealed opaque envelopes. | Not reported how allocation administered. Trial may be described as “randomised” but with no further details. | Investigator involved in treatment allocation or treatment allocation clearly not masked. |
| Blinding of participants and personnel | Clearly stated that participants and personnel not aware of which treatment received. | Described as “double‐blind” with no information on who was masked. | Open‐label or no information on masking. We assume that in absence of reporting on this outcome, patients and personnel were not masked. |
| Blinding of outcome assessors | Clearly stated that outcome assessors were masked. | Described as “double‐blind” with no information on who was masked. | Open‐label or no information on masking. We assume that in absence of reporting on this outcome, assessors were not masked. |
| Incomplete outcome data | Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. | Follow‐up not reported or missing data > 20% (i.e. follow‐up < 80%) but follow‐up equal in both groups. | Follow‐up different in each group and/or related to outcome. |
| Selective outcome reporting | All outcomes in protocol and/or trial registry entry are reported. | No access to protocol or trial registry entry. | Outcomes in protocol and/or trial registry entry selectively reported. |
| Other sources of bias Note: we did not identify any important sources of other bias so this domain is omitted from the risk of bias tables. | No other source of bias. | Trial stopped early due to poor recruitment. Baseline imbalance, but not clear that it is important. | Trial stopped early because of outcome. Important baseline imbalance that might have an effect on the results. |
| Study | Country | Open‐label | Funding sources | Declaration of interest | Trial registration | Abstract only | |
| 1 | Canada | Yes | Non‐industry | Reported; no CoI | NCT00335439 | No | |
| 2 | Canada | No | Non‐industry | Reported; no CoI | NCT01395069 | No | |
| 3 | Japan | No | Not reported | Reported; no CoI | Not registered | No | |
| 4 | USA | No | Industry | Not reported | Not registered | No | |
| 5 | Mexico | No | Not reported | Reported; no CoI | Not registered | No | |
| 6 | Greece | No | Not reported | Not reported | Not registered | No | |
| 7 | USA | No | Industry/Non‐Industry | CoI | Not registered | No | |
| 8 | Egypt | No | Not reported | Reported; no CoI | Not registered | No | |
| 9 | Japan | Yes | Not reported | Reported; no CoI | Not registered | No | |
| 10 | Italy | No | Not reported | CoI | Not registered | No | |
| 11 | South Korea | No | Non‐industry | Reported; no CoI | Not registered | No | |
| 12 | USA | No | Non‐industry | Not reported | Not registered | No | |
| 13 | China | No | Not reported | Not reported | Not registered | No | |
| 14 | USA | No | Non‐industry | Reported; no CoI | NCT00494494 | No | |
| 15 | Japan | No | Not reported | Reported; no CoI | Not registered | No | |
| 16 | Japan | No | Not reported | CoI | Not registered | No | |
| 17 | Japan | No | Not reported | Not reported | Not registered | No | |
| 18 | Greece | No | Not reported | Reported; no CoI | Not registered | No | |
| 19 | Germany | No | Not reported | Not reported | Not registered | No | |
| 20 | Italy | No | Not reported | Reported; no CoI | Not registered | No | |
| 21 | USA | No | Not reported | CoI | NCT00782717 | No | |
| 22 | Canada (8 sites) and Germany (2 sites) | No | Industry | Reported; no CoI | Not registered | No | |
| 23 | USA | No | Industry | CoI | Not registered | Yes | |
| 24 | Brazil | No | Not reported | Reported; no CoI | Not registered | No | |
| 25 | Turkey | No | Not reported | Not reported | Not registered | No | |
| 26 | Brazil | No | Not reported | Reported; no CoI | NCT02084576 | No | |
| 27 | Switzerland | No | Not reported | Not reported | Not registered | No | |
| 28 | China | Yes | Non‐industry | Not reported | Not registered | No | |
| 29 | USA | No | Industry | CoI | NCT00348244 | No | |
| 30 | USA | No | Non‐industry | Not reported | Not registered | No | |
| 31 | Turkey | No | Not reported | Reported; no CoI | Not registered | No | |
| 32 | USA | No | Not reported | Not reported | Not registered | No | |
| 33 | Sweden | No | Industry/Non‐industry | Reported; no CoI | Not registered | No | |
| 34 | China | No | Not reported | Not reported | Not registered | No | |
| CoI: conflict of interest | |||||||
| Study | Number of people randomised | Number of people randomised (missing data imputed)* | Number of eyes | Number of eyes estimated (missing data imputed)* | Number of people followed up | Number of people followed up (missing data imputed)* | Percentage follow‐up | Eyes per person enrolled in the trial | |
| 1 | 98 | 98 | 106 | 106 | ‐ | 74 | 75% | 106 eyes of 98 people | |
| 2 | 193 | 193 | ‐ | 193 | 162 | 162 | 84% | Probably one | |
| 3 | 150 | 150 | 150 | 150 | 142 | 142 | 95% | One eye | |
| 4 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probably one | ||
| 5 | 60 | 60 | 60 | 60 | 60 | 60 | 100% | One eye | |
| 6 | 145 | 145 | 145 | 145 | 138 | 138 | 95% | Probably one | |
| 7 | 100 | 100 | ‐ | 100 | ‐ | 100 | ‐ | Unclear | |
| 8 | 70 | 70 | 86 | 86 | ‐ | 86 | ‐ | 86 eyes of 70 patients | |
| 9 | 75 | 75 | 75 | 75 | 62 | 62 | 83% | One eye | |
| 10 | 281 | 281 | 281 | 281 | 229 | 229 | 81% | One eye | |
| 11 | 91 | 91 | 91 | 91 | Not reported | 91 | Not reported | One eye | |
| 12 | 500 | 500 | ‐ | 500 | 492 | 492 | 98% | Unclear | |
| 13 | 217 | 217 | 217 | 217 | ‐ | 217 | ‐ | One eye | |
| 14 | 84 | 84 | 84 | 84 | 79 | 79 | 94% | One eye | |
| 15 | 62 | 62 | 62 | 62 | 50 | 50 | 81% | Probably one | |
| 16 | 60 | 60 | 60 | 60 | 55 | 55 | 92% | One eye | |
| 17 | 72 | 72 | 72 | 72 | ‐ | 72 | ‐ | One eye | |
| 18 | 79 | 79 | 79 | 79 | ‐ | 79 | ‐ | One eye | |
| 19 | 179 | 179 | 179 | 179 | 112 | 112 | 63% | One eye | |
| 20 | 88 | 88 | 88 | 88 | ‐ | 88 | ‐ | Probably one | |
| 21 | 263 | 263 | 263 | 263 | 251 | 251 | 95% | One eye | |
| 22 | 681 | 681 | 681 | 681 | 364 | 364 | 53% | Probably one | |
| 23 | ‐ | 32 | ‐ | 32 | 32 | 32 | ‐ | Unclear | |
| 24 | 91 | 91 | 91 | 91 | 81 | 81 | 89% | Probably one | |
| 25 | 75 | 75 | 75 | 75 | 75 | 75 | ‐ | One eye | |
| 26 | 142 | 142 | 142 | 142 | 126 | 126 | 89% | One eye | |
| 27 | ‐ | 73 | ‐ | 73 | 73 | 73 | ‐ | Unclear | |
| 28 | 240 | 240 | ‐ | 240 | 167 | 167 | 70% | Unclear | |
| 29 | 546 | 546 | 546 | 546 | 478 | 478 | 88% | One eye | |
| 30 | ‐ | 201 | 231 | 231 | ‐ | 231 | 59% | 231 eyes of 210 people | |
| 31 | 189 | 189 | 189 | 189 | 179 | 179 | 95% | One eye; right eye only | |
| 32 | 37 | 37 | ‐ | 37 | ‐ | 37 | ‐ | Unclear | |
| 33 | 160 | 160 | 160 | 160 | 152 | 152 | 95% | One eye | |
| 34 | ‐ | 198 | 220 | 220 | ‐ | 220 | 100% | 220 eyes of 198 people | |
| *For studies that did not report the number randomised, we have estimated this from the number followed up. For studies that did not report the number followed up, we have estimated this from the numbers randomised. Number of eyes estimated assuming one eye per person, if not clearly stated otherwise. | |||||||||
| Study | Average age | Age range | % female | % with diabetes | % with uveitis | |
| 1 | 72 | 45 to 92 | 61% | 21% | 1% | |
| 2 | 72 | 50 to 88 | 54% | ‐ but low risk population | "low risk population" | |
| 3 | 66 | ‐ | 56% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 4 | ‐ | ‐ | ‐ | ‐ but people with DR excluded | 0% people with uveitis excluded | |
| 5 | 72 | 51 to 88 | 64% | 20% | 0% people with uveitis excluded | |
| 6 | 74 | ‐ | 40% | 10% | 0% people with uveitis excluded | |
| 7 | 73 | ‐ | 55% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 8 | ‐ | ‐ | 37% | 100% | ‐ | |
| 9 | 69 | 37 to 84 | 45% | 100% | 0% people with uveitis excluded | |
| 10 | 68 | ‐ | 52% | ‐ | ‐ | |
| 11 | 67 | ‐ | 55% | 26% | ‐ | |
| 12 | 69 | 37 to 97 | 57% | ‐ | ‐ | |
| 13 | 72 | ‐ | 63% | 100% | ‐ | |
| 14 | 72 | 44 to 90 | 54% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 15 | 66 | ‐ | 54% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 16 | 65 | 48 to 82 | 46% | 9% | 0% people with uveitis excluded | |
| 17 | 72 | 41 to 86 | 71% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 18 | 77 | ‐ | 66% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 19 | 73 | ‐ | 55% | ‐ but people with DR excluded | 0% people with uveitis excluded | |
| 20 | 74 | ‐ | 64% | 0% people with diabetes excluded | ‐ | |
| 21 | 67 | 32 to 87 | 63% | 100% | 0% people with uveitis excluded | |
| 22 | 68 | 39 to 100 | 53% | ‐ | 0% people with uveitis excluded | |
| 23 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 24 | 67 | ‐ | 47% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 25 | 61 | ‐ | 39% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 26 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 27 | 69 | ‐ | 52% | ‐ but people with DR excluded | 0% people with uveitis excluded | |
| 28 | 73 | 46 to 92 | 54% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 29 | 70 | ‐ | 53% | ‐ | ‐ | |
| 30 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 31 | 65 | ‐ | 40% | 0% people with diabetes excluded | 0% people with uveitis excluded | |
| 32 | ‐ | ‐ | ‐ | 100% | ‐ | |
| 33 | 69 | ‐ | 65% | ‐ | ‐ | |
| 34 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| DR: diabetic retinopathy | ||||||
| Study | Type of cataract surgery | Comparison | NSAIDs | Steroid | Placebo in comparator group | Type of placebo | |
| 1 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5% | Prednisolone 1% | No | ‐ | |
| 2 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5%, Nepafenac 0.1% | Prednisolone 1% | Yes | Sterile saline drops | |
| 3 | Phacoemulsification | NSAIDs versus steroids | Diclofenac 0.1% | Betamethasone 0.1% | No | ‐ | |
| 4 | Phacoemulsification | NSAIDs versus steroids | Diclofenac 0.1% | Prednisolone 1% | No | ‐ | |
| 5 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 0.1% | Dexamethasone (combined with tobramycin) | No | ‐ | |
| 6 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5% | Dexamethasone 0.1% (combined with tobramycin 0.3%) | No | ‐ | |
| 7 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | Yes | Vehicle | |
| 8 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Dexamethasone 0.1%, | No | ‐ | |
| 9 | Phacoemulsification | NSAIDs versus steroids | Bromfenac | Betamethasone (with fradiomycin sulfate) followed by fluorometholone | No | ‐ | |
| 10 | ECCE | NSAIDs versus steroids | Diclofenac 0.1% | Dexamethasone 0.1% | Yes | Not specified | |
| 11 | Phacoemulsification | NSAIDs versus steroids | Bromfenac 0.1%, Ketorolac 0.4% | Prednisolone acetate 1% | No | ‐ | |
| 12 | ECCE and phacoemulsification | NSAIDs plus steroids versus steroids | Indomethacin | Dexamethasone (in combination with neomycin sulfate, polymyxin B sulfate) for 4 days followed by dexamethasone alone for 4 weeks followed by fluorometholone for at least 6 months | Yes | Vehicle | |
| 13 | Phacoemulsification | NSAIDs plus steroids versus steroids | Diclofenac 1% | Dexamethasone (combined with tobramycin) | No | ‐ | |
| 14 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 0.1% | Prednisolone 1% | No | ‐ | |
| 15 | Phacoemulsification | NSAIDs versus steroids | Diclofenac 0.1% | Fluorometholone 0.1% | No | ‐ | |
| 16 | Phacoemulsification | NSAIDs versus steroids | Nepafenac 0.1% | Fluorometholone 0.1% | No | ‐ | |
| 17 | Phacoemulsification | NSAIDs plus steroids versus steroids/NSAIDs versus steroids | Bromfenac 0.1% | Betamethasone 0.1%, fluorometholone | No | ‐ | |
| 18 | Phacoemulsification | NSAIDs plus steroids versus steroids | Diclofenac 0.1% | Dexamethasone 0.1% (combined with chloramphenicol 0.5%) | No | ‐ | |
| 19 | ICCE | NSAIDs plus steroids versus steroids | Diclofenac 0.1% | Dexamethasone | Yes | Not specified | |
| 20 | ECCE | NSAIDs plus steroids versus steroids | Diclofenac | Dexamethasone (combined with tobramycin) | Yes | Not specified | |
| 21 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 1% | Prednisolone | Yes | Vehicle | |
| 22 | ECCE | NSAIDs plus steroids versus steroids | Flurbiprofen 0.03% Indomethacin 1% | Prednisolone | Yes | Vehicle | |
| 23 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | No | ‐ | |
| 24 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | Yes | Dextran 70/hypromellose | |
| 25 | ECCE | NSAIDs plus steroids versus steroids | Diclofenac 0.1% | Dexamethasone 1% | No | ‐ | |
| 26 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4%, Nepafenac 0.1% | Prednisolone 1% | Yes | Artificial tears | |
| 27 | ECCE/ICCE | NSAIDs plus steroids versus steroids | Indomethacin 1% | Dexamethasone (combined with either chloramphenicol or neomycin) | Yes | Vehicle | |
| 28 | Phacoemulsification | NSAIDs plus steroids versus steroids | Bromfenac 0.1% | fluorometholone 0.1% and dexamethasone 0.1% | No | ‐ | |
| 29 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | Yes | Artificial tears | |
| 30 | ICCE | NSAIDs plus steroids versus steroids | Indomethacin 1% | Steroids given as part of standard care, not specified exactly what | Yes | Vehicle | |
| 31 | Phacoemulsification | NSAIDs plus steroids versus steroids | Indomethacin 0.1% | Prednisolone 1% | No | ‐ | |
| 32 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5% | Prednisolone 1% | Yes | Artificial tears | |
| 33 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 0.1% | Dexamethasone 0.1% | Yes | Artifical tears | |
| 34 | Phacoemulsification | NSAIDs plus steroids versus steroids | Pranoprofen | Dexamethasone (combined with tobramycin) | No | ‐ | |
| ECCE: extracapsular cataract extraction | |||||||
| Poor vision outcome due to MO | Quality of life/patient satisfaction | Central retinal thickness | Adverse effects reported | CMO | Inflammation | BCVA | Additional outcomes | ||
| Study | Follow‐up | No analysis; only one study reported this | |||||||
| 1 month | Yes | OCT used but CMO not defined | Change in total macular volume | ||||||
| 1 month | COMTOL questionnaire | Mean change reported but not possible to calculate SD | LogMAR | Change in total macular volume; change in average macular cube thickness | |||||
| 8 weeks | Yes | Fluorescein angiography using Miyake 1977 classification (at 5 weeks only) | Laser flare‐cell photometry, mean value of anterior chamber flare (photons/millisecond) | LogMAR, final value | |||||
| 1 month | Laser flare‐cell photometry, mean value of anterior chamber flare reported (photons) but was not possible to calculate SD | ||||||||
| 6 weeks | Quote: "None of the patients developed clinically significant macular oedema associated with vision loss" | Final value | Yes | Only reported CMO associated with vision loss | "Inflammatory cells greater than 1+ during first week of postoperative visits" | Total macular volume | |||
| 6 weeks | Fundoscopy and Amsler grid test Quote: "no evidence of clinically significant CME" | Yes | "No evidence of clinically significant CME was detected in any patient via fundoscopy and the Amsler grid test" | Corneal oedema or Tyndall reaction or conjunctival hyperaemia | LogMAR, final value | ||||
| 3 months | Yes | "Clinically significant CME" but otherwise not defined, at 2 weeks only | "Mean inflammation score" but was not possible to calculate SD | LogMAR, final value but could not extract data on SD | |||||
| 12 weeks | Clinical examination, unclear if OCT‐verified | ||||||||
| 6 weeks | Final value | Yes | Anterior chamber flare values, photon count per millisecond | LogMAR, final value | |||||
| 140 days | Yes | Fluorescein angiography using Miyake 1977 classification | |||||||
| 1 month | Change | Yes | "Inflammatory score" (sum of anterior chamber cells and flare grade" | Change in macular volume | |||||
| Between 2.5 and 12 months | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | ||||||
| 1 month | Final value | OCT, "clinically apparent" CME otherwise not defined | Snellen acuity only, not included in analyses | ||||||
| 2 months | Change from baseline | Yes | LogMAR | Change in foveal thickness, change in macular volume | |||||
| 5 weeks | Fluorescein angiography using Miyake 1977 classification | Unit of measurement unclear | Snellen acuity only, not included in analyses | ||||||
| 5 weeks | Final value | Yes | Fluorescein angiography using Miyake 1977 classification | Flare (photons/millisec), final value | Change in logMAR BCVA, categorical 3+, 2, 1 lines increase and no change | ||||
| 2 months | Yes | "Obvious CMO confirmed by OCT" | Aqueous flare (photons/millisecond) | LogMAR, final value | |||||
| 1 month | Final value | LogMAR, final value | |||||||
| 180 days | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | ||||||
| 6 months | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | ||||||
| 90 days | Change from baseline | Yes | ">= 30% increase in central subfield macular thickness from baseline" | Flare mentioned but data not reported | Corrected BCVA loss of more than 5 letters from day 7 postop | ||||
| 6 months | Days 21 to 60, MO = positive angiography and visual acuity <= 20/40 | Yes | Fluorescein angiography using classification*** | Snellen acuity but not reported by treatment group | |||||
| 30 days (3 months mentioned but not reported) | Reported but no mean/SD | Proportion with > 10% increase in retinal thickness | |||||||
| 5 weeks | Final value | Yes | Fluorescein angiography using Miyake 1977 classification | LogMAR | |||||
| 2 months | Fluorescein angiography 0 no leakage (CME absent),1 oedema less than perifoveal, 2 mild perifoveal oedema, 3 moderate perifoveal oedema (approx. 1 disc diameter), 4 severe perifoveal oedema plus drop of 1 line of Snellen acuity since second postoperative week defined as "clinically significant" | ||||||||
| 12 weeks | Final value | Yes | LogMAR, final value (at 30 days only) | ||||||
| 12 weeks | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | ||||||
| 2 months | OCT‐confirmed CMO with "visual impairment" (not specified cutpoint) | Final value | Yes | "CME was defined as central retinal | Mean photon count values | LogMAR, final value | |||
| 4 weeks | OCT‐confirmed CMO with visual acuity < 6/9 | Yes | Clinical and OCT‐based | ||||||
| 1 year | CMO on fluorescein angiography with visual acuity < 6/60 | Yes | Fluorescein angiography, evidence but not defined | ||||||
| 3 months | "Slight fluorescein leakage | LogMAR, final value | |||||||
| 12 weeks | |||||||||
| 6 weeks | Yes | OCT‐verified but not defined | Mean anterior chamber flare reported in figure but no SD | LogMAR, final value | Change in total macular volume | ||||
| 1 month | OCT‐verified but not defined | Tyn granule + | |||||||
| BCVA: best corrected visual acuity | |||||||||
| Study | Follow‐up | Number of people followed up | Adverse effects |
| 1 month | 74 | Quote: "There were 3 dropouts in the treatment group related to ketorolac corneal toxicity, most notably pain attributed to the drops." | |
| 1 month | 162 | Quote: “One patient in the ketorolac group was hospitalized with a cardiovascular event and could not complete the follow‐up. Finally, 1 patient on nepafenac had side effects of ocular redness and irritation and could not continue with the study.” | |
| 8 weeks | 142 | 2 "complications" not specified. | |
| 1 month | NR | Adverse effects not reported. | |
| 6 weeks | 60 | Quote: "There were no serious treatment‐related adverse events or toxicity related to the use of nepafenac 0.1%." | |
| 6 weeks | 138 | Quote: "All patients reported pain and ocular discomfort lower than 1/10 on the visual analog scale at all time points." | |
| 2 weeks | 100 | Quote: "Use of ketorolac 0.4% for 1 or 3 days provided decreased levels of patient discomfort intraoperatively and postoperatively. Intraoperatively, 3 days of ketorolac 0.4% provided significantly lower discomfort scores than with 1‐hour and placebo dosing (P < 0.001). One day of ketorolac 0.4% also provided significantly reduced intraoperative discomfort scores than with 1‐hour dosing (P = 0.001) and placebo dosing (P < 0.001). Postoperatively, 3 days of ketorolac 0.4% provided significantly lower discomfort scores than 1‐hour dosing or control dosing (P < 0.001) (Figure 5). In addition, patients randomised to 1 or 3 days of ketorolac 0.4% were significantly less likely to require additional intravenous anesthesia (8% in each group) than patients in the control group (40%) (P = 0.008). Twenty percent of patients in the 1‐hour group required additional anesthesia for pain control." | |
| 12 weeks | 86 | Adverse effects not reported. | |
| 6 weeks | 62 | Quote: "No adverse events were noted in either group." | |
| 140 days | 229 | Quote: "No major adverse effects were noted in either group." "Subjective tolerance of the two treatments was good and remained similar throughout the study, although a trend towards increased burning was seen in the diclofenac group." | |
| 1 month | 91 | Quote: "There were no adverse events except for a mild burning sensation in one patient in the ketorolac group; the symptom was tolerable and did not lead to discontinuation of the medication." | |
| between 2.5 and 12 months | 492 | Quote: "There were no complications that could be ascribed to the use of topical indomethacin other than minor stinging and burning noted by the patients." | |
| 1 month | 217 | Adverse effects not reported. | |
| 2 months | 79 | Quote: "There were no adverse events reported by patients using nepafenac." | |
| 5 weeks | 50 | Adverse effects not reported. | |
| 5 weeks | 55 | NSAIDs: 6 adverse effects: decreased lacrimation, conjunctivitis allergic, abnormal sensation in eye, vomiting (2), constipation. Steroid group: 9 adverse effects: decreased lacrimation, conjunctivitis allergic, retinal haemorrhage, keratoconjunctivitis sicca, chorioretinopathy, influenza, insomnia, diarrhoea, humeral fracture. | |
| 2 months | 72 | Adverse effects not reported. | |
| 1 month | 79 | Adverse effects not reported. | |
| 180 days | 112 | Quote: "Diclofenac group: two patients were feeling burning after application of eye drops during the stationary care, for placebo: none. In both groups burning was reported later on in the examinations." | |
| 6 months | 88 | Quote: "Treatment regimens were well tolerated with no evidence of relevant side effects." | |
| 90 days | 251 | Quote: "No patient deaths were reported during the study. Overall, 13 patients reported other serious adverse events, none of which were related to treatment. Three of the serious adverse events reported in the vehicle group (cardiac failure congestive, coronary artery occlusion, and pancreatitis) led to patient discontinuation; no other serious adverse events led to discontinuation in either treatment group. Separate from the three patients who discontinued due to serious adverse events, four other patients discontinued study participation due to nonserious adverse events. Of these nonserious events, two reported instances of punctate keratitis (one in each treatment group) were assessed as being related to the study drugs. No instances of targeted adverse events (defined as corneal erosions) were reported during the study. Two reports of punctate keratitis and a single report of corneal epithelium defect were assessed as being related to treatment with nepafenac. A single report of punctate keratitis was assessed as being related to treatment with vehicle. No other ocular or nonocular adverse events reported in the study were assessed as being related to the study drugs. In both treatment groups, corneal staining and intraocular pressure were each generally similar at the presurgical baseline and at the day 90 visit (or early exit). Additionally, no safety issues or trends were identified based upon changes from baseline in fundus parameters (retina/macula/choroid and optic nerve) and ocular signs (inflammatory cells, aqueous flare, corneal oedema, and bulbar conjunctival injection). The study results indicate no new clinically relevant risks associated with increasing the dosing of nepafenac from 14 days to 90 days, even in the higher‐risk diabetic patient population." | |
| 6 months | 364 | Quote: "During the study, the mean severity of foreign‐body sensation, pain, photophobia, and tearing did not become more than mild (1 +) in any treatment group. This was also true of burning and stinging following treatment instillation (Figure 4). The severity of burning and stinging was significantly greater in the flurbiprofen group on days 4‐20 and 21‐60 and in the indomethacin group on days 1‐3, 4‐20, 21‐60, and 61‐120 than in the vehicle group. At day 1‐3, moderate to severe burning and stinging were reported by 7.0% (16/230) of the patients treated with flurbiprofen, 9.7% (23/237) of the patients treated with indomethacin, and 3.1% (7/224) of the patients treated with vehicle." | |
| 30 days (3 months mentioned but not reported) | 32 | Adverse effects not reported. | |
| 5 weeks | 81 | One patient withdrew because of burning. | |
| 2 months | 75 | Adverse effects not reported. | |
| 1 month | 126 | Quote: "There were no adverse side effects in either group." | |
| 12 weeks | 73 | Quote from translation: "40% reported a short burning after using indomethacin eye drops, only rare in patients of the placebo group. One patient had 6 weeks after treatment an allergic blepharitis due to indomethacin. Long‐term: 52 patients were followed for 6 months and 34 patients one year. 4 patients with indomethacin had visual acuity reduction because of a clinically new cystoid edema; 2 of these patients had spontaneous healing after 4‐6 weeks, the other 2 edema cases did not resolve. 2 patients had a new senile macula pathology, and 2 patients had a retinal detachment due to aphakia. Placebo: 2 patients still had an edema after 12 weeks, while one patient developed a new edema later." | |
| 2 months | 167 | Quote: "No drug‐related adverse events were identified." | |
| 4 weeks | 478 | Quote: "The most commonly reported adverse events (investigator self‐report) in the ketorolac/steroid group were burning/stinging/tearing (4/268). Transient elevations in intraocular pressure (IOP) were the most commonly reported adverse event in the steroid group (3/278). There were two serious adverse events, both in the steroid group: one patient developed endophthalmitis and one patient died (cause determined to be unrelated to the study medication)." | |
| 1 year | 231 | Adverse effects not reported. | |
| 3 months | 179 | Adverse effects not reported. | |
| 12 weeks | 37 | Adverse effects not reported. | |
| 6 weeks | 152 | Quote: "Mild to moderate punctuate epithelial defects of the cornea were found in both groups 3 weeks after treatment.Statistically significantly more patients in the nepafenac group than in the control group had corneal fluorescein staining (20 [26.7%] versus 8 [10.4%]) (PZ.0119). Headache was reported by 3 patients (4.0%) in the nepafenac group and 2 patients (2.6%) in the control group (PZ.9750). No other systemic or local untoward effects were recorded during 3 weeks of treatment in either study group." | |
| 1 month | 220 | Adverse effects not reported. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Poor vision due to MO Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 5 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.76] |
| 1.2 12 months | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.09, 20.37] |
| 2 Central retinal thickness Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 FInal value | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total macular volume Show forest plot | 6 | 570 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 4 Macular oedema Show forest plot | 21 | 3638 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.49] |
| 5 Inflammation Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Inflammation (flare) Show forest plot | 2 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐2.30, ‐0.52] |
| 7 BCVA Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Final value | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Central retinal thickness Show forest plot | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐22.64 [‐38.86, ‐6.43] |
| 2 Macular oedema Show forest plot | 5 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.18, 0.41] |
| 3 Inflammation (flare) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 BCVA Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

